Abstract
Purpose
To establish whether hypothalmic-pituitary-adrenal axis suppression is possible secondary to long-term topical ophthalmic corticosteroid use in patients who have undergone penetrating keratoplasty (PKP).
Methods
Patients who had undergone a PKP and had been using corticosteroid-based eye drops continuously for more than 6 months, with no history of concomitant steroid (oral, inhaled, or cutaneous) use, were included within the study. A low-dose short Synacthen (LDSST) test was performed in each patient followed later by a short Synacthen test (SST). The mean SST and LDSST after 30 min were calculated along with their corresponding 95% confidence intervals (CIs). Correlation between both baseline SST and baseline LDSST with duration of treatment was determined using Spearman's correlation.
Results
In all, 20 patients were included within the study. The mean duration treatment was 28.2 months (range 11–96 months). All patients had normal baseline cortisol levels in both SST and LDSST tests. The mean 30 min SST was 753.8 nmol/l (95%CI: 696.6 nmol/l, 811.0 nmol/l) and no patients displayed inadequate adrenal response. The mean 30 min LDSST was 709.8 nmol/l (95%CI: 665.1 nmol/l, 754.5 nmol/l) and only one patient had an inadequate adrenal response. There was no correlation between baseline SST or LDSST and duration of treatment.
Conclusions
This study found no evidence that patients using continuous long-term corticosteroid eye drops after PKP experienced inadequate adrenal response. We did not find any evidence of a negative correlation between length of treatment and SST or LDSST measurements at baseline.
Keywords: penetrating keratoplasty, adrenal insufficiency, topical corticosteroids
Introduction
Topical corticosteroid treatments have been widely used within the management of many conditions over the past 50 years, they provide therapeutic local effects whilst reducing the systemic bioavailability when compared with oral and parenteral administration.1 Despite this, recent reports suggest that the topical and inhaled use of corticosteroids can lead to suppression of the hypothalmic-pituitary-adrenal (HPA) axis2, 3, 4, 5 that is, exogenous, supraphysiological corticosteroid levels suppress both corticotrophin-releasing factor production in the hypothalamus and adrenocorticotrophic hormone production in the pituitary gland. This suppression, depending on its severity, may significantly reduce cortisol production by the adrenal glands (leading to adrenal atrophy), causing systemic effects that can lead to confusion, hyponatreamia, and life-threatening hypotension in the event of a trauma, surgery, or illness, known as an ‘adrenal crisis'.2
In addition, suppression of the HPA axis secondary to topical corticosteroid use is also well documented in children,6 including the use of ophthalmic preparations.7 Animal studies have also shown that ophthalmic topical corticosteroid therapy has the potential to cause adrenocortical suppression.8
Several studies have sought to identify whether topical steroids used for ocular pathologies cause suppression of the HPA axis.9, 10 In 1968, Burch et al9 found reduced adrenal corticosteroid production in four patients receiving 0.1% dexamethasone drops on a short-term basis. Krupin et al10 demonstrated partial adrenal suppression (reduced serum cortisol levels with normal HPA response on testing) in patients treated with 0.1% dexamethasone drops over 6 weeks.
The long-term use of topical corticosteroids is often necessary in patients who have undergone penetrating keratoplasty (PKP) to prevent rejection. The purpose of this study is to identify whether there is a link between the prolonged continuous use of topical ophthalmic corticosteroid preparations and adrenal suppression or insufficiency after corneal transplantation.
Materials and methods
Patients were recruited prospectively from the corneal transplantation service in a tertiary referral eye department in the United Kingdom between January and November 2001. Patients who had been using prednisolone 1%, rimexolone 1%, prednisolone 0.5% or fluorometholone (FML) 0.1% eye drops continuously for >6 months, with no history of concomitant steroid (oral, inhaled, or cutaneous) use were included within the study. After consent was obtained for investigation of adrenal suppression, all patients had measurements of serum cortisol (09:00 hours) followed by a low-dose short Synacthen test (LDSST), and then a short Synacthen test (SST) over 1 week after the initial test.11
Low-dose short synacthen test—At 09:00 hours, a blood sample LDSST 0 was taken at 0 min (baseline cortisol level), followed by an intravenous injection of tetracosactrin (1 μg). A further blood sample, LDSST 30 was taken at 30 min after the injection.
SST—At 09:00 hours, a blood sample SST 0 was taken at 0 min (baseline cortisol level), followed by an intravenous injection of tetracosactrin (250 μg). A further blood sample, SST 30 was taken at 30 min after the injection.
Normal baseline cortisol values were taken as ≥190 nmol/l. Adrenal suppression or insufficiency was defined as a response of ≤550 nmol/l at SST 30 or LDSST 30.11
Plasma cortisol measurements were carried out by a technician, using a commercially available immunoassay (Bayer ADVIA Centaur, Siemens Medical Solutions Diagnostics, Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA).
