Abstract
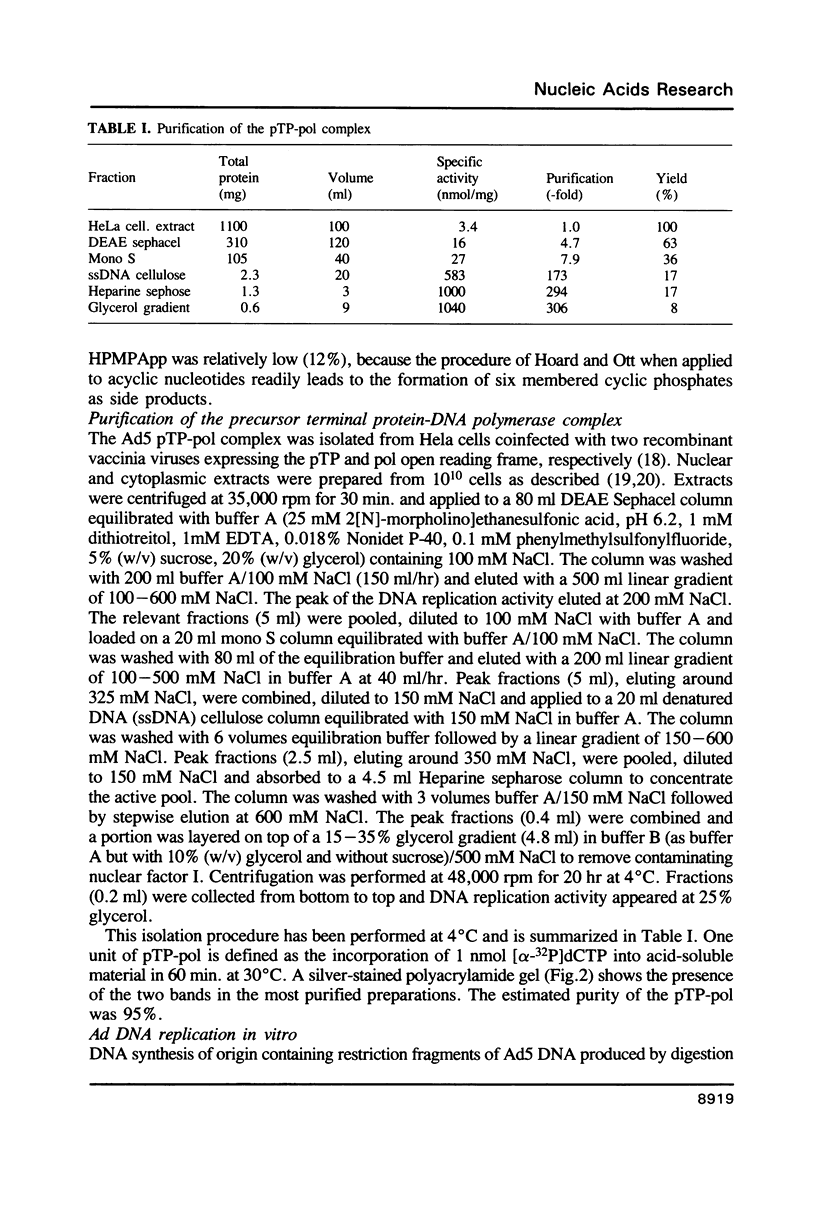
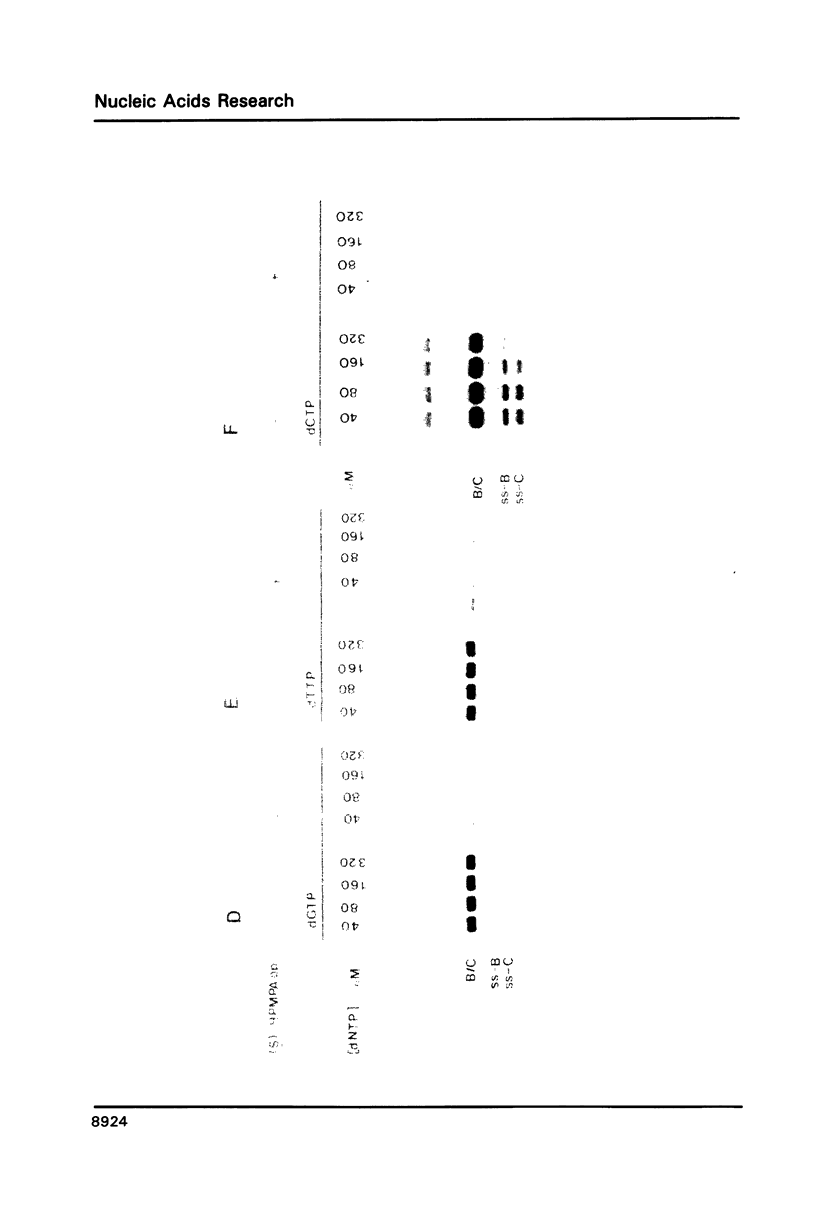
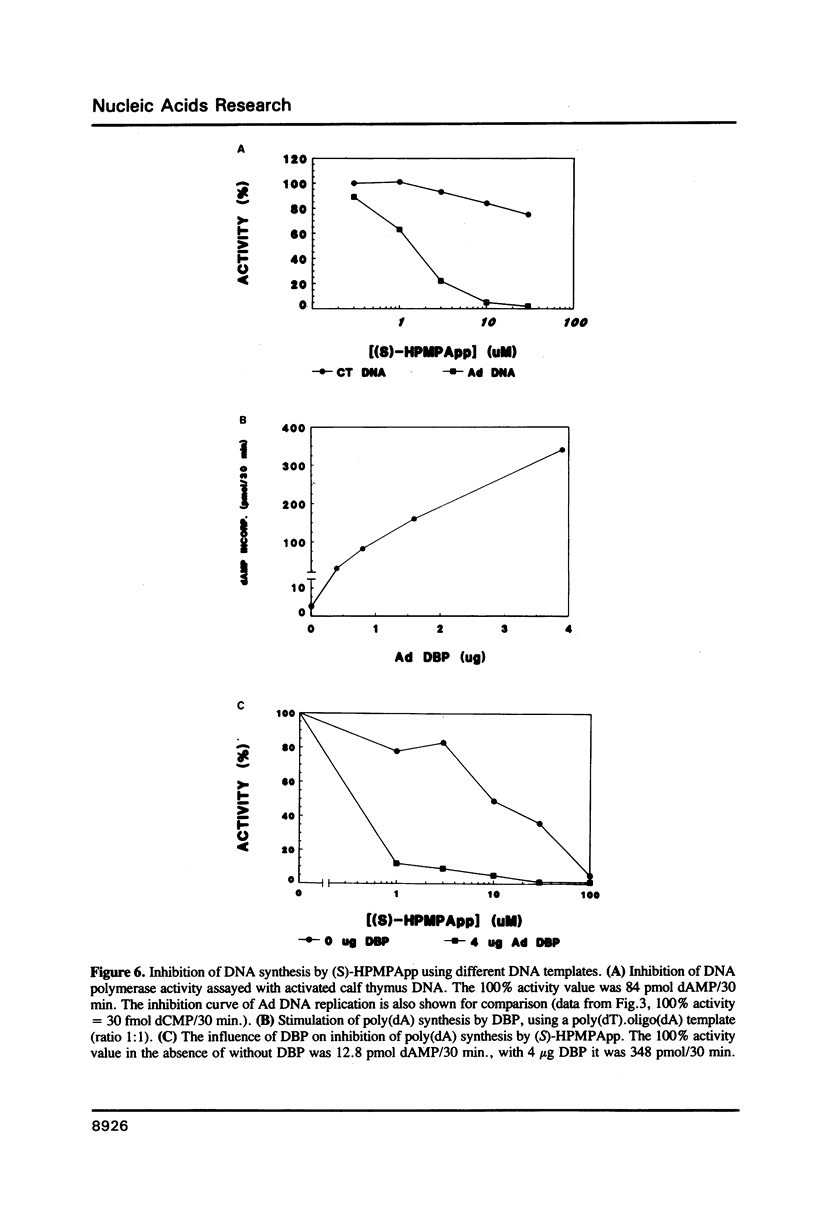
The acyclic adenosine analogue (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine [S]-HPMPA) is a potent and selective inhibitor of adenovirus (Ad) replication in cell culture. We studied the mechanism of inhibition using a reconstituted in vitro DNA replication system. The diphosphoryl derivative (S)-HPMPApp, but not (S)-HPMPA, inhibited the DNA replication of origin containing fragments strongly. The inhibitory effect was exerted at the level of elongation, while initiation was resistant to the drug. Remarkably, the elongation of short strands was only slightly impaired, while inhibition was maximal upon synthesis of long DNA fragments. (S)-HPMPApp appeared to be competitive with dATP, suggesting that the Ad DNA polymerase is the prime target for the drug. We purified the Ad DNA polymerase in complex to the precursor terminal protein to homogeneity from cells infected with overproducing recombinant vaccinia viruses. Employing gapped DNA or poly(dT).oligo(dA) templates, only a weak inhibition was observed. However, inhibition was strongly enhanced in the presence of the adenovirus DNA binding protein (DBP). We interpret this to mean that the increased processivity of the polymerization reaction in the presence of DBP leads to increased drug sensitivity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arzuza O., García-Villalón D., Tabarés E., Gil-Fernández C., De Clercq E. Inhibition of African swine fever virus DNA synthesis by (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine. Biochem Biophys Res Commun. 1988 Jul 15;154(1):27–32. doi: 10.1016/0006-291x(88)90644-4. [DOI] [PubMed] [Google Scholar]
