Abstract
Rationale
Intravascular neutrophil recruitment and activation are a key pathogenic factor that contributes to vascular injury. Intravenous immunoglobulin (IVIG) has been shown to have a beneficial effect in systemic inflammatory disorders; however, the mechanisms underlying IVIG’s inhibitory effect on neutrophil recruitment and activation are not understood.
Objective
We studied the mechanisms by which IVIG exerts protection from neutrophil-mediated acute vascular injury.
Methods and Results
We examined neutrophil behavior in response to IVIG in vivo using real time intravital microscopy. We found that an antibody that blocks both FcγRIII and its inhibitory receptor counterpart, FcγRIIB, abrogated the inhibitory effect of IVIG on leukocyte recruitment and heterotypic RBC interactions with adherent leukocytes in wild-type mice. In the context of sickle cell disease, the blockade of both FcγRIIB and III abrogated the protective effect of IVIG on acute vaso-occlusive crisis caused by neutrophil recruitment and activation. Analysis of FcγRIIB- and FcγRIII-deficient mice revealed the predominant expression of FcγRIII on circulating neutrophils. FcγRIII mediated IVIG-triggered inhibition of leukocyte recruitment, circulating RBC capture, and enhanced Mac-1 activity, whereas FcγRIIB was dispensable. In addition, FcγRIII-induced IVIG anti-inflammatory activity in neutrophils was mediated by recruitment of Src homology 2 (SH2)-containing tyrosine phosphatase-1 (SHP-1). Indeed, the protective effect of IVIG on leukocyte recruitment and activation was abrogated in SHP-1-mutant mice.
Conclusions
FcγRIII, a classical activating receptor, has an unexpected inhibitory role on neutrophil adhesion and activation via recruitment of SHP-1 in response to IVIG. Our results identify SHP-1 as a therapeutic target in neutrophil-mediated vascular injury.
Keywords: neutrophils, vascular injury, FcγRIII, IVIG, SHP-1
Introduction
Accumulation and recruitment of polymorphonuclear neutrophils is a key pathogenic factor in the development of microvascular obstruction in cardiovascular disease, including sickle cell disease (SCD).1, 2 Neutrophils are the major leukocyte subset recruited to inflamed venules and the adherence of activated neutrophils to endothelial cells is a critical step, which leads to reduction of the microcirculatory blood flow, ischemia, hypoxia and tissue damage.3–6 Therefore, pharmacological approaches to inhibit neutrophil recruitment and activation represent important strategies to prevent vascular injury.
Intravenous immunoglobulin (IVIG) is a unique immune-modulating therapy that has a variety of effects on the immune system depending on the underlying pathogenesis of given disease.7 The protective actions of IVIG in autoimmune diseases have been quite characterized including modulation of IgG Fc receptor expression, alteration of cytokine levels, complement inhibition, and modification of B cell and T cell functions.7, 8 However, the molecular mechanisms by which IVIG exerts inhibition of neutrophil recruitment and activation in systemic acute inflammation remain to be understood. Direct observation of leukocyte recruitment by intravital microscopy has revealed that IVIG inhibits selectin-mediated leukocyte rolling and β2 integrin-dependent leukocyte adhesion to endothelium.9–12 In SCD mice, IVIG reverses acute VOC by inhibiting neutrophil adhesion to the endothelium and abrogating the direct interactions between adherent leukocytes and circulating RBCs.9, 11
Fcγ receptors (FcγRs) for IgG are expressed on a wide variety of hematopoietic cells, linking cellular and humoral immunity. The family of FcγRs has been categorized into two different classes: the activating (FcγRI, FcγRIII, and FcγRIV) and inhibitory (FcγRIIB) receptors. Engagement of activating FcγRs associated with the common γ-chain triggers effector cell responses, such as antibody-dependent cell-mediated cytotoxicity, phagocytosis, reactive oxygen production, and release of inflammatory mediators, while the inhibitory FcγRIIB mediates the inhibition of activating FcγR-induced signal cascade.13 During inflammation, FcγRs play important roles in leukocyte recruitment and activation. FcγRIII mediates neutrophil tethering and adhesion in response to immune complexes in autoimmune disease.14, 15 β2 integrins, particularly Mac-1, cooperate with FcγRs to sustain neutrophil adhesion.16 In addition, the common γ-chain containing immunoreceptor tyrosine-based activation motifs (ITAMs) is involved in the initial signaling events that are required to initiate E-selectin-mediated neutrophil slow rolling and outside-in signaling through β2-integrins in neutrophils.17, 18 However, the mechanisms by which IVIG engagement to FcγRs modulates intravascular neutrophil recruitment and activation in the context of inflammation are incompletely defined.
Here, we elucidate the mechanism by which IVIG exerts inhibition of intravascular accumulation and activation of neutrophils to localized inflamed area in vivo using real-time intravital microscopy. We show that engagement of IVIG to activating FcγRIII, but not the inhibitory FcγRIIB, inhibits leukocyte recruitment, abrogates heterotypic adherent leukocyte-RBC interactions, and reduces Mac-1 activity. In addition, we identify the protein tyrosine phosphatase SHP-1 as a critical downstream mediator involved in the FcγRIII-mediated inhibitory effects of IVIG on leukocyte recruitment and activation.
Methods
Mice
Berkeley SCD mice [Tg(Hu-miniLCRα1GγAγδβS) Hba−/− Hbb−/−] have been previously described.19 Fcgr2b−/−20 and Fcgr3−/−21 mice generated by gene targeting, were purchased from The Jackson Laboratory (Bar Harbor, ME). Homozygous mice for the motheaten viable (mev) mutation were established by mating C57BL/6J-Ptpn6me-v/J (+/mev) heterozygous breeding pairs obtained from The Jackson Laboratory (Bar Harbor, ME). Additional details of fully chimeric SCD and mev/mev mice refer to the online supplemental materials. All experimental procedures performed on mice were approved by the Animal Care and Use Committee of Mount Sinai School of Medicine and Albert Einstein College of Medicine.
Intravital microscopy and image analyses
The cremasteric muscle was prepared as described online supplemental method. Either IVIG (800 mg/kg) or an equivalent volume of control human albumin was intravenously infused by programmable syringe pump (PHD 4400, Harvard Apparatus, Holliston, MA) at the rate of 667 µL/kg/min 3 h after intrascrotal injection of 0.5 µg TNF-α. Then, 20 min after IVIG or control albumin exposure, 8 to 12 venules were videotaped over a period of 60 min, with each venule recorded continuously for at least 2 min. To block endogenous FcγRIIB/III, we injected i.v. 1 mg/kg anti-FcγRIIB/III or control isotype rat IgG2b, before administration of either IVIG or control albumin. Bright-field intravital microscopy was performed using video recordings and all data were analyzed by playback assessment of video-captures as described online supplemental method.
Hemodynamic measurements
Arteriolar and venular diameter was measured with a video caliper before and after administration of either IVIG or control albumin. Centerline red cell velocities (VRBC) were determined for each venule in real time using an optical Doppler velocimeter (Texas A&M, College Station, TX). Wall shear rate and blood flow rate were calculated as described online supplement methods.
