Abstract
Background
Several arrhythmogenic mechanisms have been inferred from animal heart failure (HF) models. However, the translation of these hypotheses is difficult due to lack of functional human data. We aimed to investigate the electrophysiological substrate for arrhythmia in human end-stage non-ischemic cardiomyopathy.
Methods and Results
We optically mapped the coronary-perfused left ventricular wedge preparations from human hearts with end-stage non-ischemic cardiomyopathy (HF, n=10) and non-failing hearts (NF, n=10). Molecular remodeling was studied with immunostaining, Western blotting, and histological analyses. HF produced heterogeneous prolongation of action potential duration (APD) resulting in the decrease of transmural APD dispersion (64±12 ms vs 129±15 ms in NF, P<0.005). In the failing hearts, transmural activation was significantly slowed from the endocardium (39±3 cm/s versus 49±2 cm/s in NF, P=0.008) to the epicardium (28±3 cm/s versus 40±2 cm/s in NF, P=0.008). Conduction slowing was likely due to Cx43 downregulation, decreased colocalization of Cx43 with N-cadherin (40±2% versus 52±5% in NF, P=0.02), and an altered distribution of phosphorylated Cx43 isoforms by the upregulation of the dephosphorylated Cx43 in both the subendocardium and subepicardium layers. Failing hearts further demonstrated spatially discordant conduction velocity alternans which resulted in nonuniform propagation discontinuities and wavebreaks conditioned by strands of increased interstitial fibrosis (fibrous tissue content in HF 16.4±7.7 versus 9.9±1.4% in NF, P=0.02).
Conclusions
Conduction disorder resulting from the anisotropic downregulation of Cx43 expression, the reduction of Cx43 phosphorylation, and increased fibrosis is likely to be a critical component of arrhythmogenic substrate in patients with non-ischemic cardiomyopathy.
Keywords: congestive heart failure, repolarization, conduction velocity, optical mapping, cardiomyopathy
Introduction
End-stage heart failure (HF) is characterized by the substantial pathophysiological remodeling of cardiac function including alterations within a host of ion channels,1, 2 intracellular calcium cycling,3, 4 cell-cell coupling proteins,5 and ultrastructural abnormalities such as interstitial fibrosis6 and cellular hypertrophy.7 These changes underlie electrophysiological (EP) abnormalities, predisposing a patient to deadly arrhythmias.2, 8 Despite advances in the characterization of the ionic and molecular remodeling that occurs in the context of HF, the exact role of these changes in the genesis of electrical instability and arrhythmias at the intact multicellular tissue network level is still poorly understood. Numerous animal models of HF have thus been developed to investigate the mechanisms of arrhythmogenesis.9, 10 However, there exists insufficient EP data from human hearts due to the limited availability of live, human cardiac tissue for EP research with basic state-of-the-art imaging methods.
An electrophysiological hallmark of cells and tissues isolated from hypertrophied and failing hearts is a prolonged action potential duration (APD), reflecting delayed terminal repolarization of the cardiac myocyte. Canine models of non-ischemic dilated cardiomyopathy have shown a nonuniform prolongation of APD across the ventricular wall which exaggerates transmural APD gradient and forms a substrate for reentrant arrhythmias.9, 10 Despite the prolongation of the QT-interval observed in patients with HF, recent studies revealed a decrease in transmural APD gradient in failing human hearts.11, 12 This suggests the existence of additional factors which contribute to the formation of the transmural heterogeneities of repolarization.
Several animal models of non-ischemic dilated cardiomyopathy were utilized to characterize these conduction changes as well as to investigate their arrhythmic consequences and underlying mechanisms.5, 10, 13 Poelzing et al demonstrated the significant slowing of conduction velocity (CV) in a heterogeneous fashion with a prominent delay shown at the subepicardium.10 The cellular mechanisms underlying this conduction slowing in HF include the decreased expression of connexin 43 (Cx43), the principal ventricular gap junction protein, as well as its dephosphorylation and redistribution.5, 13 Increased interstitial fibrosis and ultrastructural abnormalities are additional hallmarks of HF.6, 7, 14 We aimed to translate these findings in animal models to human HF to determine which factors are likely to contribute to arrhythmogenesis in these patients.
In order to investigate transmural heterogeneities of activation and repolarization and their potential role in HF-related arrhythmias, we used high-resolution transmural optical mapping of transmembrane potential. To control for factors of regional heterogeneity, acute ischemia, and chronic ischemic injury, the current study was conducted in non-ischemic end-stage cardiomyopathy human hearts obtained during transplantation. As a control, we used non-failing (NF) donor hearts, which were rejected for transplantation. We aimed to characterize HF-associated changes in impulse propagation, Cx43 expression and phosphorylation, and the disruption of the extracellular matrix by fibrosis.
Methods
An expanded Material and Methods section can be found in the online data supplement.
Patients groups
The study was approved by the Washington University Institutional Review Board. Failing hearts (HF, n=10, Online Table I) with non-ischemic end-stage cardiomyopathy, and without history of myocardial infarct, were obtained at the time of cardiac transplantation performed at the Barnes-Jewish Hospital, Washington University School of Medicine. Non-failing donor hearts with normal LV function (NF, n=10, Online Table I) were provided by the Mid-America Transplant Services (Saint Louis, MO). Additional 10 human hearts (n=5/group) which were not used for optical mapping experiments were selected for histology staining (Online Table V).
Explanted hearts were cardioplegically arrested and cooled to 4–7°C in the operating room following crossclamping of the aorta. The arrested heart was maintained at 4–7°C to preserve tissue during the 15–20 minute delivery from the operating room to the research laboratory.
Experimental preparation
We isolated wedges (n=8/group) from the posterior-lateral left ventricular free wall supplied by left marginal artery as previously described in canine15, 16 and human hearts (see Online Figure IA–B).3, 11 Additionally, in five human hearts (HF=2; NF=3), we optically mapped the epicardial surface of the coronary-perfused left posterior ventricular free wall preparations (see Online Figure IC–D).17, 18 Tissue from one donor's heart was used for both preparations. Preparations were perfused (arterial pressure of 60–70 mmHg) with oxygenated Tyrode solution composed of (in mmol/l): 128.2 NaCl, 4.7 KCl, 1.19 NaH2PO4, 1.05 MgCl2, 1.3 CaCl2, 20.0 NaHCO3, and 11.1 glucose, and gassed with 95% O2-5% CO2; pH=7.35±0.05; 37°C.
The wedges were immobilized with 10 μM Blebbistatin (Tocris Bioscience, Ellisville, MO) and then stained with 4 μM di-4-ANEPPS (Molecular Probes, Eugene, OR). Optical mapping was carried out with a MiCAM Ultima-L CMOS camera (SciMedia, USA Ltd., CA) system.9 Spatial resolution was 200–300 μm/pixel.