- Baba M., Mori S., Shigeta S., De Clercq E. Selective inhibitory effect of (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and 2'-nor-cyclic GMP on adenovirus replication in vitro. Antimicrob Agents Chemother. 1987 Feb;31(2):337–339. doi: 10.1128/aac.31.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Gutiérrez J., Lázaro J. M., Bernad A., Salas M. Replication of phage phi 29 DNA in vitro: role of the viral protein p6 in initiation and elongation. Nucleic Acids Res. 1986 Jun 25;14(12):4923–4937. doi: 10.1093/nar/14.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L. Eukaryotic DNA replication. Annu Rev Biochem. 1986;55:733–771. doi: 10.1146/annurev.bi.55.070186.003505. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Holý A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P. C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986 Oct 2;323(6087):464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Walker R. T. Chemotherapeutic agents for herpesvirus infections. Prog Med Chem. 1986;23:187–218. doi: 10.1016/s0079-6468(08)70343-6. [DOI] [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Russell W. C. Recognition mechanisms in the synthesis of animal virus DNA. Biochem J. 1989 Feb 15;258(1):3–16. doi: 10.1042/bj2580003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. C., DeClercq E., Pagano J. S. Novel acyclic adenosine analogs inhibit Epstein-Barr virus replication. Antimicrob Agents Chemother. 1987 Sep;31(9):1431–1433. doi: 10.1128/aac.31.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum J. O., Field J., Hurwitz J. The adenovirus DNA binding protein and adenovirus DNA polymerase interact to catalyze elongation of primed DNA templates. J Biol Chem. 1986 Aug 5;261(22):10218–10227. [PubMed] [Google Scholar]