In vivo analysis of Mac-1 activity
Albumin-coated fluospheres were intravenously injected into mice prepared for intravital microscopy as described in detail online supplemental method. Images were captured for at least 30 s in the brightfield and FITC (for yellow-green fluospheres) channels and analyzed them with SlideBook software (Intelligent Imaging Innovations). Adherent leukocytes were visually identified in the brightfield channel and the number of fluospheres associated to each leukocyte was counted. The average number of albumin-coated fluospheres bound to adherent intravascular leukocytes in a given 100 µm-long venular segment was used as a measure of Mac-1 activity, and was obtained from the formula: fluospheres / WBC = total number of leukocyte-associated beads per venular segment / number of adherent leukocytes per venular segment, as previously described.22
Flow cytometry analyses
Blood samples were collected into sterile tubes containing 2 mM ethylenediaminetetraacetic acid (EDTA) and lysed in 0.8% NH4Cl lysis buffer and the remaining nucleated cells were washed twice in PBS containing 2 mM EDTA and 0.5% BSA (PEB buffer). Primary blood leukocytes were stained by incubation with fluorescently-labeled or biotinylated antibodies specific to mouse or corresponding with isotype controls. Biotinylated monoclonal antibody (mAb) was detected by incubation with Cy5-conjugated streptavidin (Jackson ImmunoResearch Laboratories). Stained samples were acquired with a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) and an LSRII (BD) and then analyzed with FlowJo software (Tree Star, Inc.). Leukocytes and neutrophils were gated on the basis of low forward-scatter and high side-scatter characteristics.
Immunoprecipitation and western blotting
Proteins were extracted from bone marrow neutrophils (BMNs) isolated from WT and Fcgr2b−/−mice, and the association of SHP-1 with FcγRIIB/III was analyzed by immunoprecipitation and western blotting as described online supplement methods.
Statistical analyses
All data are presented as mean ± SEM and analyzed using unpaired, 2-tailed Student’s t test or nonparametric Mann-Whitney U test, as appropriate. A P value of less than 0.05 was considered statistically significant.
Results
Low affinity Fcγ receptors alter intravascular cell interactions in wild-type animals
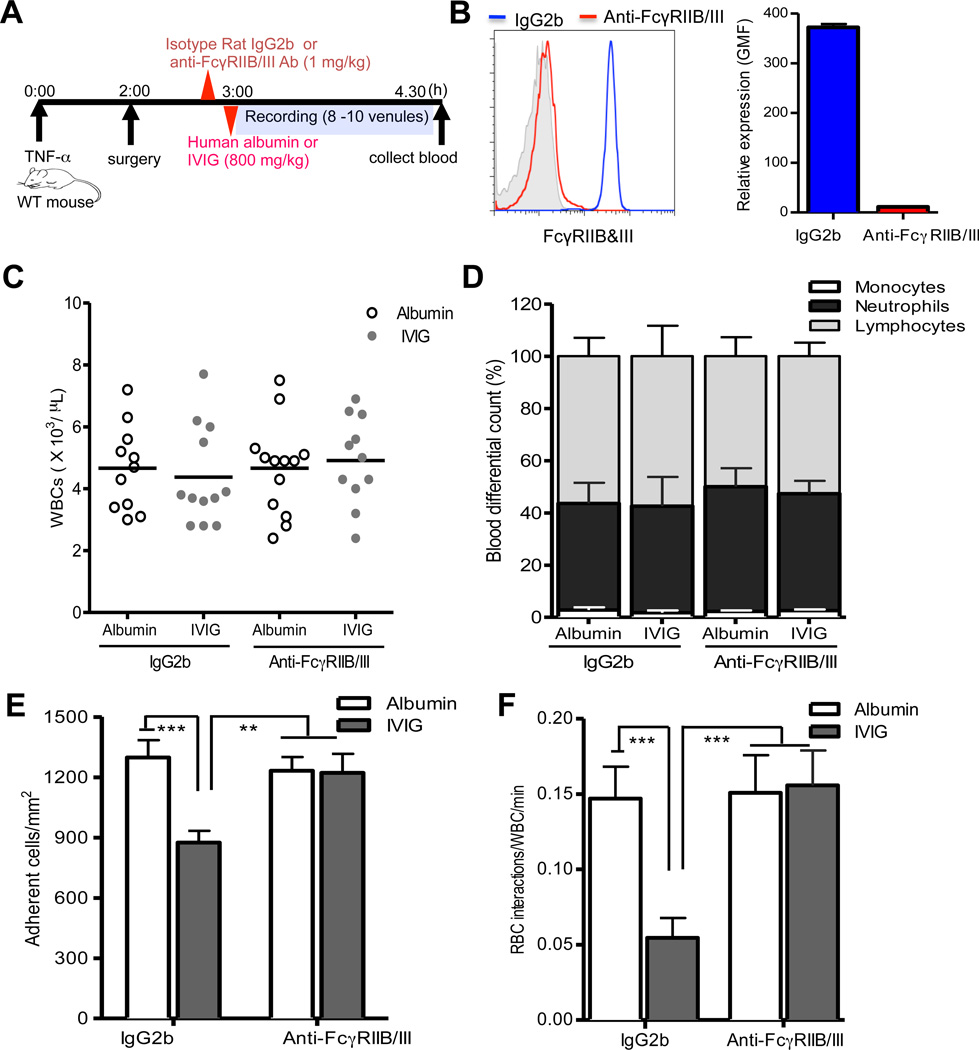
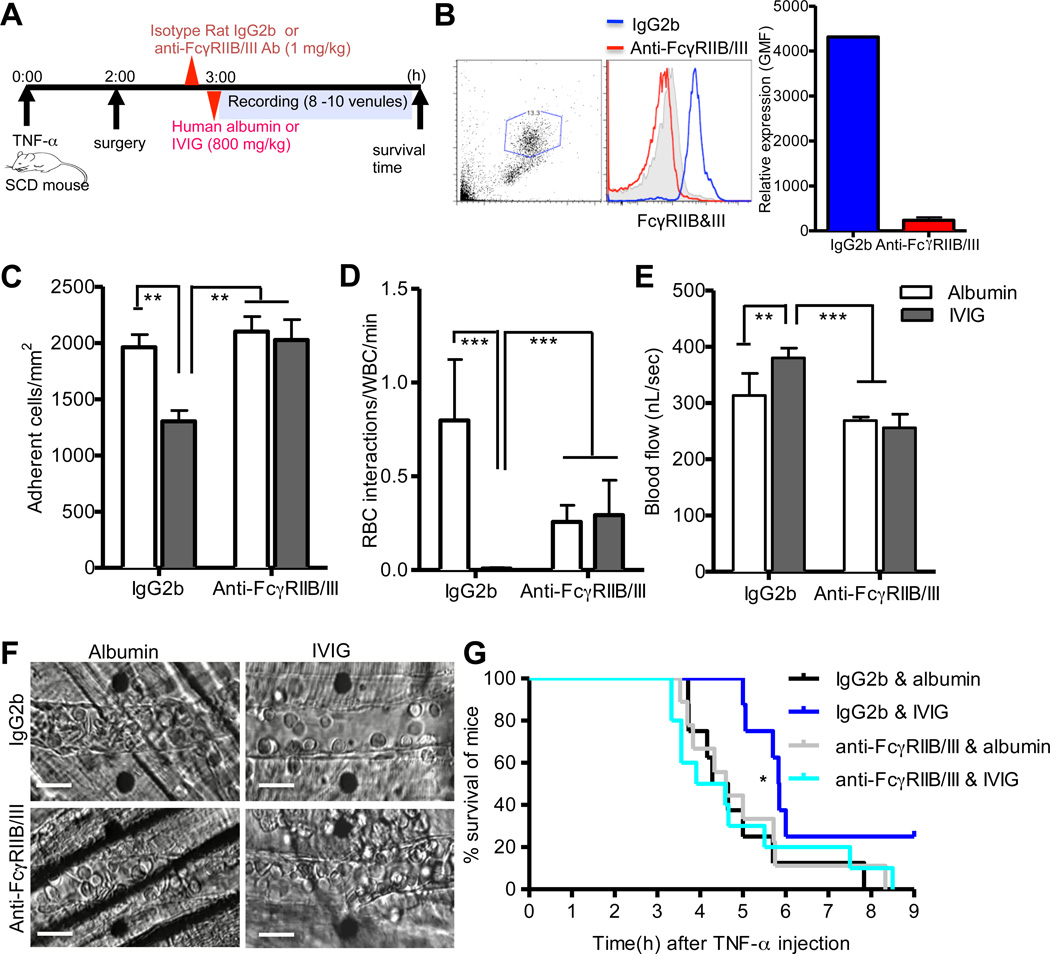
Low affinity activating FcγRIII and its inhibitory counterpart, FcγRIIB, have been suggested to be involved in IVIG-mediated immunomodulation in diverse murine models of autoantibody-triggered immune diseases.23–25 We thus investigated whether FcγRIIB/III were required for IVIG’s suppressive effects on leukocyte recruitment in systemic acute inflammation in wild-type mice. To test this, either mAb against FcγRIIB/III or control isotype rat IgG2b was injected to block endogenous FcγRIIB/III before administration of IVIG or control human albumin into WT C57BL/6 mice treated systemically with TNF-α (Figure 1A). Injection of FcγRIIB/III mAb was sufficient to saturate endogenous FcγRIIB/III antigens on neutrophils by >98%, compared to control IgG2b injection (Figure 1B). We then evaluated the effects of IVIG on leukocyte recruitment by direct observation using intravital microscopy in the microvasculature of the WT mice. We infused human albumin to control for potential changes in intravascular oncotic pressures from high-dose IVIG.9, 11 IVIG administration did not alter the number of circulating leukocytes and the percentage of major leukocyte subsets, including lymphocytes, neutrophils and monocytes, compared to control albumin administration in the presence and absence of endogenous FcγRIIB/III expression on leukocytes (Figure 1C and D). In addition, IVIG administration did not cause vasodilation or alter hemodynamic parameters (i.e. blood flow, centerline velocity and shear rate) compared albumin-infused control group in wild-type mice (Online Figure I, A and B, and Online Table I). Interestingly, IVIG administration significantly inhibited leukocyte adhesion to endothelium (~30% reduction, 875 ± 54 versus 1,298 ± 68 adherent wbcs/mm2; P<0.001), whereas FcγRIIB/III blockade negated its inhibitory effect on leukocyte adhesion to endothelium (1,221 ± 95 versus 1,232 ± 68 adherent wbcs/mm2) compared to control albumin administration in microvessels with identical average venular diameter (Figure 1C and Online Table I).
