Abstract
Odorant-evoked activity contributes to olfactory epithelium organization and axon targeting. We examined the consequences on gene expression of a genetic disruption of the channel responsible for olfactory transduction. Genes encoding calcium-binding EF-hand motifs, were among the most highly regulated transcripts consistent with the central role of Ca2+ influx in neuronal depolarization. Several genes encoding integral membrane proteins are also highly regulated. One gene, Lrrc3b, was regulated more than 10-fold by odorant activity. Changes in expression occur within thirty minutes and are maintained for several hours. In genetic disruptions of Lrrc3b, a Lrrc3b-promoter-driven reporter adopts the activity-regulated expression of the endogenous gene. Individual olfactory glomeruli have a wide spectrum of activity levels that can be modulated by altering odor exposure. The Lrrc3b reporter mouse permits direct assessment of activity in identified glomeruli. In stable odorant environments, activity-regulated proteins provide a characteristic signature that is correlated with the olfactory receptor they express.
Keywords: olfaction, activity-dependent gene expression
Introduction
The development, organization and maintenance of the mammalian olfactory system requires activity-independent and dependent cues. The contribution of odorant receptors (ORs) to the initial patterning of connections between the epithelium and the olfactory bulb (OB) is widely appreciated (Wang et al. 1998; Feinstein et al. 2004). In vision, spontaneous and stimulus-evoked neuronal activities play significant roles in the initial organization and patterning during a restricted critical period of development (Shatz 1990; Meister et al. 1991; Goodman and Shatz 1993). Although odor-evoked activity contributes only modestly to the initial establishment of the olfactory map (Brunet et al. 1996; Zou et al. 2004), it plays a critical role in the regulation of olfactory receptor neuron (ORN) survival and the maintenance of ORN axon projections to the OB (Zhao and Reed 2001). This process is presumably important in the ongoing ORN replacement and rewiring that occurs throughout life. To further define the role of activity-dependent processes in olfactory organization and development, it is critical to understand how odor-evoked activity produces long-lasting changes in the ORNs.
ORs signal through tissue-specific downstream components, including an adenylyl cyclase (ACIII), a G-protein subunit (Golf), and a cyclic nucleotide-gated (CNG) ion channel. Mice genetically deficient for any of these components are essentially devoid of odorant-evoked activity but maintain a structurally intact olfactory epithelium (Brunet et al. 1996; Belluscio et al. 1998; Wong et al. 2000). The expression of ORs, Golf and ACIII are essential for proper axon targeting, and cAMP is suggested to direct aspects of this process (Col et al. 2007; Imai and Sakano 2007; Maritan et al. 2009) Additionally, odorant-evoked cAMP stimulates the MAPK/CREB pathway and resulting CRE-mediated gene transcription increases OSNs survival (Watt et al. 2004). The ORNs from CNG channel-deficient mice retain the ability to generate odor-evoked cAMP responses and target normally to OB suggesting distinct roles for cAMP and the subsequent channel-mediated ion influx in cellular processes. In an environment when cells compete with adjacent wild type cells, channel-deficient ORNs display odorant-dependent defects in survival and maintenance of glomerular projections (Zhao and Reed 2001). These studies suggest that odor environment cues modify the cellular composition of the olfactory epithelium (OE) and the pattern of innervation of specific glomeruli in the OB.
We are interested in elucidating the mechanism by which odor-evoked activity alters the properties of individual ORNs and causes long-lasting changes in olfactory organization. We used micro-array analysis to compare OE gene expression in wild type mice and mice in which a knock-out of the CNG channel gene blocks odorant-evoked activity and identified genes regulated by odorant activity. One class of activity-regulated genes encodes calcium-binding proteins, consistent with the role of Ca2+ in signal transduction (Restrepo et al. 1996) and the critical importance of regulating its intracellular concentration. We also identified a gene, Lrrc3b, encoding an integral membrane protein whose expression in ORNs is regulated by odorant-evoked activity. A YFP reporter under Lrrc3b promoter control reveals a pattern of fluorescence in the OB reflecting the activity of each OR in its natural environment.
METHODS
1. RNA profiling
RNA isolation and expression profiling
RNA samples from adult OE were analyzed on microarrays. Total RNA from OE of six mice of each genotype were individually isolated with TRIzol (Invitrogen) and RNeasy minikit (QIAGEN). Material from three animals was pooled to generate the two samples of each genotype that were used to probe the microarrays. The sample processing was performed by the JHMI Microarray Core hybridization unit (Baltimore, MD) following standard Affymetrix protocols (Affymetrix, San Jose, CA). Briefly, five micrograms of total RNA was used to synthesize first-strand cDNA with an oligo(dT24) plus T7-promoter primer (Proligo LLC) and SuperScript Choice system (Invitrogen), purified by phenol-chloroform extraction, and biotinylated anti-sense cRNA was generated by transcription with a BioArray RNA High Yield transcript labeling kit (ENZO, Inc.).
Biotinylated cRNA was fragmented (94°C, 35 min) and hybridized (10μg) to the Affymetrix murine genome U74 Gene- Chip array (16h, 45°C with constant rotation (60 rpm)). The chip was washed and stained with a streptavidin-PE conjugate, and amplified with goat IgG and biotinylated goat anti-streptavidin antibody, followed by a second staining step with the streptavidin-PE conjugate. Fluorescence was detected with the Hewlett-Packard G2500 GeneArray scanner, and image analysis of each GeneChip sample was performed with the Micro Array Suite 5.0 software (Affymetrix), using standard default settings. Affymetrix MAS 5.0 software was used for initial probe level data analysis, including extraction and normalization of signal intensities, calculation of detection P values, and assignment of detection calls. Data were then processed in Microsoft Excel.
All RNA samples had optimal rRNA ratios (1:2 ratio of 18S to 28S) and clean run patterns. To assess the quality control of the hybridization and scanning the following parameters were studied: scaling factor, background, percentage of present calls, housekeeping genes (GAPDH), and presence or absence of internal spike controls. To assess consistency between replicates, we observed the percentages of differential calls (up or down regulated) between pairwise comparisons. Initial expression results were based on pairwise comparisons between the different experimental conditions represented by the samples. Transcripts that showed at least a twofold change in expression level between experimental sample and control sample were considered significant. For duplicated samples, the results were filtered independently for significance on each of the four pairwise comparisons. Transcripts that were significantly different in at least two of four iterative comparisons were selected for the final candidate list.
Odor preparation and odor exposure
Odorants were prepared by mixing 1% heptaldehyde or 1% isoamyl acetate in mineral oil. Animals were exposed to odorants individually in clean cages with wire mesh lids for the exposure times indicated in each experiment. The odors were presented on a solution-saturated cotton swab placed inside the cage near the lid. For clean air experiments, mice were placed in plastic chamber and filtered, compressed air (10 liter/minute) was introduced continually during the residence time (24 hours).