Histology and Immunofluorescence labeling
Immunofluorescence labeling was performed as previously described.17, 19, 20 To measure fibrosis tissue content, a paraffin embedded fresh tissue was sectioned and stained with Masson's trichrome (International Medical Equipment, San Marcos, CA, USA). For immunolabeling, a fresh-frozen tissue was sectioned and stained with commercially available antibodies: Rb-Cx43 (1:1000; Sigma, St. Louis, MO) and Ms-N-Cadherin (1:200; Sigma, St. Louis, MO). Images were collected using a Nikon C1/80i confocal microscope and quantitatively analyzed using ImageJ (National Institutes of Health) as previously described.20
Western blot analysis
Immediately after the delivery of the heart to our research laboratory, tissue samples were dissected from the LV endocardium (<1 mm) and epicardium (<1 mm) and frozen in liquid nitrogen. Western blotting was performed as previously described.21 The protein homogenates were incubated against Rb-Cx43 polyclonal antibody (1:4000; Sigma, St. Louis, MO), Ms-N-cadherin (1:250; Sigma, St. Louis, MO) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:100000; Sigma, St. Louis, MO).
Statistical analysis
Comparisons were made between HF and NF, and among the subepicardium, midmyocardium, and subendocardium. Subepicardium was defined as the region within 2mm from the epicardial surface; midmyocardium was the 2mm-wide midmyocardial layer; and subendocardium was the 2mm-wide layer adjacent to the endocardial surface. Data were analyzed with linear mixed random effects ANOVA models where subject was random and nested within the failing group. In the models, main effects and interactions included heart failure in combination with each of the following effects: cycle length or transmural layer. In each analysis, the heart failure effect was tested by contrasts at each level of the second effect (above) using variance components from the mixed models and no adjustment for multiple comparisons. Some models had unequal variance that was modeled with additional variance parameters corresponding to S1S1 for Figures 1A, 1B, 3A, 3B, and 3C, Tissue Layer for Figure 2A, combination of Tissue Layer and Failing for Figure 2B, and combinations of S1S1 and Failing for Figure 3D. In the figures, vertical bars were 95% confidence intervals calculated from variance components of the mixed random effects models. Type 3 and Type 1 statistics summary is shown in Online Table VI. A value of P<0.05 was considered statistically significant.
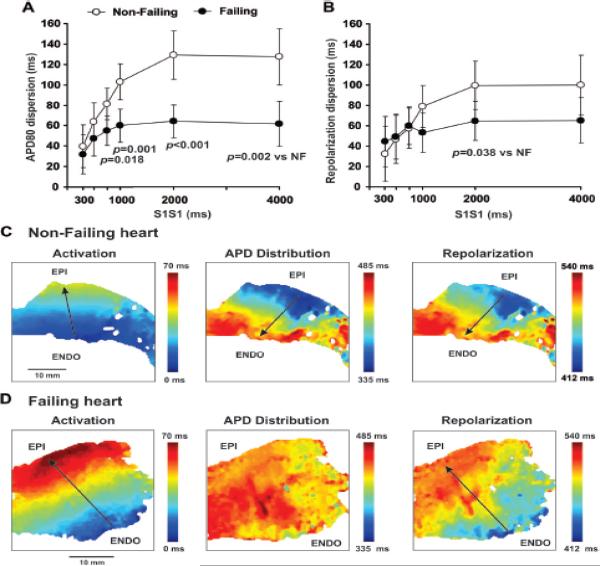
Figure 1.
Transmural APD80 (A) and repolarization (B) dispersion measured at different CLs (S1S1) in non-failing and failing human preparations. Typical examples of activation, APD distribution and resulting repolarization sequences for non-failing (C) and failing (D) human wedges recorded at 2,000 ms pacing CL. Vectors show a sequence of changes of each parameter.
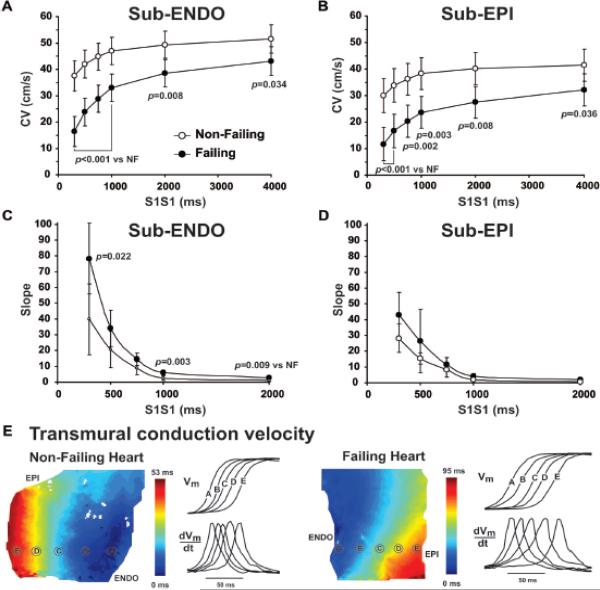
Figure 3.
Conduction velocity (CV) restitution curves calculated for sub-endocardium (A) and subepicardium (B) in non-failing and failing human hearts. Corresponding slopes are presented on panels C and D for subendocardium and subepicardium, respectively. E: Typical examples of transmural activation in non-failing and failing human wedge preparations. Near the isochronal maps, OAP upstrokes and their derivatives from equally spaced sites are presented. Vm, membrane potential.
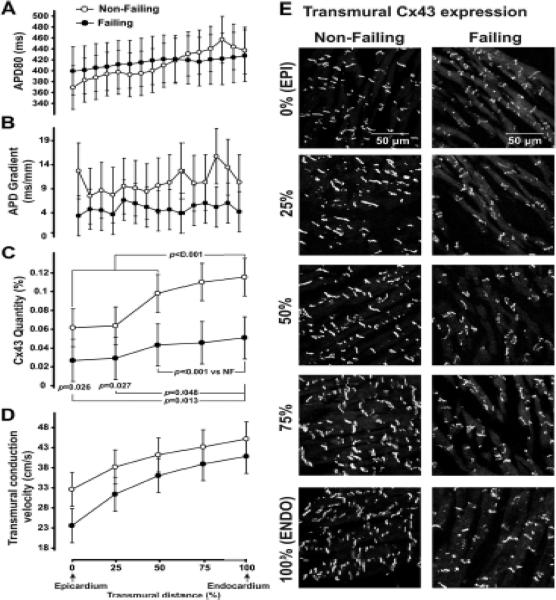
Figure 2.
