Abstract
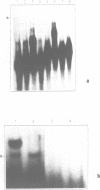
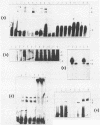
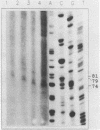
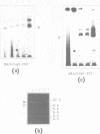
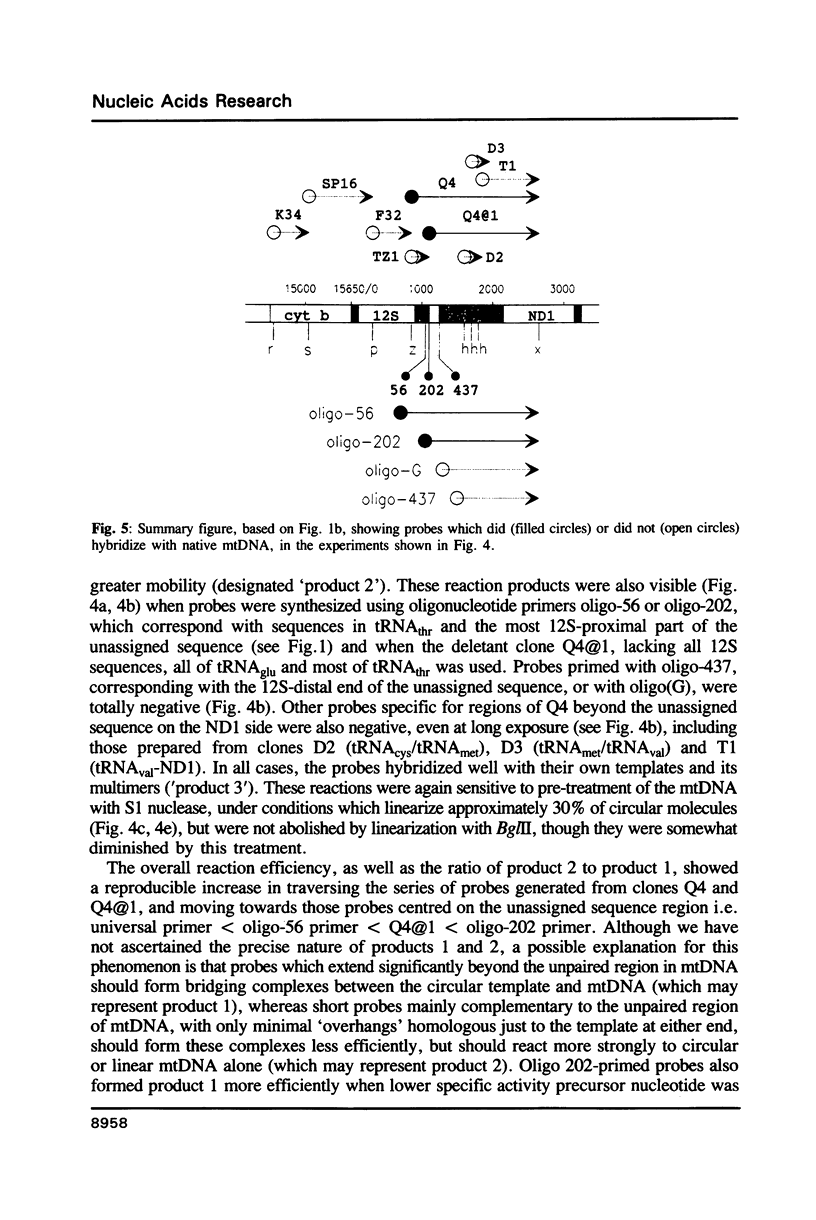
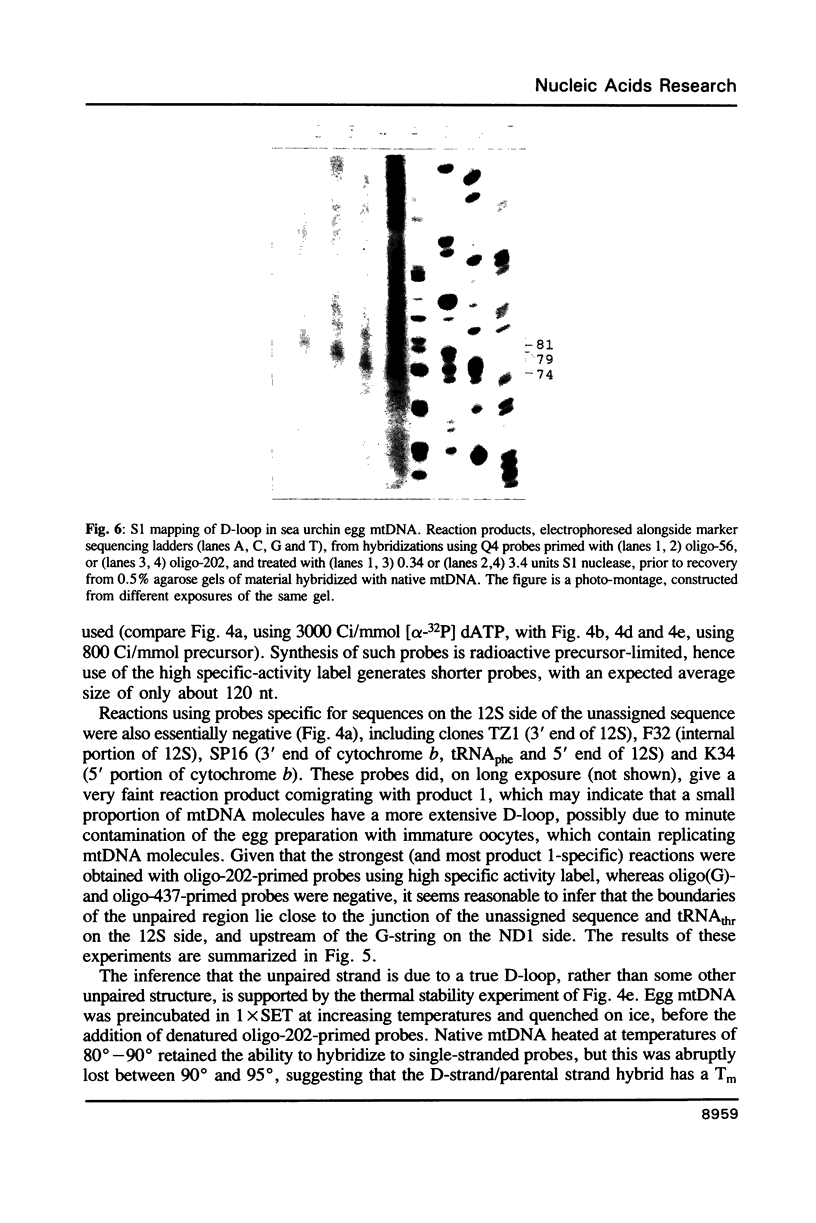
Based on solution hybridization using single-stranded probes, native mitochondrial DNA extracted from sea urchin eggs contains a displacement-loop (D-loop) of approximately 70-80 nt. This maps to the single extended unassigned sequence of the genome, between the genes for tRNA(thr) and tRNA(pro), which also appears to contain the origin of first-strand replication. The D-loop commences at or close to a site of supercoil-dependent S1 nuclease hypersensitivity, adjacent to a run of 20 consecutive C residues, terminates near to the boundary of tRNA(thr), and appears to be composed at least partly of RNA, based on the sensitivity of the assays to RNase H. These experiments imply that the mechanisms of replication initiation in sea urchin and vertebrate mtDNAs are very similar, and suggest that the developmental restriction on mtDNA synthesis in eggs and embryos is maintained at the level of D-loop extension.
