Abstract
Purpose
To determine the influence of diabetes, diabetic retinopathy (DR), and other factors on macular thickness, measured using optical coherence tomography (OCT), in a population-based sample.
Methods
Data from the population-based Singapore Indian Eye Study were analyzed. We measured macular thickness using Stratus OCT Fast Macular Thickness scan protocol in 228 participants with diabetes mellitus (including 167 without DR, 44 with mild DR, 17 with moderate or severe DR) and 72 non-diabetic controls without macular oedema or other macular lesions. Analysis was done on right eyes.
Results
The mean age of participants was 60.1±10.1 years, with 53.8% men. Macular thickness measurements did not differ significantly between diabetic participants with no or mild DR and non-diabetic participants. Diabetic participants with moderate or severe DR had greater foveal and temporal outer macula thickness compared with those with no or mild DR (P=0.003). In a multivariate linear regression model, older age (P=0.009), male gender (P=0.005), and lower spherical equivalent (P=0.001) were other factors associated with greater foveal thickness in all participants after controlling for body mass index, glycosylated haemoglobin, total cholesterol, and mean systolic blood pressure.
Conclusion
This population-based study showed that diabetic participants with moderate or severe DR had thicker foveal measurements, even in the absence of diabetic macula oedema, than non-diabetic controls. Other factors that influenced macular thickness measurements were age, gender, and spherical equivalent. These data may aid the interpretation of OCT findings in persons with diabetes and DR.
Keywords: optical coherence tomography, diabetic retinopathy, macula
Introduction
Optical coherence tomography (OCT) is a non-invasive imaging modality now extensively used to measure retinal thickness.1, 2 Despite widespread clinical use, there are only few studies from the general population on the distributions and correlations of OCT-measured retinal thickness, mostly in white ethnic groups.3, 4, 5 There are also few studies on normative OCT measures in major patient groups, such as persons with diabetes.6, 7, 8 Normative data in general populations are essential to allow pathological changes to be compared, identified, and characterized. It is particularly important to establish normative values in persons with diabetes with nearly 400 million such individuals worldwide by 2030.9
Previous studies on macular thickness measurements in persons with diabetes, obtained using OCT and other instruments such as the retinal thickness analyzer, have reported variable findings.6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Biallosterki et al7 reported that thinner pericentral macular thickness in patients with minimal diabetic retinopathy (DR) compared with controls, and hypothesized that this was due to neuronal loss in the earliest stage of DR.20 In contrast, Lattanzio et al12 found that the macular thickness in subjects with diabetes without DR was thicker (by more than 40 μm) than that in non-diabetic controls. Others have reported no difference in macular thickness between subjects with diabetes with minimal or no DR and non-diabetic controls.18 Importantly, all previous studies on macular thickness in diabetic persons recruited the participants from hospitals and universities, hence were limited by potential selection bias (eg, patients with better (or worse) diabetes control may have been more likely to be included).6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 At present, there are no population-based published data on the macular thickness in diabetic individuals with and without DR, and how macular thickness parameters compare with normal non-diabetic controls.
In this study, we described and compared macular thickness using Stratus OCT (Carl Zeiss Meditec, Dublin, CA, USA) in persons with or without diabetes in a population-based study in Asian Indians, and identified factors that affected macular thickness measurements.
Methods
Subjects
We analyzed data from the Singapore Indian Eye Study, a population-based cross-sectional survey of ethnic Indians aged 40–80 years living in the south-western part of Singapore. The subjects were selected using an age-stratified (by 10-year age group) random sampling method, from a computer-generated list provided by the Ministry of Health, Singapore. The methodology and objectives of the study population have been reported in detail elsewhere.21, 22 Of the eligible 4497 participants, 3400 (response rate 75.6%) participated in the study, conducted from May 2007 through December 2009. Participants underwent a standardized interview, systemic and ocular examination, and laboratory investigations.
As part of the study between June 2008 and April 2009, we recruited 283 participants with diabetes mellitus and 88 non-diabetic controls for OCT measurement. OCT scans were obtained for all participants with diabetes and selected non-diabetic controls. Of the 371 participants, we excluded 38 participants with co-existing macular pathology (including any evidence of diabetic macular oedema, epiretinal membrane formation, or myopic maculopathy) and a history or signs of previous retinal laser treatment. In all, 33 participants who did not have fundal photographs or had poor quality OCT scans of signal strength <6 were further excluded. This left 300 (228 subjects with diabetes and 72 non-diabetic controls) in the final analysis.
OCT
OCT scanning was performed by trained technicians using Stratus OCT (Carl Zeiss Meditec). Macular thickness measurements were obtained from the right eye of the participants after pupil dilatation, using tropicamide 1% and phenylephrine hyprochloride 2.5%. The Fast Macular Thickness scan protocol was used, which acquires six 6-mm linear scans oriented 30° apart in a radial spoke-like pattern in a continuous automated sequence. Each of the six linear scans is composed of 128 equally spaced transverse axial scans. The reproducibility of retinal thickness measurements using the Fast Macular Thickness mapping protocol of Stratus OCT is high in normal eyes.23 The Stratus OCT software generated a topographical map of the macula, which is composed of nine sectorial thickness measurements in three concentric circles with diameters of 1, 3 and 6 mm, as defined by the Early Treatment of Diabetic Retinopathy Study (ETDRS).24 The inner and outer rings were segmented into quadrants. Foveal thickness was defined as the average thickness in the central 1 mm diameter, according to the ETDRS layout.24 Only the OCT scans with signal strength ≥6 were included in this analysis.
