Abstract
Regenerative medicine approaches offer attractive alternatives to standard vascular reconstruction; however, the biomaterials to be used must have optimal biochemical and mechanical properties. To evaluate the effects of biomaterial properties on vascular cells, heparinized poly(ethylene glycol) (PEG)-based hydrogels of three different moduli, 13.7 kPa, 5.2 kPa, and 0.3 kPa, containing fibronectin and growth factor were utilized to support the growth of three human vascular cell types. The cell types exhibited differences in attachment, proliferation, and gene expression profiles associated with the hydrogel modulus. Human vascular smooth muscle cells demonstrated preferential attachment on the highest modulus hydrogel, adventitial fibroblasts demonstrated preferential growth on the highest modulus hydrogel, and human umbilical vein endothelial cells demonstrated preferential growth on the lowest modulus hydrogel investigated. Our studies suggest that the growth of multiple vascular cell types can be supported by PEG hydrogels and that different populations can be controlled by altering the mechanical properties of biomaterials.
Keywords: PEG hydrogels, vascular cells, modulus, cell proliferation, gene expression
Introduction
Vascular reconstructive surgery has become routine for treating cardiovascular disease, which is a leading cause of death in the industrialized world. Autologous arteries or veins are frequently used for these procedures; however, in some cases even autografts fail at rates approaching 50% in the first few years following surgery. 1,2 In addition, suitable grafts are often unavailable due to a patient’s medical condition. Despite plentiful research in this area, there continues to be a critical need for alternative methods, and the development of biomaterials that could improve the useful lifetime of autologous grafts or that could serve as tissue engineering scaffolds for graft production has great appeal. The majority of biomaterials approaches to date, which have utilized mainly synthetic polymeric conduits, have proven inadequate to yield functional cardiovascular tissue, particularly in small (< 6mm) and medium-sized (< 18 mm) blood vessels.3-5 Accordingly, enhanced cellularization of synthetic or autologous implants would improve results, but the design of materials to encourage appropriate cell growth and signaling is hampered by an incomplete understanding of the effects of material properties on cell functions.
Blood vessels comprise multiple layers populated with three principal cell types. It is important that engineered and instructive materials enable all three cell types to grow and maintain their phenotype. Vascular endothelial cells (vECs), which populate the tunica intima, provide a barrier between the blood and vessel wall and mediate many physiological processes by expressing proteins that act as vasodilators, vasoconstrictors, and growth factors.6 Vascular smooth muscle cells (vSMCs), which populate the tunica media, are responsible for the contraction and dilation of the blood vessel wall to regulate blood pressure and flow.7 Adventitial fibroblasts (vAFs), which populate the tunica adventitia, produce extracellular matrix (ECM) and are critical contributors to the structural integrity, growth, and remodeling of the blood vessel.8-10 Desirable materials that could be incorporated into engineered vessels or combined with autologous implants should have the ability to support and control phenotypes in these three cell types.
The development of a family of poly(ethylene glycol) (PEG)-based hydrogels that could support the growth of vascular cells would provide attractive alternatives either on their own or in combination with synthetic polymeric conduits or autologous tissues. PEG has been widely used and characterized as a biomaterial for tissue engineering applications11-19 in large part because of its inherent biocompatibility, resistance to protein adsorption, and the fact that it functions as a “blank slate” onto which functionalities can be conferred.20,21 Hydrogels based on PEG are unique in that they offer independent control over initial mechanical properties and the inclusion of adhesion molecules.22 In addition to adhesion peptides, 13,15,20,23,24 heparin and growth factors can be included in the hydrogel formulation to maximize both cell attachment and proliferation.25-31 We have previously reported strategies for manipulating gelation and degradation of heparin-containing, PEG-based hydrogels that are injectable.32,33 Through manipulation of reaction conditions, gels can be formed sufficiently rapidly34 and simply for administration during a clinical procedure. Importantly, the gelation methods also provide access to a range of moduli of relevance in the engineering of soft tissues.32,34,35
Hydrogel modulus is an important consideration because matrix stiffness is known to affect cell adhesion, proliferation, and differentiation.3,34,36-40 In vivo, vECs are constantly exposed to changing mechanical forces, including transmural pressure and shear stress due to blood flow, and shear stress and/or mechanical strain can modulate the expression of genes that are critical in endothelial physiology.6 Similarly, smooth muscle is subjected to dynamic mechanical stresses under normal in vivo conditions,41 and it is well known that mechanical stress can affect the phenotype of smooth muscle cells.7,42,43 Several studies have shown that hydrogel modulus can have differential effects on gene regulation in microvascular endothelial cells,44 on smooth muscle cell cytoskeletal assembly,22 metabolic activity,45 proliferation, and differentiation,3 as well as on adventitial fibroblast adhesion and proliferation.34 Other studies have shown effects of hydrogel modulus on cell proliferation in dermal fibroblasts,36 neural stem cells,37-39 and chondrocytes.40 Thus, understanding changes in gene expression, in addition to changes in cell proliferation, in response to biopolymers with different mechanical properties can provide guidance in the choice of materials for tissue engineering and regenerative medicine applications, as well as strategies to control the behavior of complex multicellular structures. The current study was thus undertaken to assess the differential effects of hydrogel modulus on cell proliferation, morphology, and gene expression of vascular endothelial, smooth muscle, and fibroblast cells, to provide more detailed information relevant to the engineering of therapeutic biomaterials for treating cardiovascular disease.
Materials and Methods
Maleimide-functionalized LMWH synthesis
Low-molecular weight heparin (LMWH, Mw 3,000 g/mol, Celsus, Cincinnati, OH) was reacted with N-(2-aminoethyl) maleimide trifluoroacetate salt (AEM, Sigma-Aldrich, St. Louis, MO) as previously shown.32 Briefly, LMWH was dissolved to a concentration of 10mg/ml in 0.1M MES buffer pH 6.0. 1-Hydroxybenzotriazole hydrate (HOBT, Sigma-Aldrich, 9eq LMWH), AEM (5 eq to LMWH) and N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC HCL, Acros, Morris Plains, NJ, 7 eq to LMWH) were added to the LMWH solution and stirred at room temperature for 5hrs. The completed reaction was exhaustively dialyzed using 1,000 MWCO membranes (SpectraPor, Rancho Dominguez, CA) against 1M NaCl and 18mΩ de-ionized water. The final product was lyophilized to give a white solid (80% yield). 1H NMR characterization of the product indicated a degree of maleimide functionalization of approximately 1.5 per LMWH chain. 1H NMR (400 MHz, D2O): δ = 6.81 (CO-CH=CH-CO, m), δ = 3.90-3.50 (CH2-CH2-NH, m), δ = 5.43-3.38 (heparin, m).