Quantitative RT- PCR
Invitrogen LUX (Light Upon eXtension) fluorogenic primers were used to quantify Lrrc3b message by RT-PCR. A forward fluoregenic FAM-labeled primer (CAACCTGGACTCGTG ATTATGCAGAGG [FAM] TG] and a reverse unlabeled primer (ATGGAGAGGGAGCGG GACA) were used to assess Lrrc3b abundance. JOE-labeled GAPDH primers (Invitrogen) were used for sample normalization.
Total RNA from OE was isolated using TRIzol reagent (Gibco BRL), and DNase I treated (15 min, 22C). For real-time PCR, 0.1μg of each sample RNA was added to a tube containing 10 μl each of LUX forward primer and reverse primer, 20 μl RNaseOUT, and .5 μl ThermoScript Plus/Platinum Taq enzyme in a 25 μl total volume. Amplification was performed on a SmartCycler (Cepheid) with an annealing temperature of 52 °C for 50 cycles. For each experiment, a standard dilution curve was used to convert threshold cycle to a log RNA concentration.
2. Northern and in situ hybridization analysis
Total RNA from OE was fractionated by gel electrophoresis and probed with 32P-labeled S100A5, Olfactomedin, Lrrc3b, Ephrin A5, Calretinin, and actin coding fragments. The intensity of each band was recorded by phosphorimager.
In situ hybridization for S100A5, olfactomedin, calretinin, Lrrc3b, PEP-19, and ephrin A5 mRNAs in OE were performed with a digoxigenin-labeled RNA probes. The coding sequences for each gene were subcloned in pBluescript KS II (Strategene), linearized, and antisense and sense probes generated with T3 and T7 RNA polymerase.
Tissues were isolated from mice (12 weeks) following perfusion with 4% paraformaldehyde in PBS. Fixed tissues were treated with 20% sucrose, 250 mM EDTA (24 hr, 4°C), and frozen in OCT (Tissue-Tek). Cryosections (20-40μM) were mounted on micro-slides (Fisher Scientific) post-fixed in Bouin’s solution (Sigma, St. Louis, MO, USA) or 4% paraformaldehyde (PFA) for 10 minutes at room temperature (RT) and washed in PBS (3 times, 5 min each). Proteinase K treatment (20ug/ml, RT for 10 min), was followed by a PBS wash and additional post-fixation in Bouin’s solution or 4%PFA (10 min, RT). The tissue sections were then washed in PBS (3 times, 5 min each) followed by water (5 min) and acetylated with triethanolamine/acetic anhydride/HCl solution. Finally, the tissues were washed 3 times in PBS before prehybridization and probe addition. In situ hybridization was performed essentially as described (Schaeren-Wiemers and Gerfin-Moser, 1993). NBT/BCIP was used to visualize the hybridization signal.
3. Generation of Lrrc3b KO mice
Targeting vectors were constructed with 129/Sv mouse genomic DNA fragments (Stratagene). The Lrrc3b coding sequence was replaced with a tauYFP transgene, while retaining all of the 5′ and 3′ cis-regulatory elements. The tauYFP transgene was generated by substitution of a BamHI-EcoRI YFP fragment (Venus YFP/pCS2) for the lacZ sequence in a tau-LacZ SV40 polyadenylation site reporter (Mombaerts 1996). The targeting vector Lox-NeoR-Lox (LNL) cassette (Zhao and Reed 2001) for selection of neomycin resistant ES clones was removed by mating chimeric male mice to cre-transgenic females (Schwenk et al. 1995). Sequences and methods for generating the constructs are available from the authors. Targeting constructs were linearized with FseI, electroporated into 129/Sv ES cells (JHMI Transgenic Facility), and homologous integration events identified by southern blot analysis. Genetically modified ES cells were injected into C57/BL6 blastocysts and implanted into psuedopregnant females to generate chimeric mice.
4. Immunofluorescence and imaging
Generation of S100A5 and Lrrc3b antibodies
Peptides corresponding to the 18 C-terminal residues of the deduced mouse S100A5 protein and amino acids 47-64 of mouse Lrrc3b protein were synthesized, coupled to bovine serum albumin using glutaraldehyde, and injected into rabbits to generate polyclonal antisera as previously described (Davis and Reed 1996). Antisera were affinity purified with the immunizing peptides on an Affigel-10 column.
Immunohistochemistry
Immunohistochemistry or direct imaging for YFP or GFP, was performed on PFA-fixed olfactory tissue sections (14-20μm). Sections were washed and then incubated for 30 minutes at room temperature with appropriate primary antibodies: rabbit anti-S100A5 Ab (1:200), rabbit anti-Lrrc3b Ab (1:1000), ACIII (rabbit, 1:1000; Santa Cruz Biotechnology), BrdU (rat, 1:100; Abcam), GFP (rabbit, 1:1000, Molecular Probes), lacZ (rabbit, 1:500, Promega), O/E (rabbit, 1:1000)(Wang and Reed 1993), OMP (goat, 1: 2000; Dr. F. Margolis), Gγ13̣̣ 1:500̣̣, Gα olf (1:1000), ClassIII β-tubulin (1:1000), Gβ2 (1:500, Santa Cruz). After washing, sections were incubated with either Alexa488- or Alexa 546-labled secondary antibodies (1:1000), or goat anti-rabbit HRP (1:1000, 4 °C for 60 min). The HRP-treated sections were incubated with streptavidin for 30 minutes and finally DAB reagent (5 min) with washing between each step. Slides were mounted with Aquamount and imaged in a Ziess Axioplan or LSM510 Meta confocal microscope that permitted spectral unmixing of GFP and YFP signals.
5. Tissue culture and protein analysis
Membrane fraction enrichment
Olfactory epithelium was dissected and homogenized in PBS with protease inhibitors. Unbroken cells,nuclei and debris were removed by sequential centrifugation at increasing speeds (500 xg, 5 min; 2000 xg, 15 min) and resulting supernatant divided into two fractions after a final spin (100,000 xg, 60 min). The pellet (membrane) fraction was separated by SDS-page and transferred to PVDF membrane.
HEK293 cell transfection
Human embryonic kidney cells (HEK293) were grown at 38 °C and 5% CO2 in growth media containing Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 2mM L-glutamine, 100U/ml penicillin, 100μg/ml streptomycin (Gibco/Life technologies) and 10% fetal bovine serum (Gemini).
Mouse Lrrc3b cDNA was cloned into pRK5-CMV, myc-tagged pRK5-CMV, or a YFP-protein fusion vector as an EcoRI and BamHI fragment. Cells were transiently transfected using the Fugene transfection protocol.
Western blotting
Transfected cells were harvested in 1ml PBS with .5 mM EDTA, spun down and resuspended in 1X Glycoprotein Denaturing Buffer, incubated at 100 °C for 10 min and then incubated (37 °C. 1 hr) after addition of G4 buffer and 10 μl of Endo H.
Whole-cell lysate or membrane-enriched OE samples were prepared for electrophoresis by adding Laemmli sample buffer to cells. Samples were thoroughly mixed, pelleted, and supernatant (25-30 μl per lane) loaded onto pre-cast 10% SDS-PAGE mini-gels (Bio-Rad). Samples were transferred to PVDF membranes (Bio-Rad) (15V, 15 min) using a semi-dry apparatus. Membranes were blocked and incubated (1xTBS, .1% Triton, 5% dry milk) with a 1:750 dilution of anti-Lrrc3b antisera. Proteins were detected by chemiluminescence.
