Abstract
Aims
Conventional late gadolinium enhancement (LGE) cardiac magnetic resonance can detect myocardial infarction and some forms of non-ischaemic myocardial fibrosis. However, quantitative imaging of extracellular volume fraction (ECV) may be able to detect subtle abnormalities such as diffuse fibrosis or post-infarct remodelling of remote myocardium. The aims were (1) to measure ECV in myocardial infarction and non-ischaemic myocardial fibrosis, (2) to determine whether ECV varies with age, and (3) to detect sub-clinical abnormalities in ‘normal appearing’ myocardium remote from regions of infarction.
Methods and results
Cardiac magnetic resonance ECV imaging was performed in 126 patients with T1 mapping before and after injection of gadolinium contrast. Conventional LGE images were acquired for the left ventricle. In patients with a prior myocardial infarction, the infarct region had an ECV of 51 ± 8% which did not overlap with the remote ‘normal appearing’ myocardium that had an ECV of 27 ± 3% (P < 0.001, n = 36). In patients with non-ischaemic cardiomyopathy, the ECV of atypical LGE was 37 ± 6%, whereas the ‘normal appearing’ myocardium had an ECV of 26 ± 3% (P < 0.001, n = 30). The ECV of ‘normal appearing’ myocardium increased with age (r = 0.28, P = 0.01, n = 60). The ECV of ‘normal appearing’ myocardium remote from myocardial infarctions increased as left ventricular ejection fraction decreased (r = −0.50, P = 0.02).
Conclusion
Extracellular volume fraction imaging can quantitatively characterize myocardial infarction, atypical diffuse fibrosis, and subtle myocardial abnormalities not clinically apparent on LGE images. Taken within the context of prior literature, these subtle ECV abnormalities are consistent with diffuse fibrosis related to age and changes remote from infarction.
Keywords: Magnetic resonance imaging, Myocardial infarction, Fibrosis, Aging, Gadolinium
Introduction
Cardiovascular magnetic resonance (CMR) using late gadolinium enhancement (LGE) can visualize myocardial infarction1 or fibrosis in non-ischaemic cardiomyopathies2 using contrast agents that distribute into the extracellular space.3 Myocardial infarction and focal fibrotic processes of non-ischaemic origin appear bright on LGE images.4
While LGE is clinically useful, reliance on relative signal intensity changes and nulling of ‘normal appearing’ myocardium make it difficult to identify subtle abnormalities such as diffuse interstitial fibrosis in the myocardium. Diffuse myocardial fibrosis involves increased collagen content5 and should change the myocardial extracellular volume fraction (ECV).
Gadolinium-enhanced MRI can quantify the ECV,3 and CMR measures of ECV correlate with histologic evidence of fibrosis.6 Furthermore, clinically feasible rapid T1 mapping by CMR has recently been developed.7 Combining these two methods to quantitatively visualize the spatial extent of myocardial ECV could have utility in a wide range of cardiovascular diseases.
The specific aims of this study were to (1) quantify the ECV in myocardial infarction and non-ischaemic myocardial fibrosis and (2) to detect sub-clinical diffuse abnormalities in ‘normal appearing’ myocardium. We hypothesized (1) that ECV imaging could quantitatively differentiate infarction and atypical LGE from ‘normal appearing’ myocardium, (2) that if myocardial fibrosis increases with age, then the ECV of myocardium would increase with age, and (3) that if infarction leads to diffuse fibrosis in remote myocardium, then ECV of remote myocardium would increase in a graded response related to the severity of overall left ventricular dysfunction. Thus, the goal was to quantitatively characterize ECV into two well-recognized clinical entities, namely myocardial infarction and non-ischaemic cardiomyopathies, and two sub-clinical entities, namely age-related myocardial fibrosis and diffuse myocardial fibrosis remote from infarction.
Methods
Selection of patients and volunteers
Between July 2009 and March 2010, 266 patients referred for clinical CMR assessment of known or suspected heart disease prospectively underwent ECV imaging. Patients were grouped in order to assess our respective hypotheses. (1) To assess whether ECV imaging could differentiate myocardial infarction or atypical LGE from ‘normal appearing’ myocardium, all patients with a prior myocardial infarction (n = 36) or clinically recognized atypical LGE (n = 30) on conventional LGE images (see below for classification criteria2) were included if affected sectors of the heart were also imaged by ECV. (2) To address the relationship between ECV and age, we prospectively selected 10 patients per decade of life between 20 and 80 years of age from among those with a clinical report, concluding that there were no abnormalities on LGE (ECV vs. age, n = 60). (3) To assess whether adverse remodelling alters myocardium remote from infarction, sectors lacking focal enhancement on the clinical report of patients with myocardial infarctions were considered ‘normal appearing’, and the ECV of these sectors was averaged and correlated against left ventricular ejection fraction. Classification of patients as displaying infarct LGE or atypical LGE was determined blinded to the results of ECV measurements and according to established criteria for assessment of focal findings on LGE as being ischaemic or non-ischaemic in origin.2 In short, infarct LGE patients had a lesion with a sub-endocardial or transmural localization that corresponded to a coronary artery perfusion territory. Atypical LGE patients had a non-ischaemic lesion which was mid-mural, epicardial, or localized to the right ventricular insertion points in the left ventricle. Healthy volunteers (n = 11) were recruited to assess the relationship between the ECV measure and time after injection of contrast agents. All patients and volunteers underwent venous blood sampling for measurement of haematocrit. Our institutional review board approved the study, and all subjects provided written informed consent.
