Abstract
Introduction
Preservatives in ophthalmic preparations are known to cause ocular surface damage. Excipients can also contribute to oxidative stress in the compromised ocular surface. We evaluated commonly used topical glaucoma medications to ascertain pH levels and the intrinsic presence of free radicals.
Methods
Samples of 27 topical glaucoma preparations were analysed for total free radical presence using a Randox Kit for total antioxidant status. Analytical grade indicator paper was used to ascertain pH levels.
Results
Free radical concentrations for these 27 glaucoma preparations ranged from 0 to 4.54 mmol/l, with a median value of 0.66 mmol/l (mean value of 0.662 mmol/l, SD 0.839). Levels of pH ranged from 4.0 to 7.4, with a median value of 6.5 (mean 6.252, SD 0.826). There was no evidence of a direct correlation between these two variables (r=0.232, P=0.275).
Conclusion
This study is the first to document the range of pH and concentrations of free radicals intrinsically present in commonly used glaucoma medications. Long-term exposure to preservatives, free radicals, and pH levels could all contribute to ocular surface damage. The effect of excipients could be responsible for patient intolerance when changing products in the compromised ocular surface.
Keywords: free radicals, preservatives, ocular surface, pH, glaucoma, topical medications
Introduction
Current glaucoma management involves long-term, or even lifelong, topical ocular hypotensive medications, designed to reduce the rate of optic neuropathy.1 Topical ocular medications consist of the drug, a drug vehicle, buffers, stabilising compounds, and often preservatives. The most commonly used preservative in glaucoma medications is benzalkonium chloride (BAK).2 Other preservatives include polyquaternium-1 (PQ-1), benzododecinium bromide (BDB), and disodium edetate (EDTA). Preservatives inhibit microbial growth, prevent contamination, and permit multi-dose application, but have been implicated in oxidative stress that contributes to ocular surface disorders.2 Intolerance with long-term topical glaucoma medications has been shown to be directly related to the number of preserved medications used.3 Cytotoxicity to the conjunctiva, corneal epithelium, and endothelium has been shown to decrease in the absence of preservatives, leading to the development of less cytotoxic, preservative-free preparations.3, 4, 5
A large proportion of patients with glaucoma have concurrent ocular surface disease, adversely affecting their quality of life.6, 7 All topical medications contain a variety of excipients and buffering agents. Excipients are generally regarded as pharmacologically inactive substances which act as a carrier for the active ingredients of a medication. Although they have no direct role in the mechanism of action of a drug, the ocular surface will still be exposed to relatively small concentrations of these excipients. As topical glaucoma therapy can last for many decades, these chemicals can still have an accumulating contribution to ocular surface damage. Free radicals have been shown to be present in topical and intracameral preparations, independent of preservatives.8, 9 Free radicals have the potential to be toxic to the corneal and conjunctival epithelium.10 The healthy ocular surface is normally protected by the antioxidant-rich tear film, but this protection is abnormal in ocular surface disease, and can be further compromised by recurrent use of topical agents.11, 12
We evaluated the pH levels and intrinsic free radical presence in commonly used topical glaucoma medications in the United Kingdom.
Materials and methods
Samples of 27 topical glaucoma preparations commonly used in the United Kingdom were analysed for total free radical presence on an Instrument Laboratory IL600 using a Randox Kit for total antioxidant status (TAS, Cat no. NX2332) with its own calibrator (RANDOX Laboratories Ltd, Crumlin, UK). The TAS kit provides an indirect measurement of free radicals. Incubation of ABTS (ferrylmyoglobin) with a peroxidase (metmyoglobin) results in production of the radical cation ABTS+, which is blue–green in colour, and can be detected at 600 nm. The presence of antioxidants in the added sample cause inhibition of this colour production to a degree that is proportional to their concentration in this two reagent assay. The results are linear up to a concentration of 5 mmol/l. The analyser used a 4-ul sample and 200 ul Buffer/Chromogen plus 40 ul diluted substrate. Analysis occurred at 37 °C. All the medications were previously unopened, in date, and stored as per the manufacturers' instructions, including in the dark as required. The containers containing the eye drops were mixed, opened, placed in autoanalyser cups, and immediately analysed for TAS as described above.