PEG–LMWH hydrogel formation
The heparin-containing hydrogels were made according to previously reported methods.34 Four-arm maleimide-functionalized poly(ethylene glycol) (PEG, f = 4, Mn 10,000 g/mol, JenKem Technology USA, Chicago, IL), and four-arm, thiol-functionalized PEG (f = 4, Mn 10,000 g/mol, Creative PEGWorks, Winston Salem, NC) were employed to form gel networks with maleimide-functionalized heparin (f = 1.5, average Mw 3,000 g/mol). Fibronectin (FN, BD Biosciences, Franklin Lakes, NJ) was also incorporated into the hydrogels via reaction with maleimide-functionalized polymers to provide cell-adhesive materials. To yield hydrogels that support growth of human aortic adventitial fibroblasts (AoAFs; Lonza, Walkersville, MD), human aortic vascular smooth muscle cells (T/G HA-vSMCs; ATCC, Manassas, VA), and mouse fibroblast cells (NIH3T3; ATCC), bFGF (60 nM; Peprotech, Rocky Hill, NJ) was added during gel formation. VEGF (60 nM; Genentech, San Francisco, CA) was added during gel formation to yield hydrogels to support the growth of human umbilical vein endothelial cells (HUVECs; Lonza). First, PEG-(maleimide)4 was mixed with FN (100 nM) in buffer (10mM sodium citrate, 150mM NaCl, pH = 4.5) for attachment of the bioactive groups to the PEG prior to hydrogel formation. PEG-(SH)4 was mixed with maleimide-functionalized LMWH (0.1 mM) and growth factor in buffer. Each component was sterilized by centrifugation through a 0.2 μm filter. These two solutions were then mixed; changes in viscoelasticity were immediately apparent. Resulting hydrogels were stored at 4°C overnight prior to further use and swelled 30 min in cell culture medium at 37°C before cell culture experiments. Hydrogels with three different moduli were prepared by altering the PEG concentration (3 wt%, 4 wt%, and 8 wt%), while keeping the volume of the hydrogels constant. Hydrogels of a concentration of 3wt% comprised 2mM PEG-(maleimide)4 and 1mM PEG-(SH)4; 4wt% hydrogels comprised 2mM PEG-(maleimide)4 and 2mM PEG-(SH)4; and 8wt% hydrogels comprised 4mM PEG-(maleimide)4 and 4mM PEG-(SH)4. 3,4 and 8wt% were selected to give a range of moduli for the cell studies relevant to tissue engineering.35,46-48
Rheological characterization of hydrogels
Hydrogel mechanical properties were measured via bulk oscillatory rheology on hydrogels formed in situ via co-injection of maleimide- and thiol-functionalized PEGs onto the Peltier plate of the rheometer. Experiments were conducted using an AR-2000 rheometer (TA Instruments, New Castle, DE) at 25 °C within the linear elastic response of the material (constant strain of 1.0%) and at a frequency (6 rad s-1). Strain-sweeps of the materials indicate that yielding occurred at strains > 20%, therefore by maintaining a maximum amplitude well below this material yielding strain, the torsional properties of the hydrogel can be monitored without disrupting the chemically crosslinked structure. A 25-mm diameter stainless steel upper parallel plate geometry was used in all experiments. Final moduli were recorded after the elastic modulus ceased to increase (~2hrs).
Cell culture
Standard media and culture conditions were employed to allow comparison to work in other labs; however, we note that clinical application may require use of a single medium for all cells. Each of the cell types utilized may be grown in alternative media comprising a 1:1:1 mixture of the standard media described here. AoAFs were cultured using Stromal Cell Growth Medium (Lonza). T/G HA-vSMCs were cultured in Kaighn’s modification of Ham’s F12 medium (F12K) containing 2 mM L-glutamine, 2.5 g/L sodium bicarbonate (Mediatech, Manassas, VA), 10 mM TES (Sigma-Aldrich), 10 mM HEPES (Sigma-Aldrich), 0.05 mg/mL ascorbic acid (Sigma-Aldrich), 0.03 mg/mL endothelial cell growth supplement (ECGS; Intracel, Frederick, MD), 10 ng/mL sodium selenite (Sigma-Aldrich), 0.01 mg/mL transferrin (BD Biosciences), 0.01 mg/mL insulin (Sigma-Aldrich), 10% fetal bovine serum (Mediatech), 100 U/mL penicillin, and 100 μg/mL streptomycin (Mediatech). HUVECs were cultured in F12K containing 2 mM L-gluatmine, 2.5 g/L sodium bicarbonate, 0.1 mg/mL heparin (Sigma-Aldrich), 0.03 mg/mL ECGS, 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. NIH3T3 mouse fibroblast cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose (Mediatech), 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. All the cells were cultured at 37°C at 5% CO2/95% air.
Microscopy
AoAFs, HUVECs, and T/G HA-vSMCs were seeded on TCPS or on the surface of 70 μL of hydrogel in 96-well plates (0.3 cm2 surface area) at a density of 10,000 cells per well. Live cells were viewed using a Leica Fluovert inverted microscope with Hoffman Modulation Contrast at 10× magnification. Cells were imaged using a Spot RT Slider camera (Diagnostic Instruments, Sterling Heights, MI) and Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Cell Attachment and Proliferation Assays
AoAFs, HUVECs, T/G HA-vSMCs, and control NIH3T3 cells were seeded on the surface of 70 μL of gel in 96-well plates at a density of 10,000 cells per well in 100 μL of medium and incubated at 37°C. Cell attachment and proliferation were assayed using Cell Titer Blue (Promega, Madison, WI), which provides a fluorescence measurement of the overall reductive capability of the cells. Two hours after seeding, 20 μL of Cell Titer Blue reagent was added to the medium of each well and incubated at 37°C for 2 hours. The medium containing the reagent was replaced with fresh medium and analyzed in triplicate using a PerkinElmer VICTOR X4 2030 Multilabel Plate Reader (PerkinElmer, Waltham, MA) at excitation/emission wavelengths of 550nm/590nm. The background fluorescence of the gel was measured prior to addition of Cell Titer Blue and this value was subtracted from the final fluorescence. Data acquired at the 2 hour time point were used to indicate the number of attached cells. Measurements were repeated after 3 days (72 hours) in culture, and the change in signal used as an indicator of increased cell number over time in culture and cell proliferative activity.