Definition of diabetes and DR
Non-fasting venous blood samples were analyzed at the National University Hospital Reference Laboratory for biochemical testing of glycosylated haemoglobin (HbA1C) and glucose. Diabetes mellitus was identified from non-fasting plasma glucose ≥11.1 mmol/l, self-reported use of diabetic medication, or physician-diagnosed diabetes.
A digital retinal camera (Canon CR-DGi with a 20 Dioptre SLR backing, Canon, Tokyo, Japan) was used to perform retinal photography after pupil dilation. Early Treatment for Diabetic Retinopathy Study (ETDRS) standard field 1 (centred on the optic disc) and ETDRS standard field 2 (centred on the fovea) retinal images were taken.25 Trained graders at the Centre for Vision Australia, University of Sydney, masked to the participant characteristics, evaluated the retinal photographs for the presence and severity of DR. Any retinopathy was defined as a severity score of level 15 and above, according to a scale graded using the ETDRS adaptation of the modified Airlie House Classification System corresponding to the presence of any of the following lesions: microaneurysms, haemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, venous beading, and new vessels.26, 27 A severity score of up to level 35 was considered as mild DR, from level 36 to 43 as moderate DR, and above 47 as severe DR. Among the 228 diabetic participants, no DR was seen in 167 participants, mild DR was present in 44 participants, moderate or severe DR was present in 17 participants. We excluded 38 participants because they had signs of macular oedema or other maculopathies.
Refraction
Subjective refraction and distance best-corrected visual acuity in logarithm of the minimum angle of resolution (Log MAR) scores were measured by trained and certified study optometrists.28 The spherical equivalent (SE) was determined by:
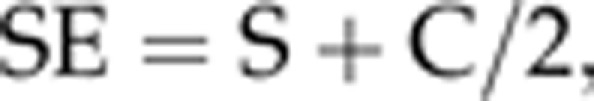 |
where S was the spherical power, and C was the cylindrical power.
Measurement of other variables
Prior to OCT imaging, detailed interviewer-administered questionnaires that collected relevant socio-demographic data (including alcohol intake, smoking, and a standardized diabetes questionnaire) and medical history were administered. The age of each participant was recorded at the time of clinic examination, when OCT imaging was performed. We evaluated the systolic and diastolic blood pressure (BP) with a digital automatic BP monitor (Dinamap model Pro 100V2; Critericon, Norderstedt, Germany), after the participants were seated for 5 minutes with legs uncrossed. A total of three measurements was taken, and the average of the two closest BP readings were taken as each participant's BP. The MABP (mean arterial BP) was calculated by this equation:
Body mass index (BMI) was calculated as the weight (in kilograms) divided by body height (in metres) squared. Biochemical analysis of random blood samples was performed on the day of the clinic examination at the National University Hospital Reference Laboratory, for total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol.
Statistical analysis
We compared the demographics between diabetic participants and controls using the chi-square test or one-way analysis of variance as appropriate. Analysis of covariance was used to compare OCT parameters between these groups: (1) diabetic participants without DR; (2) diabetic participants with mild DR; (3) diabetic participants with moderate or severe DR, adjusting for age, HbA1C, systolic BP, diabetes duration, total cholesterol, low-density lipoprotein cholesterol, and BMI. Multivariable linear regression models were constructed with foveal thickness and total macular volume as the dependent variables, and adjusting for age, gender, BMI, HbA1C, total cholesterol, SE, systolic BP, and retinopathy (with participants without diabetes as the reference). This was repeated for diabetic subjects alone, after further adjustment for duration of diabetes and the presence of retinopathy (with diabetic participants with no DR as the reference). P-values <0.05 were considered to be statistically significant. All analyses were performed with STATA version 11 (StataCorp. LP, College Station, TX, USA).
Results
Baseline characteristics of the participants are summarized in Table 1. There were significant differences in age (P<0.001), HbA1c (P<0.001), systolic BP (P=0.003), total cholesterol (P<0.001), low-density lipoprotein cholesterol (P<0.001), and BMI (P=0.036) between the four groups. Diabetic participants with moderate or severe DR had a longer duration of diabetes compared with those with mild or no DR (P<0.001).