6. Animal surgery
Animals at an early postnatal age were cooled briefly to anesthetize and one nostril was lightly cauterized with a fine tip surgical cauterizer (Henly International, TX) before being returned to their cage. Naris closure was confirmed by stereoscopic visual examination at the time of tissue harvest (PD40).
RESULTS
Altered gene expression in the CNGA2 null olfactory epithelium
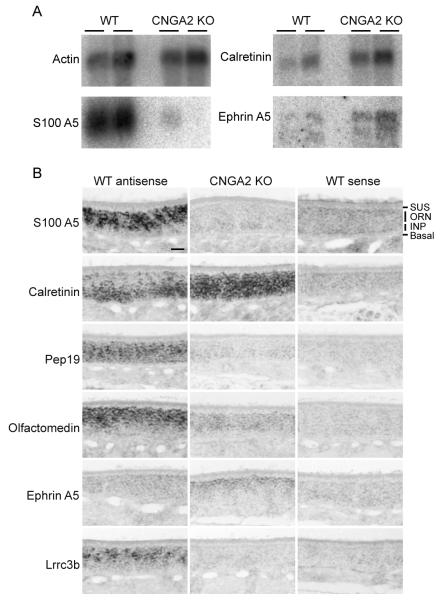
The continual exposure of animals to a rich and dynamically changing odor environment leads to a broad range of stimulus-evoked activity levels from individual olfactory neurons. We conducted a micro-array analysis in order to determine if characteristic patterns of gene expression are associated with different states of odorant-evoked activity. The Affymetrix U-74 mouse probe set was used to assess gene expression in the olfactory epithelium of wild type mice and anosmic, CNGA2-deficient mice. Genes with the greatest differences in expression between the two genotypes encode several protein classes, including transcription factors, transmembrane proteins, and calcium binding proteins (Supplemental Table 1). We confirmed the changes in expression of three of these genes by northern blot analysis (Fig. 1A).
Figure 1.
Differentially-expressed genes in olfactory epithelium (A) Total RNA from the OE of wild type and CNGA2(−/0) adult mice. Independent samples (n=2) were prepared and fractionated for Northern analysis and probed with P32-labeled S100A5, Calretinin, and Ephrin A5 coding sequences. Actin mRNA serves as a control for RNA quality and abundance. (B) In situ hybridization of differentially expressed genes. Cryosections from wild type mice (PD10) probed with antisense (left column) or sense probes (right column) were compared to antisense probe hybridization on similar sections from CNGA2 KO mice (center column). In ORNs in the wild type olfactory epithelium, S100A5 mRNA is abundant but expressed at different levels among individual ORNs. The S100A5 mRNA is dramatically decreased in CNGA2 KO ORNs. Calretinin mRNA more abundant in the ORNs of CNGA2-deficient OE and displays a distinct distribution from the immature cell-enriched pattern observed in wild type OE. The Pep19 and olfactomedin genes are both significantly down-regulated in CNGA2 KO OE while Ephrin A5 is up-regulated in these mutant mice and preferentially expressed in the most apical neuronal cells. Lrrc3b is dramatically down-regulated in KO animals and expressed at variable abundances among individual ORNs in wild type mice. scale bar = 50 microns
The divalent cation Ca2+ is a major contributor to the current mediated by odorant-activated olfactory CNG channels (Dhallan et al. 1990) and the sequestration of this ion to maintain homeostatic levels is critical to neuronal function. The mRNAs encoding Ca2+ binding proteins reflect a gene family that was highly abundant and displayed large differences in expression levels between normal and activity-deficient olfactory epithelium. The EF-hand motif, characteristic of Ca2+ binding proteins, is found in S100A5, calretinin, and Pep19. Among these Ca2+ binding proteins, S100A5 is expressed at higher levels in the wild type OE than in the OE of the X-linked, CNGA2-deficient (CNGA2(−/0)) male mice (Fig. 1A,B). In contrast, calretinin is restricted in wild type mice to immature ORNs that lie closer to the basal lamina while its expression extends to all ORNs in the CNGA2(−/0) animals (Fig. 1B). A third member of the family, PEP-19 is expressed in ORNs in both genotypes, but at significantly higher levels in the wild type OE (Fig. 1B).
We next examined another model of reduced odorant-evoked activity for similar changes in gene expression to determine if the observed changes were characteristic of altered neuronal activity or confined to the CNGA2(−/0) mouse model. Chronic unilateral naris occlusion, a procedure that blocks exposure of the epithelium to environmental odorants, was induced in newborn mice and maintained for six weeks. The expression of S100A5 protein was examined by immunocytochemistry (supplemental figure 1). On the occluded side, S100A5 protein is dramatically less abundant in the OE and in both the nerve layer and glomeruli of the OB. We conclude that changes in expression are the consequence of the loss of channel-mediated activity rather than alterations in proteins that directly associate or interact with the CNGA2 channel subunit.
Lrrc3b is down-regulated in the CNGA2 olfactory epithelium
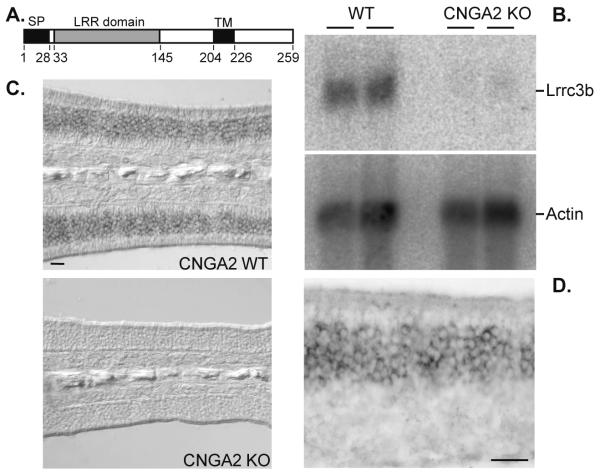
In addition to providing insights into the dynamic and spatial regulation of gene families that are engaged in similar functions (Mirnics and Pevsner 2004; Morris and Wilson 2004) microarray approaches provide the ability to reveal novel transcripts regulated in activity-dependent pathways. The most highly regulated mRNA identified in our screen was the uncharacterized EST BC019794. This transcript, encoded by the Leucine Rich Repeat Containing 3B (Lrrc3b) gene, was dramatically downregulated in CNGA2(−/0) OE. The Lrrc3b transcript encodes a 259 amino acid protein that is highly conserved in mammals. The Lrrc3b protein is predicted to span the membrane once near its carboxy-terminus and extend an extracellular 176 amino acid domain containing an amino-terminal leucine rich repeat (LRRNT) domain followed by three additional LRR segments that lack the cysteine signature. These LRR motifs may participate in protein-protein interactions as described for other LRR proteins (Fig. 2A). We confirmed that Lrrc3b expression is altered in the CNGA2 (−/0) OE by northern blot analysis (Fig. 2B) and in situ hybridization (Fig. 2C) and at higher magnification Lrrc3b expression is restricted to ORNs within the OE (Fig. 2D).