Magnetic resonance imaging methods
Imaging was performed at 1.5 T (Magnetom Avanto or Espree, Siemens Healthcare Sector, Erlangen, Germany) using a 32-channel coil. T1 quantification was performed with a Modified Look-Locker Inversion-recovery (MOLLI) sequence7 acquired during end-expiratory apnoea in a mid-ventricular short-axis and a four-chamber long-axis plane before and ∼15–20 min after a 0.15 mmol/kg intravenous dose of Gd-DTPA (gadopentate dimeglumine, Magnevist®, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA). Typical imaging parameters were: non-selective inversion pulse, steady-state free precession single-shot read out in mid-diastole, field of view of 360 × 250 mm2, matrix of 174 × 192, slice thickness of 6 mm, time to repetition/time to echo of 2.5/1.0 ms, minimum inversion time of 110 ms, inversion time increment of 80 ms, flip angle of 35°, parallel acquisition technique (PAT) factor of 2, number of inversions 2, images acquired after first inversion 3, pause 3 heart beats, and images acquired after second inversion 5.
Left ventricular volumes and ejection fraction were determined using steady-state free precession cine imaging. Late gadolinium enhancement imaging was acquired in three long-axis slices and a stack of short-axis slices using a gradient recalled echo phase-sensitive inversion recovery sequence.8
Image analysis
Quantitative parametric images of myocardial ECV were generated. A region of interest was drawn along the epicardial surface of the left ventricular myocardium on matched pre- and post-contrast MOLLI images (Leonardo Workstation, Siemens, Erlangen, Germany). Using these regions of interest, the pre- and post-contrast MOLLI images underwent non-rigid image registration to adjust for positional variation within and between breath holds. The registered MOLLI images were then used to generate pre- and post-contrast T1 pixel maps using MRmap (version 1.0, http://mrmap.sourceforge.net/).9
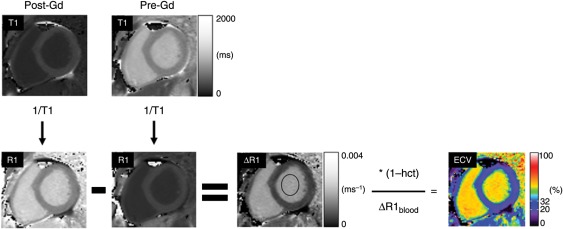
Further image processing was undertaken with ImageJ (version 1.42q, http://rsbweb.nih.gov/ij/) in accordance with established theory (see Figure 1; see Supplementary material online). R1 maps were generated by taking the reciprocal of the T1 maps on a pixel-by-pixel basis. ΔR1 maps were then generated by subtracting the pre-contrast R1 map from the post-contrast R1 map. In the ΔR1 map, a region of interest was placed in the left ventricular blood pool, and the ΔR1 map was divided by the mean ΔR1 value of the LV blood pool and multiplied by [1 − haematocrit], thus yielding a quantitative pixel map of the ECV fraction ranging from 0 to 100%. Region-of-interest measurements for infarct LGE and atypical LGE were manually delineated in regions visually identified to correspond with focal abnormalities identified in conventional LGE images acquired in the same slice position as the ECV images. ‘Normal appearing’ myocardium in these two groups was delineated in regions which appeared normal by LGE. Normal myocardium in the normal LGE group was delineated segmentally in the six midventricular short-axis segments defined by the American Heart Association 17-segment model,10 and results are presented both segmentally and as a single averaged value per individual. Inter- and intraobserver variability were tested in a sub-group of 30 patients (10 normal LGE, 10 atypical LGE, and 10 infarct LGE) where one observer measured ECV once, and a second observer measured ECV blinded to other results at two time points at least 2 weeks apart. Clinical readers of LGE images were blinded to ECV results.
Figure 1.
A flow chart describing the process of generating a composite quantitative extracellular volume fraction (ECV) image of a mid-ventricular short-axis slice through the left ventricle. The top row shows two quantitative T1 maps generated from Modified Look-Locker Inversion-recovery images acquired 15 min after (Post-Gd) and before (Pre-Gd) administration of a gadolinium (Gd)-based extracellular contrast agent. Both T1 maps are displayed with the same grey scale. The reciprocal of each pixel value is taken to generate R1 maps (bottom-left two images labelled R1). The Pre-Gd R1 map pixel values are subtracted from the Post-Gd R1 map to generate a ΔR1 map. The R1 maps and the ΔR1 map are all displayed with the same grey scale. In the ΔR1 map, the ΔR1 value of the left ventricular blood pool is measured in a region of interest (black oval). The ΔR1 map pixel values are then multiplied by one minus the haematocrit (hct) and divided by the mean of ΔR1 value of the blood pool (ΔR1blood) in order to get the composite extracellular volume fraction image, ranging in values between 0 and 100%. See Supplementary material online for details regarding the theoretical background behind the operations.
Statistical analysis
Statistical analysis was performed using SPSS version 17 (IBM, Somers, New York, USA). Data are presented as mean ± SD or median and interquartile range as appropriate. Inter- and intraobserver variability are expressed as the mean difference ± SD, in ECV percentage points, for the two measurements being compared. Statistical significance was defined as P < 0.05. Differences were determined by the two-tailed paired or unpaired t-test, as appropriate for normally distributed data. Linear regression analysis was performed using Pearson's correlation coefficient (r) for normally distributed data. Multiple comparisons were tested by one-way analysis of variance (ANOVA) with post hoc Bonferroni correction.
