Abstract
Clinical, genetic and pathological studies all demonstrate that mutations in glucocerebrosidase (GBA), which encodes the lysosomal enzyme deficient in Gaucher disease (GD), are an important and common risk factor for Parkinson disease (PD) and related disorders. Some patients with GD and Gaucher carriers develop parkinsonism. Furthermore, subjects with PD have a greatly increased frequency of GBA mutations. GBA mutation carriers exhibit diverse parkinsonian phenotypes, and have glucocerebrosidase-positive Lewy bodies. Although the mechanism for this association is unknown, we present several theories, including enhanced protein aggregation, prion transmission, lipid accumulation, and impaired autophagy, mitophagy or trafficking. Each has inherent limitations, and an unknown “second hit” might be essential. Elucidating the basis for this link will have important consequences and should provide new insights into lysosomal pathways and potential treatment strategies.
Gaucher disease and parkinsonsm: an overview
Gaucher disease (GD) is a rare autosomal recessive storage disorder caused by the deficiency of the lysosomal enzyme glucocerebrosidase (GCase) (EC.3.2.1.45), which breaks down the glycolipid glucocerebroside (GC) into glucose and ceramide. The storage of substrate primarily occurs in the cells of the reticulo-endothelial system. The classic cellular hallmarks of GD are Gaucher cells, macrophages with substrate-laden lysosomes. These macrophages accumulate in the spleen and liver causing organ enlargement, as well as inflammation. Although GD is rare, it is the most common lyosomal storage disease, and although pan-ethnic, the disorder is more common among Ashkenazi Jews, where about 1 per 855 have GD. In other populations the disease frequency is estimated as 1 per 40,000 individuals. The gene encoding glucocerebrosidase, GBA, is comprised of 11 exons, and almost 300 distinct disease-causing mutations have been reported [1]. Remarkably, there is not a strong correlation between the clinical phenotype, the molecular genotype, and the residual enzyme activity observed. Vast phenotypic variations among patients with the same genotypes have been reported, even in sib pairs and twins with the same genotype [2] [3] [4], demonstrating the complexity of this disorder and supporting a role for genetic modifiers [4] [5]. GD has classically been divided into three clinical subgroups reflecting the degree and rate of progression of involvement in the central nervous system (Box 1). More recently, the accepted classification of type 1 GD as non-neuronopathic has been challenged by the association of type 1 GD and Parkinson disease (PD).
BOX 1. The Three Types of Gaucher Disease.
Gaucher disease type 1: OMIM#230800
Type 1 is the non-neuronopathic and most common form of GD. Clinical symptoms are restricted to the hematopoietic and skeletal system and visceral organs. Commonly encountered manifestations include hepatosplenomegaly, anemia, thrombocytopenia, and bone involvement. Many subjects have few manifestations and do not reach diagnosis. Pan-ethnic in occurrence, type 1 GD is more common in Ashkenazi Jews. [1] [4].
Gaucher disease type 2: OMIM#230900
Type 2 or acute neuropathic GD is the rarest and most severe type; it most often presents in the first 6 months of life. Type 2 GD is associated with devastating and progressive neurological manifestations leading to early death. Patients develop regression of milestones, brainstem involvement, strabismus, and opisthotonus. Seizures, impaired gag reflex, and aspiration are common. Type 2 GD also has a variety of clinical presentations and can be associated with hydrops fetalis, which is characterized by an abnormal accumulation of fluid in the fetus and congenital ichthyosis, a skin disorder characterized by dry, thickened, and flaky skin. [1] [4].
Gaucher disease type 3: OMIM#2301000
Type 3 or chronic neuronopathic GD has a more slowly progressive course and manifests with a spectrum of distinct clinical symptoms. Patients with type 3 GD have a characteristic eye movement abnormality consisting of slowing of the horizontal saccades. Type 3GD can also be associated with myoclonic epilepsy, cognitive impairment or psychiatric disturbances. Patients within this subgroup develop calcifications of the aorta, hydrocephalus and/or dysmorphic features. Type 3 GD is more common in the Norbottnian region of Sweden.
During the past decade, different lines of evidence led to the recognition of an unanticipated association between mutations in GBA and the development of PD and other Lewy body disorders. This began with reports in the clinic of patients that exhibited both phenotypes. The first series of six cases was reported by Neudorfer and colleagues in 1996 [6]. Subsequently, these findings were confirmed by studies in larger cohorts of patients with GD [7] [8] [9]. Importantly, although the estimated number of patients with GD who develop PD is higher than in the general elderly population, it is clear that the vast majority of patients with GD never develop parkinsonism. Patients with GD exhibit different associated Parkinson phenotypes, ranging from patients with sporadic PD responsive to treatment with the neurotransmitter precursor L-3,4-dihydroxyphenylalanine (L-dopa), to those manifesting with an early onset form of Lewy body dementia (LBD) [10].
