Abstract
Introduction
Salt-induced hypertension in the Dahl rat is associated with increases in angiotensin II, aldosterone, free radical generation and endothelial dysfunction. However, little is known about the specific mechanism(s) associated with the end-organ damage effects of aldosterone. We hypothesised that eplerenone reduces kidney damage by blocking nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity.
Methods
Dahl salt-sensitive rats fed either a low-salt (LS) or high-salt (HS) diet were treated with aldosterone in the presence of eplerenone or apocynin. Indirect blood pressure was measured prior to start of diet and weekly thereafter. Levels of plasma nitric oxide (NO) and urinary 8-isoprostane were measured following treatment. Protein levels of selected subunits of NADPH were assessed by western blot.
Results
Eplerenone and apocynin inhibited the rise in blood pressure induced by HS and/or aldosterone. This observation was accompanied with a parallel change in kidney protein levels of NADPH oxidase 4 (NOX-4) and p22phox. Aldosterone and high salt were associated with lower NO levels and greater renal oxidative stress.
Conclusions
NADPH oxidase is associated with the vascular and renal remodelling observed in high dietary salt intake. Aldosterone-induced expression of NOX-4 plays a pivotal role in the end-organ damage effect of aldosterone, as eplerenone tended to reduce kidney damage and inhibit NOX expression.
Keywords: Aldosterone, eplerenone, hypertension, NADPH oxidase inhibitor
Introduction
Salt-induced hypertension associated end-organ damage may be due to direct effects of aldosterone on cardiac, renal and other vascular tissues. It is of paramount importance to gain an in-depth understanding of the vasculopathic effects of aldosterone, one of the major culprits associated with the renin–angiotensin–aldosterone system (RAAS), which is significantly elevated following high salt administration in Dahl rats. Salt-induced hypertension has been linked with increased oxidative stress and endothelial dysfunction, which may be due in part to angiotensin II-induced increase in aldosterone. Thus, aldosterone blockade has been shown to protect the kidneys and sharply decrease proteinuria.1 Attenuation of renal damage by eplerenone, a mineralocorticoid receptor antagonist, supports the protective effects of aldosterone blockade in hypertensive renal damage.2
The mechanism(s) associated with the vasculopathy effects of aldosterone has not been adequately investigated. Pu et al.3 reported that in rats infused with aldosterone, an increase in oxidative stress in the vascular wall is associated with impaired endothelium-dependent relaxation. Previously, we found that oxidative stress induced by buthionine-sulphoximine (BSO) caused significant elevation of nicotinamide adenine dinucleotide phosphate (NAD(P)H).4 Inhibiting NAD(P)H oxidase ameliorates the adverse myocardial effects of aldosterone, suggesting that NAD(P)H oxidase may be a source of reactive oxygen species (ROS) in response to activation of the mineralocorticoid receptor.5 Thus, we suspect that aldosterone may be directly acting on a specific subunit of NADPH oxidase.
The purpose of this study was to examine the signal pathways associated with aldosterone/salt-induced hypertension via inhibition of aldosterone at the receptor level and by use of the NADPH oxidase inhibitor, apocynin. In addition, nitric oxide (NO), 8-isoprostane and protein excretion were assessed to determine the effect of aldosterone on endothelial function, oxidative stress and renal injury respectively. Several previous studies, including our laboratory, have consistently shown that dietary salt-induced hypertension in the Dahl rat is associated with endothelial dysfunction as evidenced by a reduction in NO and prostacyclin, concomitant with increased levels of renal and cardiac angiotensin II and aldosterone.6,7 Dissecting the effects of aldosterone under low-salt (LS) and high-salt (HS) diets will advance our knowledge on how aldosterone contributes directly to sensitising tissues in the presence of salt.
Methods
Experimental design
Male Dahl salt-sensitive rats (4–5 weeks, Charles River Laboratories, Wilmington, Massachusetts, USA) were grouped three per cage in an animal facility that has 12-hour light/dark cycles with the temperature controlled at 21–23°C. Food and water were available ad libitum. Following acclimatisation, the animals were divided into two groups: (1) LS diet (0.3% NaCl; n = 24); and (2) HS diet (8% NaCl; n = 24). Within each group, the rats were placed on one of four treatment protocols: (1) no drug (control); (2) aldosterone (ALDO; 0.2 mg pellet); (3) ALDO + eplerenone (100 mg/kg/day in the drinking water); or (4) ALDO + apocynin (1.5 mM in the drinking water) for 4 weeks. Prior to treatment (basal) and weekly thereafter, body weight, systolic blood pressure and heart rate were measured.