Results
Baseline demographics
Table 1 shows the baseline characteristics of the study population. The patients classified as infarct LGE were typically referred for assessment of ischaemic heart disease. By comparison, the patients classified as having atypical LGE had a low pre-test likelihood for CAD and only 20% had prior known CAD. The patients used to study the relationship between ECV and age were prospectively selected solely based on age distribution and normal LGE findings at CMR. Many were referred to rule out ischaemic heart disease and had negative stress tests. Forty per cent had no cardiovascular medications. Over 90% did not have known CAD and, among these, the pre-test likelihood for CAD was mostly low or intermediate. The final diagnoses listed in Table 1 separate the three groups into those with myocardial infarction (infarct LGE), those with a variety of non-ischaemic aetiologies known to cause atypical LGE or had right ventricular insertion point LGE (atypical LGE), and the group with normal LGE findings (normal LGE). The 11 healthy volunteers had no history of cardiovascular disease, averaged 32 years old (range 18–52), and eight (73%) were males. Their estimated glomerular filtration rate (GFR) was >60 mL/(min/1.73 m2).
Table 1.
Baseline characteristics
| Characteristic | Infarct LGE | Atypical LGE | ECV vs. Aging |
|---|---|---|---|
| Number, n | 36 | 30 | 60 |
| Age (years) | 58 ± 12 | 52 ± 10 | 49 ± 17 |
| Male sex, n (%) | 30 (83) | 24 (80) | 31 (52) |
| Final diagnosis | |||
| Myocardial infarction | 36 (100) | 0 (0) | 0 (0) |
| Normal stress and viability | 0 (0) | 0 (0) | 31 (52) |
| Normal | 0 (0) | 0 (0) | 29 (48) |
| Hypertrophic cardiomyopathy | 0 (0) | 7 (23) | 0 (0) |
| Non-ischaemic cardiomyopathy | 0 (0) | 7 (23) | 0 (0) |
| RV insertion point enhancement | 0 (0) | 5 (17) | 0 (0) |
| Congenital heart disease | 0 (0) | 3 (10) | 0 (0) |
| Myocarditis | 0 (0) | 2 (7) | 0 (0) |
| Cancer (intracardiac) | 0 (0) | 2 (7) | 0 (0) |
| Other | 0 (0) | 4 (13) | 0 (0) |
| Race | |||
| Caucasian, n (%) | 28 (78) | 20 (67) | 40 (67) |
| African American, n (%) | 3 (8) | 5 (17) | 9 (15) |
| Asian, n (%) | 5 (14) | 3 (10) | 6 (10) |
| Native Hawaiian/Pacific Islander, n (%) | 0 (0) | 0 (0) | 2 (3) |
| Other, n (%) | 0 (0) | 2 (7) | 3 (5) |
| Ethnicity | |||
| Hispanic, n (%) | 4 (11) | 0 (0) | 5 (8) |
| Body mass index (kg/m2) | 29.4 (26.5–32.2) | 28.1 (25.8–33.3) | 25.8 (23.4–30.9) |
| Normal, <25 kg/m2, n (%) | 7 (19) | 6 (20) | 27 (45) |
| Overweight, 25–30 kg/m2, n (%) | 14 (39) | 12 (40) | 17 (28) |
| Obese, 30–40 kg/m2, n (%) | 14 (39) | 11 (37) | 13 (22) |
| Morbidly obese, >40 kg/m2, n (%) | 1 (3) | 1 (3) | 3 (5) |
| Current medication | |||
| No cardiovascular medication, n (%) | 1 (3) | 5 (17) | 24 (40) |
| Any anti-coagulant (including anti-platelet), n (%) | 32 (89) | 17 (57) | 23 (38) |
| Acetylsalicylic acid, n (%) | 31 (86) | 15 (50) | 22 (37) |
| Clopidogrel, n (%) | 18 (50) | 4 (13) | 3 (5) |
| Warfarin, n (%) | 2 (6) | 3 (10) | 1 (2) |
| Any anti-hypertensive drug, n (%) | 31 (86) | 20 (67) | 32 (53) |
| Beta-blocker, n (%) | 28 (78) | 11 (37) | 23 (38) |
| Ca-channel blocker, n (%) | 7 (19) | 1 (3) | 10 (17) |
| ACE-inhibitor, n (%) | 16 (44) | 5 (17) | 14 (23) |
| Angiotensin II receptor blocker, n (%) | 4 (11) | 7 (23) | 8 (13) |
| Diuretic, n (%) | 7 (19) | 5 (17) | 9 (15) |
| Other, n (%) | 2 (6) | 1 (3) | 2 (3) |
| Any cholesterol-lowering drug, n (%) | 28 (78) | 16 (53) | 23 (38) |
| Statin, n (%) | 27 (75) | 15 (50) | 20 (33) |
| Other, n (%) | 6 (17) | 1 (3) | 8 (13) |
| Any nitrate, n (%) | 12 (33) | 0 (0) | 4 (7) |
| Fast- or intermediate-acting, n (%) | 5 (14) | 0 (0) | 4 (7) |
| Long-acting, n (%) | 9 (25) | 0 (0) | 3 (5) |
| Any anti-diabetic drug, n (%) | 11 (31) | 5 (17) | 6 (10) |
| Insulin, n (%) | 3 (8) | 3 (10) | 1 (2) |
| Other, n (%) | 9 (25) | 5 (17) | 5 (8) |
| Risk factors | |||
| Family history of CAD, n (%) | 12 (33) | 4 (13) | 11 (18) |
| Hypertension, n (%) | 22 (61) | 20 (67) | 32 (53) |
| Dyslipidaemia, n (%) | 30 (83) | 17 (57) | 25 (42) |
| Diabetes, n (%) | 11 (31) | 6 (20) | 6 (10) |
| Former smoker, n (%) | 11 (31) | 8 (27) | 13 (22) |
| Current smoker, n (%) | 4 (11) | 0 (0) | 4 (7) |
| History of coronary artery disease | |||
| Prior known myocardial infarction, n (%) | 25 (69) | 2 (7) | 2 (3) |
| Prior PCI, n (%) | 13 (36) | 4 (13) | 3 (5) |
| Prior CABG, n (%) | 10 (28) | 3 (10) | 1 (2) |
| Total pre-test known CAD, n (%) | 28 (78) | 6 (20) | 4 (7) |
| Total pre-test unknown CAD, n (%) | 8 (22) | 24 (80) | 56 (93) |
| Estimated GFR (mL/min per 1.73 m2) | 74 (61–88) | 73 (59–80) | 78 (66–94) |
| Creatinine (mg/dL) | 1.10 (0.90–1.30) | 1.10 (1.01–1.20) | 1.00 (0.83–1.12) |
Data are presented as mean ± SD or median and interquartile range as appropriate. Creatinine and estimated GFR were not available and are thus not presented for one infarct LGE patient, two atypical LGE patients and one normal LGE patient. CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Cardiac magnetic resonance imaging findings
The findings at cardiac MRI are listed in Table 2. The distribution of myocardial infarcts was dominated by the left anterior descending coronary artery perfusion territory, followed by the right and left circumflex coronary arteries, respectively. The amount of left ventricular myocardium encompassed by LGE was similar between the infarct and atypical LGE groups (P = 0.29). Notably, 17% of the atypical LGE lesions involved the region where the right ventricle inserts into the left ventricle as the only finding in an otherwise unremarkable examination.11
Table 2.