Table 1. Demographic characteristics of participants.
| Non-Diabetic Controls (n=72) |
Diabetes |
P-valuea | |||
|---|---|---|---|---|---|
| No retinopathy (n=167) | Minimal/mild DR (n=44) | Moderate/severe DR (n=17) | |||
| Age, years | 54.2 (8.2) | 60.8 (9.9) | 60.3 (9.9) | 59.4 (7.9) | <0.001 |
| Men, % | 39 (54.2) | 89 (53.3) | 27 (61.4) | 9 (52.9) | 0.813 |
| Mean arterial blood pressure, mm Hg | 97.07 (11.63) | 96.22 (9.34) | 98.16 (14.36) | 99.16 (2.49) | 0.569 |
| Spherical equivalent (right eye) | −0.16 (1.88) | −0.31 (2.20) | 0.27 (1.13) | 0.30 (2.43) | 0.291 |
| HbA1c, % | 5.96 (0.57) | 7.29 (1.21) | 7.61 (1.60) | 8.29 (2.00) | <0.001 |
| Diabetes duration, year | — | 8.23 (7.65) | 12.34 (8.23) | 15.06 (9.85) | <0.001 |
| Systolic blood pressure, mm Hg | 131.83 (17.97) | 136.87 (16.54) | 143.08 (21.68) | 143.94 (18.23) | 0.003 |
| Total cholesterol, mmol/l | 5.30 (0.92) | 4.61 (0.95) | 4.51 (1.102) | 4.47 (1.36) | <0.001 |
| LDL cholesterol, mmol/l | 3.55 (0.85) | 2.87 (0.81) | 2.75 (0.75) | 2.70 (1.04) | <0.001 |
| HDL cholesterol, mmol/l | 1.09 (0.32) | 1.03 (0.31) | 1.01 (0.32) | 0.98 (0.25) | 0.404 |
| Body mass index, kg/m2 | 25.45 (5.72) | 27.46 (5.22) | 27.88 (5.74) | 26.43 (3.38) | 0.036 |
| Smoking status, yes vs no | 10 (13.9) | 17 (10.2) | 7 (15.9) | 3 (17.6) | 0.606 |
Data is mean (SD) or count (%).
P-value from Chi-square test/Fishers' exact test or one-way ANOVA as appropriate across all groups.
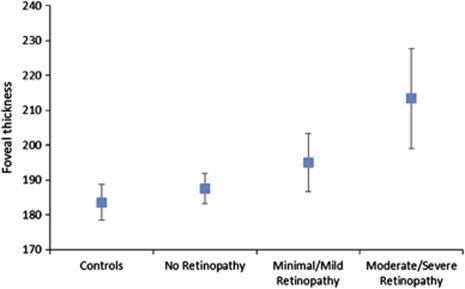
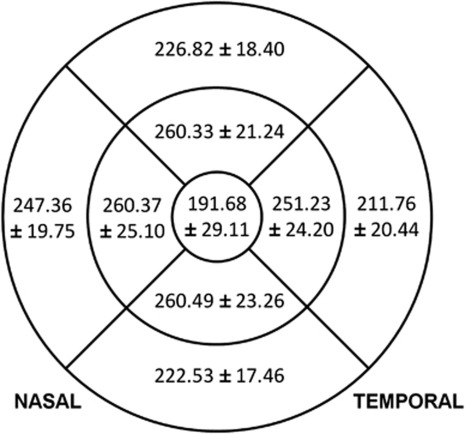
Diabetic participants with moderate or severe DR had greater foveal thickness (213.36, 95% confidence interval (CI) 199.03, 227.70 μm) and temporal outer macula thickness (218.93, 95% CI 209.18, 228.67 μm) compared with those with mild DR (194.98, 95% CI 186.62, 203.34, and 215.72, 95% CI 210.19, 221.25 μm, respectively) and no DR (187.51, 95% CI 183.21, 191.80, and 209.21, 95% CI 206.38, 212.03 μm, respectively) (P=0.003 and P=0.045, respectively) (Table 2, Figure 1). The mean and SD of macular thickness in participants with diabetes are shown in Figure 2.
Table 2. Comparison between Diabetic Participants without Diabetic Retinopathy (DR) and severity of Diabetic Retinopathy.
| Macular measurements |
Diabetes |
P-valuea | ||
|---|---|---|---|---|
| No retinopathy (n=167) | Minimal/mild DR (n=44) | Moderate/severe DR (n=17) | ||
| Foveal thickness, μm | 187.51 (183.21, 191.80) | 194.98 (186.62, 203.34) | 213.36 (199.03, 227.70) | 0.003 |
| Temporal inner macula, μm | 249.30 (245.49, 253.12) | 254.23 (246.75, 261.70) | 260.31 (247.15, 273.48) | 0.212 |
| Superior inner macula, μm | 259.21 (255.83, 262.59) | 264.09 (257.47, 270.72) | 259.56 (247.89, 271.23) | 0.437 |
| Nasal inner macula, μm | 259.29 (255.43, 263.16) | 264.80 (257.22, 272.37) | 258.42 (245.07, 271.77) | 0.425 |
| Inferior inner macula, μm | 259.52 (255.84, 263.20) | 265.64 (258.43, 272.85) | 258.42 (245.72, 271.13) | 0.309 |
| Temporal outer macula, μm | 209.21 (206.38, 212.03) | 215.72 (210.19, 221.25) | 218.93 (209.18, 228.67) | 0.045 |
| Superior outer macula, μm | 225.22 (222.40, 228.06) | 231.90 (226.35, 237.45) | 227.52 (217.74, 237.29) | 0.119 |
| Nasal outer macula, μm | 246.29 (243.27, 249.30) | 248.64 (242.73, 254.56) | 247.57 (237.14, 257.99) | 0.788 |
| Inferior outer macula, μm | 221.19 (218.50, 223.88) | 226.08 (220.82, 231.35) | 224.25 (214.97, 233.52) | 0.267 |
| Total macular volume, mm3 | 6.56 (6.48, 6.63) | 6.70 (6.56, 6.85) | 6.67 (6.41, 6.93) | 0.211 |
Data are adjusted mean (95%CI).