Gene expression profiling
AoAFs, HUVECs, and T/G HA-vSMCs were seeded on the surface of 200 μL of gel or directly onto the tissue culture polystyrene (TCPS) in 24-well plates (2 cm2 surface area). After 72 hours in culture, the cells were trypsinized and RNA was isolated using RNeasy kits according to the manufacturer’s protocol (Qiagen, Valencia, CA). Each sample contained cells pooled from eight separate wells to ensure sufficient RNA for gene expression analysis. Pooled designs are often used in array experiments out of necessity.49-51 Studies have shown that expression measurement from RNA pools are similar to averages of individuals that comprise the pool and that pooling dramatically improves accuracy for very small designs.50,52 RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). cDNA synthesis and Real-Time PCR were performed according to protocols from the manufacturer of the RT-PCR Arrays used (SABiosciences, Frederick, MD). Briefly, 250 ng of RNA was reverse transcribed using an RT2 First Strand Kit and the resulting cDNA sample was mixed with RT2 SYBR Green/ROX qPCR Master Mix and loaded into a Human Angiogenesis 384-well PCR Array containing optimized primers (SABiosciences); 89 genes and 7 control wells were analyzed in total per sample. Real-Time PCR Detection was performed on an ABI7900HT and the thermal cycling profile was: 95°C for 10 min., followed by 40 cycles of 95°C for 15 sec. and 60°C for 1 min. Each cell line was analyzed separately. Primer efficiencies were determined according to the method proposed by Rutledge and Stewart.53 Briefly, the normalized fluorescence values for each reaction were fit to a sigmoidal curve via linear regression of cycle efficiency (LRE). The y-intercept of the best fit linear regression line (the Emax) was used as the reaction-specific primer efficiency. Overall primer efficiency for each primer set was calculated as the average reaction-specific efficiency calculated across all reactions using the same primers. Reactions with no appreciable amplification and reactions where the calculated reaction-specific efficiency varied more than 25% from the average were excluded from calculations. Median overall primer efficiencies were used to calculate relative expression ratios using the Pfaffl method54 with one TCPS sample selected at random as the control and HPRT1 as the reference gene. Ratios were transformed (log base 2) and the average and standard deviation were determined for cells grown on TCPS (n = 5 pooled samples comprising 40 cultures). For pooled samples grown on hydrogels (n = 1 comprising 8 cultures for each modulus), any transformed ratio more than two standard deviations above or below the TCPS average was differentially expressed at the 95% confidence level and was flagged as being significantly different.55 A Ct value greater than 35 represents single molecule template detection;56 therefore, a Ct value greater than 35 was considered noise and genes for which all Ct values were greater than 35 were excluded. Heat maps were created using Tigr MultiExperiment Viewer (TMeV). Functionally related groups of significant genes were determined using the National Institute of Allergy and Infectious Diseases’ Database for Annotation, Visualization and Integrated Discovery (DAVID) using the list of all 89 genes on the array as the background.
Results and Discussion
Hydrogel design and characterization
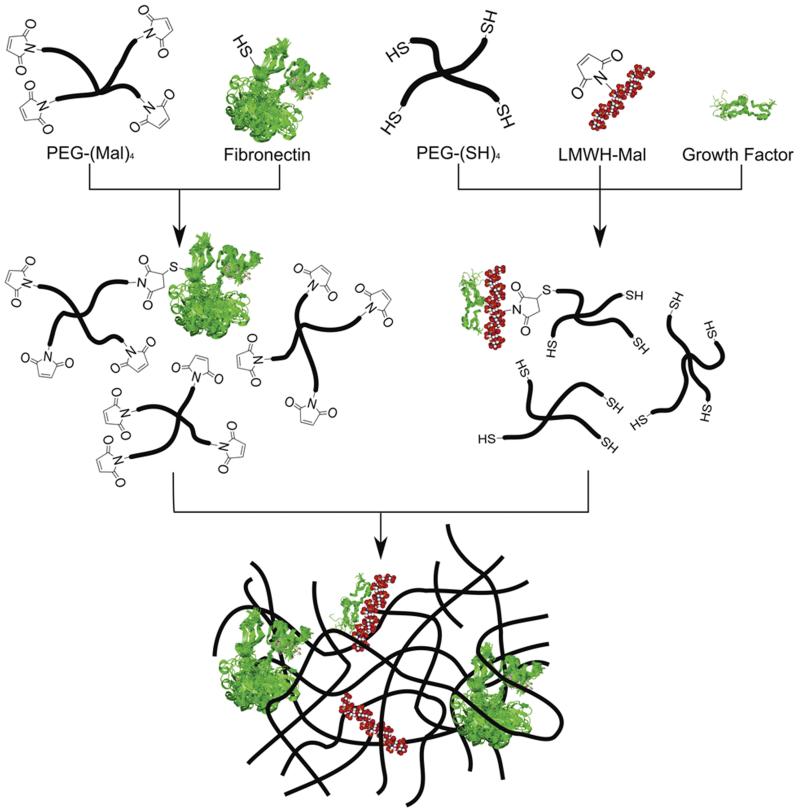
In the current study, we expand on our previous work involving the characterization of the growth of cardiovascular cells on PEG-based hydrogels.34 PEG-based hydrogels were chosen because of their hydrophilicity, biocompatibility, and chemical flexibility. The hydrogels were modified to contain low molecular weight heparin, which can sequester growth factors and modify the mechanical properties of the hydrogel networks through noncovalent interactions.57-60 The mechanical properties of heparinized hydrogels can also be readily manipulated via variations in polymer architecture, with gels of a variety of compositions exhibiting useful mechanical properties.33,61 Four-arm PEGs with termini carrying either maleimide or thiol were chosen to allow rapid crosslinking of the matrix as well as incorporation of LMWH. The LMWH was functionalized with maleimide at low degrees of substitution34 and then pre-reacted with thiol-functionalized four-arm star PEG (10,000 g mol−1) (Figure 1). Growth factor was also added to this mixture depending on the cell type (with AoAF, T/G HA-vSMC, and NIH3T3 cells receiving bFGF and HUVEC cells receiving VEGF). bFGF and VEGF have been shown to induce angiogenesis and wound healing in animal models and in clinical trials.62-67 Fibronectin, which promotes cell adhesion68 and was indicated to preferentially promote cell adhesion over RGD-containing peptides in our previous work,34 contains a free cysteine residue available to covalently react with the PEG-(maleimide)4,69,70 and was pre-reacted with maleimide-functionalized four-arm star PEG (10,000 g mol−1) to preclude significant diffusion of fibronectin within the gels. Spectrophotometric evaluation of solutions used to wash the hydrogels after formation indicated that essentially all of the fibronectin and the LMWH used during hydrogel synthesis was retained in the gels after the wash. The modulus of the hydrogels was altered by varying the total amount of PEG and was determined via oscillatory rheometry.