Figure 2.
Lrrc3b expression is altered in the CNGA2 (−/0) olfactory epithelium (A) Schematic diagram of the predicted Lrrc3b protein. The signal peptide (SP), LRR domain and transmembrane domain (TM) are indicated along with the corresponding amino acid positions. (B) Northern analysis of two independent OE RNA samples from wild type and CNGA2(−/0) animals probed for Lrrc3b message. Lrrc3b is more abundant in wild type tissue than in CNGA2(−/0) OE. The actin mRNA control reveals similar gel loading and RNA quality for each lane. (C) In situ hybridization with a Lrrc3b antisense probe on wild type and CNGA2(−/0) OE in adult mice. scale bar = 50 microns (D) Higher magnification image of Lrrc3b in situ signal confirms the restricted expression and variable levels in ORNs. scale bar = 25 microns.
Lrrc3b is a plasma membrane protein
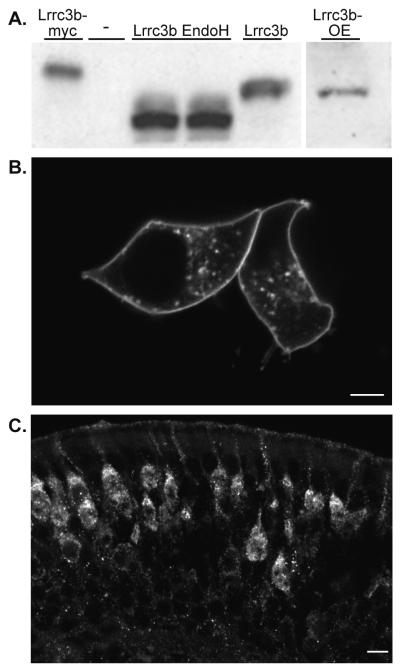
Purified antisera against amino acids 47-64 of the Lrrc3b protein specifically reacted with recombinant Lrrc3b protein expressed in HEK293 cells and recognized a similar size protein (35kDa) in OE membrane preparations (Fig. 3A). Treatment of lysates from HEK293 cells with EndoH produced a smaller, 25kDa band, suggesting that the native Lrrc3b protein is glycosylated.
Figure 3.
Lrrc3b protein is localized to the plasma membrane and glycosylated (A) Purified anti-peptide Lrrc3b antisera (amino acids 47-64) recognizes a similar size (35kDa) protein in Lrrc3b-transfected HEK293 cell lysates (lane 5) and in native OE membrane preparations (lane 6). The antisera-reactive band migrates at a higher apparent molecular weight in lysates from myc-tagged Lrrc3b transfected cells (lane 1). Endoglycosidase H treatment of the Lrrc3b-transfected lysates (lanes 3 and 4) reduced the apparent size of the Lrrc3b product (25kDa) indicating the presence of glycosylation on the HEK-293 expressed and native protein. (B) HEK293 cells transfected with a Lrrc3b-YFP fusion protein, visualized by intrinsic YFP fluorescence, display intense signal at the plasma membrane and in a punctate pattern in the cytoplasm. scale bar = 5 microns (C) The native Lrrc3b protein, visualized with the peptide antisera is predominantly localized to dendrites and the apical peri-nuclear endoplasmic reticulum and Golgi regions. scale bar = 10 microns.
The cellular distribution of Lrrc3b protein was next examined in heterologous expression systems and native tissue by immunohistochemistry and fusion to fluorescent reporter proteins. HEK293 cells transfected with a Lrrc3b::YFP (yellow fluorescent protein) fusion protein display intense signal at the plasma membrane and in a punctuate pattern in the cytoplasm (Fig. 3B). In OE, Lrrc3b protein expression is restricted to ORNs (Fig. 3C) and is asymmetrically distributed with intense staining visible in the dendrite, the dendritic knob, and the apical peri-nuclear endoplasmic reticulum and Golgi compartment. There is no staining in ORN proximal axons or in axon bundles. The intense staining observed in some mature ORNs and low levels detected in other ORNs may reflect the intrinsic odor-induced activity experienced by each cell.
Genetic analysis of Lrrc3b
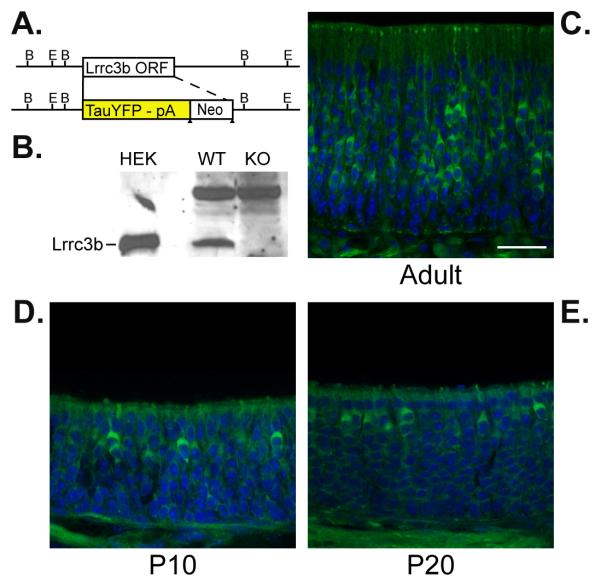
We produced a gene disruption of the Lrrc3b gene and simultaneously introduced a tau-YFP reporter in place of the Lrrc3b initiation codon and entire coding region (Lrrc3bΔYFP Fig. 4A). Western blot analysis on membrane-enriched olfactory epithelium extracts confirmed the absence of Lrrc3b protein in homozygous targeted mice (Fig 4B). The reporter expression in Lrrc3b(ΔYFP/+) mice mimics the endogenous Lrrc3b pattern with YFP intrinsic fluorescence observed in mature olfactory neurons at P10, P20 and adult mice (Fig 4C-E). Lrrc3b(ΔYFP/ YFP) mice are viable and fertile, and have a grossly normal OE and bulb. In Lrrc3b(ΔYFP/ΔYFP) mice, the expression patterns of odorant signaling and other olfactory-specific proteins are indistinguishable from wild type mice. Similarly, the zonal distribution, projection, and targeting of P2-taulacZ ORNs in the Lrrc3b(ΔYFP/ΔYFP) background is normal, as are the physiological responses to odorants measured by electro-olfactogram recording (data not shown).
Figure 4.