P-value from ANCOVA of means comparison between groups (adjusting for age, hba1c, systolic blood pressure, diabetes duration, total cholesterol, low density lipoprotein cholesterol, and body mass index).
Figure 1.
Boxplots of foveal thickness in the study participants.
Figure 2.
Mean and standard deviations of macular thickness (μm) by sector in the right eye of each subject in 228 diabetic participants from the Singapore Indian Eye Study, with optical coherence tomography using the Fast Macular Thickness scan protocol.
In the multivariable linear regression model of foveal thickness in all participants and in participants with diabetes, older age (P=0.009 and P=0.014, respectively), male gender (P=0.005 and P=0.007, respectively), lower SE (P=0.001 and P=0.019, respectively), and the presence of moderate or severe DR (P=0.001 and P<0.001, respectively) were associated with increased foveal thickness. (Table 3A ). In the multivariable linear regression model of macular volume in all participants and in participants with diabetes, older age (P<0.001 and P=0.001, respectively), male gender (P=0.003 and P=0.037, respectively), greater SE (P=0.015 and P=0.007, respectively), and the presence of moderate or severe DR (P=0.015 and P=0.041, respectively) were associated with increased macular volume (Table 3B). The presence of diabetes and mild or no DR did not significantly affect foveal thickness or total macular volume measurements compared with participants without diabetes. Among participants with diabetes, the duration of diabetes, HbA1C, and the presence of mild DR did not significantly affect foveal thickness or total macular volume measurements compared with those without DR (Table 3). All models were also adjusted for BMI, total cholesterol, and systolic BP.
Table 3. Multivariable linear regressions of Foveal Thickness and Macular Volume.
| Factors |
All persons (n=300) |
Persons with diabetes (n=228) |
||
|---|---|---|---|---|
| Mean difference (95% CI)a | P-value | Mean difference (95% CI)a | P-value | |
| A. Multiple linear regression of Foveal thickness | ||||
| Age, per year | 0.46 (0.12, 0.81) | 0.009 | 0.60 (0.12, 1.08) | 0.014 |
| Gender, female vs male | −8.82 (−14.94, −2.70) | 0.005 | −10.62 (−18.30, −2.30) | 0.007 |
| BMI | −0.20 (−0.79, 0.39) | 0.503 | −0.16 (−0.91, 0.58) | 0.672 |
| HbA1C | 1.14 (−1.44, 3.73) | 0.385 | 1.54 (−1.39, 4.47) | 0.302 |
| Total cholesterol | −1.90 (−5.17, 1.38) | 0.255 | −2.74 (−6.82, 1.33) | 0.185 |
| Spherical equivalent | −2.61 (−4.10, −1.12) | 0.001 | −2.21 (−4.05, −0.38) | 0.019 |
| Diabetes duration | — | — | −0.20 (−0.73, 0.32) | 0.443 |
| Systolic blood pressure | 0.09 (−0.08, 0.26) | 0.279 | 0.15 (−0.06, 0.36) | 0.152 |
| Retinopathy | ||||
| Non-diabetics and no retinopathy | Reference | — | — | |
| Diabetics with no retinopathy | −2.43 (−11.16, 6.29) | 0.584 | Reference | |
| Diabetics with minimal/mild | 5.52 (−5.84, 16.87) | 0.340 | 8.01 (−1.55, 17.56) | 0.100 |
| Diabetics with moderate/severe | 27.03 (10.76, 43.31) | 0.001 | 30.38 (14.98, 45.78) | <0.001 |
| B. Multiple linear regression of total macular volume | ||||
| Age, per year | −0.01 (−0.02, −0.01) | <0.001 | −0.01 (−0.02, −0.01) | 0.001 |
| Gender, female vs male | −0.16 (−0.27, −0.05) | 0.003 | −0.14 (−0.28, −0.01) | 0.037 |
| BMI | −0.01 (−0.02, 0.003) | 0.176 | −0.01 (−0.02, 0.003) | 0.126 |
| HbA1C | 0.03 (−0.02, 0.07) | 0.258 | 0.03 (−0.02, 0.08) | 0.304 |
| Total cholesterol | −0.007 (−0.063, 0.058) | 0.810 | −0.03 (−0.10, 0.04) | 0.419 |
| Spherical equivalent | 0.03 (0.01, 0.06) | 0.015 | 0.04 (0.01, 0.07) | 0.007 |
| Diabetes duration | — | — | 0.004 (−0.005, 0.013) | 0.414 |
| Systolic blood pressure | −0.001 (−0.004, 0.002) | 0.643 | 0.0001 (−0.0035, 0.0038) | 0.941 |
| Retinopathy | ||||
| Non-diabetics and no retinopathy | Reference | — | — | |
| Diabetics with no retinopathy | 0.02 (−0.13, 0.17) | 0.780 | Reference | |
| Diabetics with minimal/mild | 0.16 (−0.03, 0.36) | 0.100 | 0.12 (−0.05, 0.29) | 0.156 |
| Diabetics with moderate/severe | 0.35 (0.07, 0.63) | 0.015 | 0.28 (0.01, 0.55) | 0.041 |
Abbreviations: CI, confidence interval; DR, diabetic retinopathy; HbA1C, glycosylated hemoglobin.