Indirect blood pressure measurement in conscious rats
A non-invasive tail cuff acquisition system (The CODA 2 System, Kent Scientific, Torrington, Connecticut, USA) was used to measure systolic blood pressure and heart pulse rate.
Collection and storage of blood samples
Blood samples (2.0 ml), for NO measurement, were collected into chilled heparinised tubes. Blood samples (5-10 mL) were with-drawn via cardiac puncture (under ketamine/xylazine i.v.) prior to sacrifice and transferred to heparinised tubes. All blood samples were then centrifuged at 3000 × g for 25 min at 4°C. All plasma samples were frozen in aliquots and stored at −80°C until assayed.
Tissue harvesting
Immediately following collection of blood by cardiac puncture, both kidneys were harvested from all animals, frozen in liquid nitrogen and stored at −80°C. The ratio of kidney wet weights to body weight was calculated.
Measurements of NO, and urinary protein, sodium and 8-isoprostane
Plasma NO (as nitrates + nitrites) levels (μM) were measured by a microplate assay using the Greiss reagent (Active Motif Carlsbad, California, USA). After 4 weeks of treatment, fluid excretion was analysed by placing rats in metabolic cages for 24 hours with continued treatment and liquids, as well as food ad libitum. Urinary 8-isoprostane, a marker for oxidative stress, and urinary protein excretion were analysed using an enzyme-linked immunosorbent assay (EIA). Urinary sodium was measured with an Electrolyte 10+ Analyzer (Nova Biomedical, Waltham, Massachusetts, USA). Sodium concentration of the urine was divided by urine volume collected in the 24-hr period to derive excretion/day.
Western blot analysis
Tissue samples of kidneys were homogenised in ice-cold T-PER lysis buffer containing a protease inhibitor cocktail (Pierce, Rockford, Illinois, USA). Homogenates were pelleted at 3000 × g for 5 minutes at 4°C, and supernatants were collected for western blotting. Protein concentrations were determined using the Bradford protein assay (Bio-Rad, Hercules, California, USA). Tissue lysates (30 μg) were separated by 4–12% Bis-Tris gels (Invitrogen, Carlsbad, California, USA) and electrotransferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were incubated overnight at 4°C with specific antibodies to p22phox, p47phox or NADPH oxidase 4 (NOX-4) (Santa Cruz Biotechnology, Santa Cruz, California, USA). After incubation with the secondary antibody, immune complexes were detected using the enhanced chemiluminescence method. Proteins levels from western blots were evaluated by quantifying the band intensities using NIH Image version 1.6.3 software. Densitometry units were normalised to actin. Each lane in a blot represented tissue lysate from one rat. Fold changes between treatment groups were calculated from individual blots, and mean changes were derived from replicates.
Statistical analysis
Values are reported as mean ± standard error (SEM), where n refers to the number of rats used. Statistical significance (p < 0.05) was evaluated using analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test.
Results
Systolic blood pressure
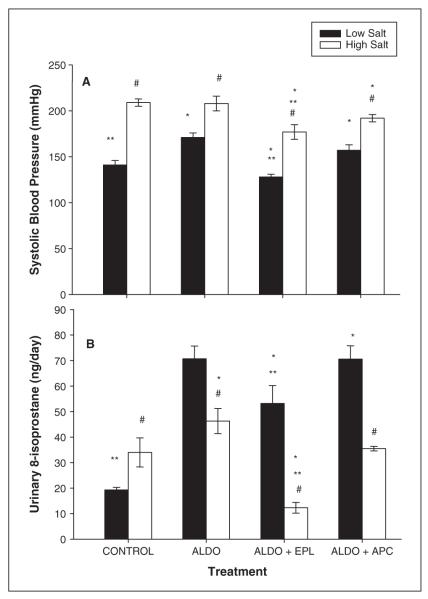
The effect of eplerenone and apocynin on ALDO-augmented blood pressure was evaluated in salt-sensitive rats fed a LS or HS diet (figure 1A). As shown in figure 1A, animals on a LS diet treated with aldosterone only showed a significant increase in systolic blood pressure (SBP) compared to controls (171 ± 5 vs. 141 ± 5 mmHg, respectively). Eplerenone blocked the increase in SBP induced by aldosterone (128 ± 3 mmHg). Also, SBP was slightly but significantly reduced by apocynin (157 ± 6 mmHg) when compared to aldosteroneonly treated rats. In contrast, rats fed the HS diet with ALDO showed no significant change in blood pressure when compared to the HS control rats (208 ± 8 vs. 209 ± 4 mmHg, respectively; figure 1A). Eplerenone inhibited the increase in SBP induced by aldosterone and/or a HS diet (177 ± 8 mmHg). In the HS-fed rats, treatment with apocynin for 4 weeks slightly reduced blood pressure (192 ± 4 mmHg) when compared to high salt with or without aldosterone treatment.