Figure 1.
Schematic of hydrogel formation.
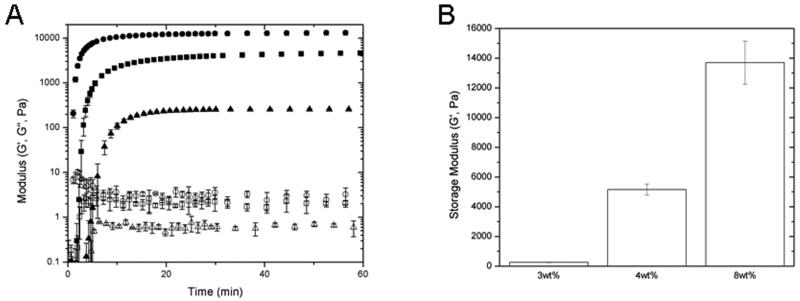
The storage modulus (G’) and the loss modulus (G”) were measured as a function of time at constant frequency (ω = 6 rad s−1) to determine approximate times to gelation as well as to indicate final moduli (Figure 2). Figure 2A displays the evolution of the storage (G’) and loss (G”) modulus versus time. These materials exhibit fast reaction rates forming highly elastic networks with minimal viscous components as soon as 204, 66, 48 seconds for the 3, 4 and 8 wt% gels respectively. The maleimide-thiol reaction rate is pH-dependent and proceeds at fast rates near physiological pH. Higher pH values resulted in spontaneous hydrogel formation upon mixing, resulting in non-uniform gels and sporadic final modulus measurements; therefore, in these experiments the onset of gelation was retarded by using pH 4.5 to allow enough time for homogeneous mixing of solutions. The average moduli for the 3, 4 and 8 wt% hydrogels were determined to be 0.3, 5.2, and 13.7 kPa (Figure 2B). This range of moduli was chosen because of its relevance for in situ injection of materials as soft tissue additives or substitutes71 and owing to their demonstrated impact on microvascular cells.44 As expected due to their relatively low concentrations, the incorporation of LMWH and/or fibronectin exhibited no measureable changes in gel moduli compared to gels composed of PEG alone (as assessed via comparison of the rheology of gels containing and lacking LMWH and/or fibronectin; data not shown), and non-specific protein adsorption to the PEG component of these hydrogels is minimal.72 After swelling in medium, there were no significant differences in final gel volumes across the moduli (data not shown); therefore, LMWH and fibronectin concentrations were held constant within the optimal range determined in previous studies.34,73 As a result, specific adsorption of heparin- or fibronectin-binding molecules should be consistent across all hydrogels.57 Thus, these materials provide a relatively consistent biochemical background and enable assessments of variation of cell behavior as a function of modulus.
Figure 2.
Oscillatory rheology results illustrating the formation kinetics of hydrogels prepared with star PEGs. (A) Modulus (log scale) is given as a function of gelation time for 8wt% (●), 4wt% (■), 3wt% (▲) gels represented with closed symbols for storage modulus (G’) and open for loss modulus (G”). (B) The final moduli of the 8 wt%, 4 wt%, and 3 wt% hydrogels were determined to be 13.7 kPa, 5.2 kPa, and 0.3 kPa, respectively. Plots are an average of three repeats with standard deviation shown by error bars.
Morphology of cells grown on hydrogels
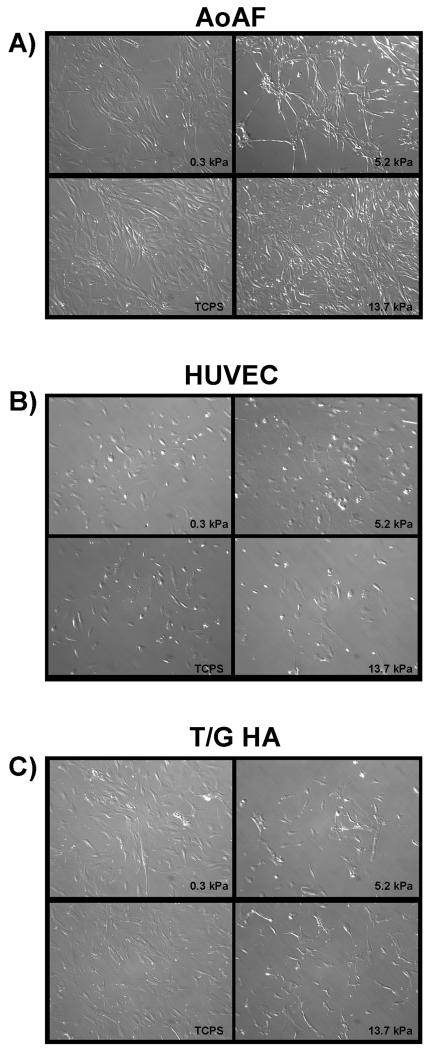
To study vascular cell responses to growth on hydrogels of different moduli, three established human cell lines were chosen to represent the three cell types predominant in blood vessels: human aortic adventitial fibroblasts (AoAFs), human umbilical vein endothelial cells (HUVECs), and human aortic vascular smooth muscle cells (T/G HA-vSMCs). The morphology of each cell type was assessed on all three hydrogels and tissue culture polystyrene (TCPS) as a control. AoAF cells (Figure 3A) displayed typical fibroblast morphology with regions of extensively spread cytoplasm, while T/G HA-vSMCs (Figure 3C) were larger and had more processes. Conversely, HUVECs (Figure 3B) were more rounded and displayed the typical cobblestone appearance of endothelial cells in culture. None of the cell types displayed significant changes in cell area (p = 0.12 for AoAFs, p = 0.69 for HUVECs, p = 0.48 for T/G HA-vSMCs) or ellipticity (p = 0.29 for AoAFs, p = 0.40 for HUVECs, p = 0.60 for T/G HA-vSMCs) when cultured on hydrogels as opposed to TCPS (Supplementary Figure 1).