Cardiac MRI findings
| Infarct LGE | Atypical LGE | ECV vs. Age | P-value | |
|---|---|---|---|---|
| Number, n | 36 | 30 | 60 | |
| LV ejection fraction (%) | 50 (45–56) | 60 (52–63) | 62 (57–67) | <0.001 |
| LV end-diastolic volume index (mL/m2) | 89 (76–101) | 72 (61–88) | 80 (67–88) | 0.26 |
| LV end-systolic volume index (mL/m2) | 45 (32–54) | 31 (21–41) | 29 (23–37) | 0.02 |
| LV stroke volume index (mL/m2) | 44 (39–47) | 43 (34–49) | 50 (41–54) | 0.01 |
| LV mass index (g/m2) | 58 (50–68) | 54 (45–62) | 48 (40–56) | 0.01 |
| LGE size, %LV mass | 19 (7–31) | 12 (6–24) | – | 0.29 |
| LGE LAD, n (%) | 16 (44) | – | – | |
| LGE LCx, n (%) | 6 (17) | – | – | |
| LGE RCA, n (%) | 14 (39) | – | – | |
| LGE RV insertion, n (%) | – | 12 (40) | – |
LGE abnormalities were classified as being in the left ventricle (LV) in either the perfusion territory of the left anterior descending coronary artery (LAD), left circumflex coronary artery (LCx) or right coronary artery (RCA), or the right ventricle (RV) insertion point, mid-mural or epicardial portion of the left ventricle. Due to incomplete cine imaging acquisition, LV functional measures are not presented for four infarct LGE patients one atypical LGE patient and two normal LGE patients. Data are presented as median and interquartile range.
Extracellular volume fraction of infarction
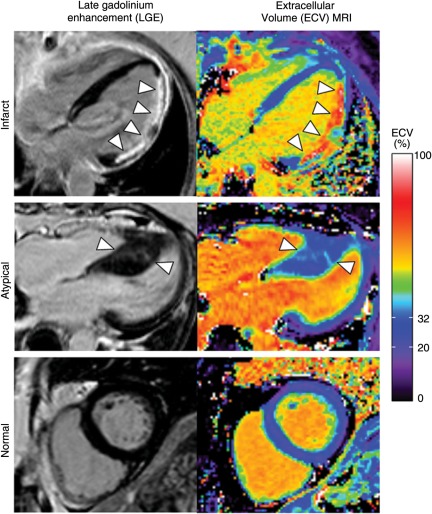
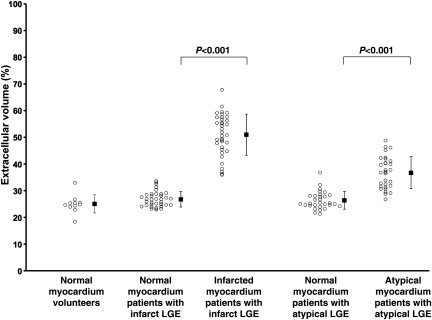
Figure 2 illustrates corresponding LGE images and quantitative ECV images. Note that in the infarct LGE patient, how the quantitative colour scale for ECV imaging provides quantitative information on the ECV throughout the left ventricle. Figure 3 shows the quantitative results of ECV values for normal and infarcted myocardium in the infarct LGE patients. On average, the ECV of infarcted myocardium was 51 ± 8% and that of the ‘normal appearing’ myocardium was 27 ± 3% (P < 0.001). There was no overlap between the infarct ECV and the normal myocardium ECV values, indicating ECV imaging can quantitatively discriminate between these tissues.
Figure 2.
Late gadolinium enhancement (left) and quantitative extracellular volume fraction images (right) in a patient with prior myocardial infarction (Infarct) in the lateral wall (arrowheads), a patient with hypertrophic cardiomyopathy associated with atypical enhancement (Atypical) in the septum (arrowheads), and a patient with normal late gadolinium enhancement findings (Normal). Differences in extracellular volume fraction in the blood pool are attributable to differences in haematocrit. In the patient with a lateral infarct, most of the septum appears normal except a small patch of late gadolinium enhancement in the basal septum. The colour scale ranges from 0 to 100% extracellular volume. The normal range (mean ± 2 SD) of ‘normal appearing’ myocardium in our normal population is indicated in the colour scale (20–32%).
Figure 3.
Extracellular volume fraction of infarcted myocardium (Infarct LGE) and regions of Atypical LGE were significantly higher than ‘normal appearing’ myocardium.