Adjusted for adjusted for all parameters in the table.
Discussion
In this population-based study, we found that persons with diabetes with moderate or severe DR had increased foveal and temporal outer macula thickness compared with those with no or mild DR and persons without diabetes. Age, gender, and SE were other factors that significantly affected macular volume and foveal thickness. However, there were no significant differences in macular thickness measurements between diabetic persons with no or mild DR and persons without diabetes. Furthermore, among participants with diabetes, the duration of diabetes and HbA1C did not significantly affect macular thickness measurements.
Our results provide new population-based data on the macular thickness measurements in persons with diabetes, which have not been previously reported. Our finding that the severity of DR significantly affects macular thickness measurements even in the absence of diabetic macular oedema is also novel. We showed that diabetic individuals with moderate or severe DR had increased fovea and temporal outer macular thickness compared with those who had no DR and non-diabetic individuals, though a few earlier studies have described a generalized increase in macular thickness in persons with diabetes.12, 16, 29 In contrast, there were no significant differences in fovea thickness and macular volume measurements between diabetic persons with mild or no DR and non-diabetic persons. This may be explained by an alteration of the blood–retinal barrier in moderate and severe DR,30 which may facilitate an increase in the vascular permeability of perifoveal and macular capillaries. Another possible mechanism for increased foveal thickness in persons with moderate or severe DR is interstitial oedema secondary to perifoveal capillary loss, which has been found to occur in the course of DR.31 Hudson et al32 have reported that macular capillary blood flow was lower in areas of diabetic macular oedema and this reduction was more evident in the temporal compared with the nasal macula (P=0.01), which is consistent with our finding that the temporal outer macula thickness was increased in persons with moderate or severe DR. These vascular changes may be related to the severity of DR even in the absence of diabetic macular oedema. Additional studies are required to verify these hypotheses. A meta-analysis evaluating the diagnostic accuracy of OCT for detecting macular oedema in people with DR found that OCT can detect macular thickening earlier than clinical examination, but many such cases did not progress to clinically detectable macular oedema and require photocoagulation.33
The findings of previous studies on macular thickness measurements in diabetic individuals were inconsistent, with some studies reporting a decreased6, 7, 19, 34 or increased12, 16, 29 macular thickness, whereas other studies reported similar macular thickness measurements compared with non-diabetic controls.8, 18 There are a few possible reasons for these discrepancies. Firstly, some studies included persons with diabetic macular oedema,11, 17 whereas others only recruited diabetic individuals with no or minimal DR.15, 18 Secondly, earlier studies were hospital- or university-based, hence were subject to selection bias,6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 which are minimized in a population-based study. Thirdly, most of the previous studies did not adjust for age, gender, ethnicity or refractive error,6, 7, 11, 29 which are possible confounders for retinal thickness measurements. Lastly, macular thickness was assessed by instruments other than the OCT in some of the earlier studies, such as the retinal thickness analyzer, and these imaging modalities may not be comparable to Stratus OCT.10, 29, 35, 36, 37
We identified age, gender, and SE as factors that significantly affected macular measurements using Stratus OCT, which is consistent with the results of previous studies.10, 13, 18, 38, 39, 40, 41 However, our results also indicate that more myopic individuals have an increased foveal thickness but reduced macular volume, which substantiate earlier reports.40, 41 Lam et al40 hypothesized that the thicker fovea could be a sign of early vitreoretinal traction in highly myopic eyes. Previous studies reported that women have thinner macular measurements,13, 30, 38, 39 which is consistent with our results. This may explain why certain macular conditions, such as macular hole, occur more frequently in women. We showed that older age is associated with increased OCT measurements of the fovea. This supports the findings of Kashani et al,18 and may be caused by the presence of interstitial oedema from capillary dropout with age, Angiographic evidence is required to substantiate this hypothesis. However, our results also indicate that although foveal thickness is greater with increasing age, macular volume is reduced. This adds to the growing evidence that the macular thinning that occurs with aging is non-uniform.42 In our study, the duration of diabetes and HbA1C levels did not significantly affect macular thickness measurements. In contrast, previous studies have reported increased macular thickness with higher HbA1C levels,43, 44 whereas a longer duration of diabetes was associated with thinner macula measurements in the absence of macula oedema.45, 46 However, these studies were not population-based, hence were susceptible to selection bias. In addition, the level of HbA1C and the duration of diabetes are likely confounded by the severity of DR, which was not adjusted for in these earlier studies.