At the same time an aliquot of each medication was taken and the pH immediately measured using analytical grade pH indicator strips 0–6, VWR Cat no. 1.09531.001; pH indicator strips 4–7, VWR Cat no. 1.09542.0001; pH indicator strips 2.5–4.5, VWR Cat no. 1.09541.0001; and pH indicator strips 6.5–10, VWR Cat no. 1.09543.0001. After the initial pH was identified, the pH was clarified by using the indicator paper with the narrower range. The pH analysis was carried out independently by two experienced technicians and the value reported was an average of the two measures. There were no differences in both values. Accuracy of the indicator paper was confirmed with standard stock chemical solutions being tested as a control and calibrated with a pH meter. The pH meter was not directly used for analysis because of insufficient available volumes of all the medications.
Products sampled included prostaglandin analogues (latanoprost, travaprost, bimatoprost, and tafluprost), carbonic anhydrase inhibitors (brinzolamide, dorzolamide), combination therapies (trade names Ganfort, Xalacom, Duotrav, Cosopt, Azarga, and Combigan), a variety of commonly available β-blockers, and other miscellaneous glaucoma medications. See Table 1 for formal product information, active ingredients, preservatives, excipients, and trademarks. Data were tested for normality using the Anderson–Darling test.
Table 1. Results and formal product information and trademarks for commonly used glaucoma medications in the United Kingdom.
| Name | Manufacturer | Active ingredients | Preservatives | Stated excipients | TAS (mmol/l) | pH |
|---|---|---|---|---|---|---|
| Azarga | Alcon | Brinzolamide/timolol maleate 10+5 mg/ml suspension | BAK EDTA | Benzalkonium chloride, mannitol (E421), carbopol 974P, tyloxapol, disodium edetate, sodium chloride, hydrochloric acid and/or sodium hydroxide (for pH adjustment), and purified water | 0.31a | 6.8 |
| Azopt | Alcon | Brinzolamide 10 mg/ml suspension | BAK EDTA | Benzalkonium chloride, mannitol (E421), carbomer 974P, tyloxapol, edetate disodium, sodium chloride, hydrochloric acid/sodium hydroxide (to adjust pH), and purified water | 0.74a | 6.8 |
| Betagan | Allergan | Levobunolol hydrochloride 0.5%w/v | BAK EDTA | Benzalkonium chloride, disodium edetate, poly(vinyl alcohol), sodium chloride, sodium phosphate dibasic heptahydrate, potassium dihydrogen phosphate, sodium metabisulphite (E223), sodium hydroxide or hydrochloric acid to adjust pH, and purified water | 0.15 | 6.5 |
| Betoptic 0.25% | Alcon | Betaxolol hydrochloride 0.25%w/v suspension | BAK EDTA | Disodium edetate, sodium chloride, benzalkonium chloride, sodium hydroxide, hydrochloric acid, and purified water | 0.31a | 6.8 |
| Combigan | Allergan | Brimonidine tartrate/timolol maleate 2+5 mg/ml | BAK | Benzalkonium chloride, sodium phosphate, monobasic monohydrate, sodium phosphate, dibasic heptahydrate, hydrochloric acid or sodium hydroxide to adjust pH, and purified water | 0.25 | 6.8 |
| Cosopt | MSD | Dorzolamide hydrochoride/timolol maleate 20+5 mg/ml | BAK | Benzalkonium chloride, hydroxyethyl cellulose, mannitol (E421), sodium citrate (E331), sodium hydroxide (E524) for pH adjustment, and water for injections | 0.45 | 5.8 |