- Maudgal P. C., De Clercq E., Huyghe P. Efficacy of (S)-HPMPA against thymidine kinase-deficient herpes simplex virus-keratitis. Invest Ophthalmol Vis Sci. 1987 Feb;28(2):243–248. [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Stillman B. W. Adenovirus terminal protein precursor. Partial amino acid sequence and the site of covalent linkage to virus DNA. J Biol Chem. 1982 Nov 25;257(22):13499–13506. [PubMed] [Google Scholar]
- Stunnenberg H. G., Lange H., Philipson L., van Miltenburg R. T., van der Vliet P. C. High expression of functional adenovirus DNA polymerase and precursor terminal protein using recombinant vaccinia virus. Nucleic Acids Res. 1988 Mar 25;16(6):2431–2444. doi: 10.1093/nar/16.6.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. The mechanism of adenovirus DNA replication and the characterization of replication proteins. Curr Top Microbiol Immunol. 1984;109:53–73. doi: 10.1007/978-3-642-69460-8_2. [DOI] [PubMed] [Google Scholar]
- Tsernoglou D., Tucker A. D., Van der Vliet P. C. Crystallization of a fragment of the adenovirus DNA binding protein. J Mol Biol. 1984 Jan 15;172(2):237–239. doi: 10.1016/s0022-2836(84)80042-x. [DOI] [PubMed] [Google Scholar]
- Votruba I., Bernaerts R., Sakuma T., De Clercq E., Merta A., Rosenberg I., Holý A. Intracellular phosphorylation of broad-spectrum anti-DNA virus agent (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and inhibition of viral DNA synthesis. Mol Pharmacol. 1987 Oct;32(4):524–529. [PubMed] [Google Scholar]
- van der Vliet P. C., van Dam D., Kwant M. M. Adenovirus DNA replication in vitro is stimulated by RNA from uninfected HeLa cells. FEBS Lett. 1984 Jun 4;171(1):5–8. doi: 10.1016/0014-5793(84)80449-4. [DOI] [PubMed] [Google Scholar]