Effect of Cx43 expression on the transmural APD gradient and CV. A: Average APD profiles measured at CL=2,000 ms are plotted as a function of transmural distance from the epicardium (in % of a wedge depth). B: Spatial derivative of APD profile presented on panel A. Heart Failure P=0.035. C: Average Cx43 quantity measured from transmural muscle layers spanning from epicardium to endocardium. D: Transmural CV measured at different transmural layers. Heart Failure P=0.036 and Tissue Layer P<0.0001. E: Cx43 expression is shown at different transmural layers.
Results
Transmural APD and Repolarization Dispersion Remodeling
In agreement with previous studies,3, 11 we observed an extension of the transmural APD throughout the failing LV. However, HF resulted in heterogeneous APD prolongation, which was more pronounced at the subepicardium than in the midmyocardium and subendocardium (see Online Table II). As a result, transmural APD dispersion was significantly decreased in failing hearts compared with non-failing hearts at all pacing CLs (Figure 1A). The average functional refractory period was also lengthened in failing hearts (393±17 ms vs. 246±15 ms for HF and NF respectively, P<0.001). Figures 1C and 1D illustrate typical examples of activation, APD distribution, and the resulting repolarization sequence in NF and HF wedge preparations, respectively. Repolarization time was calculated as the sum of activation time and APD. In a NF heart with a 150 ms intrinsic epi-to-endo APD gradient, the 42-ms transmural activation delayed subepicardial repolarization and did not affect subendocardial repolarization. Thus a 120-ms transmural repolarization gradient was produced, which was primarily governed by the APD distribution pattern. In this case, transmural gradients in activation and repolarization are opposite (marked by black arrows on maps in Figure 1C). In contrast, the failing heart had a relatively homogeneous transmural APD distribution and a slow, 70-ms, transmural activation which significantly delayed repolarization of both midmyocardium and subepicardium. This resulted in an endo-to-epi repolarization sequence primarily governed by the activation sequence rather than the APD distribution pattern. Such slow activation thereby caused a repolarization sequence propagating in the same direction as the activation sequence (black arrows on maps in Figure 1D). Summarized values of the repolarization times are presented in Online Table III. Interestingly, the average value of the repolarization gradient for all the failing hearts did not differ from that of the NF hearts at all pacing CLs except 2000 ms (Figure 1B).
Relationship between transmural gradient of repolarization and Cx43 expression
To examine the role of cell-to-cell coupling in HF-associated EP remodeling, we correlated transmural local APD and CV with the local level of Cx43 expression. Figure 2A–C shows the summarized APD profiles, local APD gradients, and local CV measured at different transmural layers in the non-failing and failing wedges. In NF hearts, the sharpest transition in transmural APD occurred between the endocardium and deeper layers of tissue where M-cell islands are thought to be presented.9, 11, 22 However, these islands of cells with significantly prolonged repolarization were not observed in failing hearts.11 This results in a relatively flat APD distribution and the paradoxical decrease of total APD dispersion (see also Online Figure II). Correspondingly, the APD gradient (Figure 2B) defined as the derivative of the profile from Figure 2A is significantly decreased during HF while simultaneously, the overall Cx43 signal (Figure 2D) is also reduced in HF compared with NF in each transmural layer. Typical examples of the transmural expression of Cx43 in representative NF and HF wedges are shown in Figure 2E. Within each transmural layer, Cx43 localizes to the longitudinal ends of the individual myocytes in both non-failing and failing hearts, consistent with normal cellular gap junction localization.5, 10, 23, 24 This pattern was observed in all experiments. As evident from Figure 2, cellular uncoupling does not correlate with the smoothed local APD gradient observed in failing hearts.
Conduction Slowing in Failing Hearts
As shown in Figure 2C–D, one of the expected consequences of the observed downregulation of Cx43 expression is conduction slowing. Transmural CV was heterogeneously slowed in failing hearts, and to a greater extent at the subepicardium (2mm-wide layer from the epicardial surface) rather than in the subendocardium (2mm-wide layer from the endocardial surface, 40% vs. 34%, respectively). The frequency dependence of CV (CV restitution curve) measured at the subendocardium and subepicardium during a dynamic restitution protocol is shown in Figure 3A–B. As is the case in animal studies,5, 10 subendocardial CV was faster than subepicardial CV in both HF and HF preparations, by 24±3%, and 29±4%, respectively (P<0.001). In addition, CV restitution curve slopes (Figure 3C–D) were found to be greater at the subendocardium rather than at the subepicardium in both HF and NF hearts. HF-associated remodeling therefore resulted in an increase in the steepness of CV restitution curves during fast pacing in both subendo- and subepicardium. Typical examples of transmural activation during endocardial point stimulation are shown in Figure 3E. Transmural conduction is significantly reduced in HF, especially in subepicardial layers, indicated by the crowding of isochrones at the subepicardium. It is also evident from the superimposed action potential upstrokes which are located closer (indicating faster propagation) in NF than in the HF myocardium. Representative examples of activation maps measured at slow and fast pacing are shown in Online Figure III. The transmural distribution of local CV vectors and their values are shown in Online Figure IV.
Cellular excitability
To estimate the role of reduced excitability in conduction slowing, we measured optical action potential upstroke velocity from OAPs recorded in both failing and non-failing hearts.5 We did not observe any significant differences in (dF/dt)max in either the subendocardium (3.04±0.32 V/s vs. 3.35±0.38 V/s in HF vs. NF, P=0.544) or the subepicardium (2.78±0.28 V/s vs. 2.75±0.26 V/s in HF vs. NF, P=0.940) when compared between the NF and HF group. There were also no significant differences between the subendocardium and subepicardium (P=0.219 and P=0.557 in NF and HF respectively).