Adding another layer of complexity were reports that even Gaucher carriers appeared to have a higher frequency of PD. Family studies focusing on probands with GD revealed that approximately 25% of families had a close relative who developed PD [11] [12]. Centers around the globe then began to explore the frequency of GBA mutations among their patients with PD. The first studies were relatively small, but suggested that GBA mutations were present in as many as 12% of autopsied subjects with a diagnosis of PD [13] and 33% of Ashkenazi Jewish patients with PD from Israel [14]. Over the next few years, multiple other studies were conducted in clinics around the world [15], each showing an increased frequency of GBA mutations in PD probands versus controls. However, most studies lacked sufficient numbers to attain true statistical significance. The finding finally reached wide acceptance after a large collaborative international multicenter study of over 5000 patients with PD and an equal number of matched controls demonstrated an odds ratio for carrying a GBA mutation in Parkinson subjects of over 5, making mutations in GBA the number one genetic risk factor for PD [16]. These results were reinforced by further large studies on patients from various ethnicities [17] [18] [19] [20] [21] [22] [23]. An increased frequency of GBA mutations was also observed in subjects with familial PD [22] [17], as well as cohorts with early onset symptoms [24] [25]. Additionally, GBA mutations are more frequent in patients with LBD [26] [10].
Generally, studies indicate that GBA mutations are associated with an earlier onset of PD manifestations, with a mean age at diagnosis five years younger than individuals without mutations [16]. Moreover, associated cognitive impairment is noted more frequently. Otherwise, GBA-associated parkinsonism resembles sporadic or idiopathic PD with regards to presence of tremor, bradykinesia, and rigidity, and also in preliminary imaging studies [27] [28].
Both PD and LBD are synucleinopathies, which are characterized by the presence of Lewy bodies in different areas of the brain (substantia nigra, cerebral cortex, and hippocampus) and the loss of dopaminergic neurons [29]. The main components of Lewy bodies and Lewy neurites are globular protein inclusions containing oligomerized, insoluble, alpha-synuclein (α-syn) and about 550 various other proteins [30]. Mutations in the alpha-synuclein gene (SNCA), as well as duplications and triplications of SNCA, are associated with PD [31] [32, 33] [34]. α-Syn is associated with presynaptic membranes, serving as a regulator of vesicle size, membrane curvature [35], and neurotransmitter release through the mediation of soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) complex assembly [36]. The neuropathology in individuals with PD carrying GBA mutations does not differ from other synucleinopathies. α-Syn positive Lewy bodies are found in the brains of patients with GBA mutations and PD, although not in patients with GD without parkinsonian manifestations[37, 38].
These findings have opened up a new field of research probing the mechanism for this association. The link between GBA mutations and the risk for developing PD and DLB is now being explored at the cellular level. In this review, we summarize the current state of research investigating the potential role of GCase and GC in neurodegeneration, and the different theories proposed to explain this association.
Does mutant glucocerebrosidase contribute directly to α-synuclein aggregation?
One of the first theories proposed for the link between GD and PD was a gain- of- function model, in which GBA mutations, resulting in misfolded mutant protein, contribute to the enhanced aggregation of α-syn directly, by a biochemical interaction with α-syn (Figure 1, arrow 7). Such a mechanism would require co-localization of α-syn and mutant glucocerebrosidase in protein aggregates and Lewy bodies in subjects with GBA mutations and PD. A recent immunohistochemistry study [37] provides support for this model. Brain samples from three individuals with GD and four GBA mutation carriers were studied. At autopsy, the diagnosis of PD and/or Lewy body dementia was confirmed. Moreover, immunofluorescence laser scanning confocal microscopy revealed that in those subjects homozygous for GBA mutations, 90% of the Lewy bodies showed a positive signal for GCase. In these patients, significant co-localization between GCase and α-syn was also detected in Lewy neurites and α-syn positive inclusions. Such co-localization was also observed in GBA heterozygotes with PD, although the extent of co-localization ranged from 90–33%, with a mean of 75%. In patients with PD without GBA mutations, less than 10% of the Lewy bodies were positive for GCase (in this case, the wild-type form). These data support a gain-of-function mechanism, in which mutant GCase might indeed directly contribute to α-syn aggregation and subsequent Lewy body formation, thereby enhancing PD-associated pathology.
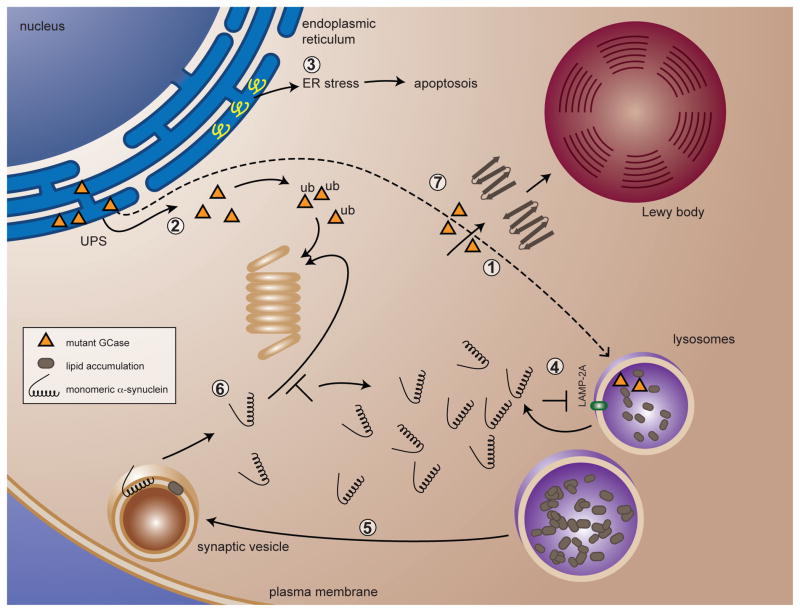
Figure 1. Putative models for α-syn turnover pathways that could be affected in Gaucher disease.