Figure 1. FcγRIIB/III mediate IVIG-induced inhibition of leukocyte recruitment and RBC interactions.
(A) Experimental scheme. WT mice (n= 11–14 per group) were injected with either mAb FcγRIIB/III or isotype rat IgG2b (1 mg/kg) followed by IVIG or control albumin administration (800 mg/kg) 3 h after administration of TNF-α (0.5 ug). Leukocyte behaviors were analyzed in cremasteric venules for 1 h. (B) Blood from WT mice was collected after intravital microscopy experiment and surface expression of FcγRIIB/III on the neutrophil population gated on the basis of side and forward scatter properties was examined by flow cytometry after PE-conjugated anti-FcγRIIB/III staining. (C) Numbers of circulating leukocytes. (D) The percentages of monocytes, neutrophils and lymphocytes. (E) Adherent leukocytes in venules. (F) Number of circulating RBC-adherent leukocyte interactions per min. Bars represent mean ± SEM. **P<0.01, ***P<0.001 vs albumin.
The microvascular obstruction is a complex multicellular process involving endothelial activation, leukocyte adhesion to endothelium, and the direct interaction of circulating red blood cells (RBCs) and adherent leukocytes (WBCs).26 In previous studies, we demonstrated that interactions between adherent leukocytes and RBC carrying normal hemoglobin (nRBC) occurred in inflamed venules under relatively low shear rates (< 500 s−1).22 We thus examined whether IVIG infusion could inhibit the interactions between adherent leukocyte and nRBCs. IVIG administration significantly reduced heterotypic interactions between adherent leukocytes and circulating nRBC interactions (67% reduction, 0.05 ± 0.01 versus 0.15 ± 0.02 RBC-WBC interactions/min; P<0.001), while FcγRIIB/III blockade abrogated its inhibitory action on heterotypic interactions (0.16 ± 0.02 versus 0.15 ± 0.02 RBC-WBC interactions/min) under similar hemodynamic conditions (Figure 1D and Online Table II). These results demonstrate a clear requirement for FcγRIIB/III for the protective actions of IVIG on leukocytes recruitment and activation during acute inflammation, suggesting that IVIG might modulate their expression or function.
FcγRIII, but not FcγRIIB, is a major Fcγ receptor on neutrophils
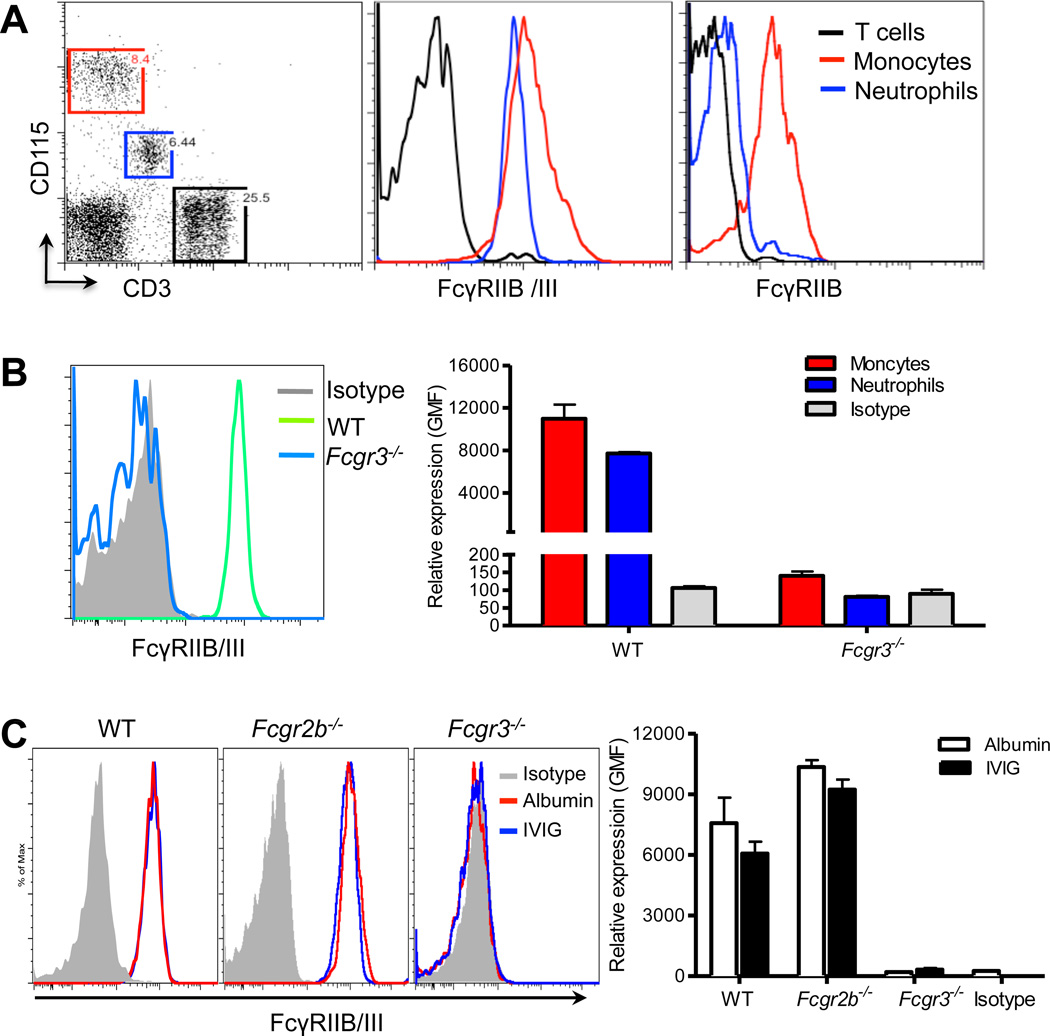
In previous studies, we found that IVIG specifically targets the recruitment of neutrophils, which make up the majority of adherent leukocytes in inflamed venules.3, 9 We therefore examined the cell surface expression of both FcγRIIB and FcγRIII on circulating neutrophils using myeloid lineage-specific markers (i.e. Gr-1, CD115 and F4/80). Gr-1hi and CD115low neutrophils were labeled by a dual-specific antibody to FcγRIIB/III, but barely by one directed at FcγRIIB, compared to expression levels of positive CD115hi monocyte and negative CD3pos T cell controls (Figure 2A). Because the high degree of homology between FcγRIIB and FcγRIII has prevented the generation of specific antibodies against individual low affinity Fcγ receptors, we have used FcγRIII-deficient (Fcgr3−/−) mice to examine further the surface expression of FcγRIIB on neutrophils. In Gr-1hi CD115low neutrophils, the binding of the antibody recognizing FcγRIIB/III in Fcgr3−/− mice was virtually undetectable, compared to WT mice (Figure 2B and Online Figure II), suggesting that mouse neutrophils constitutively express FcγRIII but little or no FcγRIIB in the steady state.