Cx43 distribution
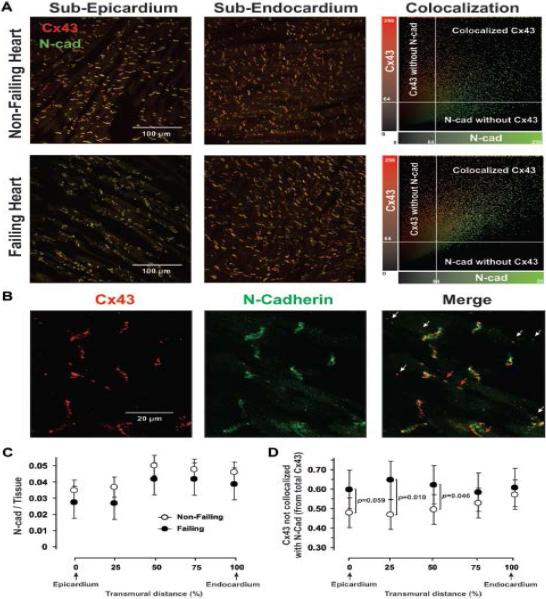
To estimate the spatial distribution of Cx43 in human end-stage HF, we used a structural protein, N-cadherin, as a marker of intercalated disks of myocytes. We calculated the co-localization of Cx43 with N-cadherin using double-immunostaining as described previously.19 The results of these experiments are shown in Figure 4. The expression of N-cadherin was significantly decreased in the subepicardium layer but was unchanged in both the midmyocardium and subendocardium (Figure 4A and C). We found that there was greater Cx43 and N-cadherin colocalization in the subepicardium of NF hearts (52 ± 5%) as compared with failing hearts (40 ± 2%, P=0.016) but no significant difference between these groups with respect to the subendocardium (43 ± 5% vs 39 ± 2%, P=0.189) (Figure 4A). It is evident from representative examples taken at 100× magnification (Figure 4B) that, although some lateralized Cx43 co-localizes with N-cadherin (red arrows), there is some Cx43 that does not (white arrows). These Cx43 are located outside the intercalated discs on the lateral myocyte membrane or within the myocytes and may represent the trafficking of Cx43.25 The amount of Cx43 that is not co-localized with N-cadherin (relative to total Cx43) was higher on the subepicardium (by 25–38% with regard to non-failing hearts, P=0.027) and was unchanged on the subendocardium (higher by 7% with regard to non-failing hearts, P=0.493) (Figure 4D). On average, in failing hearts the total amount of Cx43 that is co-localized with N-cadherin was decreased by 2.9-fold in the subepicardial layers and 2.5-fold in the subendocardial layers relative to NF hearts.
Figure 4.
Immunostaining for Cx43 and N-Cadherin. A, Left: 20x typical examples of double-staining for Cx43 (in red) and N-Cadherin (in green) in subepicardium and subendocardium of non-failing and failing human hearts. A, Right: Colocalization of Cx43 and N-Cadherin, showing that voxels of high Cx43 intensity also have a high N-Cadherin signal. Intensity level of 64 units (25%) was used as a threshold. B: 100x examples with splitted red (Cx43) and green (N-Cadherin) channels. Red arrows indicate Cx43 co-localized with N-cadherin; white arrows – Cx43 which is not co-localized with N-cadherin. C and D: Summarized data for transmural expression of N-Cadherin (relative to cardiac tissue, C) and Cx43 that is not co-localized with N-Cadherin (relative to total amount of Cx43, D) at different transmural layers (in % of wedge depth). For panel C, Heart Failure P=0.085 and Tissue Layer P<0.0001.
Cx43 phosphorylation
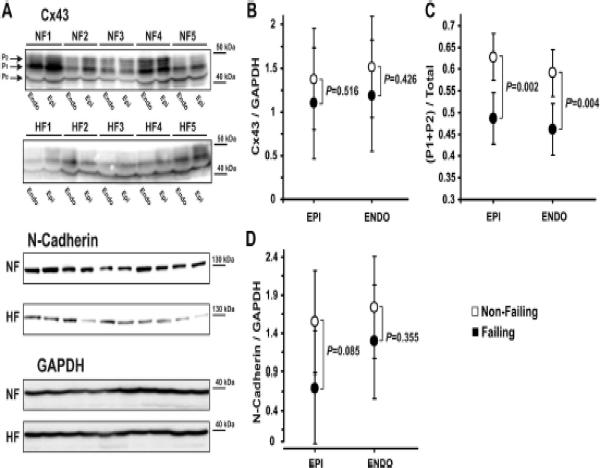
Immunostaining data was confirmed with western blot Cx43 and N-cadherin protein measurements (Figure 5A). The polyclonal Cx43 antibody recognized several phosphorylated Cx43 isoforms. As such, multiple bands, corresponding to lower-molecular-weight and lower phosphorylated, P0, higher-molecular-weight and higher phosphorylated, P1 and P2, isoforms of Cx43 (Figure 5A, top panels), migrated at ≈ 43 kDa (42 to 46 kDa). The total expression of Cx43 (normalized to GAPDH) was significantly reduced in both the subendocardium (by 40%) and subepicardium (by 33%) in failing hearts as compared to non-failing hearts (Figure 5B).
Figure 5.
Western blot analysis. A: Subepicardial (Epi) and subendocardial (Endo) expression of Cx43 (two top panels), N-Cadherin (middle panel), and GPDH (bottom panel). Different phosphorylated bands for Cx43 protein were observed: the higher-molecular-weight and higher phosphorylated isoforms (P1 and P2) and the lower-molecular-weight and dephosphorylated isoform (P0). B: Average data for Cx43 expression are presented on panel A. C: Redistribution of phosphorylated isoforms of Cx43 during HF in subepicardium and subendocardium. D: Average data for N-Cadherin expression are presented on panel A.
We also noticed an altered phosphorylation status of Cx43 isoforms. We distinguished the faster-migrating, lower-molecular-weight (42 kDa) band, which has been shown to represent the dephosphorylated (P0) isoform,21, 26 from the slower-migrating higher-molecular-weight (43–46 kDa) bands that – in contrast to P0 – represent phosphorylated isoforms of Cx43 (P1 and P2). In failing hearts, we observed a shift from the higher phosphorylated isoforms of Cx43 to the dephosphorylated Cx43 isoform in both the subendocardium and subepicardium layers (Figure 5A, top panels). The sum (P1 + P2) was downregulated by 23% and 22% (Figures 5C), whereas P0 was upregulated by 38% and 32% in the subepicardium and subendocardium, respectively. The absolute protein levels of Cx43 isoforms with respect to the amount of tissue loaded (normalized to GAPDH) are shown in Online Figure V. We determined that the proportion of higher to lower phosphorylated Cx43 changed from 63:37 (1.7:1) in non-failing hearts to 49:51 (0.96:1) in HF. The ratio of dephosphorylated to phosphorylated Cx43 increased by 79% and 68% in both subepicardium and subendocardium. The downregulation of N-cadherin expression was also confirmed by Western blotting (Figure 5A, middle panels). We observed a decrease in the N-cadherin protein level intensity with respect to the amount of protein loaded (Figure 5D).