Our results have research implications and are also clinically significant. First, our data support previous studies, which showed that mild macular thickening on OCT may not correspond to overt oedema recognized by slit lamp biomicrosopy.12, 47 This has been termed ‘subclinical macular oedema',48, 49 and has been described in up to 25% of eyes without clinically significant macular oedema.47 Bhavsar and Subramaniam48 found that a significant number of patients with subclinical macular oedema ultimately progress to clinically significant macular oedema compared with controls, with a 15% increase in the odds of progression with each 10 μm increase in central subfield macular thickness (OR 1.15, CI 1.03–1.28; P=0.01). It may therefore be important to monitor such diabetic individuals more closely so that potential vision-threatening macular oedema can be detected earlier. Second, it is well known that diabetes may also accelerate retinal neuronal cell death,50 resulting in macular atrophy. However, this is inconsistent with our results, which have shown an increase in macular thickness measurements in persons with diabetes. Further studies using spectral-domain OCT to measure the specific retinal layers are necessary to verify this hypothesis. Third, our data provide normative values of macular thickness in diabetic eyes. Such normative values are important in the interpretation of previous clinical trials evaluating DR and diabetic macular oedema using Stratus OCT, and may also be applicable in designing future research questions using spectral-domain OCT. Our results that diabetic persons with moderate or severe DR have an increased foveal thickness, even in the absence of macular oedema, should be taken into consideration when determining the upper limits of normal for eligibility into such trials.14 These findings further provide insights into the pathogenesis of early changes in DR. Our results have also confirmed that it is essential to adjust for age, gender, and SE when conducting analyses of OCT measurements, whereas most earlier studies assessing macular thickness values in persons with diabetes did not adjust for these confounding factors.6, 7, 11, 29
The main strength of our study is the population-based design, which minimizes the selection bias inherent in previous hospital- and university-based studies.6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Other strengths include detailed information on the severity of DR and possible confounders, such as BP and SE, and the use of a standardized diabetes questionnaire. However, some limitations should be considered in the interpretation of our findings. We did not distinguish between type 1 or type 2 diabetes, which vary in pathophysiology and treatment, hence might also vary in macular thickness measurements. An additional limitation of our study is the lack of data pertaining to the diagnosis of glaucoma in the participants, which is a possible confounder in the assessment of macular thickness.51, 52
In summary, our results provide population-based data that would be useful in the interpretation of macular thickness values in persons with diabetes. We have shown that macular thickness measurements are increased in moderate or severe DR even when diabetic macular oedema is absent. Additional studies using spectral-domain OCT are indicated to extend these results, and more advanced retinal segmentation methods should be used to investigate whether specific retinal layers are preferentially affected by diabetes.
Acknowledgments
This study was funded by the National Medical Research Council (NMRC), NMRC/STaR/0003/2008 and Biomedical Research Council (BMRC), 08/1/35/19/440, Singapore.
Author contributions
Design of study (CCAS, CYC, REM, RL, PM, TA, TWY); conduct of study (CYC, REM, RL, TYW), statistical analysis (CCAS, WW), preparation of manuscript (CCAS, CYC, REM, RL, WW, PM, TA, TYW). Written informed consent was obtained from each participant and the study conducted adhered to the Declaration of Helsinki. Ethics approval was obtained from the Singapore Eye Research Institute Institutional Review Board.
The authors declare no conflict of interest.
References
- Huang D, Swanson E, Lin C, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangelder G, Van der Heijde R, Polak B, Ringens P. Precision and reliability of retinal thickness measurements in foveal and extrafoveal areas of healthy and diabetic eyes. Invest Ophthalmol Vis Sci. 2008;49:2627–2634. doi: 10.1167/iovs.07-0820. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Holmstrom G, Alm A, Larsson E. A population-based study of macular thickness in full-term children assessed with Stratus OCT: normative data and repeatability. Acta Ophthalmol. 2009;87:714–715. doi: 10.1111/j.1755-3768.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- Huynh S, Wang X, Burlutsky G, Rochtchina E, Stapleton F, Mitchell P. Retinal and optic disc findings in adolescence: a population-based OCT study. Invest Ophthalmol Vis Sci. 2008;49:4328–4335. doi: 10.1167/iovs.07-0699. [DOI] [PubMed] [Google Scholar]