Figure 2. FcγRIII, but not FcγRIIB, is expressed on neutrophils.
(A) Circulating leukocytes from WT mice were stained for FcγRIIIB/III, CD115, CD3 expression after RBC lysis. Neutrophils (blue) were gated as CD115low, monocytes (red) as CD115hi, T cell population (black) as CD3pos. (B) Blood was collected from WT (green, n=3) and Fcgr3−/− (blue, n=6) mice and then stained for Gr-1, CD115, and FcγRIIB/III expression. FcγRIIB/III expression was analyzed by gating on the neutrophil population with Gr-1hi and CD115low and monocyte population with Gr-1low-hi and CD115hi. Representative histogram of FcγRIIB/III expression and control isotype (gray, left panel) and quantification of the geometric mean of fluorescence (GMF) (right panel). (C) Representative expression of FcγRIIIB/III (left panel) on neutrophil population (Gr-1hi/ CD115low) from TNF-α treated mice deficient in the FcγRIB, FcγRIII or control WT mice (n= 2–3 per group) 1 h after administration of IVIG or control albumin. Gray histograms represent isotype control. Quantification of FcγRIIIB/III expression levels from WT, Fcgr2−/− and Fcgr3−/− mice (right panel).
It has been proposed that the benefits of IVIG may result from the induction of expression of inhibitory FcγRIIB on tissue-infiltrating macrophages, thereby raising the threshold required for triggering activating FcγRs in autoimmune disease.23, 27 Thus, we sought to determine whether low affinity FcγRIIB/III expression on neutrophils is changed after IVIG administration. We found that the expression levels of FcγRIIB/III on neutrophils did not change 1 h after administration of IVIG or control albumin into control WT, FcγRIIB-deficient (Fcgr2b−/−), and Fcgr3−/− mice (Figure 2C). These results indicate that IVIG administration does not induce inhibitory FcγRIIB expression on neutrophils and that the activating FcγRIII is a major receptor in murine neutrophils.
IVIG inhibition of leukocyte recruitment and activation is mediated by FcγRIII
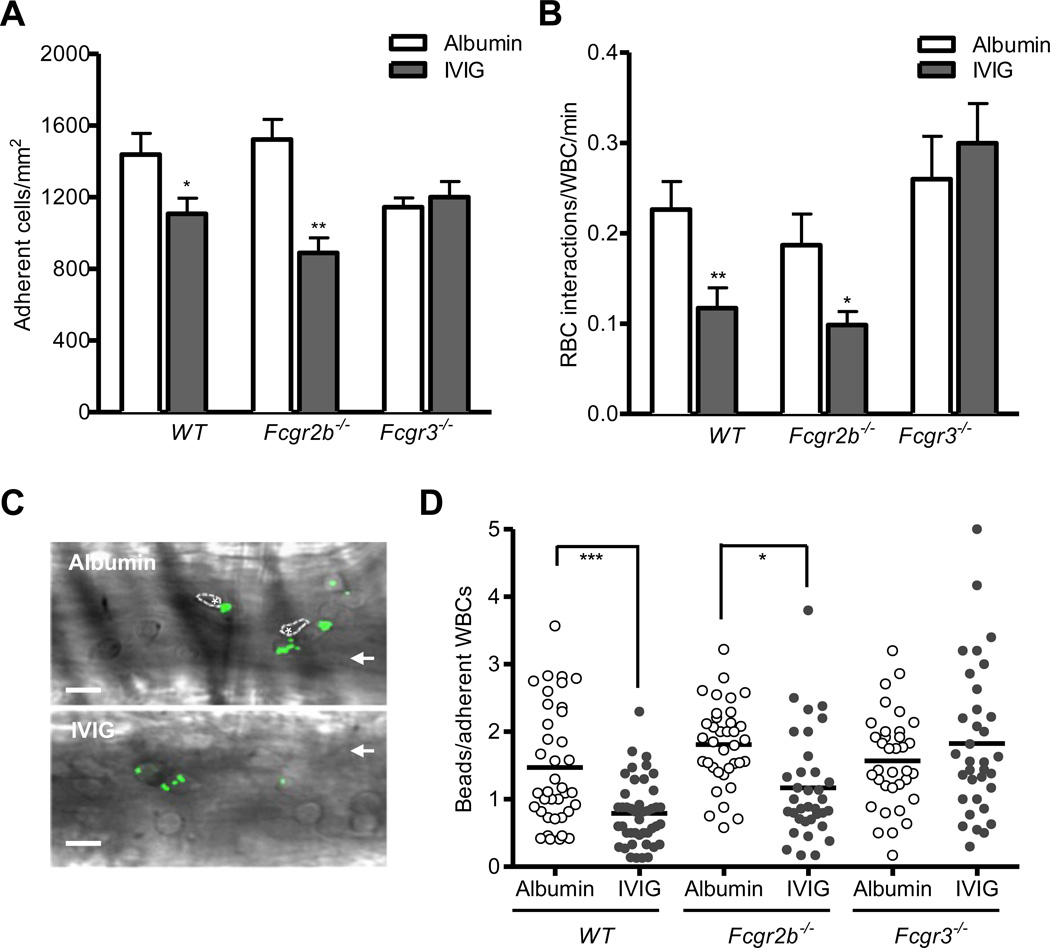
In mice, both inhibitory FcγRIIB and activating FcγRIII are important for IVIG-mediated amelioration of inflammation.23–25, 27 To determine the differential contribution of FcγRIIB and FcγRIII to IVIG-mediated protection, we assessed leukocyte recruitment and heterotypic interactions between adherent leukocytes and circulating RBCs after IVIG or control albumin administration in the context of systemic acute inflammation in control WT, Fcgr2b−/−, or Fcgr3−/− mice. As compared to control albumin, IVIG administration significantly reduced leukocyte adhesion to endothelium by approximately 30–40% in both control WT (1108 ± 88 versus 1439 ± 117 adherent wbcs/mm2, P<0.05) and Fcgr2b−/− mice (890 ± 84 versus 1523 ± 112 adherent wbcs/mm2, P<0.01). Strikingly, its inhibitory effect on leukocyte recruitment was absent in Fcgr3−/− mice (1201 ± 87 versus 1145 ± 51 adherent wbcs/mm2; Figure 3A and Online Table III). Furthermore, IVIG administration significantly reduced RBC interactions with adherent leukocytes by 50% under relatively low shear rates (< 500 s−1) in both control WT (0.12 ± 0.02 versus 0.23 ± 0.03, RBC-WBC interactions/min; P<0.01) and Fcgr2b−/− (0.10 ± 0.02 versus 0.19 ± 0.03 RBC-WBC interactions/min; P<0.05) mice but not Fcgr3−/− (0.30 ± 0.04 versus 0.26 ± 0.05, RBC-WBC interactions/min) mice compared to control albumin administration (Figure 3B and Online Table IV).