Structural changes and interstitium
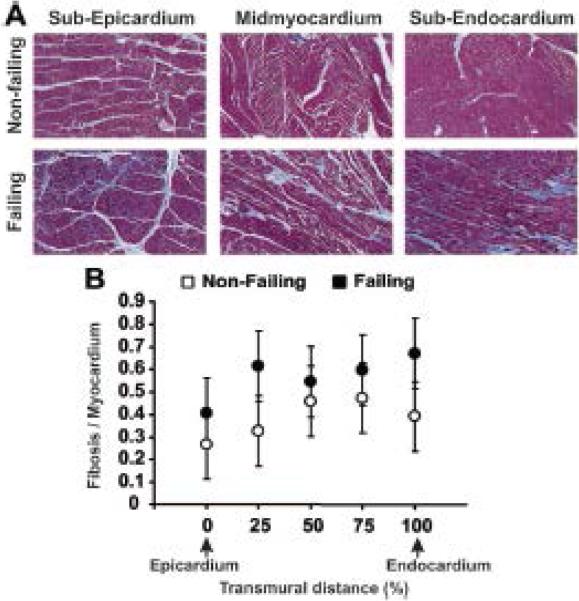
Many human studies have reported increased interstitial fibrosis during HF, often accompanied by ultrastructural abnormalities.6, 7, 14 In the present study, detailed quantitative image analysis of multiple myocardial sections revealed a statistically significant increase in the percentage of fibrous tissue content per cardiac tissue in failing hearts compared with non-failing hearts (Figure 6). Detailed histology figures are shown in Online Figures VI and VII.
Figure 6.
Masson Trichrome staining of transmural sections from the human left ventricle. A: Sample images from non-failing and failing human hearts taken from the subepicardium, midmyocardium, and subendocardium using a 10x magnification are presented. B: Average ratio of cardiac tissue to connective tissue measured at different transmural layers in non-failing and failing human preparations. Failing effect across tissue layers – P=0.023.
Conduction abnormalities in HF
There is significant evidence in human studies that ventricular tachyarrhythmias are prevalent in patients with HF.8 In order to investigate the mechanisms of arrhythmogenesis during HF, we optically mapped five human hearts from the epicardial surface of the coronary-perfused left posterior ventricular free wall preparations (Figure 7A).
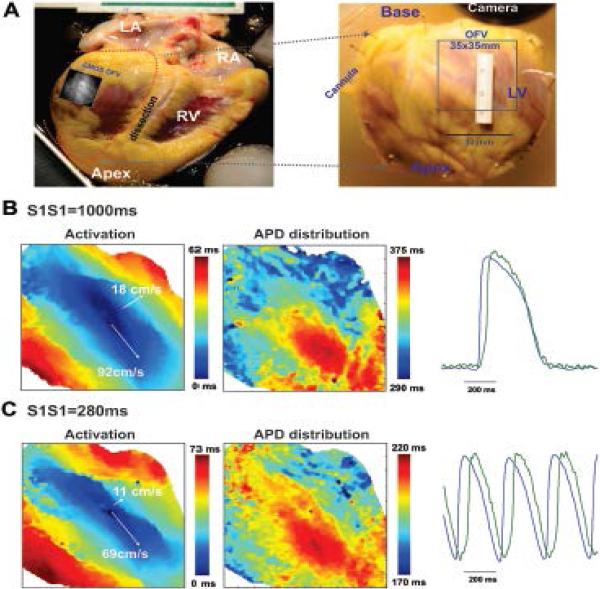
Figure 7.
Optical mapping of the epicardial surface of a non-failing human heart. A: Epicardial optical mapping of the isolated, left ventricle free wall preparation (marked by dotted line on the left panel) was conducted. Optical field of view (OFV: 35 mm by 35 mm) is denoted by square. Activation and APD distribution patterns are shown for slow (B, S1S1=1,000 ms) and fast (C, S1S1=280 ms) pacing. The gradual shortening of pacing interval depressed conduction velocity along the transverse direction and resulted in increased conduction anisotropy without any conduction abnormalities. LA and RA – left and right atria; LV and RV – left and right ventricle.
During constant pacing at CL=1,000 ms in NF hearts, ventricular preparations had complex but steady state activation patterns determined by the myocardial fiber orientation. In the LV free wall preparations, we were able to measure both longitudinal and transversal conduction velocities. In the wedge preparations, transmural conduction velocity represents a combination of longitudinal and transversal conduction velocities, with a high impact of transversal conduction (compare data from Figure 3A–B and Online Table IV). The longitudinal and transverse CV's were CVL=92±4 cm/s and CVT=22±2 cm/s, respectively (Figures 7B). The gradual shortening of the pacing interval from 1,000 ms to the functional refractory period (288±31 ms) lead to a heterogeneous slowing of both CV (CVL=73±4 cm/s and CVT=11±3 cm/s) and an increase in conduction anisotropy (CVL/CVT ratio from 4.2±0.4 to 7.2±1.4) (Figure 7C). For more details, see Online Table IV.
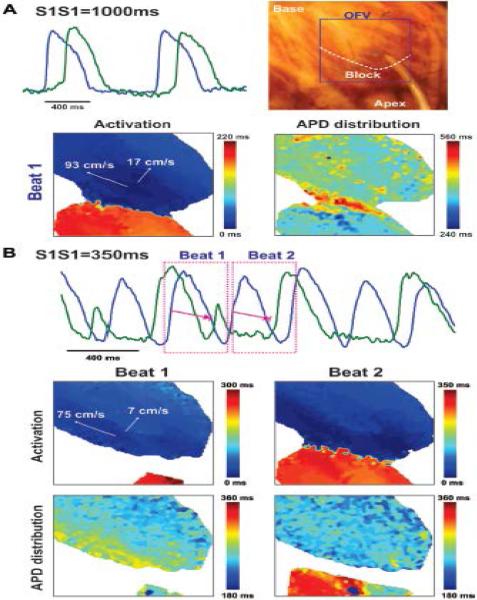
In contrast to non-failing hearts, failing hearts had a higher conduction anisotropy during both slow (CL=1,000 ms) and fast pacing (5.8±0.8 and 9.7±1.3, respectively) due to significantly slower CV along the transverse direction (16±2 cm/s vs. 22±2 cm/s, in HF and NF respectively) (Figure 8A). A progressive increase in the stimulation frequency resulted in discordant CV alternans, non-uniform conduction block in the transverse direction, and wavebreaks (Figure 8B). Failing hearts were also characterized by a significantly higher APD dispersion, resulting from heterogeneous activation. The area of transversal conduction block coincided with the location of strands of extensive interstitial fibrosis presented in failing hearts. Such considerable interstitial fibrosis resulted not only in transversal conduction block, but also in the occurrence of discordant APD alternans. As shown in Figure 8B, the APD in regions located before conduction block (blue) and after (green) oscillate out-of-phase. During such discordant APD alternans, some regions of tissue alternate in a long-short-long pattern (blue APD), whereas other regions simultaneously alternate in a short-long-short pattern (green APD). When the amplitude of these APD alternans grows large enough (APD=360 ms in the region located after the conduction block versus pacing interval S1S1=350 ms, beat 3 on Figure 8B), AP failure occurs, causing conduction block in this area (no green AP on the optical recording).
Figure 8.