Lrrc3b::tauYFP expression in genetically modified mice. (A) Schematic diagram of the tau-YFP reporter knock-in genomic structure. The reporter and neo selectable marker replace the single exon that contains the complete Lrrc3b coding region. (B) Membrane-enriched OE extract from wild type mice (lane 2) contains a protein band that comigrates with HEK293-expressed Lrrc3b protein (lane 1) and is absent in Lrrc3b(ΔYFP/ΔYFP) mice (lane 3). Lrrc3b protein is visualized with affinity purified Lrrc3b antisera. A cross-reacting band of higher molecular weight does not change in abundance. (C) Visualization of intrinsic YFP fluorescence confirms the variation in intensity among ORN cell bodies in Lrrc3b(ΔYFP/+) adult OE. Fewer intensely labeled cells are observed in Lrrc3b(ΔYFP/+) olfactory epithelium at PD10 (D) and PD20 (E). scale bar = 25 microns
Regulation of Lrrc3b expression by odor-evoked activity
We next asked whether Lrrc3b expression was altered in a dynamic fashion in addition to the previously documented chronic changes. We initially used in situ hybridization to determine whether the abundance of Lrrc3b in the OE of wild type mice after exposure to different odor environments. While we could detect activity-dependent message changes by in situ hybridization (supplemental Fig 2), the magnitude of these changes in total OE RNA, measured quantitatively by real time PCR (Supplemental Fig 2), was not sufficiently robust to establish a reliable time course.
We used a genetically modified mouse model (UbI7) to examine the odor-dependent expression of Lrrc3b in the OE. In this mouse model, a bicistronic genetic construct comprised of the olfactory marker protein (OMP) gene and the mouse I7 OR (mORI7), leads to ubiquitous expression of I7 OR, previously shown to be octanal and heptaldehyde-responsive (Zhao et al. 1998), in all mature ORNs. These mice have uniform, low levels of I7 expression in addition to a normal pattern and repertoire of endogenous receptors. Accordingly, the UbI7 mouse allows for the uniform stimulation of a broad ORN population and enhanced physiological response to a specific odorant (R. Reed unpublished). We performed Lrrc3b in situ hybridization on UbI7 mice. A robust heptaldehyde-dependent Lrrc3b signal is observed after 30 minutes of exposure (Fig 5A). Maintenance of UbI7 and wildtype animals in filtered clean air for 24 hours significantly suppressed the intensity of the signal observed in the cage air environment (Fig 5A). The robust signal was dependent on the cognate I7 odorant and ubiquitous expression of the I7 OR (Fig 5A lower panels). Experiments to correlate c-fos induction in ORNs (Norlin et al. 2005) with Lrrc3b expression by double label in situ were precluded by the relatively weak c-fos induction in these cells and the more rapid time course of the former inducible gene.
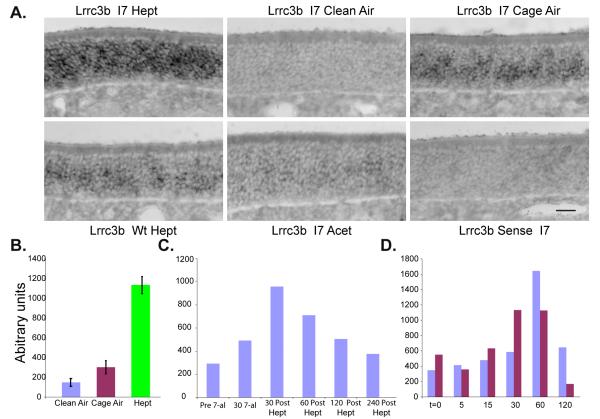
Figure 5.
Lrrc3b is regulated by odorant-evoked activity. (A) Lrrc3b in situ hybridization on OE tissue sections from UbI7 mice housed in different odor conditions. The Lrrc3b signal observed in the normal odor environment (Cage Air) is lower than the mRNA levels after heptaldehyde (Hept) exposure (30 min). Prolonged maintenance in a clean air environment (Clean Air) for 24 hr reduced Lrrc3b signal. Stimulation of UbI7 mice with acetophenone (Acet) or wild type mice with heptaldehyde supports the stimulus specificity of the signal. scale bar = 25 microns (B) Changes in Lrrc3b mRNA in UbI7 mice using the paradigm described in (A) with the I7 ligand heptaldehyde (Hept) were quantified by quantitative RT-PCR. Values reflect the independent measurement of RNA from mice (n=3) exposed to each condition. (C) The rate of recovery of Lrrc3b mRNA levels after a 30 minute heptaldehyde exposure and return to clean air was determined by RT-PCR at time points from 30 to 240 minutes. Results from a representative experiment are shown. (D) Under conditions of constant heptaldehyde exposure, Lrrc3b mRNA levels return to baseline within 120 minutes. Data obtained from two representative experiments are presented.
We next exposed UbI7 mice to heptaldehyde and quantified activity-regulated Lrrc3b expression by RT-PCR. After a 30 minute odorant exposure, Lrrc3b levels in the OE were up-regulated approximately five-fold (Fig. 5B). Animals maintained for 24 hours in clean air had Lrrc3b message levels that were two-fold lower than normally-maintained mice (Fig. 5B). When UbI7 mice were exposed to heptaldehyde for 30 minutes and then transferred to clean air, Lrrc3b mRNA levels peaked 30-60 minutes after the initial exposure, and returned to near-baseline levels 4 hours later (Fig. 5C). Interestingly, during continual heptaldehyde exposure, Lrrc3b levels returned to baseline within two hours, as all ORNs presumably desensitized to odorant stimulation (Fig. 5D). These data indicate that Lrrc3b expression displays robust and rapid changes reflecting the quality and duration of odor stimulation in the environment.
YFP as a marker of active ORNs
We generated the Lrrc3b::tauYFP reporter mice to facilitate the identification of cells that express the native Lrrc3b protein. The activity-dependent regulation of Lrrc3b mRNA transcription suggested that the level of the fluorescent YFP reporter in each cell would be in proportion to the activity of each cell. There is a wide range of YFP signal intensities in individual ORNs including some that express YFP below the threshold of detection. Reporter fluorescence is also visible in axon bundles below the basal lamina of the OE, and in the olfactory nerve layers of the OB (Figs. 4,6). The spectrum of activity levels in ORN cell bodies therefore are likely reflected and maintained in the axons and their termini.
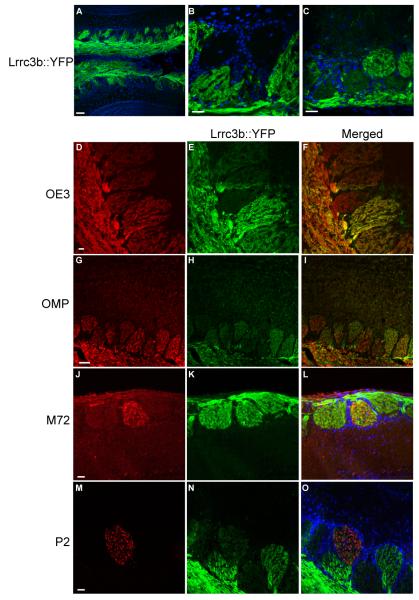
Figure 6.