Figure 1.
Effect of eplerenone and apocynin on systolic blood pressure and urinary 8-isoprostane levels. Figure 1A represents the systolic blood pressure after 4 weeks on either a low-salt (LS) or a high-salt (HS) diet, with or without (control) treatment with aldosterone in the presence of eplerenone or apocynin. Figure 1B shows urinary 8-isoprostane levels following 4 weeks on the treatment protocols.
Data are represented as mean ± SEM for six animals per group. Significant difference (p < 0.05) from the LS group’s systolic blood pressure is denoted by #; * denotes significant difference (p < 0.05) from the control group (no drug treatment: low and high salt); ** denotes significant difference (p < 0.05) from aldosterone treatment only.
Body weights were measured weekly in these rats. The LS-fed rats had significantly higher body weights than their HS-fed counterparts as shown in table 1. Treatment with ALDO, eplerenone and/or apocynin produced no significant change in body weight when compared to control rats within the diet groups (table 1).
Table 1.
Body weight and urinary analysis from Dahl salt-sensitive rat following 4-week treatment protocol
| Groups | Body Weight (g) |
Albumin excretion (μg/day) |
Na+ excretion (mmol/day) |
|---|---|---|---|
| Low-salt group | |||
| Control | 330 ± 3 | 226 ± 15# | 3.0 ± 0.2 |
| Aldosterone | 332 ± 5 | 341 ± 10 | 3.5 ± 0.1 |
| Eplerenone | 343 ± 7 | 299 ± 21#, ** | 2.5 ± 0.3# |
| Apocynin | 338 ± 5 | 288 ± 14#, ** | 4.0 ± 0.6 |
| High-salt group | |||
| Control | 291 ± 12* | 429 ± 17* | 20 ± 1* |
| Aldosterone | 279 ± 19* | 392 ± 18 | 28 ± 3*‡ |
| Eplerenone | 297 ± 5* | 369 ± 9‡ | 5 ± 1*,‡,+ |
| Apocynin | 321 ± 12 | 380 ±16‡ | 23 ± 3* |
p < 0.05, vs. low-salt - aldosterone;
p < 0.05, vs. low-salt - control
p < 0.05, vs. high-salt - control;
p < 0.05, vs. low-salt group
p < 0.05, vs. high-salt - aldosterone
Effect of ALDO on urinary 8-isoprostane
Renal ROS production was determined by measuring urinary 8-isoprostane levels, a marker for oxidative stress. After 4 weeks on dietary salt, urinary 8-isoprostane was significantly higher in the HS-fed rats compared to the LS group (34 ± 5.7 vs. 19 ± 1.0 ng/day, respectively). Treating with ALDO increased urinary 8-isoprostane in both dietary groups (70.7 ± 5 ng/day for LS rats and 46.3 ± 4.9 ng/day for the HS rats). This increase in 8-isoprostane was significantly reduced by eplerenone treatment (figure 1B; 53.2 ± 7 ng/day for LS rats and 12.3 ± 2 ng/day for HS rats). Apocynin treatment had no significant effect on aldosterone-induced production of 8-isoprostane in these rats.
Effect of ALDO on urinary protein and sodium excretion
Urinary protein excretion is an index of renal injury. Table 1 lists results of urinary excretion of albumin and sodium from the various treatment groups in this study. The excretion of albumin and sodium was significantly higher in the HS rats compared to the LS-fed control rats. Treating with ALDO produced no further increase in albumin excretion in the rats fed a HS diet; however, in this study, ALDO treatment in the LS rats produced a significant increase in urinary excretion of albumin when compared to the LS control rats. Treatment with eplerenone or apocynin caused a small but significant decrease in the ALDO-induced excretion of albumin in the LS group and significantly reduced the HS-induced increase in albumin excretion (table 1). Urinary sodium (table 1) tended to increase in response to aldosterone in both diet groups. The excretion of sodium in the HS rat was sixfold higher than that observed for the LS rat. Treatment with eplerenone significantly reduced sodium excretion induced by aldosterone and high salt.