| Cosopt unit dose | MSD | Dorzolamide hydrochoride/timolol maleate 20+5 mg/ml | None | Hydroxyethyl cellulose, mannitol (E421), sodium citrate (E331), sodium hydroxide (E524) for pH adjustment, and water for injections | 0.50 | 5.8 |
| Duotrav | Alcon | Travoprost/timolol 40+5 mg/ml | BAK EDTA | Mannitol, trometamol, polyoxyethylene hydrogenated castor oil 40, boric acid, disodium edetate, hydrochloric acid (to adjust pH), and purified water | 0.00 | 5.5 |
| Ganfort | Allergan | Bimatoprost/timolol maleate 300 ug/ml+ 5 mg/ml | BAK | Benzalkonium chloride, sodium chloride, sodium phosphate dibasic heptahydrate, citric acid monohydrate, hydrochloric acid or sodium hydroxide (to adjust pH), and purified water | 0.66 | 7.4 |
| Iopidine 0.5% | Alcon | Apraclonidine hydrochloride 5 mg/ml | BAK | Benzalkonium chloride, sodium acetate (trihydrate), sodium chloride, hydrochloric acid and/or sodium hydroxide, and purified water. | 4.54 | 5.3 |
| Lumigan 0.01% | Allergan | Bimatoprost 0.1 mg/ml | BAK | Benzalkonium chloride, sodium chloride, sodium phosphate dibasic heptahydrate, citric acid monohydrate, hydrochloric acid or sodium hydroxide (to adjust pH), and purified water | 0.03 | 7.1 |
| Lumigan 0.03% | Allergan | Bimatoprost 0.3 mg/ml | BAK | Benzalkonium chloride, sodium chloride, sodium phosphate dibasic heptahydrate, citric acid monohydrate, hydrochloric acid or sodium hydroxide (to adjust pH), and purified water | 0.89 | 7.4 |
| Minims metipranolol | Bausch & Lomb | Metipranolol 0.1% w/v | None | Hydrochloric acid BP, sodium chloride PhEur, sodium hydroxide, purified water PhEur | 0.02 | 5.0 |
| Minims pilocarpine 2% | Bausch & Lomb | Pilocarpine nitrate 2%w/v | None | Purified water | 0.75 | 4.0 |
| Pilogel | Alcon | Pilocarpine hydrochloride 4%w/v | BAK EDTA | Benzalkonium chloride, carbomer 940, disodium edetate, sodium hydroxide and/or hydrochloric acid, and purified water. | NAa | 5.0 |
| Brimonidine tartrate | Genus | Brimonidine tartrate 2 mg/ml | BAK | Benzalkonium chloride, poly (vinyl alcohol), sodium chloride, sodium citrate, citric acid monohydrate, purified water, hydrochloric acid (for pH adjustment) or sodium hydroxide (for pH adjustment) | 0.78 | 6.1 |
| Saflutan | MSD | Tafluprost 15 mg/ml | EDTA | Glycerol, sodium dihydrogen phosphate dehydrate, disodium edetate, polysorbate 80, hydrochloric acid and/or sodium hydroxide for pH adjustment, and water for injections | 0.70 | 5.3 |
| Teoptic 1% | Novartis | Carteolol hydrochloride 1%w/v | BAK | Benzalkonium chloride, sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic, and water for injection | 0.82 | 6.5 |
| Timoptol-LA 0.25% | MSD | Timolol maleate 2.5 mg/ml | BDB | Gellan gum, trometamol, mannitol E421, and water for injection. Benzododecinium bromide (0.012%) is added as preservative | 0.70 | 6.5 |
| Timoptol-LA 0.5% | MSD | Timolol maleate 5 mg/ml | BDB | Gellan gum, trometamol, mannitol E421, and water for injection. Benzododecinium bromide (0.012%) is added as preservative | 0.70 | 6.5 |
| Timolol 0.25% | Martindale | Timolol maleate 0.25w/v | BAK | Sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, benzalkonium chloride, sodium hydroxide and/or hydrochloric acid (to adjust pH), and purified water. | 0.72 | 7.1 |