When GBA is mutated, there is impaired localization of misfolded GCase to the lysosome (1). Instead misfolded GCase undergoes ERAD via the UPS pathway through parkin-mediated ubiquitination (2). Accumulation of natural parkin substrates in the ER could cause ER stress followed by eventual cell death (3). The impaired localization of mutant GCase to the lysosome causes the substrate GC, and eventually other glycolipids, to accumulate in the lysosome (1). α-syn is degraded in part via CMA; altered lipid metabolism might interfere with the CMA pathway, resulting in impaired breakdown of α-syn (4). Altered lipid metabolism and accumulation in lysosomes can affect the composition of lipid microdomains on other endosomal vesicular compartments, causing elevated α-syn levels and aggregates (5). This can occur through impaired breakdown via the UPS (6) or via the CMA pathway (4). Mutant GCase might then contribute to α-syn aggregation and subsequent Lewy body formation (7).
An alternative explanation for increased GCase in the Lewy bodies of PD patients with GBA mutations could be offered by the “misfolded trap” mechanism: this model posits that soluble oligomers, in this case α-syn, can bind and trap misfolded proteins [39]. It is plausible that soluble α-syn oligomers trap mutant GCase, which eventually reaches the Lewy body where these oligomers might be converted to insoluble fibrils.
Do GBA mutations alter the lysosomal/autophagic pathway?
In lysosomes, degradation of proteins, lipids and organelles occurs through distinct autophagic pathways including macroautophagy, microautophagy and Chaperone-Mediated Autophagy (CMA) [40] (Box 2). It is established that proteins associated with PD such as α-syn are degraded in part via CMA (Figure 1, arrow 4), whereas α-syn mutants A53T and A30P bind to lysosome-associated membrane protein 2A (LAMP-2A), but fail to translocate into the lysosomal lumen for breakdown, blocking further CMA-mediated degradation of substrates [41]. In transgenic mice over-expressing wild type α-syn, LAMP-2A and heat shock cognate 70 (HSC70) are up-regulated, thus increasing the elimination of α-syn through lysosomal clearance [42]. Additionally, a postmortem study of seven brain samples from subjects with PD and eight control brains showed that LAMP-2A and HSC70 were significantly reduced in the substantia nigra and amygdala in PD brains, and that autophagy-related proteins appeared to accumulate in Lewy bodies [43].
BOX 2. Autophagy and Mitophagy.
Macroautophagy
In this intracellular process, double-membraned autophagosomes engulf lipids, proteins, and damaged organelles in several steps. The outer membranes of autophagosomes fuse with lysosomes to form the autophagolysosome, whose contents are eventually broken down by acid hydrolases. The formation of double-membrane autophagosomes is regulated by the mammalian target of rapamycin (mTOR) through either mTOR-dependent or mTOR-independent signaling. The mTOR complex is an energy housekeeping protein complex and a negative regulator of autophagy. Upon starvation, mTOR is inhibited, and UNC51-Like Kinase (ULK) 1-Autophagy (ATG) 13-focal adhesion kinase family interacting protein of 200 kD (FIP200) signaling promotes autophagosome formation. By contrast, the mechanisms underlying mTOR-independent signaling, a process which involves cytosolic homeostasis of calcium, cyclic adenosine monophosphate (cAMP), and inositol triphosphate (IP3), remain unclear. Well-characterized regulators of autophagosome formation and maturation include beclin-1 and Light Chain (LC3). Upon cell starvation, beclin-1forms a complex with Vacuolar Protein Sorting 34 (VPS34), VPS15, and ATG14, which is then targeted to autophagosomes. During autophagosome formation, the cytosolic form of LC3-I is converted, with the aid of ATG3 and ATG7, into lipid-conjugated LC3-II. The decreased concentration of soluble LC3-I reflects the number of autophagosomes in a cell (reviewed in [74] [75]).
Microautophagy
During microautophagy, a process well studied in yeast, cytosolic soluble material, or organelles, are recruited for breakdown by invagination followed by budding of the lysosomal membrane into the lumen. Yeast mutants defective in genes involved in microautophagy helped to elucidate the pathway involved in formation of microautophagic vesicles, but in mammalian cells the process remains poorly understood [76]. A very recent study uncovers the existence of a similar mechanism to lysosomal microautophagy in late endosomes named endosomal-microautophagy (e-MI). During e-MI cytosolic proteins undergo in bulk or HSC70-mediated selective internalization through membrane invagination during biogenesis of multivesicular bodies (MVB). Once internalized into intraluminal vesicles (ILVs) the proteins can undergo degradation or ILVs [77]
Chaperone-mediated autophagy (CMA)
CMA, a process described only in mammals, selectively delivers proteins with the KFERQ consensus sequence directly across the lysosomal membrane via the specific receptor, lysosomal-associated membrane protein 2A (LAMP2A), aided by chaperones including HSC70. Cytosolic HSC70 forms a complex with proteins destined for CMA-mediated breakdown; this complex is targeted to the lysosomal membrane where it interacts with monomeric LAMP2A. This promotes LAMP2A oligomerization, a process which is required for membrane translocation of the CMA substrate. Once the substrate has crossed the membrane, lysosomal lys-HSC70 induces the disassembly of LAMP2A from the oligomeric complex, thus releasing the proteins destined for breakdown into the lumen of the lysosome [78]. A very recent study that used a co-immunoprecipitation assay discovered two new crucial LAMP2A regulators that regulate the translocation machinery: glial fibrillary acidic protein (GFAP) stabilizes high molecular weight LAMP-2A complexes, whereas elongation confirmed factor 1 alpha (EF1α) regulates GFAP–LAMP2A in a Guanosine triphosphate (GTP)-binding dependent manner [79].