Effect of ALDO on plasma NO
Plasma levels of the vasodilator NO were greater in the LS-compared to the HS-fed rats (figure 2). When ALDO was given to the rats on a LS diet, plasma NO levels were reduced by 35%. In this LS-fed group, treatment with eplerenone or apocynin had no significant effect on plasma NO levels as measured by total nitrate + nitrite. However, rats fed the HS diet in the presence of eplerenone reduced plasma levels of NO by 54% when compared to the control group (HS diet without drug treatment).
Figure 2.

Effect of eplerenone and apocynin on plasma nitric oxide levels in Dahl salt-sensitive rats on an aldosterone/salt diet. Data are represented as mean ± SEM for six animals per group. # Denotes a significant difference (p < 0.05) from control (on diet with no drug treatment) value. * Denotes significant difference (p < 0.05) from the low-salt group.
Effect of ALDO and drug treatment on protein expression of NADPH subunits
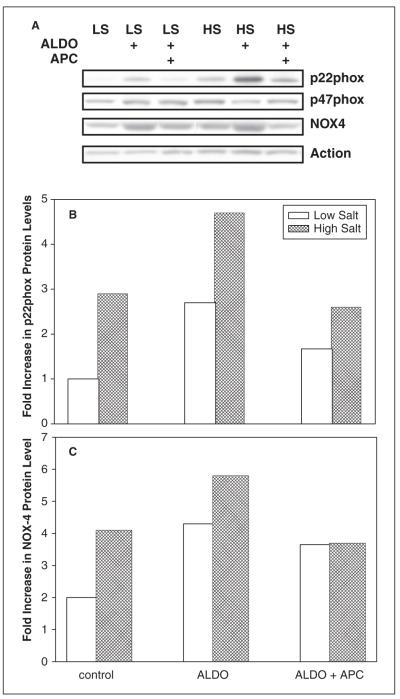
Protein levels of NADPH oxidase subunits were also assessed in these rats. Aldosterone/salt treatment induced a 2–3.5-fold increase in NOX-4 expression when compared to the LS group. Both eplerenone and apocynin blocked the ALDO and HS-induced increase in NOX-4 expression (figures 3 and 4). Similarly, protein expression of p22phox was increased almost twofold in hypertensive rats as a result of high salt or ALDO treatment, but reversed by either eplerenone or apocynin. In contrast, none of these treatments had a significant effect (<25% change) on p47phox protein levels.
Figure 3.

Representative western blots of aldosterone/salt-induced protein levels of nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX-4) subunit of NADPH following treatment with eplerenone. Figure 3B is a histogram reflecting the data in figure 3A.
LS: low salt, HS: high salt.
Figure 4.
Representative western blots (A) of aldosterone/salt-induced protein levels of nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX-4), p22phox and p47phox subunits of NADPH following treatment with apocynin. Figure 4B–C is histogram reflecting the data in figure 4A for p22phox and NOX-4 respectively.
LS: low salt, HS: high salt.
Discussion
This study examined the effect of eplerenone and apocynin on aldosterone/salt-induced hypertension. Administration of aldosterone to non-hypertensive rats for 4 weeks resulted in a progressive increase in blood pressure. Eplerenone therapy significantly lowered blood pressure in the aldosterone-induced hypertensive rats. In contrast, apocynin treatment did not entirely block the aldosterone/salt-induced hypertension but noticeably reduced the rise in blood pressure. Furthermore, eplerenone and apocynin treatment clearly blocked the aldosterone and salt-induced increase in selected components of the NADPH oxidase, namely p22phox and NOX-4 kidney expression levels. This finding correlates with the inhibition of renal ROS production as measured by 8-isoprostane following treatment with eplerenone.
Salt content of the diet appears to be crucial for the hypertensive effect of aldosterone. In fact, aldosterone increased blood pressure in the presence of a LS diet, but not in the presence of a HS diet. We suspect that the addition of aldosterone to the HS group was moderate compared with the increase in endogenous aldosterone induced by a HS diet. We previously reported that the administration of high dietary salt to the Dahl salt-sensitive rat results in an increase in angiotensin II that causes an increase in aldosterone levels.7 Previous studies have shown that salt loading is associated with an inadequate suppression of aldosterone production and increased aldosterone secretion in response to angiotensin II.8 Therefore, it is conceivable that the increase in the endogenous aldosterone and angiotensin II in hypertensive rats may eclipse the effect of exogenous aldosterone, thus producing no further increase in blood pressure when salt intake is high. Changes in blood pressure are a common observation after either aldosterone administration or mineralocorticoid receptor antagonism.9 In the present study, we show that eplerenone inhibits aldosterone-induced hypertension completely in the LS diet group; however, eplerenone response in the HS group was moderate. These findings indicate that the rise in blood pressure during a HS intake diet may be primarily due to angiotensin II acting on the AT1 receptor, as aldosterone can increase local production of angiotensin II by upregulating angiotensin converting enzyme.10,11 Activation of the AT1 receptor increases NADPH oxidase activity. It is also of interest to note that apocynin impeded the progression of high blood pressure in the aldosterone/salt-treated rats suggesting that specific components of the NADPH oxidase pathway may be involved. These findings are in agreement with Tian et al.,12 who reported that apocynin lowered long-term arterial pressure in Dahl salt-sensitive rats fed a HS diet.