| Timolol 0.5% | Martindale | Timolol maleate 0.5w/v | BAK | Sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, benzalkonium chloride, sodium hydroxide and/or hydrochloric acid (to adjust pH), and purified water. | 0.66 | 6.8 |
| Timoptol 0.25% | MSD | Timolol maleate 2.5 mg/ml | None | Disodium phosphate dodecahydrate (may be replaced by equivalent amounts of the dihydrate or anhydrous form), sodium dihydrogen phosphate dehydrate (may be replaced by equivalent amounts of the monohydrate), sodium hydroxide, and water for injection | 0.68 | 6.8 |
| Travatan | Alcon | Travoprost 40 ug/ml | EDTA PQ-1 | Polyquaternium-1, polyoxyethylene hydrogenated castor oil 40 (HCO-40), boric acid (E284), mannitol (E421), sodium chloride, propylene glycol (E1520), sodium hydroxide and/or hydrochloric acid (to adjust pH), and purified water | 0.67 | 6.5 |
| Trusopt | MSD | Dorzolamide hydrochloride 20 mg/ml | BAK | Benzalkonium chloride, hydroxyethyl cellulose, mannitol (E421), sodium citrate (E331), sodium hydroxide (E524) for pH adjustment, and water for injections | 0.58 | 5.8 |
| Xalatan | Pfizer | Latanoprost 50 mg/ml | BAK | Sodium chloride, benzalkonium chloride, sodium dihydrogen phosphate monohydrate, anhydrous disodium phosphate, and water for injections | 0.00 | 6.8 |
| Xalacom | Pfizer | Latanoprost/timolol 50+5 mg/ml | BAK | Sodium chloride, benzalkonium chloride, sodium dihydrogen phosphate monohydrate, disodium phosphate anhydrous, hydrochloric acid solution, sodium hydroxide solution, and water for injections | 0.61 | 6.1 |
Excipients obtained where available from electronic Medicines Compendium, and/or product information, accessed on April 2011.
Nb. Minims metipranolol has been discontinued in the United Kingdom.
Suspension that may affect reading. Pilogel was not appropriate for use with analyser due to viscosity therefore not tested for TAS.
Results
BAK was the most common preservative, found in 70% of the products. In total, 30% contained EDTA. Evaluation of the excipients detailed in the product information revealed the majority of the β-blocker-based medications contained sodium hydroxide and/or hydrochloric acid to buffer the pH.
The reproducibility and accuracy of the TAS assay were excellent, with intra-assay CV of 0.38% and inter assay CV of 0.74% (standard acceptable laboratory CVs are <5%). All the measurements occurred on the same day, which contributed to the high-quality control of the assay.
Free radical concentrations for the 27 glaucoma preparations ranged from 0 to 4.54 mmol/l, with a median value of 0.66 mmol/l (mean value of 0.662 mmol/l, SD 0.839). The data were not normally distributed (Anderson–Darling P<0.005) and Iopidine 0.5% was seen to be an outlier (4.54 mmol/l). The highest result for the prostaglandin analogue group was bimatoprost 0.3 mg/ml (0.89 mmol/l). Interestingly, the result for bimatoprost 0.1 mg/ml was much less (0.03 mmol/l). The highest result for the β-blockers was carteolol (0.82 mmol/l) and Ganfort for the combination therapies (0.66 mmol/l; Tables 1 and 2). For comparative purposes, 0.5% hydrogen peroxide had a TAS reading of 1.39 mmol/l and 0.1% hydrogen peroxide was 0.04 mmol/l.8, 9
Table 2. Glaucoma medications arranged by intrinsic total antioxidant status (TAS) concentration.