Pacing-induced discordant conduction alternans and occurrence of the transverse conduction block in a failing human heart. Epicardial optical mapping revealed a considerable conduction delay in the transversal direction (A). Progressive shortening of the pacing CL from 1,000 ms (A) to 350 ms (B) led to occurrence of beat-to-beat discordant conduction alternans with 2:1 conduction block along the transverse direction. OAPs corresponding to the reconstructed activation and APD distribution maps are marked by pink dotted rectangles. Anatomical location of the block line is denoted by the white dotted curve on the heart photo on the panel A.
Discussion
Ventricular arrhythmia is one of the primary causes of death in humans with HF.8 Despite major advances in the characterization of ionic and molecular remodeling that occurs in humans during HF, the exact role of these changes in the formation of an EP substrate for arrhythmia and arrhythmogenesis itself are still poorly understood. In the present study, high-resolution optical mapping was performed in the human wedge preparation to assess activation and repolarization pattern remodeling in end-stage nonischemic cardiomyopathy. We showed that CV slowing in HF is a critical component of arrhythmogenesis. We also found that CV abnormalities can affect conduction anisotropy and dispersion of repolarization, thereby forming a substrate for arrhythmia induction. Slowed impulse propagation was correlated with cellular and molecular determinants of myocardial conduction.
Conduction Abnormalities and Arrhythmic substrate in HF
Unidirectional conduction block and slow conduction are prerequisites for reentry, and require some extent of spatial heterogeneity. This EP heterogeneity has been attributed to either spatial dispersion in refractory period,9, 27 local differences in excitability, nonuniformity in the amount of excitatory current generated by the cell membrane, or anisotropy.28 Despite our increased understanding of repolarization changes during HF, the degree to which conduction abnormalities play a role in arrhythmogenesis in HF remains unknown.
The significance of conduction delays in HF has been highlighted in clinical reports.29 It was observed that some HF patients exhibited a prolonged QRS duration and/or a delay in the low-frequency terminal portion of the QRS complex in their signal-averaged electrocardiograms. Such delays have been recognized to be predictors of sudden cardiac death.30 Despite emerging evidence linking conduction delays to complex arrhythmias and increased susceptibility of sudden death in human non-ischemic cardiomyopathy, this connection remained indirect.
In the present study, we examined end-stage non-ischemic cardiomyopathic human hearts to specifically characterize conduction changes and investigate their arrhythmic consequences and underlying mechanisms. In failing hearts, we observed that regional changes in tissue properties result in the nonuniform anisotropy of impulse propagation. This subsequently promotes conduction block, typically in the transverse direction (Figure 8), and could be associated with reentrant arrhythmias known as anisotropic reentry.31 Similar results revealed the preferential occurrence of conduction block in the transverse direction, which appeared to be more vulnerable regardless of whether intercellular coupling or active generator properties were impaired.32 These results were also obtained in the rabbit ventricles under hypothermic conditions, when the degree of intercellular coupling and cellular excitability were reduced,19 and under the direct superfusion of the sheep epicardial muscle33 or the whole rabbit heart31 by the electrical uncoupler heptanol.
In the present study, we also found that conduction abnormalities can affect repolarization patterns, and thus alter the repolarization gradient. This in turn could form arrhythmic substrates. APD distribution primarily reflects the intrinsic repolarization properties of myocytes determined by the expression of proteins responsible for the time-dependent repolarizing ionic currents. The repolarization of the coupled myocardium, however, is dependent upon the complex interplay between both intrinsic ionic properties and extrinsic factors such as propagation34 and cell-to-cell coupling.35 In our earlier studies,34, 36 we demonstrated that APD base-to-apex gradient governs repolarization and refractoriness patterns in the guinea pig heart because activation is relatively fast, and therefore the conduction delay is significantly shorter compared to the global APD gradient. In this case, transmural gradients in activation and repolarization should be opposite as we demonstrated for non-failing human hearts (Figure 1C). However, if transmural activation is relatively slow, such as during end-stage HF, it can influence the sequence of repolarization and can exaggerate the dispersion of repolarization. Similarly, in all studied failing hearts, even with homogeneous APD distributions, such slow activation produced a significant repolarization gradient governed by activation. Slow transmural activation was observed in all failing hearts in both subepicardium and subendocardium (Figure 3A–B).
Mechanisms Underlying Conduction Slowing in HF
To investigate cellular and molecular mechanisms underlying conduction slowing in human HF, we assessed the roles of reduced excitability, fibrosis in the extracellular matrix, and cell-to-cell coupling regulated by the gap junction protein expression, distribution, and phosphorylation.
We did not observed any changes in action potential upstroke velocity in failing hearts (Figure 3F). Although the local conduction slowing within a single pixel could influence the action potential upstroke velocity, such measurements can help to indirectly estimate the impact of cellular excitability in conduction slowing as observed in our study. Similar results were also shown by Akar et al. in a tachycardia pacing induced canine HF model.5 In the same canine HF model, the Na+-current density measured in the ventricular myocytes has been shown to be unchanged,37 suggesting that cellular excitability may not be responsible for CV slowing. On the other hand, Valdivia et al. using a similar canine pacing model of HF as well as explanted failing human hearts demonstrated a 39% and 57% decrease in the peak INa density, respectively in canine and human hearts. No changes in the kinetics of the current were demonstrated.38 At the same time, using quantitative measurement of mRNA, they showed no differences in the expression of different α subunit isoforms (NaV1.1, 1.3, 1.5) or β1 and β2 subunits. These conclusions correspond with a previous study done by Kaab et al39 and also with the recent study by Soltysinska et al.1 This may suggest the presence of completely non-functional channels at the surface membrane; however, their contribution to HF-associated CV slowing remains a subject of debate.
Previous reports have demonstrated that Cx43 is reduced in a variety of animal models of HF5, 10 and in humans.21, 23, 24 In the present study, relative Cx43 quantity in EP-characterized failing myocardium was likewise reduced by 58 ± 7% (averaged through all transmural muscle layers) compared with non-failing hearts. This result corresponds well with previous reports which showed a 50% reduction of Cx43 protein expression and 40% reduction of Cx43 mRNA in human congestive HF.10, 40 With unchanged (dV/dt)max in failing hearts, this finding indicates that CV slowing in the subepicardium is attributable to a localized reduction of intercellular coupling in subepicardial layers, rather than depressed excitability.