Figure 3. FcγRIII mediates IVIG-induced inhibition of leukocyte adhesion and Mac-1 activity.
IVIG or control albumin (800 mg/kg) was administrated 3 h after TNF-α injection (0.5 µg) in WT, Fcgr2b−/−, and Fcgr3−/−mice (n= 5-7 per group). Cremasteric venules were analyzed by intravital microscopy for 1 h. (A) Adherent leukocytes in venules. (B) Number of circulating RBC-adherent leukocyte interactions per min. Bars represent mean ± SEM. *P<0.05, **P<0.01 vs albumin. (C) Representative images of fluosphere bound to leukocytes in venules from WT mice. Images were acquired in the brightfield and FITC channel. Asterisks represent RBCs that interact with adherent leukocytes and arrows indicate the direction of flow. Scale bar, 20 µm. (D) Binding of albumin-coated fluospheres to leukocytes in WT, Fcgr2b−/−, and Fcgr3−/− mice. Mice prepared for intravital microscopy were intravenously injected with 109 albumin-coated fluosphere 10 min after administration of either IVIG or control albumin. Each dot represents the average number of fluospheres bound per leukocyte within individual venules (n= 35–45 venules from four mice per group). *P<0.05, ***P<0.001, Mann-Whitney test.
Activated Mac-1 integrin microdomains at the leading edge of neutrophils, a phenomenon that is triggered by E-selectin expressed on the inflamed endothelium drives vascular damage in SCD through heterotypic interactions.22 We thus assessed the effect of IVIG on Mac-1 activity in adherent leukocytes using a previously validated in vivo fluosphere bead-binding assay.22 This specifically assay measure Mac-1 activity on adherent neutrophils, since albumin-coated fluospheres captured by adherent Gr-1posF4/80neg neutrophils have been shown to be absent in Mac-1-deficient (Itgam−/−) mice.22 Overlay of images acquired in bright field and fluoresecence channels revealed that the capture of albumin-coated fluorescent beads by adherent leukocytes was strongly inhibited in IVIG-infused mice (Figure 3C). In parallel with the reduction in heterotypic interactions with RBCs seen after IVIG treatment, IVIG administration significantly reduced Mac-1 activity in leukocytes compared to control albumin administration in both control WT and Fcgr2b−/− mice. However, IVIG-induced reduction of Mac-1 activity was abrogated in Fcgr3−/− mice (Figure 3D). Taken together, these results indicate that FcγRIII mediates IVIG-induced inhibition of leukocyte adhesion to endothelium, heterotypic RBC interactions with adherent leukocytes, and Mac-1 integrin activation.
Involvement of SHP-1 in the inhibitory effect of IVIG on leukocyte recruitment
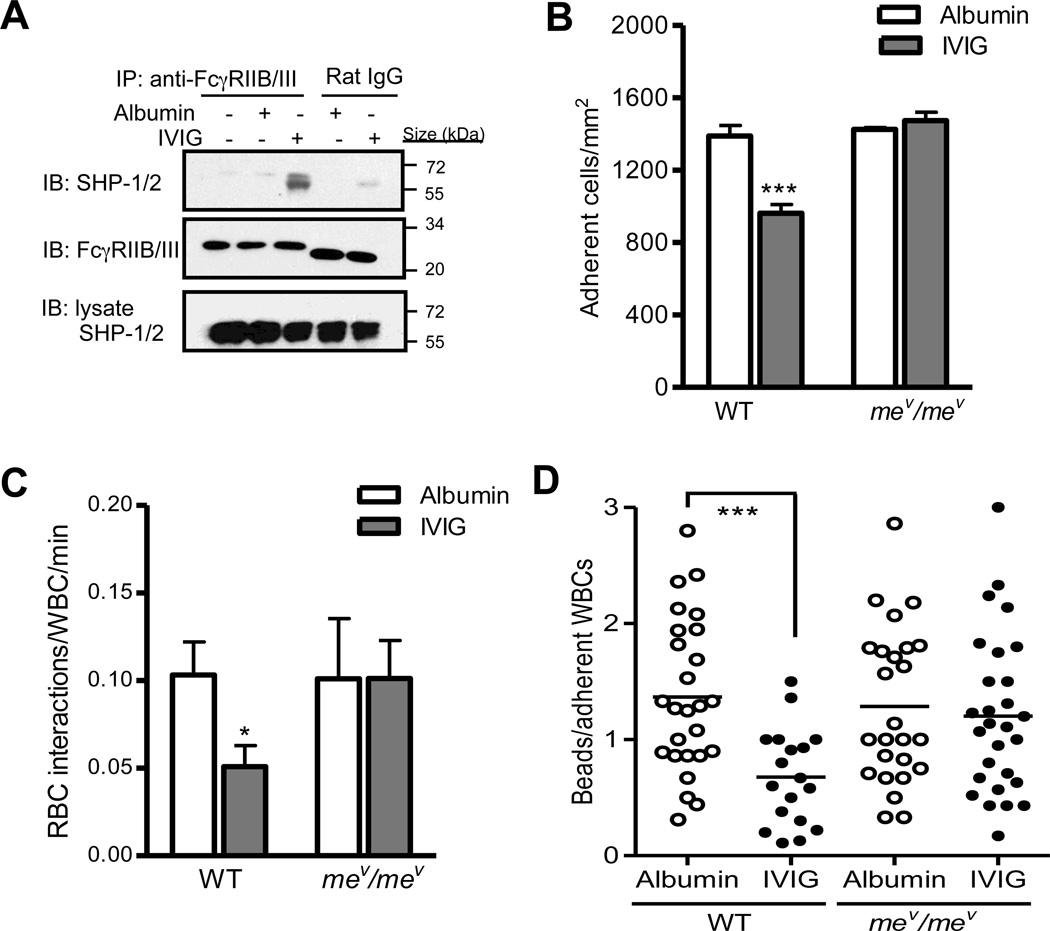
Previous studies have provided evidence that activating FcγRs associated with ITAMs can induce inhibitory signaling mediated by recruitment of Src homology 2(SH2)-containing tyrosine phosphatase-1 (SHP-1).28, 29 The tyrosine phosphatase SHP-1 can act as a negative regulator that dephosphorylates multiple immunoreceptor-regulated substrates, leading to cell inactivation.30 We hypothesized that IVIG induces the recruitment of SHP-1 to FcγRIII in neutrophils, thereby inactivating ITAM-induced activating signal cascade through dephosphorylation. To assess whether SHP-1 is recruited to FcγRIII in response to IVIG, bone marrow neutrophils (BMNs) isolated from control WT mice were incubated with either IVIG or control albumin, and then analyzed the association of SHP-1 with FcγRIIB/III. As compared to either resting or albumin-treated BMNs, IVIG treatment induced the recruitment of SHP-1 to FcγRIIB/III in neutrophils from WT mice (Figure 4A). In addition, IVIG-induced SHP-1 recruitment to FcγRIIB/III was also observed in neutrophils from Fcgr2−/− mice (Online Figure III). This indicates SHP-1 associates with FcγRIII in response to IVIG, suggesting that SHP-1 may be involved in FcγRIII-mediated inhibitory pathways induced by IVIG.
Figure 4. Abrogation of IVIG-induced anti-inflammatory activity in mev/mev mice.
(A) SHP-1 association with FcγRIIB/III in response to IVIG in neutrophils. Bone marrow neutrophils (BMNs) isolated from control WT mice (n= 3) were incubated with IVIG or albumin (6.7 mg/ml) at 37 °C for 15 min, and then lysates were prepared. Lysates were immunoprecipitated with anti-FcγRIIB/III or control isotype rat IgG2b followed by immunoblotting (IB) with anti-SHP-1/2 ab. (B) Adherent leukocytes in venules. (C) Number of circulating RBC-adherent leukocyte interactions per min. Bars represent mean ± SEM. *P<0.05, ***P<0.001 vs albumin. (D) Binding of albumin-coated flurosphere to leukocytes10 min after either IVIG or control albumin administration in control chimeric WT or mev/mev mice. Each dot represents the average number of fluospheres bound per leukocyte within individual venules (n= 20–28 venules from 4 mice per group). ***P<0.001 vs albumin, Mann-Whitney test.