Mitophagy
Mitophagy is a form of autophagy that eliminates damaged mitochondria. Both parkin and the mitochondrial-targeted serine-threonine kinase PTEN-induced putative kinase 1 (PINK1) are regulators of mitophagy; mutations in these genes are associated with recessive familial PD. Under normal conditions with intact mitochondrial potential, PINK1, which has a mitochondrial import sequence, is translocated from the outer membrane to the inner membrane of the mitochondria, where it is rapidly degraded by protease presenilin-associated rhomboid-like protein (PARL). In impaired mitochondria with disrupted membrane potential, full-length PINK1 is not translocated to the inner membrane and instead accumulates on the outer mitochondrial membrane, with subsequent recruitment of cytosolic parkin to the mitochondrial membrane [80]. Mitophagy is subsequently induced by parkin-dependent polyubiquitination of mitochondrial proteins. The ubiquitin- and LC3-binding protein p62 is recruited to the mitochondrial membrane and mediates aggregation of damaged mitochondria. [81].
The CMA pathway has been proposed as a therapeutic target for PD and LBD [44]. Constitutive expression of beclin-1 induces macroautophagy, and in neuronal cells over-expressing α-syn, as well as in brains from SNCA transgenic mice, beclin-1 expression results in autophagy, lysosomal activation, reduction of α-syn accumulation, and reduced synaptic and dendritic pathology [45].
No studies on autophagic pathways in models of GD with PD have been published. However, alterations in autophagy have been reported in some, but not all, models of GD. In fibroblasts from patients with GD, beclin-1 and LC3-II protein levels remain unchanged after starvation compared to control fibroblasts [46]. Saposin C (Sap C) is an activator protein that enhances the activity of GCase, aiding its interaction with GC. By perturbing the lysosomal lipid membrane bilayer, Sap C exposes GC to facilitate its breakdown by GCase. Interestingly, in four fibroblast cell lines derived from patients with atypical GD with different SAP C mutations, LC3-II levels were significantly up-regulated compared to control fibroblasts after starvation, as well as following treatment with lysosomal proteases [47]. Additionally, Sap C null mice homozygous for the GCase V394L amino acid substitution are a model for neuronopathic GD [48]. Electron microscopy revealed an accumulation of undigested material in axons in brain sections of these mice. Accumulation of the macroautophagic markers LAMP-2 and p62 suggests impairment of autophagy due to storage of the GCase substrates GC and glucosylsphingosine. It was speculated that impaired autophagy causes the degeneration of axons and the subsequent pathogenesis of neuronopathic GD in this mouse model [49]. These observations remain to be confirmed in brains of patients with neuronopathic GD.
Do GBA mutations cause dysfunctional mitophagy?
Dysfunctional mitochondria are involved in the pathogenesis of several neurodegenerative diseases, but the experimental evidence for a role in altered mitochondria dynamics is especially strong in PD. Although mitochondria in the neurons of the substantia nigra are reported to have a higher somatic mitochondrial DNA mutation rate compared to other brain regions [50], overall there is no significant difference in the amount of somatic mutations in patients with PD compared to aged-matched controls [51]. However, an increase in mitochondrial DNA damage has been linked to the development of PD [52]. The elimination of damaged mitochondria is needed in post-mitotic neurons because progressive accumulation of damaged mitochondria might lead to eventual cell death. Macroautophagy plays a role in the elimination of damaged mitochondria in a tightly regulated process called mitophagy (Box 3). In vitro models demonstrate that both wild type and A53T α-syn can localize to the inner membrane of mitochondria, and interfere with mitochondrial function by interacting with complex I of the electron transport chain [53]. In vivo, in transgenic mice overexpressing α-syn A53T in dopaminergic neurons, A53T α-syn localizes to the mitochondria both as a monomer and oligomer. Oligomerization is favored in the presence of proteasome inhibitors, which leads to inhibition of complex I function, and an increase in mitophagy in midbrain dopaminergic neurons [54]. No experimental evidence is available regarding mitochondrial damage, mitophagy and the degree of localization of Parkinson protein 2 (Parkin), PTEN-induced putative kinase 1 (PINK) or α-syn in PD patients with GBA mutations.
BOX 3. Prion Transmission.
α-Synuclein as a prion
α-Syn aggregates might have a prion-like mechanism of cell-to-cell transmission. This hypothesis is supported by the Braak staging scheme for parkinsonism, whereby α-syn aggregates progressively, before and after visible symptoms appear. This proposed pattern of disease progression is compatible with prion transmission. This theory was expanded and it was suggested that α-syn acts as a prion when it spreads between neurons in the gut and nose, to the temporal lobe and caudal brainstem, respectively [82] [83]. It was shown that α-syn aggregates can be transmitted between neurons, possibly between filamentous neuronal connections. Indeed, when transplanted in vivo, mouse cortical neuronal stem cells were shown to contain GFP-labeled α-syn from the host brain. Similar results were found in cell culture [84]. Although α-syn aggregates acting like prions is an attractive model, evidence exists that refutes this hypothesis. Only artificial in vitro and in vivo models have been used to show intercellular α-syn transfer. Evidence for the uptake of α-syn by post-mitotic dopaminergic neurons in animal models is lacking (Reviewed by [69]).