| Name | TAS mmol/l |
|---|---|
| Duotrav | 0.00 |
| Xalatan | 0.00 |
| Minims metipranolol | 0.02 |
| Lumigan (0.01%) | 0.03 |
| Betagan | 0.15 |
| Combigan | 0.25 |
| Azarga | 0.31 |
| Betoptic (0.25%) | 0.31 |
| Cosopt | 0.45 |
| Cosopt unit dose | 0.50 |
| Trusopt | 0.58 |
| Xalacom | 0.61 |
| Ganfort | 0.66 |
| Timolol (0.5%) | 0.66 |
| Travatan | 0.67 |
| Timoptol (0.25%) | 0.68 |
| Saflutan | 0.70 |
| Timoptol-LA (0.25%) | 0.70 |
| Timoptol-LA (0.5%) | 0.70 |
| Timolol (0.25%) | 0.72 |
| Azopt | 0.74 |
| Minims pilocarpine (2%) | 0.75 |
| Brimonidine tartrate | 0.78 |
| Teoptic (1%) | 0.82 |
| Lumigan (0.03%) | 0.89 |
| Iopidine (0.5%) | 4.54 |
| Pilogel | NA |
Levels of pH ranged from 4.0 to 7.4, with a median value of 6.5 (mean value of 6.25, SD 0.83). The pH distribution also failed the normality test (P=0.028) and was skewed, with a tail to the right. Minims Pilocarpine 2% was seen to be an outlier (pH 4). Iopidine 0.5% again had an extreme result (pH 5.3). Tafluprost had the lowest pH of the prostaglandin group, (5.3) Duotrav for the combination therapies (5.5), followed by Cosopt (5.8; Tables 1 and 3)
Table 3. Glaucoma medications arranged in order of pH.
| Name | pH |
|---|---|
| Minims pilocarpine (2%) | 4.0 |
| Minims metipranolol | 5.0 |
| Pilogel | 5.0 |
| Saflutan | 5.3 |
| Iopidine (0.5%) | 5.3 |
| Duotrav | 5.5 |
| Cosopt | 5.8 |
| Cosopt unit dose | 5.8 |
| Trusopt | 5.8 |
| Xalacom | 6.1 |
| Brimonidine tartrate | 6.1 |
| Betagan | 6.5 |
| Travatan | 6.5 |
| Timoptol-LA (0.25%) | 6.5 |
| Timoptol-LA (0.5%) | 6.5 |
| Teoptic (1%) | 6.5 |
| Xalatan | 6.8 |
| Combigan | 6.8 |
| Azarga | 6.8 |
| Betoptic (0.25%) | 6.8 |
| Timolol (0.5%) | 6.8 |
| Timoptol (0.25%) | 6.8 |
| Azopt | 6.8 |
| Lumigan (0.01%) | 7.1 |
| Timolol (0.25%) | 7.1 |
| Ganfort | 7.4 |
| Lumigan (0.03%) | 7.4 |
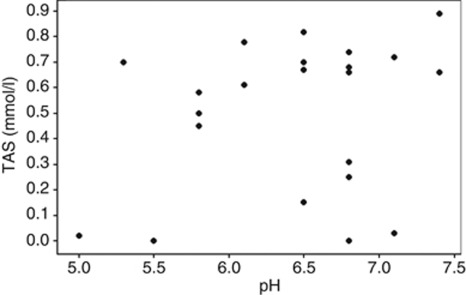
After removing both outliers (Iopidine 0.5% and Minims Pilocarpine 2%), there was no statistical evidence of a direct correlation between free radical concentration and pH (r=0.232, P=0.275). (Figure 1).
Figure 1.
Graph correlating pH with TAS result and showing spread of results. There was no statistical evidence of a direct correlation between free radical concentration and pH (r=0.232, P=0.275).