Full text
PDF


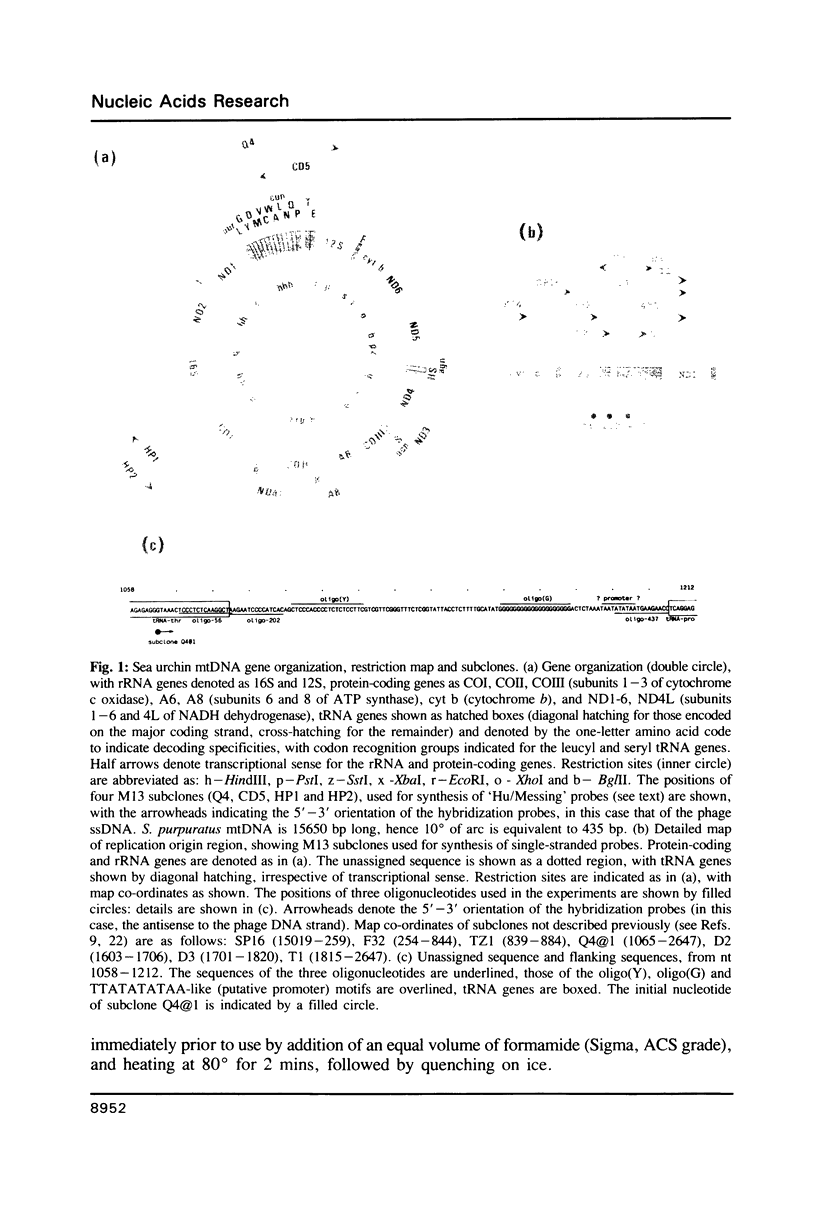

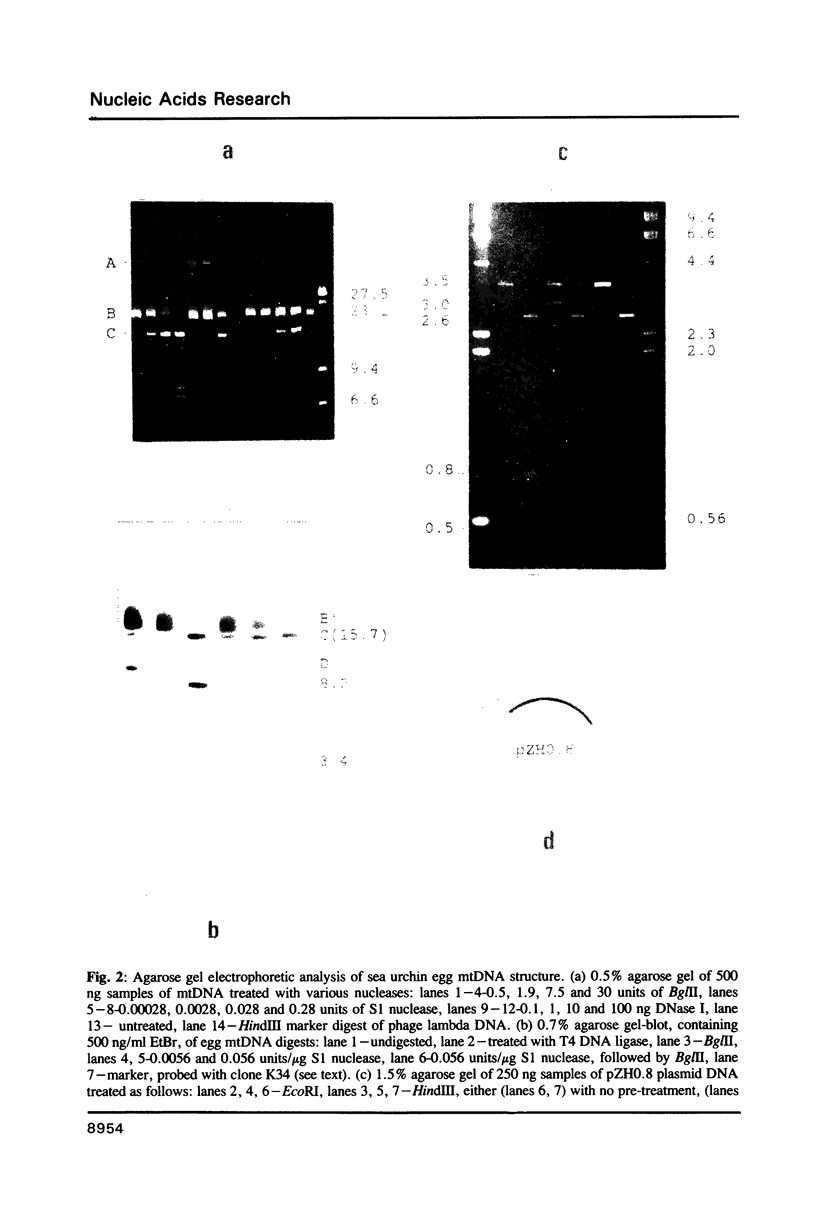

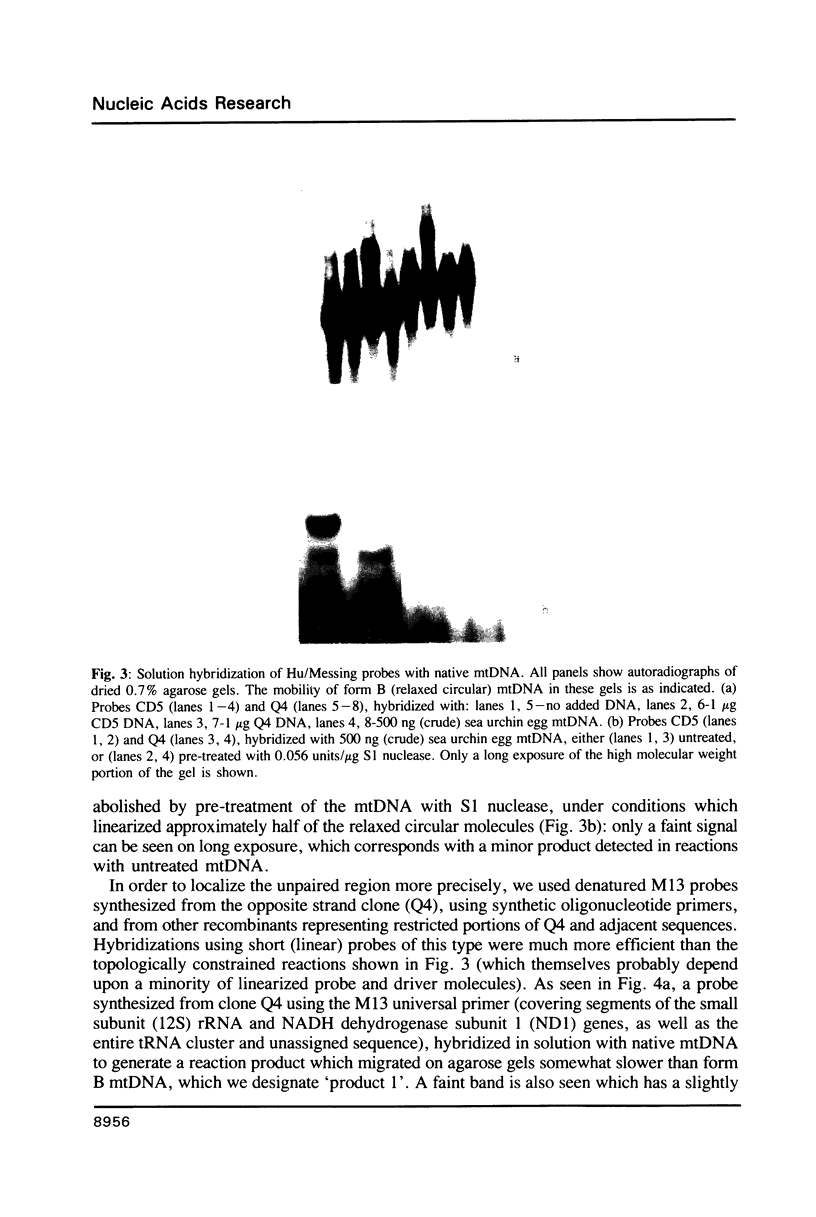
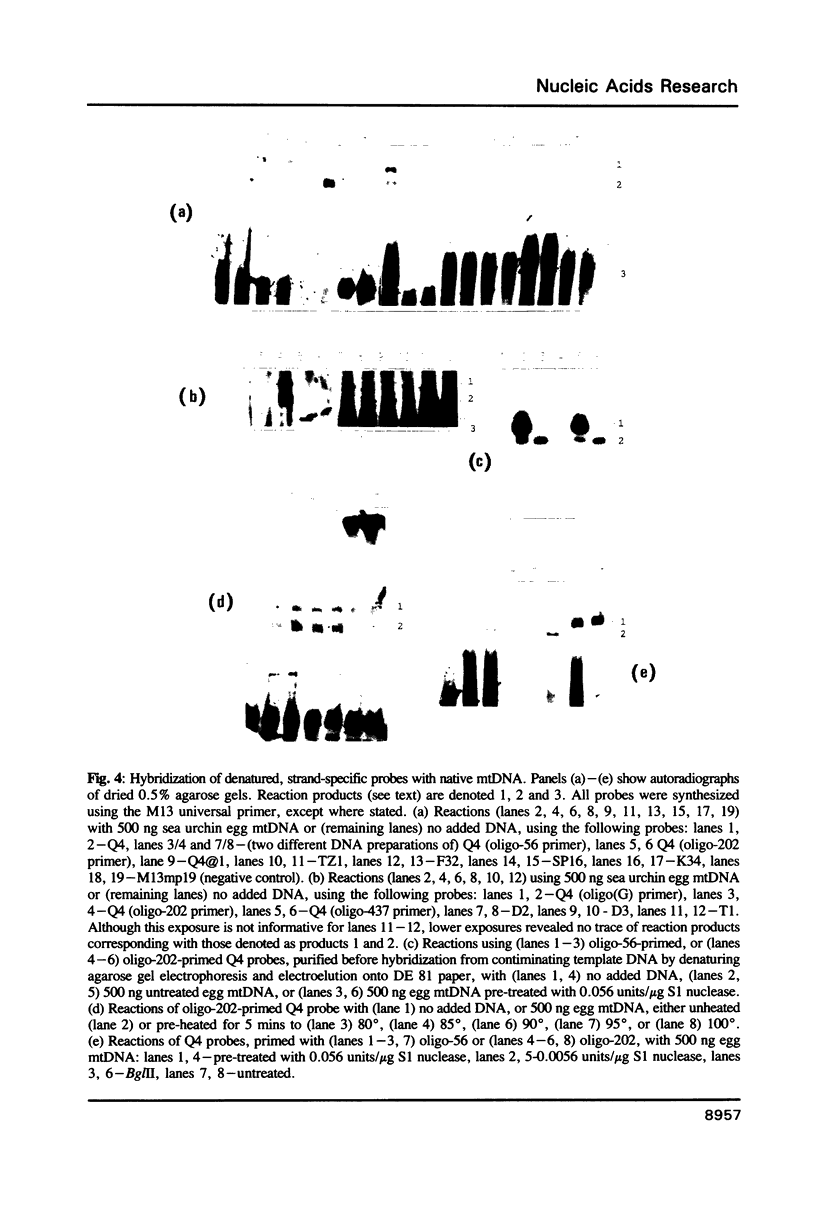






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci G., Chérif-Zahar B., Bernardi G. The initiation of DNA replication in the mitochondrial genome of yeast. EMBO J. 1984 Sep;3(9):2115–2120. doi: 10.1002/j.1460-2075.1984.tb02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Mouse RNAase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondrial RNA substrate. Cell. 1989 Jan 13;56(1):131–139. doi: 10.1016/0092-8674(89)90991-4. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc Natl Acad Sci U S A. 1985 Jan;82(2):351–355. doi: 10.1073/pnas.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Fisher R. P., Clayton D. A. Roles for a promoter and RNA processing in the synthesis of mitochondrial displacement-loop strands. Biochim Biophys Acta. 1987 Jul 14;909(2):85–91. doi: 10.1016/0167-4781(87)90029-7. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Hauswirth W. W., Clayton D. A. Replication priming and transcription initiate from precisely the same site in mouse mitochondrial DNA. EMBO J. 1985 Jun;4(6):1559–1567. doi: 10.1002/j.1460-2075.1985.tb03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Clayton D. A. A tridecamer DNA sequence supports human mitochondrial RNA 3'-end formation in vitro. Mol Cell Biol. 1988 Oct;8(10):4502–4509. doi: 10.1128/mcb.8.10.