Informed consent was obtained from all subjects after the nature of the procedure had been fully explained. The tenets of the Declaration of Helsinki were followed and registration was obtained with the Royal Victoria Infirmary for a prospective audit of all corneal transplantation surgery outcomes. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
The mean SST and LDSST after 30 min were calculated. The corresponding 95% confidence intervals (CIs) were calculated to determine the range that the mean was likely to fall in and whether this included 550. Correlation between both baseline SST and LDSST with duration of treatment was determined using Spearman's correlation.
Statistical analyses were performed using Stata 11 (StataCorp LP, College Station, TX, USA), and P<0.05 was considered statistically significant.
Results
In all, 20 patients were included within the study. There was a similar frequency of males and females in the data set, but age and treatment type varied considerably from patient to patient (Table 1). The mean duration of treatment was 28.2 months (range 11–96 months). The mean baseline SST and LDSST were 451.0 nmol/l and 458.9 nmol/l, respectively, with corresponding 95% CIs that indicated the ranges the means were likely to fall in were both >190 nmol/l (95%CI: 403.1–495.1 and 95%CI: 415.0–502.7, respectively). All patients had normal baseline cortisol levels (≥190 nmol/l).
Table 1. Patient baseline characteristics.
| Characteristic | Number (%) |
|---|---|
| Treatment type | |
| Prednisolone 1% | 13 (65%) |
| Rimexolone 1% | 5 (25%) |
| Fluorometholone 0.1% | 2 (10%) |
| Sex | |
| Male | 9 (45.0) |
| Female | 11 (55.0) |
| Original pathology | |
| Fuchs dystrophy | 4 (20.0) |
| Pseudophakic bullous keratopathy | 8 (40.0) |
| Keratoconus | 4 (20.0) |
| Interstial keratitis | 1 (5.0) |
| Acanthamoeba keratitis | 1 (5.0) |
| Herpes simplex keratitis | 1 (5.0) |
| Macular dystrophy | 1 (5.0) |
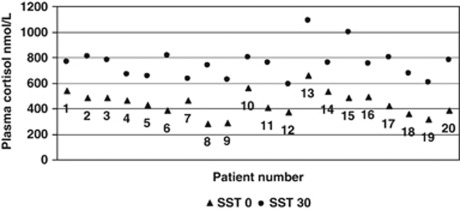
The mean 30-min SST was 753.8 nmol/l, with a 95% CI that suggests the range the mean is likely to fall in is >550 nmol/l (696.6 nmol/l, 811.0 nmol/l). On the basis of these 30-minute SST cortisol levels, all patients had adequate adrenal function, >550 nmol/l (Figure 1).
Figure 1.
Short synacthen test results.
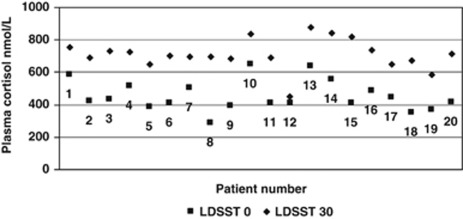
Similarly, the mean 30-min LDSST was 709.8 nmol/l with a 95% CI indicative that the range the mean is likely to fall in is >550 nmol/l (665.1 nmol/l, 754.5 nmol/l). However, one patient did show an inadequate adrenal response with a 30-min LDSST of only 449 nmol/l (Figure 2).
Figure 2.
Low dose short synacthen test results.
There was no significant correlation (Spearman's rank (rho, ρ)) between baseline SST or LDSST and the duration of treatment (ρ=0.04, P=0.87 and ρ=0.14, P=0.56, respectively).
Discussion
An intact HPA axis is essential for the appropriate production of cortisol in response to stress.1 The insulin tolerance test (ITT) is an established method for assessment of the integrity of the HPA axis but is potentially dangerous, resource intensive, and contraindicated in the elderly, epilepsy, and ischaemic heart disease.12 In light of this, SST grew in popularity and studies have consistently demonstrated a high correlation between results obtained from both ITT and SST.13
SST was originally designed as a test for primary adrenal failure and delivers a supraphysiological dose (250 μg) of tetracosactrin, which may lead to some falsely high results in cases of insidious adrenal insufficiency.14 In light of this, we choose to include a LDSST, which delivers a more physiological dose of tetracosactrin (1 μg) and may identify more subtle forms of adrenal insufficiency. The results of the LDSST have been shown to correlate highly with both ITT and SST, whilst producing less false-positive results.15
One patient was identified as having adrenal insufficiency by the LDSST. This patient had been using prednisolone 1% drops once per day to prevent corneal graft rejection, for >6 months. The patient had been otherwise well with no signs or symptoms to suggest clinically apparent adrenal insufficiency, and has developed no subsequent problems since testing. All remaining patients had a normal adrenal response, and in addition we did not find any evidence of a negative correlation between the length of treatment and SST or LDSST measurements at baseline.