GBA mutations and impaired ER-associated degradation (ERAD)
At the endoplasmic reticulum (ER), misfolded proteins are detected by the ER quality control machinery, which then attaches polyubiquitin chains, formed through sequential additions at ubiquitin residue K48 by an E3 ubiquitin ligase [55]. The potential involvement of the ERAD and the ubiquitin-proteasome (UPS) degradation pathways in the development of PD remains obscure because data from animal models treated with proteasome inhibitors has been controversial. In one study, adult rats treated with proteasome inhibitors developed clinical features of PD and the loss of dopaminergic neurons in the substantia nigra. These results were partially confirmed by two groups, although one was unable to detect α-syn inclusions in treated rats. Three other groups, using proteasome inhibitors in rats and primates, failed to observe any clinical or pathological features of PD [56]. In another study, a conditional substantia nigra knock-out mouse model for the 26S proteasomel did induce Lewy-like inclusions and significant neurodegeneration [57].
Another avenue of evidence for the involvement of ERAD and UPS dysfunction in PD involves mutations in PARK2, the gene encoding parkin, a cytosolic protein E3 ubiquitin ligase, which is associated with an autosomal recessive juvenile form of PD. [58]. Over a dozen substrates of parkin-mediated ubiquination have been identified, but it is still unclear whether parkin is involved in K48-specific ubiquitination (which marks cells for proteasome-mediated degradation). Emerging evidence indicates that parkin is associated with the outer membrane of damaged mitochondria, where it is involved in mitophagy, a process, when de-regulated, contributes to the development of PD [59].
Limited evidence is available on the involvement of ERAD in GD or PD associated with GBA mutations. It has been reported that in fibroblasts derived from patients with GD, mutant GCase undergoes ERAD; it was proposed that disease severity might correlate with the degree of ER retention [60] [61]. For the GCase amino acid substitution L444P, which is associated with severe type 3 GD when homozygous, Ambroxol, a pharmacological GCase chaperone, enhanced the removal of the mutant enzyme from the ER with sporadic increased enzymatic activity [61].
Only one report is available regarding the link between GD and PD that involves ERAD. Using immunoprecipitation assays, it showed that both wild type and N370S GCase interact with parkin; however, only mutant GCase undergoes K48-mediated poly-ubiquitination and subsequent proteasome-mediated degradation (Figure 2). Mutant, non-functional (“ligase-dead”) parkin interacts with mutant GCase, but does not mediate its breakdown. The authors proposed that mutant GCase, rather than the wild type protein, was a substrate for parkin-mediated ERAD degradation (Figure 2 and Figure 1, arrow 2). Mutant GCase interaction with parkin could block interactions with other parkin substrates, interfering with their UPS-mediated breakdown, which could lead to ER stress and eventual cell death (Figure 2 and Figure 1, arrow 3) [62].
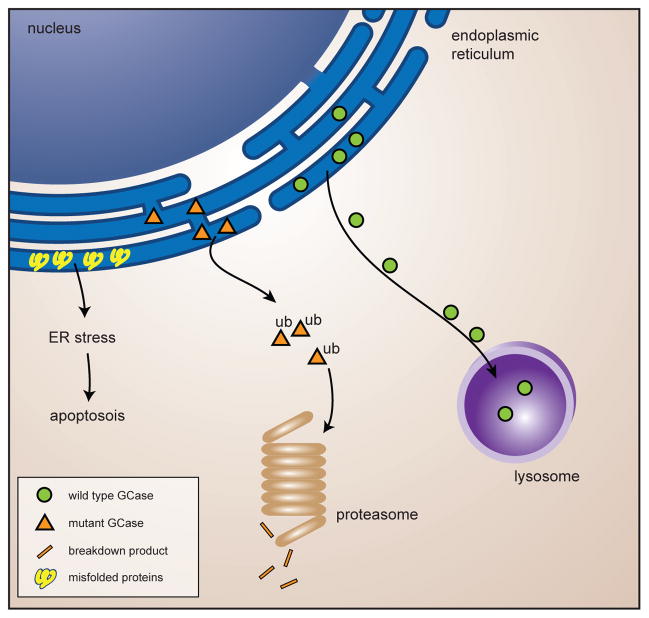
Figure 2. Impaired ERAD-mediated breakdown of GCase.
Wild type GCase enzyme gets transported to the lysosomes. Mutant misfolded GCase enzyme undergoes parkin-mediated poly-ubiquitination and subsequent proteasome-mediated degradation. The presence of mutant GCase might cause build-up of other parkin substrates causing ER-stress and eventual apoptosis of the neurons.
GBA mutations and altered lipid metabolism
Both in vitro and in vivo data show that α-syn directly interacts with lipid membranes under physiological, as well as pathological, conditions. α-Syn binds preferentially to anionic lipid-containing membranes, where it assumes an alpha-helical conformation, and under certain conditions, shows an increased tendency to aggregate. Lipid composition and curvature are important factors for the affinity of α-syn binding. Altered lipid composition, due to aging or changes in lipid metabolism, could significantly alter α-syn binding to membranes. It is known that α-syn interacts with pre-synaptic vesicles and assists in the maintenance of pools of pre-synaptic vesicles available for release [63] [64]. It also plays a key role in the formation and regulation of SNARE complexes, the regulatory machinery for fusion between the presynaptic vesicle and the plasma membrane [36]. Breakdown of α-syn occurs in part via the CMA pathway, and, interestingly, it was shown that LAMP-2A protein levels were down-regulated at the lysosomal membrane with age, pointing to reduced CMA efficacy with aging. This reduction probably stems from decreased levels of cholesterol in lipid raft microdomains, where LAMP-2A normally associates [65].