It is now well known that NAD(P)H oxidase is a major source of ROS in the vasculature and kidney.13,14 Several studies indicate that aldosterone increases ROS production.15,16 Virdis et al.17 showed that vascular NAD(P)H oxidase activity and ROS production were increased in aldosterone/salt-treated hypertensive rats. In the present study, aldosterone/salt treatment is associated with increased kidney expression of the NOX-4 and p22phox components of NADPH oxidase. These data suggest that at least some of the aldosterone/salt-induced effect in the kidney is mediated through the NAD(P)H oxidase pathway. Salt-induced hypertension did not significantly alter p47phox protein levels, in contrast to Tian et al.12 who found an increase in p47phox mRNA levels. However, we do not know whether the phosphorylation status of p47phox protein was affected by HS or aldosterone treatment. The present study also showed that increased NOX-4 expression induced by aldosterone/salt was prevented by treatment with eplerenone. These data are consistent with those of previous studies that reported that increased vascular NAD(P)H oxidase in aldosterone/salt-treated rats and other pathological conditions are reduced by treatment with spironolactone and eplerenone.15,17,18 Furthermore, the increased expression of NOX-4 by aldosterone/salt and the subsequent blockade by eplerenone correlates with the reduction in urinary 8-isoprostane levels, which is often used as an index of oxidative stress in vivo.19 These findings are consistent with previous studies in Dahl salt-sensitive rats, which show that 8-isoprostane levels are significantly elevated in HS treatment compared to Dahl salt-resistant rats.19
Other studies have shown that administration of apocynin to hypertensive Dahl rats can reduce renal damage, oxidative stress and inflammation.12 Further investigation on the effect of apocynin on aldosterone/salt-treated rats was assessed by measuring NOX-4 expression, a constitutively expressed protein of the NAD(P)H oxidase. Apocynin appeared to reverse the increased protein expression of NOX-4 associated with high blood pressure. NOX-4 is the major catalytic component of the NAD(P)H oxidase in the kidney and endothelium, and requires p22phox for activation. However, ROS production does not appear to require the interaction of cytoplasmic activator proteins such as p47phox, p67phox or Rac1, unlike other isoforms of NOX such as p91phox.20,21 The production of ROS associated with treatments that induce high blood pressure is therefore likely to involve oxidation of various molecules including proteins and lipids. Apocynin is believed to impede the assembly of the subunit components of the NADPH oxidase complex. This pharmacological inhibitor of superoxide production appeared to reverse the effects of aldosterone on protein expression of selective components of the NADPH complex. However, apocynin is unlikely to act directly at the transcription level, and the effect on the observed protein levels may operate by other mechanisms. Taken together, these findings suggest that therapeutic targeting of NOX-4 might present an alternative approach to ameliorate salt and aldosterone-induced hypertension.22
These effects in the vasculature would be expected to increase the bioavailability of NO, thereby improving endothelial-dependent and nitrate-dependent vasorelaxation. However, in this study, eplerenone did not increase plasma NO levels; in fact, the NO levels were reduced. This observation is in direct contrast to our previous findings, which showed an increase in NO levels following treatment with eplerenone.23 In the previous study, eplerenone was given in absence of aldosterone/salt treatments. However, the assay for plasma NO may not be a true representation of the tissue bioavailability of NO in these rats. However, the assay used measures total nitrates and nitrites and is an indirect measurement of the bioactive form of NO. Thus, we are in the process of measuring tissue levels of NO and other vasoactive agents. It should be noted that although slight, the NADPH oxidase inhibitor apocynin increased plasma NO levels.