Extracellular volume fraction of atypical late gadolinium enhancement
Figure 2 also illustrates a patient with hypertrophic cardiomyopathy complicated by atypical LGE. The ECV corresponding to regions of atypical LGE in the septum of this patient was in the range 35–40%, which was more than 3SD higher than normal. On average, atypically enhanced myocardium had an ECV of 37 ± 6%, whereas ‘normal appearing’ myocardium in these patients had an ECV of 26 ± 3% (P < 0.001, Figure 3). All atypical LGE lesions had a higher ECV than ‘normal appearing’ myocardium in the same patient.
Extracellular volume fraction of ‘normal appearing’ myocardium in relation to age
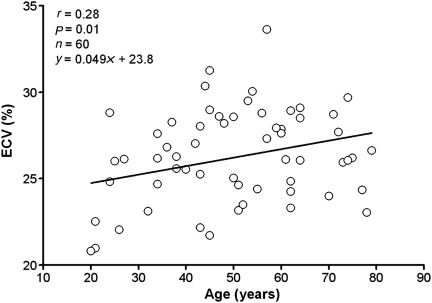
In patients with no clinically detected abnormalities by LGE (normal LGE), the ECV of the normal myocardium was 26 ± 3% (P = 0.35 and P = 0.86 vs. ‘normal appearing’ myocardium in both infarct LGE and atypical LGE, respectively). There was little variation in the ECV of normal myocardium on a segmental basis: anteroseptal, 27.3 ± 3.1%; inferoseptal, 26.8 ± 3.3%; inferior, 26.8 ± 3.3%; inferolateral, 25.8 ± 3.3%; anterolateral, 25.1 ± 3.2%, anterior, 25.3 ± 3.1%. The ECV of ‘normal appearing’ myocardium did increase with age in this group of normal LGE patients (r = 0.28, P = 0.01, n = 60, Figure 4). Notably, the ECV of ‘normal appearing’ myocardium in these patients was not predicted by the presence of hypertension (P = 0.43), dyslipidaemia (P = 0.49), current or former smoking (P = 0.25), or diabetes (P = 0.16).
Figure 4.
Extracellular volume fraction of myocardium increases as a function of age. Directionally, these findings are consistent with an age-related increase in diffuse fibrosis.
Extracellular volume fraction in ‘normal appearing’ myocardium remote from infarction
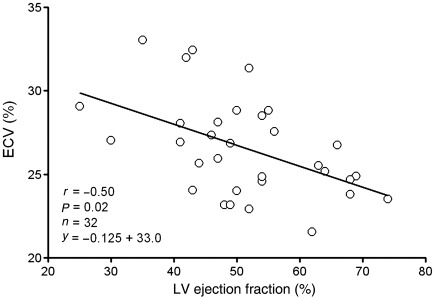
We also postulated that if diffuse fibrosis occurs in ‘normal appearing’ myocardium remote from myocardial infarction, in which case the ECV of ‘normal appearing’ myocardium would increase as left ventricular ejection fraction worsens. Our findings confirm that ECV imaging can characterize these changes remote from infarction, since for infarct LGE patients, the ECV of ‘normal appearing’ myocardium increased as left ventricular ejection fraction decreased (r = −0.50, P = 0.02, Figure 5). By comparison, the ECV of the infarcted region did not vary with ejection fraction (r = −0.14, P = 0.23).
Figure 5.
Extracellular volume fraction of ‘normal appearing’ myocardium is inversely related to ejection fraction in patients with myocardial infarction. These findings are consistent with adverse post-infarct remodelling in myocardium remote from infarction.
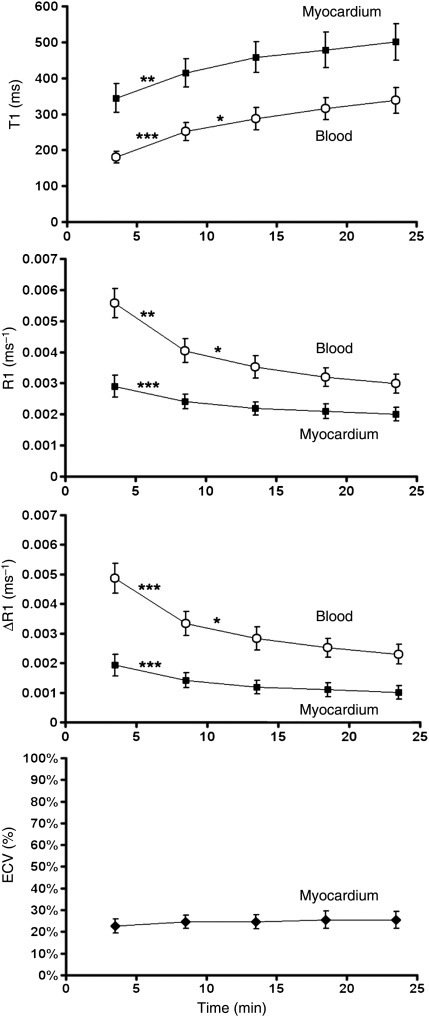
Time-independence of extracellular volume fraction measurement
Figure 6 shows the change in T1, R1, ΔR1, and ECV over time after the injection of an intravenous contrast bolus in 11 healthy volunteers. The data show that the T1 of both myocardium and blood increase over time as contrast is cleared from the blood via renal clearance. Thus, both R1, which is the reciprocal of T1, and ΔR1, which is proportional to contrast agent concentration, decrease over time after contrast injection. Notably, ECV remains constant over time after contrast injection.
Figure 6.