In addition to Cx43 downregulation during HF, we observed the local disorganization of gap junctions. It has been shown that some pathological conditions can cause relocalization of Cx43 proteins from the intercalated discs to the lateral membrane. This lateralization is associated with a loss of gap junction coupling between myocytes, resulting in conduction block and reentrant arrhythmias. In the present study, we used a structural protein, N-cadherin, as a marker of intercalated disks of myocytes, which indicates a transverse-oriented localization of Cx43, and calculated colocalization of Cx43 with N-cadherin. Cardiac N-cadherin is an integral part of the intercalated disc junction, essential for the adherens junctions in myocyte, Cx43 delivery to cell-cell contact, and thus the establishment of Cx43 intercellular channels.41 Conditional deletion of this important adherens junctional protein in the adult mouse heart leads to a complete dissolution of the intercalated disc structure and a significant decrease in the Cx43 expression. In that mouse model, N-cadherin deletion results in dilated cardiomyopathy, conduction slowing and higher conduction anisotropy and thus spontaneous ventricular tachycardia and arrhythmic death.42 The intracellular coassembly of connexins and cadherins is required for gap junction and adherens junction formation, a process that likely underlies the intimate association between electrical gap junction and mechanical adherens junction formation.43 Our results obtained by co-labeling Cx43 with N-cadherin (Figure 4) show that some lateralized Cx43 cannot be a part of functional gap junctions. These Cx43 are located outside of intercalated discs on the lateral myocyte membrane or within the myocytes, perhaps representing a trafficking of Cx43.25 It is difficult to determine whether these immunolabelled structures represent functional gap junctions or gap junction subunits, disassembled by lateralization, or internalization. Whatever the case, the consequence of such an alteration could be a profound change in uniform anisotropy with potential to give rise to arrhythmogenicity.
Cx43 is a phosphoprotein, with multiple phosphorylation sites for both serine/threonine, as well as for tyrosine phosphorylation.44 It has been shown that Cx43 is differentially phosphorylated at a dozen or more serine residues throughout its life cycle.44 The regulation of Cx43 by phosphorylation is a profoundly complex subject, with some sites being positive regulators, leading to increased conductance, whereas others negatively regulate or close the channel. Cx43 phosphorylation regulates its properties including assembly, trafficking, turnover, electrical and metabolic coupling.45 Factors and conditions altering the phosphorylation pattern of Cx43 can also alter its properties and, by extension, affect heart function. In the present study, we observed the re-distribution of phosphorylated and denphosphorylated Cx43 isoforms: higher-molecular-weight and higher phosphorylated (P1 and P2) Cx43 isoforms were downregulated by ~23%, whereas the lower-molecular-weight and dephosphorylated (P0) isoform was upregulated by ~35% (Figures 5).
Cx43 lateralization around cardiac myocytes was reported in human dilated46 and hypertrophic40 cardiomyopathies, both of which were associated with fibrosis. Lateralization of Cx43 is believed to be associated with non-functional and/or de-phosphorylated states of Cx43. However, there is no direct evidence that these Cx43 proteins are non-functional. At this point, it is important to notice the study by Wiegerinck et al. on the explanted hearts of patients with end-stage of HF.47 Using the rotigaptide, a compound belonging to a group of antiarrhythmic substances derived from AAP10,48 authors obtained an increase in ventricular conduction on the epicardium and decrease in the percentage of sites with conduction slowing throughout the mapped area. The mechanism by which rotigaptide improves gap junctional conductance is probably related to phosphorylation of Cx43 at Ser297 and at the PKC-sensitive site Ser368.49 Treatment with rotigaptide may cause phosphorylation of the dephosphorylated Cx43 and therefore increase conduction in hearts of patients with HF.47 Despite these observations as well as our results, further studies must be done to directly determine to what extent these changes in distribution and phosphorylation contributed to altered conduction and arrhythmogenesis.
Interstitial fibrosis may promote the basis for conduction block and reentry by introducing impedance mismatches and altering the path and safety factor of propagation.47 Strands of myocardium separated from each other by interstitial fibrosis along the longitudinal axis of the myocytes (Figure 6A) may form tissue discontinuities, which results in higher conduction anisotropy and a decrease in conduction safety as observed in failing hearts (Figure 8). Moreover, Cx43 lateralization may be influenced by the remodeling of the extracellular matrix. The presence of interstitial fibrosis has been documented in the ventricles of patients with ischemic as well as dilated cardiomyopathies and is thought to contribute significantly to conduction abnormalities and arrhythmias in humans.
Clinical relevance and prospective
Our study provides an integrative investigation of arrhythmogenic substrate in end-stage HF due to non-ischemic cardiomyopathy in humans. We show for the first time in the same human hearts a relation among multiple levels of EP signaling: from protein expression and phosphorylation, protein-protein colocalization, to transmural conduction and repolarization. Numerous bits and pieces of often contradictory findings have been presented mainly from animal models of HF. Here we present evidence in support of some previous animal and human findings at the cellular and molecular levels, and contrary to some other finings in animal models. We show that conduction disorder is a critical component of arrhythmogenic substrate in patients with non-ischemic cardiomyopathy. We demonstrate that conduction abnormalities lead to nonuniform propagation discontinuities and reentry conditioned by structural disorder. Moreover, we show that Cx43 downregulation, dephosphorylation and lateralization are the likely causes of such conduction abnormalities.
Supplementary Material
Acknowledgments
The authors thank Ai-Li Cai for technical support.
Funding Sources: This work was supported by NIH R01 grants HL085369, HL067322.
Non-standard Abbreviations and Acronyms
- HF
heart failure
- EP
electrophysiology
- M-cells
midmyocardial cells with prolonged repolarization
- LV
left ventricle
- CL
cycle length
- AP
action potential
- APD
action potential duration
- OAP
optical action potential
Footnotes
Journal Subject Codes: [10] Cardio-renal physiology/ pathophysiology; [148] Heart failure - basic studies; [110] Congestive Heart Failure; [155] Physiological and pathological control of gene expression; [37] CV surgery: transplantation, ventricular assistance, cardiomyopathy.