In conclusion, the protective effect of a mineralocorticoid receptor blocker is clearly distinct from the effects of a NADPH oxidase inhibitor in the Dahl salt-sensitive and suggests that these agents can have differential effects on the cardiovascular system. Thus, the mechanism underlying these differences seems to involve opposing effects on the regulation of NADPH oxidase.
Acknowledgments
Funding
This work was supported, in part, by NIH/NIDDK (grant number 1SC1DK082385-01). Facilities and support services were partially funded by NIH/NCRR/RCMI (grant number G12-RR03034).
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Cortinovis M, Perico N, Cattaneo D, Remuzzi G. Aldosterone and progression of kidney disease. Ther Adv Cardiovasc Dis. 2009;3:133. doi: 10.1177/1753944708100409. [DOI] [PubMed] [Google Scholar]
- 2.Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani JA, MacMahon EG. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002;43:4828–4836. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- 3.Pu Q, Neves MF, Virdis A, Touyz RM, Schiffrin EL. Endothelin Antagonism on Aldosterone-Induced Oxidative Stress and Vascular Remodeling. Hypertension. 2003;42:49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. [DOI] [PubMed] [Google Scholar]
- 4.Bayorh MA, Ganafa AA, Socci RR, Eatman D, Silvestrov N, Abukhalaf IA. Effect of Losartan on oxidative stress-induced Hypertension. Am J Hypertension. 2003;16:387–392. doi: 10.1016/s0895-7061(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, et al. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics. 2004;16:194–203. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 7.Bayorh MA, Ganafa AA, Emmett N, Socci RR, Eatman D, Fridie IL. Alterations in aldosterone and angiotensin II Levels in salt-induced hypertension. Clin Exp Hypertension. 2005;4:355–367. [PubMed] [Google Scholar]
- 8.Schlaich MP, Klingbeil AU, Jacobi J, Delles C, Schneider MP, Schmidt BM, et al. Altered aldosterone response to salt intake and angiotensin II infusion in young normotensive men with parental history of arterial hypertension. J Hypertension. 2002;20:117–124. doi: 10.1097/00004872-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE Inhibition or Aldosterone Blockade Is Blood Pressure–Dependent. Hypertension. 2003;41:201–206. doi: 10.1161/01.hyp.0000049881.25304.73. [DOI] [PubMed] [Google Scholar]
- 10.Harada E, Yoshimura M, Yasue H, Nakagawa O, Nakagawa M, Harada M, et al. Aldosterone induces angiotensin-converting-enzyme gene expression in cultured neonatal rat cardiocytes. Circulation. 2001;104:137–139. doi: 10.1161/01.cir.104.2.137. [DOI] [PubMed] [Google Scholar]
- 11.Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, et al. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148:1688–1696. doi: 10.1210/en.2006-1157. [DOI] [PubMed] [Google Scholar]
- 12.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, et al. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Harding P, Garvin JL, Juncos R, Peterson E, Juncos LA, et al. Isoforms and functions of NAD(P)H oxidase at the macula densa. Hypertension. 2009;53:556–563. doi: 10.1161/HYPERTENSIONAHA.108.124594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, et al. Possible Contributions of Reactive Oxygen Species and Mitogen-Activated Protein Kinase to Renal Injury in Aldosterone/Salt-Induced Hypertensive Rats. Hypertension. 2004;24:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 16.Patni H, Mathew JT, Luan L, Franki N, Chander PN, Singhal PC. Aldosterone promotes proximal tubular cell apoptosis: role of oxidative stress. Am J Physiol Renal Physiol. 2007;293:F1065–F1071. doi: 10.1152/ajprenal.00147.2007. [DOI] [PubMed] [Google Scholar]
- 17.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Duquaine D, King S, Pitt B, Patel P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation. 2002;105:2212–2216. doi: 10.1161/01.cir.0000015854.60710.10. [DOI] [PubMed] [Google Scholar]
- 19.Meng S, Roberts LJ, 2nd, Cason GW, Curry TS, Manning RD., Jr. Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R732–R738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 20.Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multi-component Nox1 and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Brandes RP, Schröder K. Differential vascular functions of Nox family NADPH oxidases. Curr Opin Lipidol. 2008;19:513–518. doi: 10.1097/MOL.0b013e32830c91e3. [DOI] [PubMed] [Google Scholar]
- 23.Bayorh MA, Mann G, Walton M, Eatman D. Effects of enalapril, tempol, and eplerenone on salt induced hypertension in Dahl salt-sensitive rats. Clin Exp Hypertension. 2006;28:121–132. doi: 10.1080/10641960500468276. [DOI] [PubMed] [Google Scholar]