Discussion
Preservatives
Long-term use of preserved topical medications has been associated with dry eye syndrome, allergy, ocular surface inflammation, and filtration surgery failure.2, 3, 4, 13 BAK has a 20-h half-life in the corneal epithelium, and this retention causes chronic dose-dependent toxic cellular damage, including inflammation, reduced cell viability, and at increasing concentrations leads to apoptosis, and immediate cell necrosis.14, 15 A recent 3D toxicological model comparing the prostaglandin analogues (latanoprost, travaprost, bimatoprost, and the preservative-free tafluprost) confirmed that the cytotoxicity was primarily related to the concentration of their preservative.16 A similar relationship has been identified with topical β-blockers and anti-allergic topical preparations, with cell viability most affected by BAK concentration rather than the active component or drug action.17, 18 Recognition of this cellular damage has led to the substitution of BAK with PQ-1 in travoprost. PQ-1 has a higher molecular weight with no hydrophobic domains, therefore cannot enter mammalian cells or penetrate lipid membranes, and has a reduced in vivo cytotoxicity profile compared with BAK.19, 20, 21, 22 However, all BAK vs PQ-1 studies acknowledged that there was still some cellular damage with all products, whether preserved or not. The Goblet cell density was shown to be significantly decreased due to PQ-1, and in vivo confocal microscopy identified abnormalities compared with the control.20
Oxidative stress and free radicals
Oxidative stress is increasingly being recognised as a common pathway in ocular surface disease.2, 4, 12 Free radicals have been shown to be present in similar concentrations in both preserved and unpreserved topical and intracameral preparations.8, 9 In this study, we have identified the magnitude of the intrinsic free radical concentrations associated with topical glaucoma medications. These results are independent of preservation status, and suggest that the excipients detailed in Table 1 can contribute to both the positive and adverse effects of a topical medication. We realise this is an extremely detailed table, but it is enlightening for the ophthalmologist to be aware of the many components contained within the medications we prescribe. Although the healthy corneal and conjunctiva rapidly eliminates free radicals from the ocular surface, this protective mechanism is suboptimal in eyes with ocular surface disease.23 An unhealthy tear film and a vulnerable ocular surface can be compromised by oxidative stress generated from all the chemicals contained within eye drops.
Chemical composition (pH)
Level of pH is an important determinant of potential damage from an ocular chemical injury, and pH has been the focus of a recent pharmaceutical advertising campaign for a combination topical β-blocker and carbonic anhydrase inhibitor. A patient preference study found greater comfort associated with the physiological pH of the brinzolamide-based suspensions compared with the more acidic dorzolamide-based solutions and concluded that differences in ocular discomfort and adverse effects were likely to be attributable to the pH and formulation differences given the similarities of the active ingredients in the two combination products investigated.24, 23 In other words, excipients could have a role in patient tolerance and subsequent therapeutic compliance, independent of preservatives.
Clinical relevance
To the best of our knowledge this is the first attempt to document the pH levels of the commonly used glaucoma medications in the United Kingdom. The relatively acidic trend we found in our results probably represents necessary formulation designs, intended to optimise solubility of the active molecules. The impact of physiological pH on compliance has recently been accentuated by the makers of Azarga.25, 24 However, this claim was only compared with Cosopt, and not set in the context of a survey of the pH of all other glaucoma products. The lack of easily available information regarding the pH of commonly used glaucoma medications was the main motivation for this study.
We raise concerns that even unpreserved topical glaucoma medications may chronically injure a compromised ocular surface, due to exposure to free radicals or non-physiological pH. Stabilising excipients contained within these products can cause long-term toxicity and could affect compliance. In light of this, free-radical levels and pH warrant consideration when using multiple topical therapies in glaucoma patients. As our results appear specific to the individual products, without a clear correlation, it would be beneficial if such information was clearly stated. For example, if we consider the prostaglandin group, despite having the same BAK preservative, Xalatan has no measurable TAS result (0 vs 0.61 mmol/l) and a more physiological pH (6.8 vs 6.1) than Xalacom, probably due to the β-blocker component and the associated buffering excipients of sodium hydroxide and hydrochloric acid. The 0.01% Lumigan product has a TAS result of 0.03 mmol/l compared with 0.89 mmol/l for the 0.03% Lumigan formulation. The only preservative-free prostaglandin product, Saflutan, was the most acidic of this group (pH 5.3) and also had a TAS result of 0.7 mmol/l. Our Tables 2 and 3 list the glaucoma products according to TAS level or pH, and should educate the ophthalmologist who is striving to prescribe the most physiological products to minimise chronic damage to the ocular surface.