- Duan X, Liang Y, Friedman D, Sun LP, Wong TY, Tao QS, et al. Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult Chinese persons: the Handan Eye Study. Ophthalmology. 2010;117:1585–1594. doi: 10.1016/j.ophtha.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Asefzadeh B, Fisch B, Parenteau C, Cavallerano A. Macular thickness and systemic markers for diabetes in individuals with no or mild diabetic retinopathy. Clin Experiment Ophthalmol. 2008;36:455–463. doi: 10.1111/j.1442-9071.2008.01769.x. [DOI] [PubMed] [Google Scholar]
- Biallosterski C, van Velthoven M, Michels R, Schlingemann R, DeVries J, Verbraak F. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol. 2007;91:1135–1138. doi: 10.1136/bjo.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler N, Edwards A, Antoszyk A, Beck RW, Browning DJ, Ciardella AP, et al. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am J Ophthalmol. 2008;145:894–901. doi: 10.1016/j.ajo.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Goebel W, Franke R. Retinal thickness in diabetic retinopathy: comparison of optical coherence tomography, the retinal thickness analyzer, and fundus photography. Retina. 2006;26:49–57. doi: 10.1097/00006982-200601000-00009. [DOI] [PubMed] [Google Scholar]
- Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT) Retina. 2002;22:759–767. doi: 10.1097/00006982-200212000-00012. [DOI] [PubMed] [Google Scholar]
- Lattanzio R, Brancato R, Pierro L, Bandello F, Iaccher B, Fiore T, et al. Macular thickness measured by optical coherence tomography in diabetic patients. Eur J Ophthalmol. 2002;12:482–487. doi: 10.1177/112067210201200606. [DOI] [PubMed] [Google Scholar]
- Massin P, Erginay A, Haouchine B, Mehidi A, Paques M, Gaudric A. Retinal thickness in healthy and diabetic subjects measured using optical coherence tomography mapping software. Eur J Ophthalmol. 2002;12:102–108. doi: 10.1177/112067210201200205. [DOI] [PubMed] [Google Scholar]
- Sanchez-Tocino H, Alvarez-Vidal A, Maldonado M, Moreno-Montanes J, Garcia-Layana A. Retinal thickness study with optical coherence tomography in patients with diabetes. Invest Ophthalmol Vis Sci. 2002;43:1588–1594. [PubMed] [Google Scholar]
- Schaudig U, Glaefke C, Scholz F, Richard G. Optical coherence tomography for retinal thickness measurement in diabetic patients without clinically significant macular oedema. Ophthalmic Surg Lasers. 2000;31:182–186. [PubMed] [Google Scholar]
- Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219:379–385. doi: 10.1159/000088382. [DOI] [PubMed] [Google Scholar]
- Yang C, Cheng C, Lee F, Hsu W, Liu J. Quantitative assessment of retinal thickness in diabetic patients with and without clinically significant macular edema using optical coherence tomography. Acta Ophthalmol Scand. 2001;79:266–270. doi: 10.1034/j.1600-0420.2001.790311.x. [DOI] [PubMed] [Google Scholar]
- Kashani A, Zimmer-Galler I, Shah S, Dustin L, Do DV, Eliott D, et al. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol. 2010;149:496–502. doi: 10.1016/j.ajo.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, von Wendt G, Wanger P, Martin L. Early detection of macular changes in patients with diabetes using Rarebit Fovea Test and optical coherence tomography. Br J Ophthalmol. 2007;91:1596–1598. doi: 10.1136/bjo.2007.124461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieth E, Gardner T, Barber A, Antonetti D. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 2000;28:3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Lavanya R, Jeganathan V, Zheng Y, Raju P, Cheung N, Tai ES, et al. Methodology of the Singapore Indian Chinese Cohort (SICC) eye study: quantifying ethnic variations in the epidemiology of eye diseases in Asians. Ophthalmic Epidemiol. 2009;16:325–336. doi: 10.3109/09286580903144738. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Lavanya R, Wu R, Wong WL, Wang JJ, Mitchell P, et al. Prevalence and causes of visual impairment and blindness in an urban indian population The Singapore Indian Eye Study. Ophthalmology. 2011;118 (9:1798–1804. doi: 10.1016/j.ophtha.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Polito A, Del Borrello M, Isola M, Zemella N, Bandello F. Repeatability and reproducibility of fast macular thickness mapping with stratus optical coherence tomography. Arch Ophthalmol. 2005;123:1330–1337. doi: 10.1001/archopht.123.10.1330. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Early treatment diabetic retinopathy study design and baseline patient characteristic. ETDRS report number 7. Ophthalmology. 1991;98:741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Study Coordinating Center . Diabetic Retinopathy Study: Manual of Operations. MDRSCC: Baltimore; 1972. [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98 (5 Suppl:786–806. [PubMed] [Google Scholar]
- Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115 (11:1869–1875. doi: 10.1016/j.ophtha.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Pan CW, Wong TY, Lavanya R, Wu RY, Zheng YF, Lin XY, et al. Prevalence and risk factors for refractive errors in Indians: the Singapore Indian Eye Study (SINDI) Invest Ophthalmol Vis Sci. 2011;52 (6:3166–3173. doi: 10.1167/iovs.10-6210. [DOI] [PubMed] [Google Scholar]
- Fritsche P, Van der Heijde R, Suttorp-Schulten M, Polak B. Retinal thickness analysis (RTA): an objective method to assess and quantify the retinal thickness in healthy controls and in diabetics without diabetic retinopathy. Retina. 2002;22:768–771. doi: 10.1097/00006982-200212000-00013. [DOI] [PubMed] [Google Scholar]