In GD, diminished or loss of GCase enzymatic activity leads to the accumulation of the substrate GC in macrophages. It has been proposed that the accumulation of this substrate alters lipid metabolism in GD. A model of GD, generated by treatment of human THP-1 macrophages with the specific inhibitor of GCase, conduritol β epoxide (CBE), showed GC accumulation in lysosomes as well as other sub-cellular fractions. Secondary elevations in ceramide, dihexosylceramide, trihexosylceramide and phosphatidylglycerol levels were observed both in the lysosome and in other sub-cellular compartments where GC was elevated (Figure 1, arrows 1 and 5). It was proposed that the accumulation of excess substrate saturates the lysosomal trafficking pathway of GC, shunting it to other subcellular compartments where it accumulates specifically in lipid microdomains (rafts), causing other sphingolipids to eventually experience a similar fate. Lipid rafts are regulators of protein and lipid sorting and trafficking, and impaired lipid raft function due to altered lipid composition could interfere with membrane curvature and the normal sorting and trafficking of proteins and lipids [66] [67]. Indirect evidence was provided by experiments showing that CBE treatment of mice, as well as cultured neuroblastoma cells, led to elevated α-syn levels and α-syn aggregates (Figure 1, arrow 4 and 6) [68].
Does mutant GCase play a role in the transmission of prions?
The basis of the pathogenesis of prion diseases is protein misfolding. The misfolded protein or prion spreads in an infectious fashion between cells of the affected organism as well as between species. In the host cells, the transmitted prion stimulates the misfolding of WT proteins into the prion form. Eventually, proteins will polymerize into amyloid aggregates. Prion diseases progress gradually and are degenerative [69]. Recently, it has been suggested that α-syn might behave like a prion (Box 3). These new insights could provide another explanation for the molecular link between GD and PD. It was proposed that macrophages, which are the cells affected in GD, might be the carriers of α-syn prions. Endogenous, as well as ingested exogenous α-syn might aggregate in the presence of accumulated anionic lipids in Gaucher cells. However, this would not explain why GBA carriers also have an increased predisposition to develop PD. Therefore, it was proposed that a “second-hit” might occur in the GBA gene, producing a disease-causing somatic mutation in a subset of macrophages [70]. Over time, misfolded GCase, lipids, and aggregated (toxic) α-syn would all accumulate in the “second-hit” macrophages. The rate -limiting step in this proposed model is α-syn unloading into the extracellular environment near neurons. This could occur as a result of the cellular death of the macrophages or by enhanced transfer via exosomes, small intralumenal membranous vesicles (50nm–100nm) that are released into the extracellular environment upon exocytosis of intracellular multivesicular bodies (MVBs) (Figure 3). Indeed, infectious prions are known to be associated with exosomes [71], and it has been reported that macrophages release prion proteins via exosomes [72]. Rigorous experimental testing will be necessary to test the prion-like hypothesis of disease progression in patients with GD and Gaucher carriers with PD.
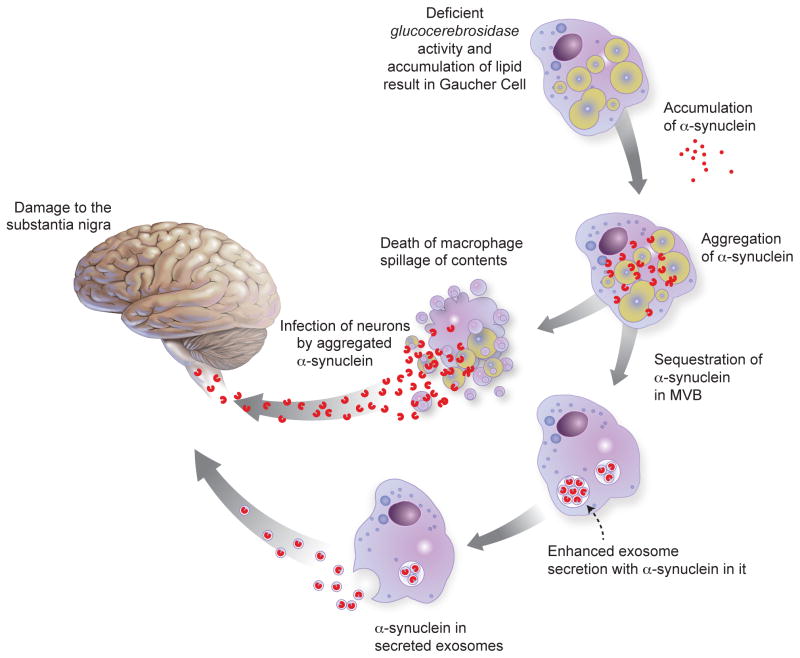
Figure 3. A theoretical model for α-syn as a prion in GBA- associated parkinsonism.