As more products become generic and are produced from alternative sources, it may be prudent for the ophthalmologist to be aware of the exact formulation being dispensed, as each may contain different excipients, have non-physiological pHs and varying levels of inherent oxidative stress. For example, some of the β-blockers have a range of pH 5 (Metipranolol) to 7.1 (Timolol 0.25%) and a TAS range of 0.02 mmol/l (Metipranolol) to 0.82 mmol/l (Teoptic 1%). The prostaglandins range from a pH of 5.3 (Saflutan) to 7.4 (Lumigan 0.03%), and a TAS range of 0 mmol/l (Xalatan) to 0.89 mmol/l (Lumigan 0.03%). Manufacturing differences of the preservatives and the excipients in generic products could be a future source of intolerance and corresponding poor compliance.
Limitations
Products with high viscosity, such as Pilogel, were not able to be analysed for ROS as the analyser used in this study can only evaluate liquids. However, topical pilocarpine was evaluated. The direct relevance of the specific results we obtained are difficult to interpret clinically without further investigation into the specific free radical exposure and their effect on cell line studies. Despite removing the outliers of Iopidine 0.5% and Minims Pilocarpine 2%, we were unable to identify a direct correlation between pH and free radical concentration in this study. Some products were very acidic, yet had negligible intrinsic free radical presence, while others had a physiological pH, with very high TAS results. This would suggest recent awareness of the pH of certain glaucoma medications and tolerance needs to be considered in the context of the whole product (active ingredient, preservatives, and excipients). However, the magnitude of the results and potential accumulative totals from long-term use still warrant consideration when prescribing for the vulnerable ocular surface. The fact that Iopidine and pilocarpine were seen to be outliers is consistent with our clinical experience, which shows poor tolerability and often short-term use of these two products.
Conclusion
Prescribing in the setting of a compromised ocular surface is complex, as the tear film and lacrimal functional unit can be exquisitely vulnerable to damage from any imbalance created by external topical agents. This study shows the range of intrinsic free radical activity in glaucoma medications. It also shows the range of pH, and identifies the products that are outside the physiological pH of the eye. Preservatives, free radicals, and pH levels can all contribute to ocular surface damage following long-term exposure. The potential accumulative damage of free radicals and pH should also be considered when prescribing glaucoma therapy with a compromised ocular surface. The effect of excipients could be responsible for patient intolerance when using generic products. Further study into excipient-related damage may further explain the ocular surface toxicity associated with chronic use of both preserved and unpreserved topical medications.

Acknowledgments
We thank Dr Alan Rotchford for his review of this manuscript.
DL and EM have received speakers' honoraria for educational meetings sponsored by Alcon. The other authors declare no conflict of interest.