The results suggest that prolonged continuous use of low-dose topical ophthalmic steroid preparations as prophylaxis against graft rejection in patients after PKP, does not cause adrenal insufficiency in adults. However, many ophthalmic conditions require the long-term use of topical steroids at higher doses than the patients within this case series have received, often in combination with systemic steroids. Such patients are at a higher risk of adrenal insufficiency, especially when reducing the dosage of long-term systemic steroids. Therefore, the contribution of the topical ophthalmic steroids to any adrenal insufficiency should be considered.
We recognise that the results of this small case series are limited and that the association between long-term ocular topical steriod use and normal adrenal function may not be representative of the general population. Larger studies are needed to help confirm the findings.
The authors declare no conflict of interest.
Footnotes
This study was previously presented in the Association for Research in Vision and Ophthalmology meeting 2008—poster presentation of Short Synacthen and Low dose Short Synacthen results.
References
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54 (1:1–15. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Brown P, Blundell G, Greening A, Crompton G. Hypothalamo-pituitary-adrenal axis suppression in asthmatics inhaling high dose corticosteroids. Respir Med. 1991;85 (6:501–510. doi: 10.1016/s0954-6111(06)80268-4. [DOI] [PubMed] [Google Scholar]
- Levin C, Maibach HI. Topical corticosteroid-induced adrenocortical insufficiency: clinical implications. Am J Clin Dermatol. 2002;3 (3:141–147. doi: 10.2165/00128071-200203030-00001. [DOI] [PubMed] [Google Scholar]
- Flynn MD, Beasley P, Tooke JE. Adrenal suppression with intranasal betamethasone drops. J Laryngol Otol. 1992;106 (9:827–828. doi: 10.1017/s0022215100120997. [DOI] [PubMed] [Google Scholar]
- Abraham G, Gottschalk J, Ungemach FR. Evidence for ototopical glucocorticoid-induced decrease in hypothalamic-pituitary-adrenal axis response and liver function. Endocrinology. 2005;146 (7:3163–3171. doi: 10.1210/en.2005-0080. [DOI] [PubMed] [Google Scholar]
- Güven A, Gülümser O, Ozgen T. Cushing's syndrome and adrenocortical insufficiency caused by topical steroids: misuse or abuse. J Pediatr Endocrinol Metab. 2007;20 (11:1173–1182. doi: 10.1515/jpem.2007.20.11.1173. [DOI] [PubMed] [Google Scholar]
- Kröger L, Kotaniemi K, Jääskeläinen J. Topical treatment of uveitis resulting in adrenal insufficiency. Acta Paediatr. 2009;98 (3:584–585. doi: 10.1111/j.1651-2227.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- Roberts SM, Lavach JD, Macy DW, Severin GA. Effect of ophthalmic prednisolone acetate on the canine adrenal gland and hepatic function. Am J Vet Res. 1984;45 (9:1711–1714. [PubMed] [Google Scholar]
- Burch P, Migeon C. Systemic absorption of topical steroids. Arch Ophthalmol. 1968;79 (2:174–176. doi: 10.1001/archopht.1968.03850040176013. [DOI] [PubMed] [Google Scholar]
- Krupin T, Mandell A, Podos M, Becker B. Topical corticosteroid therapy and pituitary-adrenal function. Arch Ophthalmol. 1976;94:919–920. doi: 10.1001/archopht.1976.03910030459003. [DOI] [PubMed] [Google Scholar]
- Rasmuson S, Olsson T, Hagg E. A low dose ACTH test to assess the function of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf) 1996;44 (2:151–156. doi: 10.1046/j.1365-2265.1996.600482.x. [DOI] [PubMed] [Google Scholar]
- Hurel SJ, Thompson CJ, Watson MJ, Harris NM, Baylis PH, Kendal-Taylor P. Audit of short synacthen and insulin stress tests in the assessment of the hypothalamo-pituitary-adrenal axis. Clin Endocrinol (Oxf) 1996;44:141–146. doi: 10.1046/j.1365-2265.1996.555381.x. [DOI] [PubMed] [Google Scholar]
- Kane KF, Emery P, Sheppard MC, Stewart PM. Assessing the hypothalamo-pituitary-adrenal axis in patients on long-term glucocorticoid therapy: the short synacthen test versus the insulin tolerance test. QJM. 1995;88 (4:263–267. [PubMed] [Google Scholar]
- Streeten DHP, Anderson GH, Bonaventyra MM. The potential for serious consequences from misinterpreting normal responses to the rapid adrenocorticotrophin test. J Clin Endocrinol Metab. 1996;81 (1:285–290. doi: 10.1210/jcem.81.1.8550765. [DOI] [PubMed] [Google Scholar]
- Abdu TAM, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab. 1999;84 (3:838–843. doi: 10.1210/jcem.84.3.5535. [DOI] [PubMed] [Google Scholar]