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soltysinska E, Olesen SP, Christ T, Wettwer E, Varro A, Grunnet M, Jespersen T. Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflugers Arch. 2009;459:11–23. doi: 10.1007/s00424-009-0718-3. [DOI] [PubMed] [Google Scholar]
- 2.Nass RD, Aiba T, Tomaselli GF, Akar FG. Mechanisms of disease: ion channel remodeling in the failing ventricle. Nat Clin Pract Cardiovasc Med. 2008;5:196–207. doi: 10.1038/ncpcardio1130. [DOI] [PubMed] [Google Scholar]
- 3.Lou Q, Fedorov VV, Glukhov AV, Moazami N, Fast VG, Efimov IR. Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure. Circulation. 2011;123:1881–1890. doi: 10.1161/CIRCULATIONAHA.110.989707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 5.Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 6.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Fractionated electrograms in dilated cardiomyopathy: origin and relation to abnormal conduction. J Am Coll Cardiol. 1996;27:1071–1078. doi: 10.1016/0735-1097(95)00612-5. [DOI] [PubMed] [Google Scholar]
- 7.Unverferth DV, Baker PB, Swift SE, Chaffee R, Fetters JK, Uretsky BF, Thompson ME, Leier CV. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. Am J Cardiol. 1986;57:816–820. doi: 10.1016/0002-9149(86)90620-x. [DOI] [PubMed] [Google Scholar]
- 8.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 9.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–645. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 10.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–1770. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 11.Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res. 2010;106:981–991. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taggart P, Sutton PM, Opthof T, Coronel R, Trimlett R, Pugsley W, Kallis P. Transmural repolarisation in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res. 2001;50:454–462. doi: 10.1016/s0008-6363(01)00223-1. [DOI] [PubMed] [Google Scholar]
- 13.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KP, Walker R, Urie P, Ershler PR, Lux RL, Karwandee SV. Myocardial electrical propagation in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;92:122–140. doi: 10.1172/JCI116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda N, Zipes DP, Wu J. Functional and transmural modulation of M cell behavior in canine ventricular wall. Am J Physiol Heart Circ Physiol. 2004;287:H2569–2575. doi: 10.1152/ajpheart.00526.2004. [DOI] [PubMed] [Google Scholar]
- 16.Voss F, Opthof T, Marker J, Bauer A, Katus HA, Becker R. There is no transmural heterogeneity in an index of action potential duration in the canine left ventricle. Heart Rhythm. 2009;6:1028–1034. doi: 10.1016/j.hrthm.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Fedorov VV, Glukhov AV, Chang R, Kostecki G, Aferol H, Hucker WJ, Wuskell JP, Loew LM, Schuessler RB, Moazami N, Efimov IR. Optical mapping of the isolated coronary-perfused human sinus node. J Am Coll Cardiol. 2010;56:1386–1394. doi: 10.1016/j.jacc.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG, Efimov IR. Effects of KATP channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol. 2011;51:215–225. doi: 10.1016/j.yjmcc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedorov VV, Glukhov AV, Sudharshan S, Egorov Y, Rosenshtraukh LV, Efimov IR. Electrophysiological mechanisms of antiarrhythmic protection during hypothermia in winter hibernating versus nonhibernating mammals. Heart Rhythm. 2008;5:1587–1596. doi: 10.1016/j.hrthm.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorov VV, Schuessler RB, Hemphill M, Ambrosi CM, Chang R, Voloshina AS, Brown K, Hucker WJ, Efimov IR. Structural and Functional Evidence for Discrete Exit Pathways That Connect the Canine Sinoatrial Node and Atria. Circ Res. 2009;104:915–923. doi: 10.1161/CIRCRESAHA.108.193193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96:54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- 22.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 23.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res. 2008;77:757–765. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, Bauer EP, Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- 25.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293:H2024–2038. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brugada J, Boersma L, Kirchhof CJ, Heynen VV, Allessie MA. Reentrant excitation around a fixed obstacle in uniform anisotropic ventricular myocardium. Circulation. 1991;84:1296–1306. doi: 10.1161/01.cir.84.3.1296. [DOI] [PubMed] [Google Scholar]
- 29.Hombach V. Electrocardiogram of the failing heart. Card Electrophysiol Rev. 2002;6:209–214. doi: 10.1023/a:1016316706195. [DOI] [PubMed] [Google Scholar]
- 30.Mancini DM, Wong KL, Simson MB. Prognostic value of an abnormal signal-averaged electrocardiogram in patients with nonischemic congestive cardiomyopathy. Circulation. 1993;87:1083–1092. doi: 10.1161/01.cir.87.4.1083. [DOI] [PubMed] [Google Scholar]
- 31.Brugada J, Mont L, Boersma L, Kirchhof C, Allessie MA. Differential effects of heptanol, potassium, and tetrodotoxin on reentrant ventricular tachycardia around a fixed obstacle in anisotropic myocardium. Circulation. 1991;84:1307–1318. doi: 10.1161/01.cir.84.3.1307. [DOI] [PubMed] [Google Scholar]
- 32.Delgado C, Steinhaus B, Delmar M, Chialvo DR, Jalife J. Directional differences in excitability and margin of safety for propagation in sheep ventricular epicardial muscle. Circ Res. 1990;67:97–110. doi: 10.1161/01.res.67.1.97. [DOI] [PubMed] [Google Scholar]
- 33.Delmar M, Michaels DC, Johnson T, Jalife J. Effects of increasing intercellular resistance on transverse and longitudinal propagation in sheep epicardial muscle. Circ Res. 1987;60:780–785. doi: 10.1161/01.res.60.5.780. [DOI] [PubMed] [Google Scholar]
- 34.Efimov IR, Ermentrout B, Huang DT, Salama G. Activation and repolarization patterns are governed by different structural characteristics of ventricular myocardium: experimental study with voltage-sensitive dyes and numerical simulations. J Cardiovasc Electrophysiol. 1996;7:512–530. doi: 10.1111/j.1540-8167.1996.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 35.Conrath CE, Wilders R, Coronel R, de Bakker JM, Taggart P, de Groot JR, Opthof T. Intercellular coupling through gap junctions masks M cells in the human heart. Cardiovasc Res. 2004;62:407–414. doi: 10.1016/j.cardiores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Efimov IR, Huang DT, Rendt JM, Salama G. Optical mapping of repolarization and refractoriness from intact hearts. Circulation. 1994;90:1469–1480. doi: 10.1161/01.cir.90.3.1469. [DOI] [PubMed] [Google Scholar]
- 37.Kaab S, Nuss HB, Chiamvimonvat N, O'Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 38.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 40.Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 41.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD, Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97:474–481. doi: 10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- 43.Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 44.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Veen TA, van Rijen HV, Jongsma HJ. Physiology of cardiovascular gap junctions. Adv Cardiol. 2006;42:18–40. doi: 10.1159/000092560. [DOI] [PubMed] [Google Scholar]
- 46.Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano EJ, Kubo S, Hayashi Y, Itoh H, Yokoyama M. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:865–870. doi: 10.1046/j.1540-8167.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- 47.Wiegerinck RF, de Bakker JM, Opthof T, de Jonge N, Kirkels H, Wilms-Schopman FJ, Coronel R. The effect of enhanced gap junctional conductance on ventricular conduction in explanted hearts from patients with heart failure. Basic Res Cardiol. 2009;104:321–332. doi: 10.1007/s00395-008-0771-7. [DOI] [PubMed] [Google Scholar]
- 48.Muller A, Schaefer T, Linke W, Tudyka T, Gottwald M, Klaus W, Dhein S. Actions of the antiarrhythmic peptide AAP10 on intercellular coupling. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:76–82. doi: 10.1007/pl00005031. [DOI] [PubMed] [Google Scholar]
- 49.Dhein S, Larsen BD, Petersen JS, Mohr FW. Effects of the new antiarrhythmic peptide ZP123 on epicardial activation and repolarization pattern. Cell Commun Adhes. 2003;10:371–378. doi: 10.1080/cac.10.4-6.371.378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.