Gaucher macrophages, which are present in patients with GD and might be acquired in carriers by a second hit, contain large lipid-laden structures owing to deficient glycolipid metabolism. In a Gaucher cell, large amounts of exogenously-ingested or endogenous α-syn could aggregate and acquire the prion form in the presence of an abundance of lipids. In a small number of patients, cellular death or enhanced transfer via exosomes could cause these Gaucher cells to unload the prion form of α-syn into the extracellular environment near neurons. Adapted with permission from Ref. [70]
Concluding remarks and future perspectives
Although it is clear that elucidating the mechanism for the link between GBA mutations and the risk for the development of parkinsonism will ultimately yield important insights, each of the pathways and models proposed to date has significant limitations.
The gain-of-function mechanism of mutant GCase and its direct role in α-syn aggregation, as well as the “misfolded trap” mechanism, both have shortcomings, given that only a fraction of patients and carriers with GBA mutations develop PD. GBA mutations therefore contribute to, but do not initiate, the development of α-syn pathology. Second, this model does not explain why carriers and patients with null GBA alleles resulting in absent GCase, such as those with c.84dupG, also develop PD. Neither theories related to lipid alterations nor malfunctions in degradation pathways adequately explain why only a fraction of individuals with GBA mutations develop PD. Moreover, the lack of substrate accumulation in GBA carriers makes the involvement of altered lipid metabolism in PD development difficult to explain. The current models can only be supported if GBA mutations, or subsequent substrate accumulation, have an enhanced effect on α-syn pathology, but are not the initiator. The prion model seems attractive because of the involvement of the macrophage, which is the diseased cell in GD, but the mandatory second hit somatic mutation in GBA which must occur in about 5 to 10% of macrophages in the carriers seems very high and may be unlikely. This model also lacks an explanation for the development of PD in only a fraction of patients with GD.
It must be stressed that only a small percentage of GBA carriers and patients with GD develop PD. Therefore, we suggest that presence of aberrant GCase and/or the subsequent alteration in enzyme activity and elevation of substrate add to the pathology of α-syn in a secondary fashion. Although much progress has been made in the past few years, the rate-limiting step and major limitation in this field of research is the lack of cell and animals models that closely represent either GD and/or PD. Recently, a conditional knock-out mouse model was developed that reflects many aspects of GD pathology [73]. However, there is no GCase produced in this model; thus if aberrant protein folding plays a role, this model, too, might be inadequate. Moreover, most animal models of PD do not develop Lewy bodies, which might be due to the short lifespan of mouse models compared to humans.
The future challenge will be to identify other risk factors that, in combination with GBA mutations, favor development of parkinsonism. Once we better understand how these factors each contribute to or interact in the pathogenesis of parkinsonian manifestations, we can then tackle the challenging task of identifying therapeutic targets that can alleviate or halt the progression of this disorder.
Acknowledgments
We thank Dr. Jim Gruschus and Dr. Grisel Lopez for critically reading the manuscript. Dr. Ehud Goldin designed Figure 1, and Darryl Leja assisted in the drafting of the figures. This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health
References
- 1.Hruska KS, et al. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 2.Biegstraaten M, et al. A monozygotic twin pair with highly discordant Gaucher phenotypes. Blood Cells Mol Dis. 2010 doi: 10.1016/j.bcmd.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ron I, Horowitz M. Intracellular cholesterol modifies the ERAD of glucocerebrosidase in Gaucher disease patients. Mol Genet Metab. 2008;93:426–436. doi: 10.1016/j.ymgme.2007.10.132. [DOI] [PubMed] [Google Scholar]
- 4.Goker-Alpan O, et al. Divergent phenotypes in Gaucher disease implicate the role of modifiers. J Med Genet. 2005;42:e37. doi: 10.1136/jmg.2004.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Neudorfer O, et al. Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 7.Tayebi N, et al. Reciprocal and nonreciprocal recombination at the glucocerebrosidase gene region: implications for complexity in Gaucher disease. Am J Hum Genet. 2003;72:519–534. doi: 10.1086/367850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherin P, et al. The neurological manifestations of Gaucher disease type 1: the French Observatoire on Gaucher disease (FROG) J Inherit Metab Dis. 2010;33:331–338. doi: 10.1007/s10545-010-9095-5. [DOI] [PubMed] [Google Scholar]
- 9.Bultron G, et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J Inherit Metab Dis. 2010;33:167–173. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goker-Alpan O, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 11.Goker-Alpan O, et al. Parkinsonism among Gaucher disease carriers. J Med Genet. 2004;41:937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halperin A, et al. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis. 2006;36:426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Lwin A, et al. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Aharon-Peretz J, et al. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler SG, et al. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol Genet Metab. 2007;91:195–200. doi: 10.1016/j.ymgme.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidransky E, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsui J, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009;66:571–576. doi: 10.1001/archneurol.2009.72. [DOI] [PubMed] [Google Scholar]
- 18.Kalinderi K, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452:87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Mata IF, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bras J, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol Aging. 2009;30:1515–1517. doi: 10.1016/j.neurobiolaging.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann J, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols WC, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72:310–316. doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan-Or Z, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 24.Eblan MJ, et al. Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord. 2006;21:282–283. doi: 10.1002/mds.20766. [DOI] [PubMed] [Google Scholar]