The relationship between T1, R1, ΔR1, and extracellular volume fraction of normal myocardium (black squares) and blood (white circles) over time after contrast injection in healthy volunteers (n = 11). R1 is equal to 1/T1. ΔR1 is the difference between post-contrast and pre-contrast R1. ΔR1 is proportional to contrast agent concentration. Extracellular volume fraction is the extracellular volume fraction and is defined as the ratio of ΔR1 of the myocardium to ΔR1 of the blood, multiplied by one minus haematocrit. See Methods and Supplementary material online for details. Extracellular volume fraction effectively remains constant over the time period after contrast injection, indicating that the relationship between contrast agent concentration in the myocardium and in the blood is in a dynamic equilibrium. Extracellular volume fraction measurement in normal myocardium inherently compensates for renal clearance and is thus not dependent on imaging time after contrast injection. Statistical differences by analysis of variance with Bonferroni correction are denoted according to the convention: ***P < 0.001, **P < 0.01, and *P < 0.05.
Intra- and interobserver variability
ECV in normal LGE had an intraobserver variability of 0.3 ± 0.2% and an interobserver variability of 0.6 ± 0.5%. ECV of focal atypical LGE had an intraobserver variability of 0.3 ± 0.8% and an interobserver variability of 0.4 ± 1.1%. ECV of focal infarct LGE had an intraobserver variability of 0.6 ± 0.6% and an interobserver variability of 0.3 ± 1.9%.
Discussion
The major finding of the study is that ECV imaging can quantitatively characterize myocardial infarction, atypical fibrosis, and diffuse myocardial abnormalities not clinically apparent on LGE images. Infarctions varied considerably in ECV, but displayed no overlap with ECV of ‘normal appearing’ myocardium. In a clinically well-characterized population, we prospectively tested two sub-clinical hypotheses. If myocardial fibrosis increases with age, then the ECV of myocardium should increase with age. Recognizing that these results are subtle (∼3% over six decades), it is rather remarkable that a sample size of 60 could define this relationship. The second hypothesis focused on the concept that myocardial remodelling should alter myocardial ECV in ‘normal appearing’ myocardium remote from myocardial infarction in a graded response related to the severity of overall left ventricular dysfunction. While these findings need independent confirmation and correlative measures, it appears that myocardial ECV imaging is able to detect subtle abnormalities that are hidden within the current LGE imaging approach of nulling ‘normal appearing’ myocardium.
Age-related changes in ‘normal appearing’ myocardium
The current study is the first to show a continuous relationship between age and the ECV of ‘normal appearing’ myocardium. The magnitude of these changes was small on average, but is nonetheless consistent with diffuse fibrosis, which has previously been shown to be increased in elderly by histological assessment of myocardial collagen content.5 Notably, the presence of hypertension, dyslipidaemia, smoking, and diabetes, as individual variables, did not influence ECV in our population.
It is not completely known what causes age-related diffuse myocardial fibrosis. It is defined as increased collagen content in the interstitium12 likely as a result of either age-related myocyte loss13 or reduced collagen degradation,14 or both. Potential mechanisms include an increased tumor growth factor beta-1 transcription with age15 and age-related decrease in matrix metalloproteinases.16 These changes lead to passive stiffness in the left ventricle17 as manifested by reduced left ventricular diastolic function with age.18 Taken together, our findings suggest that age-related changes in the ‘normal-appearing’ myocardium can be quantified in vivo.
Extracellular volume fraction remote from infarction
We found that ECV imaging could detect changes consistent with diffuse myocardium fibrosis remote from infarction. It is known that infarction can lead to adverse remodelling in the remote non-infarcted myocardium, including ventricular dilatation and progressive heart failure.19 Post-infarct remodelling involves diffuse fibrosis in myocardium remote from the infarct and has been measured histologically as the collagen volume fraction in patients.20,21 Another study showed trends in the same direction but did not have the statistical power to detect these subtle changes even by autopsy.22 The collagen volume fraction of remote myocardium was inversely related to left ventricular ejection fraction after infarction in patients.23 Prior biopsy data showed good correlation between gadolinium-enhanced MRI and collagen volume fraction in patients.6,24,25 Taken in the context of these prior studies, our results are consistent with age-related increases in myocardial diffuse fibrosis and increased myocardial collagen associated with post-infarct remodelling.
Development of diffuse fibrosis is influenced by neurohormonal factors including angiotensin-converting enzyme,26 angiotensin II,27 catecholamines,28 and aldosterone.29 Pharmacological therapy with beta-blockers30 and angiotensin-converting enzyme inbitors31 can reduce diffuse myocardial fibrosis. Our methodology may make it possible to non-invasively, serially, and quantitatively assess diffuse fibrosis as a potential endpoint for mechanistic studies of pharmacological intervention in heart failure.
Extracellular volume fraction imaging compared with related magnetic resonance imaging techniques
Extracellular volume fraction-equivalent MRI measures have been reported in acute myocardial infarction in rats3 and humans,32 chronic myocardial infarction in humans,33 aortic stenosis,6 congenital heart disease,34 and idiopathic dilated cardiomyopathy.35 The current results agree with prior measures of ECV in normal and infarcted myocardium within 2–3%.33 Thus, as a quantitative continuous variable, our measures appear to be well calibrated. Our parametric images have the added advantage of quantifying ECV at the resolution of the image, and this is a significant step beyond the previously used region-of-interest measurements.
The study shows that normal myocardium is in a dynamic equilibrium with the blood pool minutes after contrast injection, in agreement with a previous study.33 This means that contrast agent exchange between blood and normal myocardium is fast relative to renal clearance. By comparison, chronically infarcted myocardium takes 15–20 min before achieving a steady state after intravenous contrast bolus administration, but nonetheless achieves a dynamic equilibrium.33 Thus, T1 measurements can be used to accurately quantify ECV of normal myocardium ∼5 min following contrast injection, whereas ECV of chronic infarcts should be performed ∼15–20 min following bolus contrast injection. Our measurements of ECV in patients were acquired at a median of 18 min (interquartile range 16–19 min) after contrast administration and thereby well within the acceptable range. Of note, pathologies may result in small differences in post-contrast T1 in tissue, sometimes as small as 20 ms differences in T1. However, as explained in detail in the Supplementary material online, ECV depends on both pre- and post-contrast T1 in the tissue, as well as haematocrit and pre- and post-contrast T1 in the blood. Thus, solely evaluating pathology based on post-contrast T1 in a tissue may be misleading, since all the other factors contribute to the measurement of ECV, which is the measure which provides characterization of pathologies in the extracellular space in physiologically intuitive units.