We next gauged the role of SHP-1 in IVIG’s anti-inflammatory effect on leukocyte recruitment and activation by assessing motheaten viable (mev/mev) mice that have reduced phosphatase activity due to a mutation inSHPTP1.31 Neutrophils from mev/mev mice show increased oxidative production, surface expression of β2 integrins, and leukocyte adhesion in vitro,32 suggesting a significant role for SHP-1 in modulating the tyrosine phosphorylation signaling pathways that regulate neutrophil activation. To analyze the contribution of SHP-1 in the hematopoietic lineage, we generated chimeras by transplantation of WT and mev/mev bone marrow donors into WT recipients (Online Figure IV, A and B). In contrast to WT chimeras, administration of IVIG in mev/mev bone marrow chimeras did not alter leukocyte recruitment, RBC interactions with adherent leukocytes, and Mac-1 activity (Figure 4B through 4D and Online Table V and VI). Taken together, these results suggest that IVIG mediates its inhibitory effect on leukocyte recruitment and activation via recruitment of SHP-1 to FcγRIII.
FcγRIII mediate the protective effect of IVIG against acute vaso-occlusion
Acute vaso-occlusive crisis (VOC) is the most common complication of sickle cell disease (SCD) and is a major cause of morbidity and mortality for SCD patients.33 Previous studies have established that IVIG can reverse acute vaso-occlusive crisis (VOC) in a humanized murine model of SCD via specific inhibition of neutrophil recruitment and their interactions with circulating red blood cells (RBCs).9, 11 We thus tested whether engagement of the low affinity receptors FcγRIIB/III mediate the protective effect of IVIG on acute VOC in SCD similar to systemic acute inflammation in WT mice. We injected mice with a mAb against FcγRIIB/III or control isotype rat IgG2b, prior to IVIG or control human albumin administration in TNF-α-treated SCD mice (Figure 5A). Injection of 1 mg/kg FcγRIIB/III mAb was sufficient to saturate endogenous FcγRIIB/III antigens on neutrophils by >96%, compared to control IgG2b injection (Figure 5B). Consistent with previous studies,9, 11 IVIG administration significantly reduced leukocyte adhesion to endothelium (~30% reduction, 1,303 ± 96 versus 1,961 ± 123 adherent wbcs/mm2; P<0.01) and heterotypic adherent leukocyte-RBC interactions (>98% reduction, 0.01± 0.001 vs. 0.80 ± 0.3 RBC-WBC interactions/min; P<0.001) in control IgG2b-treated SCD mice (Figure 5C and D). Although IVIG administration did not cause vasodilatory response in both arteries and veins, its infusion significantly improved blood flow rates, a surrogate measure for vaso-occlusion, compared to albumin-infused control group (P<0.01, Figure 5E and Online Figure V, A and B), as well as the mean centerline RBC velocity (VRBC) and wall shear rate in venules (P<0.01, respectively; Table 1). Importantly, blockade of endogenous FcγRIIB/III abrogated IVIG’s alteration of leukocyte adhesion to endothelium (2,100 ± 134 versus 2,026 ± 181 adherent wbcs/mm2, heterotypic adherent leukocyte-RBC interactions (0.26 ± 0.06 versus 0.28 ± 0.10 RBC-WBC interactions/min), and blood flow rates (271 ± 16 versus 265 ± 15 nL/s) (Figure 5C through 5E). Furthermore, IVIG-induced inhibitory effect on leukocyte recruitment led to reduced sickling, resulting in protection from intermittent vaso-occlusion in post-capillary venules, while FcγRIIB/III blockade abrogated IVIG’s suppressive effect on acute vaso-occlusion (Figure 5F and Online Movie I to IV). In addition, IVIG significantly prolonged the survival time of SCD mice compared to albumin-infused control group (P<0.05, log-rank test), whereas FcγRIIB/III blockade abrogated the survival benefit (Figure 5G). Taken together, endogenous FcγRIIB/III blockade negated the protective effect of IVIG on acute VOC in SCD mice, thus demonstrating that the beneficial effects of IVIG during neutrophil-mediated acute injury result from the interaction with, and signaling through, FcγRIII.
Figure 5. Blockade of FcγRIIB and III abrogates the protective actions of IVIG against acute vaso-occlusion in SCD mice.
(A) Experimental scheme. Three hours after administration of TNF-α (0.5 ug), SCD mice (n= 8 per group) were injected with either anti-FcγRIIB/III monoclonal antibody (mAb) or isotype rat IgG2b, (1 mg/kg) before administration of IVIG or an equivalent volume of human albumin (800 mg/kg). (B) Blood from SCD mice was collected after intravital experiment and surface expression of FcγRIIB/III on the neutrophils was examined with PE-conjugated anti-FcγRIIB/III mAb by flow cytometry. (C) Adherent leukocytes in venules. (D) Number of circulating sickle RBC-adherent leukocyte interactions per min. (E) Blood flow rates. Bars represent mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs albumin. (F) Representative images of each group after IVIG or control albumin administration showing leukocyte recruitment and heterotypic interactions. Scale bars, 20 µm. (G) The Kaplan-Meier survival curves for individual SCD mice. P=0.03, log-rank test, IVIG vs albumin in IgG2b-treated group. P=0.93, log-rank test, IVIG vs albumin in anti-FcγRIIB/III mAb-treated group.
Table 1.
Hemodynamic parameters after IVIG or control albumin administration following injection of anti-FcγRIIB/III mAb or IgG2b control in TNF-α-treated SCD mice.
| Treatment | Mice (n) |
Venule (n) |
Venular diameter (µm) |
Centerline velocity (mm/s) |
Shear rate (s−1) |
|
|---|---|---|---|---|---|---|
| IgG2b | Albumin | 6 | 51 | 22 ± 0.6 | 1.3 ± 0.1 | 657± 53 |
| IVIG | 8 | 76 | 20 ± 0.2 | 1.9 ± 0.1 ** | 990 ± 60** | |
| Anti- FcγRIIB/III |
Albumin | 7 | 61 | 21 ± 0.2 | 1.2 ± 0.1 | 622± 27 |
| IVIG | 8 | 56 | 21 ± 0.3 | 1.2 ± 0.1 | 601 ± 44 | |
Hemodynamic parameters were analyzed from intravital microscopy recording of venules used for the results shown in Figure 5C and 5D. Data are presented as mean ± SEM.
P<0.01 vs albumin.
Discussion
IVIG is an effective therapeutic agent in a variety of autoimmune diseases or chronic inflammatory disorders. The mechanisms of IVIG action have been most thoroughly investigated in autoantibody-mediated diseases, but the exact mechanism by which IVIG prevents leukocyte recruitment and activation during acute vascular injury remains poorly understood. Using intravital microscopy, we have directly studied the roles of IVIG in intravascular neutrophil recruitment and activation in TNF-α-induced acute inflammation. Our data demonstrate that IVIG inhibits leukocyte adhesion to endothelium and heterotypic RBC interactions with adherent leukocytes by reducing Mac-1 activity on neutrophils. In addition, our studies identify FcγRIII as the FcγR required for mediating IVIG’s anti-inflammatory activity. Engagement of IVIG to FcγRIII induces recruitment of the protein tyrosine phosphatase, SHP-1, which subsequently inhibits leukocyte recruitment and activation (Online Figure VI).