Figure 3.
Cell morphology of AoAF (A), HUVEC (B), and T/G HA-vSMC (C) was assessed on TCPS and on 13.7 kPa, 5.2 kPa, and 0.3 kPa PEG-based hydrogels.
Cell attachment to and proliferation on hydrogels
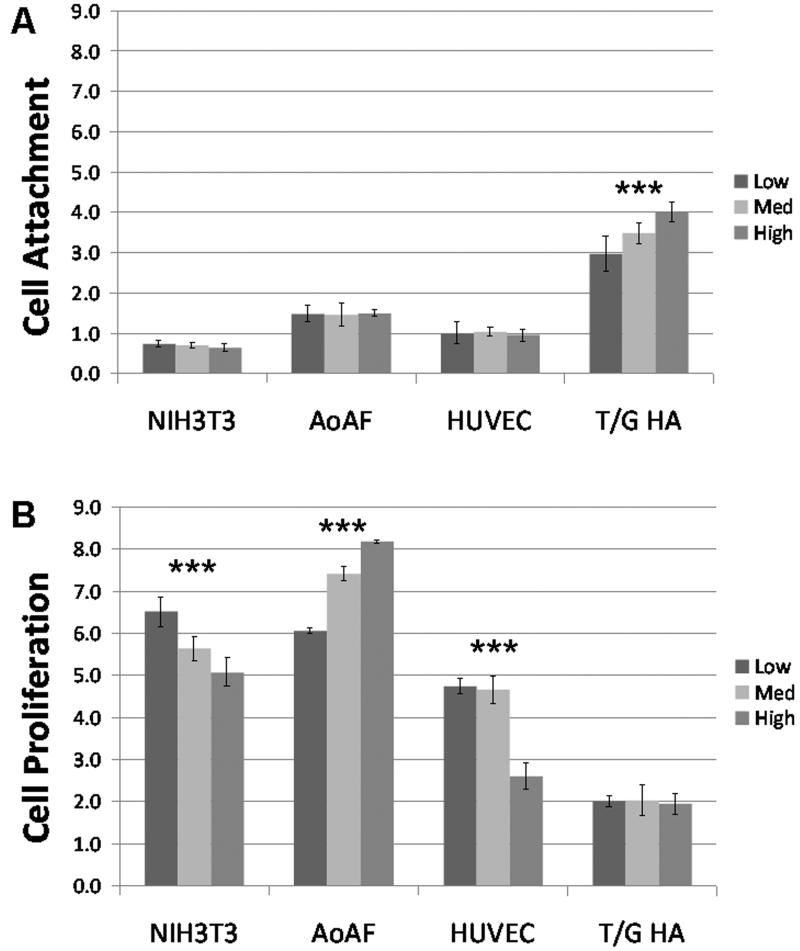
Cell attachment and proliferation were assessed using a Cell Titer Blue assay (Figure 4). NIH3T3 mouse fibroblast cells, which are a well-established cell line that has been grown on hydrogels in previous studies,68,74-77 were utilized as controls to validate the system. Cells were seeded at the same density on each modulus gel. Two hours after seeding (i.e., Day 0), the NIH3T3 cells had the same density on all three hydrogels, indicating that they adhered equally well on all hydrogels of this modulus range. By day 3, there was a significant increase in Cell Titer Blue fluorescence on all three moduli with the low modulus hydrogels showing the highest proliferation and proliferation decreasing as the modulus increased (p<0.01). These results are consistent with other studies in which NIH3T3 cells proliferated to a greater extent on lower modulus gels made of gelatin cross-linked with microbial transglutaminase77 or fibrin,78 and serve to validate our hydrogel system.
Figure 4.
Cell Titer Blue results for NIH3T3, AoAF, HUVEC, and T/G HA-vSMCs grown on heparinized PEG-based hydrogels containing fibronectin and growth factor. Mean fluorescence units (+ std dev; n = 4) are given to indicate number of viable cells attached during the first two hours of culture (A) and the change in cell number over time (B). Asterisks indicate statistically significant differences by One Way ANOVA (p<0.01).
Interestingly, cultures of human adventitial fibroblasts (AoAFs) demonstrated a proliferative response opposite to that exhibited by the NIH3T3 cells. AoAFs attached to the same degree on all three moduli but proliferated least on the hydrogel with the lowest modulus with increasing proliferation as modulus increased (p<0.01). These AoAF data are consistent with previous studies34,36 showing improved proliferation of several cell types on materials with higher modulus but serve to demonstrate that fibroblasts from different sources respond differently given the same substrata.
HUVECs, on the other hand, demonstrated similar proliferation on the low and medium modulus hydrogels but had decreased proliferation on the high modulus hydrogel (p<0.01), suggesting a non-linear response to changes in substrate stiffness. This behavior is consistent with previous studies in which HUVECs grown on peptide-based hydrogels RAD16-I (with an amino acid sequence of (RADA)4) or RAD16-II (with an amino acid sequence of (RARADADA)2) demonstrated an increased ability to form capillary-like networks as the hydrogel stiffness decreased from 735 Pa to 46 Pa.79 Intriguingly, a study of HUVEC on self-assembled β-sheet fibrillar peptide hydrogels showed significantly higher rates of proliferation on ligated (48.5 kPa) versus unligated (10.5 kPa) hydrogels. 80 Taken together, these observations demonstrate that the mechanochemical properties of hydrogels play important roles in modulating cell proliferation and suggest that the response of HUVECs to different environments may be complex and non-linear.
Finally, in our assessment of vSMCs on the PEG-LMWH hydrogels, T/G HA-vSMCs exhibited preferential attachment with increasing modulus based on Cell Titer Blue fluorescence on day 0 (p<0.01) but no apparent differences in proliferation between days 0 and 3. These results are similar to a previous study that found the proliferation of aortic SMCs in 3D aggregates did not depend significantly on the matrix stiffness (448-5804 Pa) of PEG-conjugated fibrinogen,22 although in a longer term study, aortic SMCs were suggested to proliferate to a greater extent on 423.9 kPa than 13.7 kPa PEG hydrogels after 7 days; this difference, however, was not statistically significant.3
Our results demonstrate that the proliferative response of cells to hydrogel stiffness appears to be cell-type specific and potentially non-linear; therefore, a range of moduli should be examined to determine optimal conditions for growth. Because of these differences, our PEG-based hydrogels offer a unique system in which different cell types could be localized into regions of hydrogels simply on the basis of spatial differences in hydrogel moduli.