YFP expression in the olfactory bulb of Lrrc3b(ΔYFP/+) mice. (A) YFP expression in the Lrrc3b(ΔYFP/+) OB. YFP is abundant in the olfactory nerve layer. Individual glomeruli display a wide range of signal intensity. DAPI staining of cell nuclei highlight the periglomerular cells that outline each glomerulus. The YFP signal is pseudocolored green. scale bar= 100 microns (B-C) High magnification images of olfactory glomeruli. Several strongly YFP-positive glomeruli are immediately adjacent to glomeruli that display no detectable YFP fluorescence. Regions with intermediate intensity signals are also observed. scale bar= 100 microns (D-I) All glomeruli receive innervation by ORNs based on the uniform presence of OE3 (D) or OMP (G). The variable intensity of Lrrc3b is apparent in intrinsic YFP fluorescence images of the Lrrc3b-tagged axons (E,H) and in the merged images (F,I). The OMP and OE3 signals were visualized using antibodies to lacZ in OMP(tau-lacZ/+) mice and spectral separation of GFP and YFP signals in OE3(tau-GFP/+) mice respectively. (J-O) Determination of the YFP florescence intensity of specific olfactory bulb glomeruli. Individual glomeruli innervated by M72- or P2-positive axons were identified with anti-β-gal antibody in M72(tau-lacZ/+) (J) and P2((tau-lacZ/+) (M) mice double heterozygous for the Lrrc3bΔYFP allele. The intense intrinisic YFP florescence of the glomerulus corresponding to M72 (K, L) suggests that cells expressing this OR are highly active. The glomerulus receiving input from P2-expressing ORNs are relatively inactive in the same odor environment (N,O). scale bars (D-O) = 100 microns
In the olfactory bulb (OB) of Lrrc3b(ΔYFP/+) mice, labeled axons enter the glomerular layer and display a relatively uniform signal within each glomerulus (Fig. 6A,B). This observation is consistent with innervation of a glomerulus by cells expressing a single OR species and thus displaying a similar level of odor-evoked activity and abundance of Lrrc3b-driven tauYFP. Comparison of fluorescence among glomeruli reveals a wide range of YFP intensity (Fig. 6A,B). Highly fluorescent glomeruli are distributed throughout the bulb. Additionally, there is low level YFP expression in the mitral, tufted, and granule cell populations of the OB. Interestingly, a subset of glomeruli are not innervated by YFP-positive axons (Fig. 6C). In order to confirm that these YFP-negative glomeruli receive innervation from ORNs, we crossed the Lrrc3b::tauYFP allele into O/E3tau-GFP mice to visualize all ORN axons (Fig. 6 D,E,F). As expected, all glomeruli are innervated by GFP positive axons while only a subset of these glomeruli are innervated by axons containing YFP. We observed a similar pattern in mice double heterozygous for the Lrrc3b::tauYFP mutation and a taulacZ-tagged olfactory marker protein (OMP) allele (Fig. 6 G,H,I) (Mombaerts et al. 1996). Our results indicate that a subset of structurally normal glomeruli are innervated solely by inactive, non-odorant stimulated ORNs.
The expression of a fluorescent, activity-dependent YFP reporter in the Lrrc3b(ΔYFP/+) mouse model allows the direct visualization of the relative odor-evoked activity level of ORNs expressing defined odorant receptors. We introduced the Lrrc3b::tauYFP allele into mouse lines with genetically-tagged OR loci (Fig. 6 J-O). ORNs expressing the M72 OR target to a dorsal glomerulus identified by lacZ immunochemistry in OB sections from M72(tau-lacZ/+)/Lrrc3b(ΔYFP/+) mice. The YFP intensity of the M72 glomerulus is comparable to the brightest glomeruli in the dorsal olfactory bulb. In contrast, the glomerulus targeted by P2 ORNs in P2(tau-lacZ/+) /Lrrc3b(ΔYFP/+) are only weakly YFP positive. In this manner, we are able to assess the activity level of ORNs expressing specific ORs in the normal (or an altered) odor environment.
We also examined the expression of the activity-regulated protein S100A5 in the OB by immunofluorescence to determine whether the pattern of another activity-regulated gene mimics the Lrrc3b::tauYFP reporter. We observed a pattern of S100A5 positive and S100A5 negative glomeruli, with a range of S100A5 immunostaining intensity among positive glomeruli. This pattern was similar to the pattern of YFP fluorescence in the Lrrc3b(ΔYFP/+) OB. When we examined the expression of S100A5 in the Lrrc3b(ΔYFP/+) mouse, YFP positive glomeruli also express the activity-regulated S100A5 (Supplemental figure 3). The coincident expression of these two activity regulated proteins suggests that the YFP-negative glomeruli correspond to axons from populations of ORNs that have not been stimulated by odorant, and therefore express low levels of Lrrc3b.
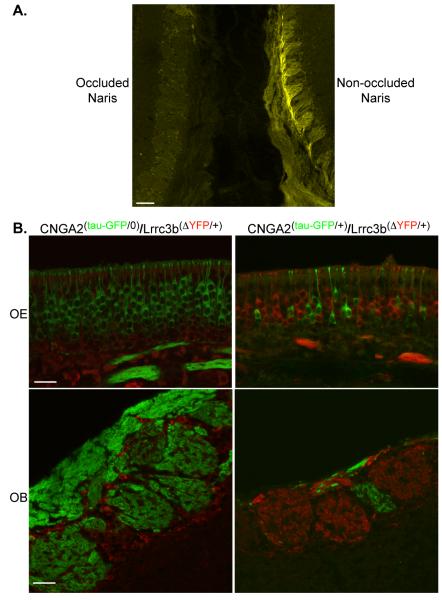
Finally, we examined the consequences of naris occlusion and genetic elimination of odor-induced activity. After unilateral naris-occlusion for two months in Lrrc3b::tauYFP mice, reporter expression is dramatically reduced on the occluded side (Fig. 7A). Similarly, YFP is undetectable in the olfactory bulb of male, anosmic CNGA2(−/0)/Lrrc3b(ΔYFP/+) mice (Fig. 7B). In female CNGA2(+/−) mice, the OE is comprised of both odorant-responsive ORNs, and inactive, CNGA2-deficient ORNs, arising from the random inactivation of the X chromosome-encoded CNGA2 channel subunit (Zhao and Reed 2001). We examined the mosaic OE of CNGA2(tau-GFP/+)/Lrrc3b(ΔYFP/+) mice, and observed that individual ORNs exclusively express either YFP or GFP, corresponding to the expression of tagged Lrrc3b gene, or a tagged, inactive CNGA2 channel, respectively (Fig. 7B). In the olfactory bulb of CNGA2(tau-GFP/+)/Lrrc3b(ΔYFP/+) mice, the glomeruli segregate into two distinct classes. Glomeruli that maintain innervation by GFP positive, CNGA2-null neurons contain no YFP positive axons. Conversely, the glomeruli that contain YFP axons lack axons from channel-deficient, GFP-labeled cells, most likely due to the activity-dependent elimination of those inactive ORNs. Interestingly, glomeruli that continue to be innervated by the GFP-positive, channel-deficient ORNs are smaller in diameter than other glomeruli in the bulb. Together, our results show that Lrrc3b expression is cell autonomous and that, as expected, inactive ORNs express low or undetectable levels of the tauYFP reporter.