The anti-inflammatory activity of high dose IVIG can be attributed to the minor fraction of dimeric or sialylated IgG in a murine model of immune thrombocytopenic purpura (ITP) that macrophages are responsible for the clearance of autoantibody-coated platelets 23, 34, 35. These studies have suggested that IVIG increases expression of the inhibitory receptor FcγRIIB on the surface of inflammatory macrophages, thereby resulting in suppression of autoantibody-triggered inflammation.23, 36 Thus, macrophages containing FcγRIIB in autoantibody-mediated disorders are the effector cells in the anti-inflammatory response mediated by IVIG. Since neutrophils represent the vast majority of adherent leukocytes in inflamed venules and are specifically targeted by IVIG in SCD 9, we have examined the surface expression of FcγRIIB and FcγRIII on IVIG-treated neutrophils. Our results show that neutrophils predominantly express FcγRIII but not FcγRIIB in the steady state. Interestingly, IVIG administration did not alter the surface expression of either inhibitory FcγRIIB or activating FcγRIII on neutrophils. Based on our data and previous studies, IVIG may therefore differentially act on distinct effector cells, or potentially affect various leukocyte subsets through different mechanisms of action.
Neutrophil recruitment during inflammation is classically attributed to a multi-step cascade involving selectin-mediated initial tethering and rolling along the vessel wall, followed by β2 integrin-mediated firm adhesion to the vascular endothelium.37 During leukocyte recruitment, IVIG modulates these adhesion molecules, including PSGL-1, Mac-1(αMβ2), and LFA-1(αLβ2) on leukocytes.10, 38 Moreover, analyses of leukocyte recruitment by intravital microscopy have also revealed that IVIG can inhibit P-selectin-dependent leukocyte rolling, E-selectin-mediated slow rolling, and β2 integrin-dependent leukocyte adhesion to the endothelium in vivo suggesting that IVIG directly targets leukocytes.9–11 The receptors and signaling pathways potentially involved in this direct inhibitory effect remain poorly understood. We found that blockade of endogenous FcγRIIB/III abrogated IVIG’s anti-inflammatory activity during leukocyte recruitment. In addition the inhibitory effects of IVIG on leukocyte recruitment and activation were abolished in Fcgr3−/−mice, indicating the requirement of FcγRIII to mediate IVIG’s anti-inflammatory activity during leukocyte recruitment.
Activating FcγRIII is associated with the common γ-chain, which contains an ITAM motif. Although ITAMs are used by multiple receptors to activate immune cells, recent studies have suggested that ITAMs can paradoxically function to propagate inhibitory signals under specific conditions.39 ITAM-mediated inhibition downstream of activating FcγRs can occur when a low-affinity ligand favors the recruitment of a signaling effector such as protein tyrosine phosphatase SHP-1 with inhibitory potential instead of activation of signal-promoting kinases.28, 29 In agreement with this contention, our data show that the protein tyrosine phosphatase SHP-1 is recruited to FcγRIII in IVIG-treated neutrophils compared to either resting or albumin-treated neutrophils, suggesting that IVIG induces recruitment of SHP-1 to FcγRIII in neutrophils.
IVIG significantly increases leukocyte rolling velocities, suggesting that it alters adhesion pathways involving E-selectin.9 Interestingly, E-selectin engagement cannot induce LFA-1-dependent slow rolling in the absence of ITAM-associated with immunoreceptor in leukocytes.40 E-selectin-mediated intracellular signaling pathways in neutrophils indeed exhibit strong similarities to that of FcγR or β2 integrin-mediated outside-in signaling. Binding of neutrophils to E-selectin on inflamed endothelium activates Src family kinases (SFKs), which in turn activates ITAM-dependent pathways, such as spleen tyrosine kinase (Syk), phosphoinositide-3-kinase (PI3K), and p38 mitogen-activated protein kinase, resulting in LFA-1-dependent slow rolling and Mac-1 activation at the leading edge of adherent neutrophils.18, 22, 40–42Since protein tyrosine phosphatases such as SHP-1 can switch off ITAM-induced activating signaling cascades,30 our results suggest the possibility that the recruitment of SHP-1 in response to IVIG inactivates ITAM-mediated signals, including E-selectin- and FcγRIII-induced pathways. This results in alteration in neutrophil responses triggered by these receptors: Mac-1 activation, reduced β2 integrin-dependent neutrophil arrest, and reduced Mac-1-dependent RBC interactions. Therefore, signaling pathways controlled by SHP-1 phosphatase may become therapeutic targets to control neutrophil functions in inflammatory disease.
Of particular relevance, we have assessed the contribution of FcγRs in IVIG-induced protection from VOC in the context of SCD. Consistent with the central role of integrin activation and RBC capture by neutrophils during vascular occlusion, blockade of FcγRIIB/III prior to IVIG delivery prevented all the beneficial effects associated with this treatment. In addition, IVIG inhibits ROS-producing neutrophils in a experimental model of transfusion-related acute lung injury (TRALI) in which mechanisms similar to those leading to vaso-occlusion promote vascular and organ injury (data not shown).22 These results suggest that other types of vascular disease could also be benefit from the insights on IVIG’s mode of action reported herein. For example, neutrophils have been recently shown to participate in chronic arterial disease, including atherosclerosis 43 or restenosis 44. One could envision that prolonged neutrophil activation, through mechanisms partly shared with the acute processes described here, contributes to vascular injury in large vessels.
In summary, this study reveals an inhibitory role for FcγRIII in response to IVIG, resulting in recruitment of SHP-1 and preventing neutrophil adhesion and activation in areas of vascular injury. Therefore, elucidating the target signals affected by IVIG-induced SHP-1 recruitment will provide novel insights for designing therapeutic strategies that prevent vascular disease.
Novelty and Significance.
What Is Known?
Immunoglobulins are potent immune modulators acting through Fcγ receptors (FcγR).
Intravenous immunoglobulins (IVIG) are widely used to treat a variety of autoimmune disease and inflammatory disorders.
IVIG inhibits neutrophil recruitment that mediates vascular damage and occlusion during sickle cell disease crises.
What New Information Does This Article Contribute?
Activating FcγRIII, rather than the classical inhibitory FcγRIIB, on neutrophils mediates IVIG-induced inhibition of neutrophil recruitment and activation.
Src homology 2 (SH2)-containing tyrosine phosphatase-1 (SHP-1) is a critical downstream mediator involved in FcγRIII-mediated IVIG’s anti-inflammatory activity.
SHP-1 is a potential therapeutic target in neutrophil-mediated vascular injury.
Summary.
The recruitment and activation of neutrophils in the microvasculature is a key pathogenic factor that contributes to vascular injury, including vaso-occlusive episodes in sickle cell disease. IVIG treatment exerts anti-inflammatory activity by reducing neutrophil recruitment, but the mechanisms underlying its modulation on neutrophil function remain unclear. Understanding the mechanisms by which IVIG dampens neutrophil recruitment and activation will provide valuable insights for designing therapeutic strategies aimed at preventing neutrophil-mediated vasculopathies. Here we show that engagement of IVIG to activating FcγRIII, but not the inhibitory FcγRIIB, inhibited neutrophil recruitment and activation. Furthermore, the FcγRIII-induced IVIG anti-inflammatory activity in neutrophils was mediated by recruitment of protein tyrosine phosphatase SHP-1. This study demonstrates an unexpected inhibitory role of FcγRIII on neutrophil adhesion and activation in response to IVIG and implicates SHP-1 in the therapeutic efficacy of IVIG. Thus, elucidating the target signals affected by IVIG-induced SHP-1 recruitment will provide novel insight for designing therapeutic strategies to prevent vascular disease.
Supplementary Material
Acknowledgments
We thank to Colette Prophete for technical assistance, and Christoph Scheiermann, Julie Lacombe, Andrew Chow, and Daniel Lucas for discussions and comments on the manuscript.