- Giebel S, Menicucci G, McGuire P, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- Sander B, Larsen M, Engler C, Lund-Andersen H, Parving H. Early changes in diabetic retinopathy: capillary loss and blood-retina barrier permeability in relation to metabolic control. Acta Ophthalmol. 1994;72:553–559. doi: 10.1111/j.1755-3768.1994.tb07179.x. [DOI] [PubMed] [Google Scholar]
- Hudson C, Flanagan JG, Turner GS, Chen HC, Rawji MH, McLeod D. Exaggerated relative nasal-temporal asymmetry of macular capillary blood flow in patients with clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89 (2:142–146. doi: 10.1136/bjo.2003.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgili G, Menchini F, Murro V, Peluso E, Rosa F, Casazza G. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2011. p. CD008081. [DOI] [PubMed]
- Verma A, Rani P, Raman R, Pal SS, Laxmi G, Gupta M, et al. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye. 2009;23:1824–1830. doi: 10.1038/eye.2009.184. [DOI] [PubMed] [Google Scholar]
- Pires I, Bernardes R, Lobo C, Soares M, Cunha-Vaz J. Retinal thickness in eyes with mild nonproliferative retinopathy in patients with type 2 diabetes mellitus: comparison of measurements obtained by retinal thickness analysis and optical coherence tomography. Arch Ophthalmol. 2002;120:1301–1306. doi: 10.1001/archopht.120.10.1301. [DOI] [PubMed] [Google Scholar]
- Weinberger D, Axer-Siegel R, Landau D, Yassur Y. Retinal thickness variation in the diabetic patient measured by the retinal thickness analyser. Br J Ophthalmol. 1998;82:1003–1006. doi: 10.1136/bjo.82.9.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa T, Kiryu J, Tsujikawa A, Dong J, Suzuma I, Takagi H, et al. Quantitative analysis of foveal retinal thickness in diabetic retinopathy with the scanning retinal thickness analyzer. Retina. 1998;18:150–155. doi: 10.1097/00006982-199818020-00009. [DOI] [PubMed] [Google Scholar]
- Kelty P, Payne J, Trivedi R, Kelty J, Bowie E, Burger B. Macular thickness assessment in healthy eyes based on ethnicity using Stratus OCT optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:2668–2672. doi: 10.1167/iovs.07-1000. [DOI] [PubMed] [Google Scholar]
- Wong A, Chan C, Hui S. Relationship of gender, body mass index, and axial length with central retinal thickness using optical coherence tomography. Eye. 2005;19:292–297. doi: 10.1038/sj.eye.6701466. [DOI] [PubMed] [Google Scholar]
- Lam D, Leung K, Mohamed S, Chan WM, Palanivelu MS, Cheung CY, et al. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci. 2007;48:376–382. doi: 10.1167/iovs.06-0426. [DOI] [PubMed] [Google Scholar]
- Wu P, Chen Y, Chen C, Chen YH, Shin SJ, Yang HJ, et al. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye. 2008;22:551–555. doi: 10.1038/sj.eye.6702789. [DOI] [PubMed] [Google Scholar]
- Neuville JM, Bronson-Castain K, Bearse MA, Jr, Nq JS, Harrison WW, Schneck ME, et al. OCT reveals regional differences in macular thickness with age. Optom Vis Sci. 2009;86 (7:E810–E816. doi: 10.1097/OPX.0b013e3181adff59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung L, Sun CC, Ku WC, Chuang LH, Chen CH, Huang BY, et al. Associations between chronic glycosylated haemoglobin (HbA1c) level and macular volume in diabetes patients without macular oedema. Acta Ophthalmol. 2010;88 (7:753–758. doi: 10.1111/j.1755-3768.2009.01711.x. [DOI] [PubMed] [Google Scholar]
- Chou TH, Wu PC, Kuo JZ, Lai CH, Kuo CN. Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye. 2009;23 (6:1360–1363. doi: 10.1038/eye.2008.279. [DOI] [PubMed] [Google Scholar]
- Asefzadeh B, Fisch BM, Parenteau CE, Cavallerano AA. Macular thickness and systemic markers for diabetes in individuals with no or mild diabetic retinopathy. Clin Experiment Ophthalmol. 2008;36 (5:455–463. doi: 10.1111/j.1442-9071.2008.01769.x. [DOI] [PubMed] [Google Scholar]
- Verma A, Rani PK, Raman R, Pal SS, Laxmi G, Gupta M, et al. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye. 2009;23 (9:1824–1830. doi: 10.1038/eye.2009.184. [DOI] [PubMed] [Google Scholar]
- Brown JC, Solomon SD, Bressler SB, Schachat AP, DiBernardo C, Bressler NM. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol. 2004;122 (3:330–335. doi: 10.1001/archopht.122.3.330. [DOI] [PubMed] [Google Scholar]
- Bhavsar KV, Subramanian ML. Risk factors for progression of subclinical diabetic macular oedema. Br J Ophthalmol. 2011;95 (5:671–674. doi: 10.1136/bjo.2010.182337. [DOI] [PubMed] [Google Scholar]
- Browning DJ, Fraser CM. The predictive value of patient and eye characteristics on the course of subclinical diabetic macular edema. Am J Ophthalmol. 2008;145 (1:149–154. doi: 10.1016/j.ajo.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Ganapathy PS, Roon P, Moister TK, Mysona B, Smith SB. Diabetes accelerates retinal neuronal cell death in a mouse model of endogenous hyperhomocysteinemia. Ophthalmol Eye Dis. 2009;1:3–11. doi: 10.4137/oed.s2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield D, Bagga H, Knighton R. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol. 2003;121:41–46. doi: 10.1001/archopht.121.1.41. [DOI] [PubMed] [Google Scholar]
- Leung C, Chan W, Yung W, Ng AC, Woo J, Tsang MK, et al. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology. 2005;112:391–400. doi: 10.1016/j.ophtha.2004.10.020. [DOI] [PubMed] [Google Scholar]