Figure 7.
Intrinsic fluorescence levels in the Lrrc3b(ΔYFP/+) mouse are altered by odor-evoked activity. (A) Fluorescence in a PD40 Lrrc3b(ΔYFP/+) mouse showing intrinsic signal in the two OBs examined near the midline. The signal is very low in the left OB of an animal subjected to a unilateral naris occlusion at PD2. The bulb from the non-occluded side (right side) displays robust YFP signal. scale bar= 100 microns (B) The OE of CNGA2(tau-GFP/+)/Lrrc3b(ΔYFP/+) female mice (upper left panel) contains an X-inactivation-induced mosaic of ORNs expressing exclusively GFP or YFP (pseudo-colored red in each panel). In the OB of these same mice (lower left panel), glomeruli are either GFP or YFP positive. Note the small size of the GFP-labeled CNGA2-deficient glomerulus. In the OE from male CNGA2(tau-GFP/0)/Lrrc3b(ΔYFP/+) mice (upper right panel), the ORNs are all inactive and uniformly express GFP but little or no YFP. The glomeruli in the OB (lower right panel) are uniformly GFP positive. YFP is undetectable in the glomerular neuropil but is present in periglomerular cells. scale bar (upper panels) = 25 microns. scale bar (lower panels) = 50 microns
DISCUSSION
Alterations in the odor environment modify the cellular composition and organization of the mature olfactory system. We compared the patterns of gene expression in wild type and anosmic mice and observed that neuronal activity contributes to gene expression changes that may underlie some aspects of these organizational, cellular and physiological changes in olfactory neurons. Interestingly, many of the modulated genes fall into categories reflecting the physiological changes that accompany neuronal activity including proteins with calcium binding domains. The changes in gene expression therefore serve as surrogates of activity in each cell. Finally, tagging of modulated genes with specific reporters that label the convergent axons in the olfactory bulb allows the assessment of activity of individual OR populations in the natural odor environment of the mouse.
The screen performed here identified genes in ORNs whose expression is regulated by changes in cyclic nucleotide-activated channel activity. Cells deficient for CNGA2 channel function lack stimulus-induced rises in intracellular Ca2+ and monovalent ions (Na+ and Cl−), but retain odor-evoked modulation of cAMP. The cAMP changes present in channel-deficient mice could modulate gene transcription through activation of the PKA/MAPK/CREB pathway. It is not surprising that the genes identified here with altered regulation in CNGA2−/− mice display similar patterns after naris-occlusion or in Gαolf(−/−) mouse models (data not shown) since in each case ion flux through the channel is eliminated. However, a comparison of gene expression profiles in other genetic models of reduced stimulus-evoked activity might shed light on distinct subsets of genes differentially regulated by each of these processes.
Management of intracellular calcium in odorant stimulated ORNs
The maintenance of homeostatic Ca2+ levels in the presence of the continual flux induced by persistent odorant signaling represents a significant challenge for the ORN. Specific binding proteins present at high levels in these cells are thought to buffer the intracellular Ca2+. Since the Ca2+ flux and the demands for buffering differ in each olfactory neuron depending on the receptors they express and the associated activity in particular odor environments, it is perhaps not surprising that these cytoplasmic proteins are dynamically regulated. In the most extreme situation, when the odorant-induced activity and Ca2+ flux are globally eliminated, as in CNG channel–deficient mice, the expression of specific classes of Ca2+-binding protein genes are dramatically altered.
In situ hybridization analysis of Ca2+ binding-protein-encoding transcript superfamily reveals at least three different patterns of expression in the OE. Most abundant among the classes, S100A5, is a member of the large family of S100 Ca2+ EF-hand proteins. The S100A5 protein binds Ca2+ with particularly high affinity (Schafer et al. 2000). Odorant activity modulates Ca2+ influx in ORNs. As a result, proteins that bind and buffer Ca2+ are highly regulated. PEP-19 is a 6kDa polypeptide that is highly expressed in select populations of neurons where it regulates Ca2+ signaling by binding to calmodulin and inhibiting CaM kinase II activity (Johanson et al. 2000). The different expression patterns of genes encoding proteins with Ca2+ binding domains is consistent with the hypothesis that odorant-evoked activity maintains Ca2+ homeostasis through the expression of specific genes in mouse OE.
Evidence for this differential regulation in native olfactory tissue comes from the considerable variability observed in S100A5 mRNA and protein abundance across individual neurons in the epithelium. A recent study by Yu and colleagues identified S100A5 expression in approximately 70% of ORNs in the OE (Yu et al. 2005). While the authors infer that S100A5 is co-expressed with a specific subset of ORNs, our data suggest that the high level S100A5 expressing cells are those that are active, odorant-stimulated ORNs and that the mosaic pattern of S100A5 expression in the OE reflects different activity levels in ORNs across the OE.
We show here that the expression of S100A5 and other molecules is related to the activity associated with a particular odorant receptor, which is highly dependent on the odor environment. The odor environment experienced by laboratory mice is likely to be considerably more restricted than what is encountered in nature. Furthermore, these activity levels in nature are likely to be highly dynamic due to the complexity of the odor environment. Sakano et al. (Serizawa et al. 2006) observed that the abundance of ephrin A5 correlates with the specific OR gene expressed in each neuron and suggested that these cell signaling molecules define the anatomical target in the olfactory bulb. Our data indicate that the ephrin A5 levels in individual cells may simply reflect the activity that is a characteristic of each OR gene in a particular odor environment. It is difficult to reconcile how a system dependent on the precise abundance of an activity-regulated cell surface molecule can accomplish precise targeting in the face of dynamic modulation.
Through altered patterns of gene transcription, odorant-evoked activity can mediate long-term cellular plasticity in ORNs and allow organisms to adapt to a changing odor environment. Odorant-evoked signal transduction elicits sensory responses on timescales that are too rapid for alterations in gene expression to play a direct role in detection and subsequent responses. Rather, by regulating gene expression in this manner, olfactory signal transduction events may be coupled to molecular mechanisms that lead to sustained changes in ORN properties within the olfactory system
Lrrc3b
We confirmed that Lrrc3b expression is downregulated in the CNGA2 OE and that Lrrc3b is regulated by odorant activity. Odorant stimulation acutely upregulates the expression of Lrrc3b in ORNs. We have not established a definitive function for Lrrc3b. The presence of an extracellular LRR domain suggests that Lrrc3b interacts with other proteins on the surface of ORNs or adjacent cells. Lrrc3b protein has a similar expression pattern to stomatin like protein 3 (SLP3), a novel member of the stomatin family with expression restricted to ORNs (Goldstein et al. 2003). SLP3, like Lrrc3b, is highly enriched in the apical perinuclear region, including the Golgi complex where membrane proteins are processed and modified. The localization of SLP3 expression suggests a role in the assembly, stabilization, translocation, or function of the odorant transduction complex. We suspect that Lrrc3b might have a similar role in pathways that convert odorant-evoked activity into cues that guide other cellular events in ORNs.