References
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma Arch Ophthalmol 2002120(6701–713.discussion 829–830. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29 (4:312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86 (4:418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin Experiment Ophthalmol. 2008;36 (6:553–559. doi: 10.1111/j.1442-9071.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- De Saint Jean M, Debbasch C, Brignole F, Rat P, Warnet JM, Baudouin C. Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells. Curr Eye Res. 2000;20 (2:85–94. doi: 10.1076/0271-3683(200002)20:2;1-d;ft085. [DOI] [PubMed] [Google Scholar]
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17 (5:350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29 (6:618–621. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- Lockington D, Macdonald E, Gregory M, Stewart P, Caslake M, Ramaesh K. Presence of free radicals in commonly used ophthalmic preparations. Br J Ophthalmol. 2010;94 (4:525–526. doi: 10.1136/bjo.2009.162495. [DOI] [PubMed] [Google Scholar]
- Lockington D, Macdonald EC, Young D, Stewart P, Caslake M, Ramaesh K. Presence of free radicals in intracameral agents commonly used during cataract surgery. Br J Ophthalmol. 2010;94 (12:1674–1677. doi: 10.1136/bjo.2009.171009. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Zhu CC, Harris DL. Relationship among oxidative stress, DNA damage, and proliferative capacity in human corneal endothelium. Invest Ophthalmol Vis Sci. 2009;50 (5:2116–2122. doi: 10.1167/iovs.08-3007. [DOI] [PubMed] [Google Scholar]
- Kuizenga A, van Haeringen NJ, Kijlstra A. Inhibition of hydroxyl radical formation by human tears. Invest Ophthalmol Vis Sci. 1987;28 (2:305–313. [PubMed] [Google Scholar]
- Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002;50 (3:341–351. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112 (11:1446–1454. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Riancho L, Warnet JM, Brignole F. In vitro studies of antiglaucomatous prostaglandin analogues: travoprost with and without benzalkonium chloride and preserved latanoprost. Invest Ophthalmol Vis Sci. 2007;48 (9:4123–4128. doi: 10.1167/iovs.07-0266. [DOI] [PubMed] [Google Scholar]
- De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40 (3:619–630. [PubMed] [Google Scholar]
- Liang H, Pauly A, Riancho L, Baudouin C, Brignole-Baudouin F. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional-reconstituted corneal epithelium system. Br J Ophthalmol. 2011;95 (6:869–875. doi: 10.1136/bjo.2010.189449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase K, Lin W, Aoyama Y, Yamamoto T, Shimazawa M, Hara H. Effects of timolol-related ophthalmic solutions on cultured human conjunctival cells. Jpn J Ophthalmol. 2010;54 (6:615–621. doi: 10.1007/s10384-010-0881-2. [DOI] [PubMed] [Google Scholar]
- Ayaki M, Iwasawa A, Yaguchi S, Koide R. Preserved and unpreserved 12 anti-allergic ophthalmic solutions and ocular surface toxicity: in vitro assessment in four cultured corneal and conjunctival epithelial cell lines. Biocontrol Sci. 2010;15 (4:143–148. doi: 10.4265/bio.15.143. [DOI] [PubMed] [Google Scholar]
- Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27 (11:837–845. doi: 10.1007/s12325-010-0070-1. [DOI] [PubMed] [Google Scholar]
- Labbé A, Pauly A, Liang H, Brignole-Baudouin F, Martin C, Warnet JM, et al. Comparison of toxicological profiles of benzalkonium chloride and polyquaternium-1: an experimental study. J Ocul Pharmacol Ther. 2006;22 (4:267–278. doi: 10.1089/jop.2006.22.267. [DOI] [PubMed] [Google Scholar]
- Aihara M, Otani SI, Kozaki J, Unoki K, Takeuchi M, Minami K, et al. Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J Glaucoma. 2012;21 (1:60–64. doi: 10.1097/IJG.0b013e3181fc8129. [DOI] [PubMed] [Google Scholar]
- Katz G, Springs CL, Craven ER, Montecchi-Palmer M. Ocular surface disease in patients with glaucoma or ocular hypertension treated with either BAK-preserved latanoprost or BAK-free travoprost. Clin Ophthalmol. 2010;4:1253–1261. doi: 10.2147/OPTH.S14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G, Riley MV. Does topical hydrogen peroxide penetrate the cornea. Invest Ophthalmol Vis Sci. 1993;34 (9:2752–2760. [PubMed] [Google Scholar]
- Manni G, Denis P, Chew P, Sharpe ED, Orengo-Nania S, Coote MA, et al. The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination vs dorzolamide 2%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2009;18 (4:293–300. doi: 10.1097/IJG.0b013e31818fb434. [DOI] [PubMed] [Google Scholar]
- Mundorf TK, Rauchman SH, Williams RD, Notivol R. A patient preference comparison of Azarga (brinzolamide/timolol fixed combination) vs Cosopt (dorzolamide/timolol fixed combination) in patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2008;2 (3:623–628. doi: 10.2147/opth.s4088. [DOI] [PMC free article] [PubMed] [Google Scholar]