Sources of Funding
This work was supported by NIH grants R01HL69438, RC1HL099545, and R01HL097700 (to P. S. F.); a Ramón y Cajal fellowship, and grants SAF2009-11037 from the Spanish Ministry of Science and Innovation and 246655 from the FP7-People-IRG Program (to A. H.); and Founders Affiliate Predoctoral Fellowship from the American Heart Association (to J-E. J.). P. S. F. was supported by an Established Investigator Award from the American Heart Association.
Non-standard Abbreviations and Acronyms
- SCD
sickle cell disease
- IVIG
intravenous immunoglobulin
- ITAMs
immunoreceptor tyrosine-based activation motifs
- SHP-1
src homology 2 (SH2)-containing tyrosine phosphatase-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Lard LR, Mul FP, de Haas M, Roos D, Duits AJ. Neutrophil activation in sickle cell disease. Journal of leukocyte biology. 1999;66:411–415. doi: 10.1002/jlb.66.3.411. [DOI] [PubMed] [Google Scholar]
- 2.Mehta J, Dinerman J, Mehta P, Saldeen TG, Lawson D, Donnelly WH, Wallin R. Neutrophil function in ischemic heart disease. Circulation. 1989;79:549–556. doi: 10.1161/01.cir.79.3.549. [DOI] [PubMed] [Google Scholar]
- 3.Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nature methods. 2007;4:219–222. doi: 10.1038/nmeth1018. [DOI] [PubMed] [Google Scholar]
- 4.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao PS, Ma XL, Lefer AM. Activated neutrophils aggravate endothelial dysfunction after reperfusion of the ischemic feline myocardium. American heart journal. 1992;123:1464–1471. doi: 10.1016/0002-8703(92)90796-x. [DOI] [PubMed] [Google Scholar]
- 6.Jang JE, Hod EA, Spitalnik SL, Frenette PS. Cxcl1 and its receptor, cxcr2, mediate murine sickle cell vaso-occlusion during hemolytic transfusion reactions. The Journal of clinical investigation. 2011;121:1397–1401. doi: 10.1172/JCI45336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annual review of immunology. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 8.Baerenwaldt A, Biburger M, Nimmerjahn F. Mechanisms of action of intravenous immunoglobulins. Expert review of clinical immunology. 2010;6:425–434. doi: 10.1586/eci.10.9. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Shi PA, Chiang EY, Frenette PS. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111:915–923. doi: 10.1182/blood-2007-04-084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill V, Doig C, Knight D, Love E, Kubes P. Targeting adhesion molecules as a potential mechanism of action for intravenous immunoglobulin. Circulation. 2005;112:2031–2039. doi: 10.1161/CIRCULATIONAHA.105.546150. [DOI] [PubMed] [Google Scholar]
- 11.Turhan A, Jenab P, Bruhns P, Ravetch JV, Coller BS, Frenette PS. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103:2397–2400. doi: 10.1182/blood-2003-07-2209. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Lukita-Atmadja W, Machen NW, Baker GL, McCuskey RS. Effect of intravenous immunoglobulin g on the tnfalpha-mediated hepatic microvascular inflammatory response. Shock. 1999;11:291–295. doi: 10.1097/00024382-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature reviews. Immunology. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 14.Coxon A, Cullere X, Knight S, Sethi S, Wakelin MW, Stavrakis G, Luscinskas FW, Mayadas TN. Fc gamma riii mediates neutrophil recruitment to immune complexes. A mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 15.Tsuboi N, Asano K, Lauterbach M, Mayadas TN. Human neutrophil fcgamma receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity. 2008;28:833–846. doi: 10.1016/j.immuni.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones SL, Knaus UG, Bokoch GM, Brown EJ. Two signaling mechanisms for activation of alpham beta2 avidity in polymorphonuclear neutrophils. The Journal of biological chemistry. 1998;273:10556–10566. doi: 10.1074/jbc.273.17.10556. [DOI] [PubMed] [Google Scholar]
- 17.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nature immunology. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. Psgl-1 engagement by e-selectin signals through src kinase fgr and itam adapters dap12 and fcr gamma to induce slow leukocyte rolling. The Journal of experimental medicine. 2008;205:2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 20.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in fc gamma rii-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 21.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired igg-dependent anaphylaxis and arthus reaction in fc gamma riii (cd16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nature medicine. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of ivig mediated through the inhibitory fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 24.Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, Nutt SL, Hu X, Ivashkiv LB. Fcgammariii-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Siragam V, Crow AR, Brinc D, Song S, Freedman J, Lazarus AH. Intravenous immunoglobulin ameliorates itp via activating fc gamma receptors on dendritic cells. Nature medicine. 2006;12:688–692. doi: 10.1038/nm1416. [DOI] [PubMed] [Google Scholar]
- 26.Frenette PS. Sickle cell vaso-occlusion: Multistep and multicellular paradigm. Current opinion in hematology. 2002;9:101–106. doi: 10.1097/00062752-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for ivig protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 28.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffie C, Henin D, Benhamou M, Pretolani M, Blank U, Monteiro RC. Identification of fcalphari as an inhibitory receptor that controls inflammation: Dual role of fcrgamma itam. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro da Silva F, Aloulou M, Skurnik D, Benhamou M, Andremont A, Velasco IT, Chiamolera M, Verbeek JS, Launay P, Monteiro RC. Cd16 promotes escherichia coli sepsis through an fcr gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nature medicine. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Somani AK, Siminovitch KA. Roles of the shp-1 tyrosine phosphatase in the negative regulation of cell signalling. Seminars in immunology. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 31.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 32.Kruger J, Butler JR, Cherapanov V, Dong Q, Ginzberg H, Govindarajan A, Grinstein S, Siminovitch KA, Downey GP. Deficiency of src homology 2-containing phosphatase 1 results in abnormalities in murine neutrophil function: Studies in motheaten mice. Journal of immunology. 2000;165:5847–5859. doi: 10.4049/jimmunol.165.10.5847. [DOI] [PubMed] [Google Scholar]
- 33.Frenette PS, Atweh GF. Sickle cell disease: Old discoveries, new concepts, and future promise. The Journal of clinical investigation. 2007;117:850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teeling JL, Jansen-Hendriks T, Kuijpers TW, de Haas M, van de Winkel JG, Hack CE, Bleeker WK. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin g dimers: Studies in experimental immune thrombocytopenia. Blood. 2001;98:1095–1099. doi: 10.1182/blood.v98.4.1095. [DOI] [PubMed] [Google Scholar]
- 35.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of ivig. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel t(h)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigal D, Vermot-Desroches C, Heitz S, Bernaud J, Alfonsi F, Monier JC. Effects of intravenous immunoglobulins (ivig) on peripheral blood b, nk, and t cell subpopulations in women with recurrent spontaneous abortions: Specific effects on lfa-1 and cd56 molecules. Clinical immunology and immunopathology. 1994;71:309–314. doi: 10.1006/clin.1994.1091. [DOI] [PubMed] [Google Scholar]
- 39.Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory itams as novel regulators of immunity. Immunological reviews. 2009;232:59–71. doi: 10.1111/j.1600-065X.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 40.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase syk is necessary for e-selectin-induced alpha(l)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of e-selectin ligands on neutrophils reveals distinct functions of psgl-1, esl-1, and cd44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, Zarbock A. Tyrosine kinase btk regulates e-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase c (plc) gamma2 and pi3kgamma pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C. Protective role of cxc receptor 4/cxc ligand 12 unveils the importance of neutrophils in atherosclerosis. Circulation research. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 44.Soehnlein O, Wantha S, Simsekyilmaz S, Doring Y, Megens RT, Mause SF, Drechsler M, Smeets R, Weinandy S, Schreiber F, Gries T, Jockenhoevel S, Moller M, Vijayan S, van Zandvoort MA, Agerberth B, Pham CT, Gallo RL, Hackeng TM, Liehn EA, Zernecke A, Klee D, Weber C. Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Science translational medicine. 2011;3 doi: 10.1126/scitranslmed.3002531. 103ra198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.