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Clayton D. A. In vitro transcription of human mitochondrial DNA: accurate termination requires a region of DNA sequence that can function bidirectionally. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6277–6281. doi: 10.1073/pnas.83.17.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Devlin R. B. Biogenesis of the mitochondrial ATPase from sea urchin embryos. J Biol Chem. 1982 Aug 25;257(16):9711–9716. [PubMed] [Google Scholar]
- Doda J. N., Wright C. T., Clayton D. A. Elongation of displacement-loop strands in human and mouse mitochondrial DNA is arrested near specific template sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6116–6120. doi: 10.1073/pnas.78.10.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. J., Jacobs H. T. Mutually exclusive synthetic pathways for sea urchin mitochondrial rRNA and mRNA. Mol Cell Biol. 1989 Mar;9(3):1069–1082. doi: 10.1128/mcb.9.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory P. J., Jr, Vinograd J. 5-bromodeoxyuridine labeling of monomeric and catenated circular mitochondrial DNA in HeLa cells. J Mol Biol. 1973 Feb 25;74(2):81–94. doi: 10.1016/0022-2836(73)90100-9. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3886–3890. doi: 10.1073/pnas.75.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from the genus Drosophila. Nucleic Acids Res. 1980 Feb 25;8(4):741–757. [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L. Mitochondrial DNA in Xenopus laevis oocytes. I. Displacement loop occurrence. Dev Biol. 1974 Jun;38(2):346–355. doi: 10.1016/0012-1606(74)90012-8. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T. Do ribosomes regulate mitochondrial RNA synthesis? Bioessays. 1989 Jul;11(1):27–34. doi: 10.1002/bies.950110108. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Chloroplast DNA from higher plants replicates by both the Cairns and the rolling circle mechanism. Nature. 1975 Aug 28;256(5520):708–711. doi: 10.1038/256708a0. [DOI] [PubMed] [Google Scholar]
- Kruse B., Narasimhan N., Attardi G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989 Jul 28;58(2):391–397. doi: 10.1016/0092-8674(89)90853-2. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto L., Kasamatsu H., Pikó L., Vinograd J. Mitochondrial DNA replication in sea urchin oocytes. J Cell Biol. 1974 Oct;63(1):146–159. doi: 10.1083/jcb.63.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Taylor K. D. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987 Oct;123(2):364–374. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- Posakony J. W., England J. M., Attardi G. Mitochondrial growth and division during the cell cycle in HeLa cells. J Cell Biol. 1977 Aug;74(2):468–491. doi: 10.1083/jcb.74.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Haniford D. B., Morgan A. R. A structural basis for S1 nuclease sensitivity of double-stranded DNA. Cell. 1985 Aug;42(1):271–280. doi: 10.1016/s0092-8674(85)80122-7. [DOI] [PubMed] [Google Scholar]
- Rinaldi A. M., Carra E., Salcher-Cillari I., Oliva A. O. The nucleus negatively controls the synthesis of mitochondrial proteins in the sea urchin egg. Cell Biol Int Rep. 1983 Mar;7(3):211–218. doi: 10.1016/0309-1651(83)90228-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi A. M., De Leo G., Arzone A., Salcher I., Storace A., Mutolo V. Biochemical and electron microscopic evidence that cell nucleus negatively controls mitochondrial genomic activity in early sea urchin development. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1916–1920. doi: 10.1073/pnas.76.4.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Solignac M., Génermont J., Monnerot M., Mounolou J. C. Drosophila Mitochondrial Genetics: Evolution of Heteroplasmy through Germ Line Cell Divisions. Genetics. 1987 Dec;117(4):687–696. doi: 10.1093/genetics/117.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5411–5421. doi: 10.1093/nar/9.20.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]