Flett et al.6 have measured an equivalent to our ECV measure using a slightly different approach. They used a contrast agent bolus followed by a slow infusion of the same agent, estimated T1 using a multiple breath-hold imaging approach, and performed measurements corresponding to ECV in regions of interest in the myocardium. Our data (Figure 6) illustrate that although the ΔR1 and thus contrast agent concentrations are constantly changing after bolus injection, the relationship between normal myocardium and blood remains in a dynamic equilibrium and thus is inherently insensitive to time of imaging over the time period 5–25 min after contrast injection. As a result, the ECV of normally perfused myocardium can be reliably measured using a bolus approach without the need for infusion, and it has also recently been shown that infusion and bolus yield equivalent results when measuring ECV.36 Furthermore, our use of single breath-hold T1 mapping combined with image registration has the advantage of providing a shorter imaging time which is more compatible with clinical routine, while providing parametric maps of ECV to illustrate the spatial distribution of ECV throughout the myocardium.
Limitations
Our cross-sectional data describe changes in myocardial ECV remote from infarction. These results are hypothesis-generating and require serial studies of remote myocardium following infarction in order to confirm the ability to detect post-infarct remodelling. The study was designed to quantitatively characterize focal lesions seen on LGE images and to the extent that it was possible, quantitatively characterize sub-clinical changes in the ECV of ‘normal appearing’ myocardium in patients with infarction or normal LGE findings. Due to the limited number of patients with specific ‘atypical’ diagnoses such as hypertrophic cardiomyopathy, we chose not to assess subtle changes of ECV in ‘normal appearing’ myocardium in the atypical group. Further studies with larger numbers of patients with non-ischaemic pathologies will be of interest to undertake with this new methodology. Our ECV imaging pixel maps are acquired at a lower spatial resolution compared with LGE imaging. This, in combination with the registration and T1 fitting process, makes it challenging to quantitatively assess small lesions, especially for sub-endocardial findings in the transition between myocardium and blood, which is particularly susceptible to the partial volume effect. Consequently, sub-endocardial findings should be interpreted with caution while taking spatial resolution and the partial volume effect into consideration. We chose to study the ECV of findings of atypical LGE defined as focal abnormalities of non-ischaemic origin seen in LGE images. This was performed in patients where atypical LGE was the only focal finding in the LGE images regardless of the clinical implications of the findings. Such abnormalities, particularly when they are small, may be difficult to diagnose with certainty in LGE images and ECV imaging may provide quantitative information for increased diagnostic confidence in this setting. However, it would also be of potential interest to study the ‘normal appearing’ myocardium of patients with a primary or secondary cardiomyopathy, and such future studies are justified. Acute myocardial infarction, with or without microvascular obstruction, does not achieve a dynamic equilibrium over the first 40 min after contrast bolus injection.32 These data are consistent with a slower exchange rate for contrast between the blood and acutely infarcted myocardium compared with renal clearance. Thus, ECV values for acutely infarcted myocardium may vary with time after contrast bolus injection and should be interpreted with caution. Furthermore, patients with reduced cardiac function may have a reduced cardiac output which could theoretically result in the delayed delivery of contrast which might require a longer time period to achieve a dynamic steady state for the relationship between myocardial and blood contrast concentrations. This has not been studied and may be of interest to address in future studies. However, reduced cardiac output will also theoretically reduce renal clearance, which potentially could counterbalance the reduced perfusion and result in no net change in time to dynamic equilibrium. Lastly, while ECV image acquisition is currently routinely feasible, the post-processing needed to generate ECV images is currently not automated, and is time-consuming, although automated approaches are under development.
Conclusions
ECV imaging by MRI is a tool for visualization and quantitative characterization of both focal and diffuse myocardial abnormalities of both ischaemic and non-ischaemic origin. Importantly, ECV imaging detects diffuse changes in the myocardium which occur with age and as a result of post-infarct remodelling—findings that are not clinically obvious on conventional late gadolinium enhanced imaging.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, USA [1 Z01 HL004607-08 CE].
Conflict of interest: Dr. Arai is a principal investigator on a US government Cooperative Research and Development Agreement (CRADA) with Siemens Medical Solutions (HL-CR-05-004).
Supplementary Material
Acknowledgments
The authors thank Pamela Vincent, RT, Christine Mancini RT, Marsha Block RN, Kathie Bronson CRNP, Tracy Lowry RN, Jennifer Henry RN and Jacquin Jones RN, for expert help with imaging and coordination of patients and volunteers.