Molecular expression analysis of cells grown on hydrogels
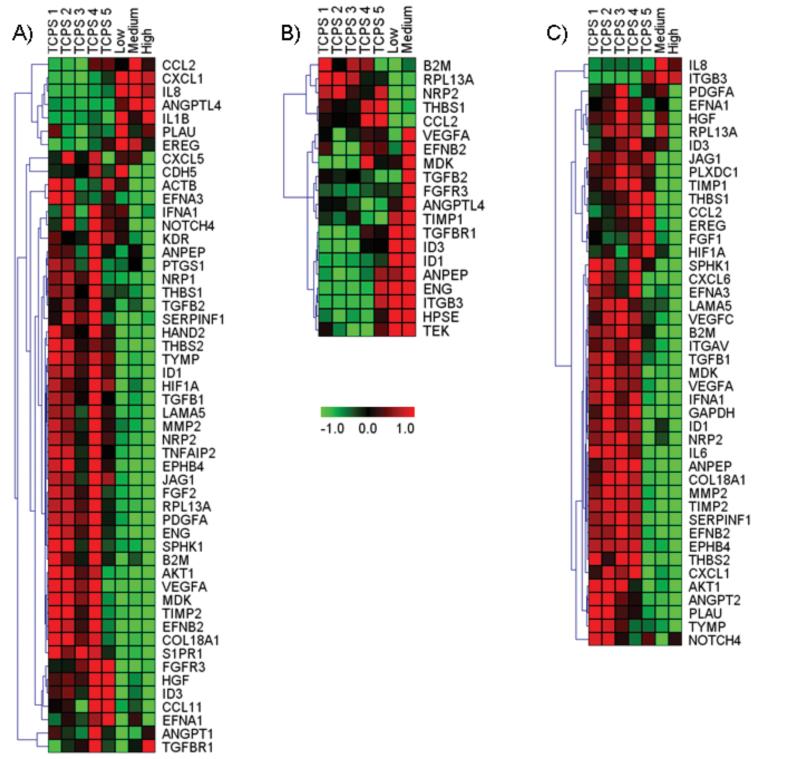
To assess the effects of hydrogel substrata on cultured cells in more detail, we assessed the levels of mRNA from 88 target genes associated with angiogenesis using real time qPCR (Figure 5, Supplementary Figure 2). Hierarchical cluster analyses of genes that were significantly altered (i.e., expression in hydrogel-cultured samples varied by more than two standard deviations from the mean levels determined for normative TCPS controls) are shown in Figure 5. Each element of the matrix represents the expression level of a gene in an individual sample with red and green reflecting increased and decreased expression, respectively. Overall, there were 63 target genes that were significantly altered in at least one condition. Of these, 9 targets were significantly altered across all three cell types. These included RPL13A, which is a ribosomal protein that is not essential for the canonical translation of mRNA to protein but is required for rRNA methylation and for translational silencing of specific mRNAs.81,82 RPL13A had decreased expression in all experimental samples measured indicating that culture on hydrogel substrata may have generally affected the control of translational activity in cells. Other targets that were down-regulated across all three cell types included B2M, NRP2, and THBS1. B2M is a serum protein that associates with major histocompatibility complex heavy chains on cell surfaces; NRP2 is a cell-surface receptor that binds semaphorins and VEGF-A;83 THBS1 is an important matricellular protein that inhibits angiogenesis and is involved in cell migration and tissue growth.84 All three of these gene products localize to cell surfaces or cell exteriors, suggesting a potential effect of hydrogel interaction with cell surfaces. The remaining 5 common targets were differentially affected depending on cell type. CCL2, which is also called MCP1, is a chemokine that is produced in the tunica adventitia during vascular inflammation and remodeling85 but also produced by vascular endothelial and smooth muscle cells under inflammatory and pro-atherogenic conditions.86 CCL2 was up-regulated in AoAF cells on the medium modulus gels but down-regulated in the HUVEC and TG/HA-vSMC preparations, suggesting that AoAFs may have adopted an inflammatory phenotype whereas the other cell types did not. The targets ANPEP, ID1, ID3, and VEGFA were all down-regulated in AoAF and TG/HA-vSMCs but up-regulated in HUVECs. ANPEP, also called CD13, is a cell surface aminopeptidase and a potent regulator of blood vessel formation; ANPEP is up-regulated in endothelial cells during angiogenesis in vivo.87 ID1 and ID 3 are HLH proteins that heterodimerize with transcription factors to inhibit their activity. They are well known mediators of endothelial cell activation in angiogenesis and are up-regulated by VEGFA stimulation.88 VEGFA is potent inducer of angiogenesis and stimulates endothelial cell proliferation, migration, and cell survival through activation of specific VEGF receptors.89 VEGF receptors have been identified on many non-endothelial cells and VEGF may act as an autocrine regulator of cell survival and function in diverse situations.89 Taken together, the results from these 4 genes suggest that the HUVECs adopted an angiogenic phenotype under the experimental conditions employed.
Figure 5.
Hierarchical cluster analysis of gene expression data. Genes whose expression was significantly different on hydrogels (low modulus = 0.3 kPa, medium modulus = 5.2 kPa, high modulus = 13.7 kPa) versus TCPS are shown for AoAF (A), HUVEC (B), and T/G HA-vSMCs (C) cells in heat maps. Columns represent each sample and rows represent each gene. Red and green in cells reflect high and low expression levels, respectively, as indicated in the scale bar (log2-transformed scale).