Visualization of odorant activity maps in the OB
One challenge for elucidating the representation of odors in the OB has been the development of mapping techniques with sufficient spatial and/or temporal resolution. Intrinsic optical imaging has revealed the OB activity patterns elicited by the exposure to individual compounds and simple mixtures. Similarly, OMP-driven expression of Synapto-pHluorin (spH) in ORNs (Bozza et al. 2004) allows visualization of ORN synaptic output. Recently these methods have been combined to reveal the dorsally-accessible odorant-evoked glomerular activity maps directly in vivo (Soucy et al. 2009). Additionally, activity-dependent expression of immediate early genes (IEGs) such as c-fos has been used for mapping studies in the glomerular layer of the mouse OB (Guthrie et al. 1993; Liu and Baker 1999; Kawamoto et al. 2003). A fluorescent-reporter-tagged activity-regulated gene, such as Lrrc3b, complements these approaches and can contribute to our understanding of odor mapping through the functional imaging of the olfactory system and the creation of accurate glomerular maps of responses to specific odorants.
The Lrrc3b(ΔYFP/+) mouse model has limited temporal resolution for visualizing an odor map given the dynamics of odorant-evoked Lrrc3b expression and translocation of the protein to the OB. However, this model can achieve high spatial resolution and a global perspective of the responses to odorants, since individual odorant-activated ORNS and their axons are fluorescently tagged. Importantly, unlike studies that examine IEG expression in bulb, the levels of YFP present in individual glomeruli reflect the direct activity of cells expressing the OR directing convergence to that location. The availability of reporter-tagged OR genes and the Lrrc3b::tauYFP allele provides a direct assessment of the activity of individual labeled glomeruli in natural odor environments. Such studies are not accessible using previously available methods for visualization OB activity inputs. Future studies could utilize the Lrrc3b(ΔYFP/+) mouse to obtain precise odor activity maps of individual, identified glomeruli or the entire glomerular ensemble in a given odor environment, or after exposure to specific odorants.
Supplementary Material
References
- Belluscio L, Gold GH, et al. Mice deficient in G(olf) are anosmic. Neuron. 1998;20(1):69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, et al. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42(1):9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, et al. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17(4):681–93. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Col JA, Matsuo T, et al. Adenylyl cyclase-dependent axonal targeting in the olfactory system. Development. 2007;134(13):2481–9. doi: 10.1242/dev.006346. [DOI] [PubMed] [Google Scholar]
- Davis JA, Reed RR. Role of Olf-1 and Pax-6 transcription factors in neurodevelopment. J Neurosci. 1996;16(16):5082–94. doi: 10.1523/JNEUROSCI.16-16-05082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhallan RS, Yau KW, et al. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347(6289):184–7. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Feinstein P, Bozza T, et al. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117(6):833–46. doi: 10.1016/j.cell.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Kulaga HM, et al. Cloning and characterization of SLP3: a novel member of the stomatin family expressed by olfactory receptor neurons. J Assoc Res Otolaryngol. 2003;4(1):74–82. doi: 10.1007/s10162-002-2039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72(Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, et al. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 1993;90(8):3329–33. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Sakano H. Molecular mechanisms of odorant receptor-instructed axonal projection. Seikagaku. 2007;79(12):1148–52. [PubMed] [Google Scholar]
- Johanson RA, Sarau HM, et al. Calmodulin-binding peptide PEP-19 modulates activation of calmodulin kinase II In situ. J Neurosci. 2000;20(8):2860–6. doi: 10.1523/JNEUROSCI.20-08-02860.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto M, Tamura M, et al. The alteration of odor-induced c-Fos immunoreactivity in the rat olfactory bulb after olfactory nerve transection. J Neurol. 2003;250(1):51–4. doi: 10.1007/s00415-003-0948-9. [DOI] [PubMed] [Google Scholar]
- Liu N, Baker H. Activity-dependent Nurr1 and NGFI-B gene expression in adult mouse olfactory bulb. Neuroreport. 1999;10(4):747–51. doi: 10.1097/00001756-199903170-00016. [DOI] [PubMed] [Google Scholar]
- Maritan M, Monaco G, et al. Odorant receptors at the growth cone are coupled to localized cAMP and Ca2+ increases. Proc Natl Acad Sci U S A. 2009;106(9):3537–42. doi: 10.1073/pnas.0813224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Wong RO, et al. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252(5008):939–43. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Pevsner J. Progress in the use of microarray technology to study the neurobiology of disease. Nat Neurosci. 2004;7(5):434–9. doi: 10.1038/nn1230. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Targeting olfaction. Curr. Op. Neurob. 1996:481–486. doi: 10.1016/s0959-4388(96)80053-5. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, et al. Visualizing an olfactory sensory map. Cell. 1996;87(4):675–86. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Morris CM, Wilson KE. High throughput approaches in neuroscience. Int J Dev Neurosci. 2004;22(7):515–22. doi: 10.1016/j.ijdevneu.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Norlin EM, Vedin V, et al. Odorant-dependent, spatially restricted induction of c-fos in the olfactory epithelium of the mouse. J Neurochem. 2005;93(6):1594–602. doi: 10.1111/j.1471-4159.2005.03159.x. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Teeter JH, et al. Second messenger signaling in olfactory transduction. J Neurobiol. 1996;30(1):37–48. doi: 10.1002/(SICI)1097-4695(199605)30:1<37::AID-NEU4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Schafer BW, Fritschy JM, et al. Brain S100A5 is a novel calcium-, zinc-, and copper ion-binding protein of the EF-hand superfamily. J Biol Chem. 2000;275(39):30623–30. doi: 10.1074/jbc.M002260200. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, et al. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23(24):5080–1. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, et al. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127(5):1057–69. doi: 10.1016/j.cell.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5(6):745–56. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, et al. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12(2):210–20. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Wang F, Nemes A, et al. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93(1):47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Wang MM, Reed RR. Molecular cloning of the olfactory neuronal transcription factor Olf-1 by genetic selection in yeast. Nature. 1993;364(6433):121–6. doi: 10.1038/364121a0. [DOI] [PubMed] [Google Scholar]
- Watt WC, Sakano H, et al. Odorant stimulation enhances survival of olfactory sensory neurons via MAPK and CREB. Neuron. 2004;41(6):955–67. doi: 10.1016/s0896-6273(04)00075-3. [DOI] [PubMed] [Google Scholar]
- Wong ST, Trinh K, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27(3):487–97. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Yu TT, McIntyre JC, et al. Differentially expressed transcripts from phenotypically identified olfactory sensory neurons. J Comp Neurol. 2005;483(3):251–62. doi: 10.1002/cne.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ivic L, et al. Functional expression of a mammalian odorant receptor. Science. 1998;279(5348):237–42. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
- Zhao H, Reed RR. X inactivation of the OCNC1 channel gene reveals a role for activity-dependent competition in the olfactory system. Cell. 2001;104(5):651–60. doi: 10.1016/s0092-8674(01)00262-8. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, et al. Postnatal refinement of peripheral olfactory projections. Science. 2004;304(5679):1976–9. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.