References
- 1.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 2.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. doi:10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 3.Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, Dae MW, Wendland MF. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211:698–708. doi: 10.1148/radiology.211.3.r99jn41698. [DOI] [PubMed] [Google Scholar]
- 4.Thomson LE, Kim RJ, Judd RM. Magnetic resonance imaging for the assessment of myocardial viability. J Magn Reson Imaging. 2004;19:771–788. doi: 10.1002/jmri.20075. doi:10.1002/jmri.20075. [DOI] [PubMed] [Google Scholar]
- 5.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. doi:10.1016/S0047-6374(01)00238-X. [DOI] [PubMed] [Google Scholar]
- 6.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. doi:10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 7.Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. doi: 10.1002/jmri.21119. doi:10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 8.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. doi: 10.1002/mrm.10051. doi:10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messroghli DR, Rudolph A, Abdel-Aty H, Wassmuth R, Kuhne T, Dietz R, Schulz-Menger J. An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med Imaging. 2010;10:16. doi: 10.1186/1471-2342-10-16. doi:10.1186/1471-2342-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. doi:10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 11.Hesselstrand R, Scheja A, Wuttge D, Arheden H, Ugander M. Enlarged right-sided dimensions and fibrosis of the right ventricular insertion point on cardiovascular magnetic resonance imaging is seen early in patients with pulmonary arterial hypertension associated with connective tissue disease. Scand J Rheumatol. 2011;40:133–138. doi: 10.3109/03009742.2010.507217. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues de Souza R. Aging of myocardial collagen. Biogerontology. 2002;3:325–335. doi: 10.1023/a:1021312027486. doi:10.1023/A:1021312027486. [DOI] [PubMed] [Google Scholar]
- 13.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart: myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 14.Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96:1–14. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- 15.Rossi P, Karsenty G, Roberts AB, Roche NS, Sporn MB, de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988;52:405–414. doi: 10.1016/s0092-8674(88)80033-3. doi:10.1016/S0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- 16.Robert V, Besse S, Sabri A, Silvestre JS, Assayag P, Nguyen VT, Swynghedauw B, Delcayre C. Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest. 1997;76:729–738. [PubMed] [Google Scholar]
- 17.Janicki JS. Myocardial collagen remodeling and left ventricular diastolic function. Braz J Med Biol Res. 1992;25:975–982. [PubMed] [Google Scholar]
- 18.Miller TR, Grossman SJ, Schectman KB, Biello DR, Ludbrook PA, Ehsani AA. Left ventricular diastolic filling and its association with age. Am J Cardiol. 1986;58:531–535. doi: 10.1016/0002-9149(86)90028-7. doi:10.1016/0002-9149(86)90028-7. [DOI] [PubMed] [Google Scholar]
- 19.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction: potential mechanisms and early predictors. Circulation. 1993;87:755–763. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 20.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 21.Volders PG, Willems IE, Cleutjens JP, Arends JW, Havenith MG, Daemen MJ. Interstitial collagen is increased in the non-infarcted human myocardium after myocardial infarction. J Mol Cell Cardiol. 1993;25:1317–1323. doi: 10.1006/jmcc.1993.1144. doi:10.1006/jmcc.1993.1144. [DOI] [PubMed] [Google Scholar]
- 22.Marijianowski MM, Teeling P, Becker AE. Remodeling after myocardial infarction in humans is not associated with interstitial fibrosis of noninfarcted myocardium. J Am Coll Cardiol. 1997;30:76–82. doi: 10.1016/s0735-1097(97)00100-9. doi:10.1016/S0735-1097(97)00100-9. [DOI] [PubMed] [Google Scholar]
- 23.Montera MW, Drumond C, Takiya C, Mesquita CT, Dohmann HF, Mady C. Correlation of myocardial interstitial collagen in the right ventricular septum with ventricular function of patients with ischemic cardiomyopathy. Arq Bras Cardiol. 2009;92:54–62. doi: 10.1590/s0066-782x2009000100009. doi:10.1590/S0066-782X2009000100009. [DOI] [PubMed] [Google Scholar]
- 24.Sibley CT, Noureldin RA, Gai N, Souto Nacif M, Mudd JO, Halushka MK, Bluemke DA. Abstract 19921: Cardiac MRI T1 mapping noninvasively predicts interstitial myocardial fibrosis in the absence of late gadolinium enhancement. Circulation. 2010;122:A19921. [Google Scholar]
- 25.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. doi:10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 26.Michel JB, Lattion AL, Salzmann JL, Cerol ML, Philippe M, Camilleri JP, Corvol P. Hormonal and cardiac effects of converting enzyme inhibition in rat myocardial infarction. Circ Res. 1988;62:641–650. doi: 10.1161/01.res.62.4.641. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–1569. doi: 10.1006/jmcc.1998.0721. doi:10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 28.Collins P, Billings CG, Barer GR, Daly JJ, Jolly A. Quantitation of isoprenaline-induced changes in the ventricular myocardium. Cardiovasc Res. 1975;9:797–806. doi: 10.1093/cvr/9.6.797. doi:10.1093/cvr/9.6.797. [DOI] [PubMed] [Google Scholar]
- 29.Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990;67:1355–1364. doi: 10.1161/01.res.67.6.1355. [DOI] [PubMed] [Google Scholar]
- 30.Hamdani N, Paulus WJ, van Heerebeek L, Borbely A, Boontje NM, Zuidwijk MJ, Bronzwaer JG, Simonides WS, Niessen HW, Stienen GJ, van der Velden J. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. Eur Heart J. 2009;30:1863–1872. doi: 10.1093/eurheartj/ehp189. doi:10.1093/eurheartj/ehp189. [DOI] [PubMed] [Google Scholar]
- 31.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 32.Klein C, Schmal TR, Nekolla SG, Schnackenburg B, Fleck E, Nagel E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2007;9:653–658. doi: 10.1080/10976640601105614. doi:10.1080/10976640601105614. [DOI] [PubMed] [Google Scholar]
- 33.Klein C, Nekolla SG, Balbach T, Schnackenburg B, Nagel E, Fleck E, Schwaiger M. The influence of myocardial blood flow and volume of distribution on late Gd-DTPA kinetics in ischemic heart failure. J Magn Reson Imaging. 2004;20:588–593. doi: 10.1002/jmri.20164. doi:10.1002/jmri.20164. [DOI] [PubMed] [Google Scholar]
- 34.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. doi:10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. doi:10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D, Shroff SG, Wong TC. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion vs. bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. doi:10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.