Within each cell type, there were also patterns of expression across the different hydrogel moduli. In the AoAF cells, 52 genes were identified all together. Growth on the low-modulus hydrogels resulted in the up-regulation of 7 and down-regulation of 29 targets. Culture on the medium-modulus hydrogels resulted in 6 genes being up-regulated and 33 down-regulated, and on the high-modulus hydrogels, 4 genes were up-regulated and 41 were down-regulated. As with the other cell types, there was significant overlap in the genes flagged across the three moduli. In particular, of the 8 total genes that were up-regulated, 4 were in common across all three moduli (ANGPTL4, CXCL1, IL1B, and IL8). One gene (EREG) was increased in both the low- and medium-modulus samples. The remaining three genes were up-regulated in only one modulus: the medium-modulus yielded CCL2 and the low-modulus samples yielded PLAU and CDH5 as being significantly increased. ANGPTL4 is involved in glucose and lipid metabolism, CXCL1, which is also called GROA, is a diffusible cytokine that attracts leukocytes to sites of inflammation.90 IL1B and IL8 are well known inflammatory mediators that are also potent effectors of cell proliferation and angiogenesis. CCL2 is another cytokine produced during inflammation,86 and PLAU is an enzyme involved in dissolution of blood clots. These data suggest that the AoAF cells may be initiating an inflammatory response when cultured on hydrogels with some slight differences associated with modulus; such an effect, if it could be temporally regulated, could be employed to improve healing around vascular grafts. There were 45 down-regulated genes in the AoAF samples. Of these, 24 were found in cultures grown on all three moduli (COL18A1, EFNB2, ENG, EPHB4, FGF2, FGFR3, HAND2, HGF, HIF1A, ID1, ID3, JAG1, LAMA5, MDK, NRP2, PDGFA, RPL13A, SERPINF1, SIPR1, TGFB1, TIMP2, THBS2, TNFAIP2, and TYMP), 8 were in common between medium- and high-modulus samples (ACTB, AKT1, CDH5, KDR, MMP2, NOTCH4, NRP1, and VEGFA), 3 were in common between low- and high-modulus samples (B2M, EFNA1, and TGFB2), and 10 appeared in cultures on only one of the moduli (ANGPT1, ANPEP, CCL11, CXCL5, EFNA3, IFNA1, PTGS1, SPHK1, TGFBR1, and THBS1). This complex pattern of expression provides substantial new information regarding the regulation of human AoAF cells, and the data are consistent with alterations to AoAF phenotype associated with culture on hydrogel of different modulus. Of particular note, CDH5 was found to be up-regulated in the AoAFs grown on low modulus hydrogels but down-regulated in the medium and high modulus cultures. CDH5 is a cadherin (cadherin5 or VE-cadherin) that was originally identified in vascular endothelial cells. Cadherins are a class of transmembrane cell surface proteins that mediate cell:cell adhesion by calcium-dependent, homotypic binding. Although the specific role of cadherin5 in fibroblasts is not well understood, it is clear that changes in cadherin expression are associated with changes in fibroblast sub-phenotype.91 For example, in fibroblast to myofibroblast transitions, there is a down-regulation of CDH2 (neural cadherin or N-cadherin) and an up-regulation of CDH11 (osteoblast cadherin or OB-cadherin).91 The dramatic difference in CDH5 expression across samples thus strongly suggests that modulus has an effect on the phenotype of AoAF cells that could be exploited to modulate fibroblast functions within engineered constructs. For example, it may be possible to promote vascular remodeling and the formation of microvascular networks within large blood vessels by controlling regional AoAF function in engineered constructs.
In the HUVEC experiments, too few cells grew on the high modulus hydrogel after 72 hours to provide sufficient RNA for analysis, indicating the undesirable environment of this hydrogel for HUVEC proliferation, and thus yielding only two sets of target genes to analyze. In total, 9 genes were up-regulated and 7 were down-regulated in the medium and low modulus gel cultures versus the TCPS controls. There was significant overlap in the set of genes that was up- or down-regulated on each modulus. Both the low and medium modulus hydrogels induced down-regulation of RPL13A and up-regulation of ID1 and ID3 in HUVECs, as mentioned above. The Id 1 and 3 proteins act in concert to down-regulate THBS1 and enhance EC migration; consistently, THBS1 mRNA levels were found to be decreased in HUVEC cultures grown on both moduli. In the medium modulus group, there were an additional 7 genes up-regulated; these were all consistent with an angiogenic response but indicated that cell migration may have been enhanced at the expense of cell proliferation on substrates of medium modulus. Four of the up-regulated genes (VEGFA, FGFR3, TIMP1, ANPEP) are generally associated with angiogenesis and may affect proliferation, migration, or both: VEGFA is a well-known pro-angiogenic factor with effects on both proliferation and migration, FGFR3 has been associated with the regulation of VEGF 92 and thereby can affect cell proliferation and migration, TIMP1 influences both migration and proliferation but has pleiotropic and context-specific effects93 as does ANPEP.87 Increases in TGFB2 and TGFBR1, on the other hand, indicate that migration may have been selectively enhanced on the medium-modulus hydrogels since TGFB2 signaling through TGFBR1 stimulates cell migration.94 TGFBR1 activation has also been associated with the maturation phase of angiogenesis and ECM assembly.95 ANGPTL4 expression is positively controlled by TGF-β signaling96 and has been associated with control of EC proliferation and migration.97 Its up-regulation supports the finding of enhanced TGFB2 signaling, which would be consistent with decreased proliferation and enhanced migration in the medium modulus samples. The interpretation of enhanced migration on the medium-modulus hydrogels is also supported by the down-regulation of THBS1, which would tend to relieve the THBS1 inhibition of cell migration.98 The decreased levels of FGF1 and PGF seen in the low-modulus group suggest direct or indirect effects of modulus on growth factor expression. Our results demonstrate the importance of tailoring moduli to control cell proliferative and migratory phenotypes and suggest that alterations in regional modulus could be used to drive the spatial organization of vascular ECs.