- 25.Wu YR, et al. Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry. 2007;78:977–979. doi: 10.1136/jnnp.2006.105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark LN, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66:578–583. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saunders-Pullman R, et al. Gaucher disease ascertained through a Parkinson’s center: imaging and clinical characterization. Mov Disord. 2010;25:1364–1372. doi: 10.1002/mds.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kono S, et al. Functional brain imaging in glucocerebrosidase mutation carriers with and without parkinsonism. Mov Disord. 2010;25:1823–1829. doi: 10.1002/mds.23213. [DOI] [PubMed] [Google Scholar]
- 29.Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia Q, et al. Proteomic identification of novel proteins associated with Lewy bodies. Front Biosci. 2008;13:3850–3856. doi: 10.2741/2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 32.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 33.Simon-Sanchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 35.Middleton ER, Rhoades E. Effects of curvature and composition on alpha-synuclein binding to lipid vesicles. Biophys J. 2010;99:2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burre J, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goker-Alpan O, et al. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120:641–649. doi: 10.1007/s00401-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong K, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Gruschus JM. Do amyloid oligomers act as traps for misfolded proteins? A hypothesis. Amyloid. 2008;15:160–165. doi: 10.1080/13506120802193746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuervo AM, et al. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 42.Mak SK, et al. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez-Erviti L, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 44.Santos RX, et al. Targeting autophagy in the brain: a promising approach? Cent Nerv Syst Agents Med Chem. 2010;10:158–168. doi: 10.2174/187152410791196350. [DOI] [PubMed] [Google Scholar]
- 45.Spencer B, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacheco CD, et al. Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum Mol Genet. 2007;16:1495–1503. doi: 10.1093/hmg/ddm100. [DOI] [PubMed] [Google Scholar]
- 47.Vaccaro AM, et al. Saposin C mutations in Gaucher disease patients resulting in lysosomal lipid accumulation, saposin C deficiency, but normal prosaposin processing and sorting. Hum Mol Genet. 2010;19:2987–2997. doi: 10.1093/hmg/ddq204. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, et al. Neuronopathic Gaucher disease in the mouse: viable combined selective saposin C deficiency and mutant glucocerebrosidase (V394L) mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits. Hum Mol Genet. 2010;19:1088–1097. doi: 10.1093/hmg/ddp580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y, Grabowski GA. Impaired autophagosomes and lysosomes in neuronopathic Gaucher disease. Autophagy. 2010:6. doi: 10.4161/auto.6.5.12047. [DOI] [PubMed] [Google Scholar]
- 50.Soong NW, et al. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat Genet. 1992;2:318–323. doi: 10.1038/ng1292-318. [DOI] [PubMed] [Google Scholar]
- 51.Simon DK, et al. Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson’s disease. Neurobiol Aging. 2004;25:71–81. doi: 10.1016/s0197-4580(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee R, et al. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devi L, et al. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chinta SJ, et al. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beal F, Lang A. The proteasomal inhibition model of Parkinson’s disease: “Boon or bust”? Ann Neurol. 2006;60:158–161. doi: 10.1002/ana.20939. [DOI] [PubMed] [Google Scholar]
- 57.Bedford L, et al. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci. 2008;28:8189–8198. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beasley SA, et al. Structure of the Parkin in-between-ring domain provides insights for E3-ligase dysfunction in autosomal recessive Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:3095–3100. doi: 10.1073/pnas.0610548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grunewald A, et al. Mutant Parkin impairs mitochondrial function and morphology in human fibroblasts. PLoS One. 2010;5:e12962. doi: 10.1371/journal.pone.0012962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 61.Bendikov-Bar I, et al. Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol Dis. 2010 doi: 10.1016/j.bcmd.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Ron I, et al. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet. 2010;19:3771–3781. doi: 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- 63.Auluck PK, et al. alpha-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- 64.Kjaer L, et al. The influence of vesicle size and composition on alpha-synuclein structure and stability. Biophys J. 2009;96:2857–2870. doi: 10.1016/j.bpj.2008.12.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiffin R, et al. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007;120:782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- 66.Hein LK, et al. Secondary sphingolipid accumulation in a macrophage model of Gaucher disease. Mol Genet Metab. 2007;92:336–345. doi: 10.1016/j.ymgme.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Hein LK, et al. Lipid composition of microdomains is altered in a cell model of Gaucher disease. J Lipid Res. 2008;49:1725–1734. doi: 10.1194/jlr.M800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manning-Bog AB, et al. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30:1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Angot E, et al. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- 70.Goldin E. Gaucher disease and parkinsonism, a molecular link theory. Mol Genet Metab. 2010;101:307–310. doi: 10.1016/j.ymgme.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fevrier B, et al. Exosomes: a bubble ride for prions? Traffic. 2005;6:10–17. doi: 10.1111/j.1600-0854.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 72.Wang G, et al. Cellular prion protein released on exosomes from macrophages binds to Hsp70. Acta Biochim Biophys Sin (Shanghai) 2010;42:345–350. doi: 10.1093/abbs/gmq028. [DOI] [PubMed] [Google Scholar]
- 73.Mistry PK, et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc Natl Acad Sci U S A. 2010;107:19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Metcalf DJ, et al. Autophagy and misfolded proteins in neurodegeneration. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kunz JB, et al. Determination of four sequential stages during microautophagy in vitro. J Biol Chem. 2004;279:9987–9996. doi: 10.1074/jbc.M307905200. [DOI] [PubMed] [Google Scholar]
- 77.Sahu R, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bandyopadhyay U, et al. Identification of regulators of chaperone-mediated autophagy. Mol Cell. 2010;39:535–547. doi: 10.1016/j.molcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin SM, et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Narendra D, et al. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6:1090–1106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hawkes CH, et al. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 83.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci U S A. 2009;106:12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]