In the T/G HA-vSMC samples, too few cells were isolated from the low modulus hydrogel after 72 hours to provide sufficient RNA for analysis. A total of 44 different genes were altered in the T/G HA-vSMCs on the hydrogels versus TCPS. On the medium-modulus hydrogels, 2 genes were up-regulated and 21 were down-regulated, and on the high-modulus hydrogels, 1 gene was up-regulated and 41 were down-regulated. In both hydrogels, IL8, which stimulates vSMC proliferation99 and migration,100 was elevated. On the other hand, IL6, which also stimulates vSMC proliferation and migration, was down-regulated suggesting that the cells were differentially activated on the hydrogels versus TCPS. In the cultures on the medium-modulus hydrogels, ITGB3 was also elevated. The ITGB3 gene product works in combination with alpha integrins (principally alpha V or alpha IIb) to mediate cell adhesion to a variety of matrix molecules. Interestingly, integrin alpha V (ITGAV) was down-regulated in both medium and high modulus samples suggesting that the hydrogel cultures had a distinct complement of integrins relative to the TCPS cultures. Among the numerous down-regulated genes on the hydrogels, 20 out of the 21 genes identified on the medium-modulus hydrogels were also down-regulated on the high-modulus hydrogel. These included 5 genes related to cell:cell and cell:matrix interactions (B2M, EFNB2, JAG1, ITGAV, and THBS1), 7 involved in enzymatic reactions (MMP2, PLAU, SPHK1, SERPINF1, TIMP2, ANPEP, and TIMP1), 7 involved with growth factor/chemokine signaling (CXCL1, CXCL6, IFNA1, IL6, TGFB1, VEGFA, and VEGFC), and one receptor (PLXCD1). Among the down-regulated genes, only one target was identified on the medium-modulus hydrogels but not the high-modulus hydrogels: NOTCH4. NOTCH4 is a cell-surface receptor involved in cell:cell signaling by heterotypic binding of cell-surface ligands. In general, it is not highly expressed in healthy adult vSMCs but becomes elevated after vascular injury.101 The relative decrease seen in the hydrogel culture may indicate recovery from injury-related pathways in the medium-modulus cultures.
Culture of TG/HA cells on high-modulus hydrogels resulted in the down-regulation of an additional 21 genes that were not flagged in our assays as being down-regulated more the 2 S.D.s in the medium-modulus samples. These included 5 genes related to cell:cell and cell:matrix interactions (EPHB4, EFNA3, EFNA1, THBS2, LAMA5, and COL18A1), 3 involved in metabolic reactions (AKT1, GAPDH, and TYMP), 7 involved with growth factor/chemokine signaling (CCL2, ANGPT2, EREG, HGF, FGF1, MDK, PDGFA, and NRP2), 1 related to hypoxia sensing (HIF1A), and 3 related to transcriptional or translational control of gene expression (ID1, ID3, and RPL13A). Together, alterations in these genes suggest significant phenotypic differences between the two moduli, especially regarding bidirectional cell:cell signaling through ephrin pathways and diffusible growth factor/chemokine signaling.
Conclusion
These studies demonstrate the ability of several cell types to adhere to and proliferate on heparinized PEG-based hydrogels of three different moduli. Importantly, responses to differences in hydrogel stiffness were cell-type specific, with NIH3T3 cells and HUVECs demonstrating greater proliferation on lower modulus hydrogels and AoAF cells demonstrating greater proliferation on higher modulus hydrogels. The three vascular cell types displayed different changes in gene expression in response to growth on hydrogels, as well. Specifically, AoAF cells expressed a pattern of mRNAs consistent with an inflammatory response when cultured on hydrogels and showed a complex pattern of gene expression that indicated differences in fibroblast phenotype when cultured on hydrogels of different moduli. The expression profiles in HUVECs suggested enhanced migration when cultured on medium versus low modulus hydrogels, as well as differential effects of hydrogel modulus on growth factor expression. T/G HA-vSMCs had expression profiles that indicated differential activation of signaling pathways in cells cultured on hydrogels versus TCPS, and displayed modulus-specific differences in cell:cell signaling. Overall, these results indicate that the mechanical properties of hydrogels can have as great of an influence on cell phenotype as the chemical properties and suggest potential applications for heparinized, PEG-based hydrogels with spatially resolved mechanical properties to encourage the organization of multiple cell types in complex multicellular constructs.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (COBRE, REA;INBRE, REA and KLK; 1 RO1 EB003172 01 KLK), the National Science Foundation (DGE-0221651, support for ADB) and the Nemours Foundation (REA and KLK). The authors thank Mary McDonald for performing the Real Time PCR efficiency calculations. Allison Bartsch, David Johnson, David Scheiblin, and Megan Viereck are thanked for their experimental assistance and discussions.
References
- 1.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28(3):616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 2.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 3.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27(28):4881–93. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Shinoka T, Breuer C. Tissue-engineered blood vessels in pediatric cardiac surgery. Yale J Biol Med. 2008;81(4):161–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang WJ, Liu W, Cui L, Cao Y. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11(5):945–57. doi: 10.1111/j.1582-4934.2007.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31(1 Pt 2):162–9. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 7.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98(6):2321–7. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, Schultz GS, Ozaki CK, Berceli SA. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007;293(1):H482–8. doi: 10.1152/ajpheart.01372.2006. [DOI] [PubMed] [Google Scholar]
- 10.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89(12):1111–21. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 11.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 12.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64(1):70–9. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 13.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modifiedPEG hydrogels for bone tissue engineering. Biomaterials. 2002;23(22):4315–23. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 14.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci U S A. 1999;96(6):3104–7. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22(22):3045–51. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 16.]Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60(1):86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 17.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26(15):2467–77. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 20.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39(2):266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89(2):1374–88. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29(17):2597–607. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–34. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Gobin AS, West JL. Val-ala-pro-gly, an elastin-derived non-integrin ligand: smooth muscle cell adhesion and specificity. J Biomed Mater Res A. 2003;67(1):255–9. doi: 10.1002/jbm.a.10110. [DOI] [PubMed] [Google Scholar]
- 25.Boontheekul T, Mooney DJ. Protein-based signaling systems in tissue engineering. Curr Opin Biotechnol. 2003;14(5):559–65. doi: 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14(5):526–32. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–67. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Pratt AB, Weber FE, Schmoekel HG, Muller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86(1):27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 29.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 30.Tae G, Scatena M, Stayton PS, Hoffman AS. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci Polym Ed. 2006;17(1-2):187–97. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL. Growth factor mediated assembly of cell receptor-responsive hydrogels. J Am Chem Soc. 2007;129(11):3040–1. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. Journal of Controlled Release. 2007;122(3):287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz KM, Baldwin AD, Kiick KL, Furst EM. Gelation of Covalently Cross-Linked PEG-Heparin Hydrogels. Macromolecules. 2009;42(14):5310–5316. doi: 10.1021/ma900766u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie T, Akins RE, Jr., Kiick KL. Production of heparin-containing hydrogels for modulating cell responses. Acta Biomater. 2009;5(3):865–75. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. Journal of Cell Biology. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28(4):671–9. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–9. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13(18):2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95(9):4426–38. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian A, Lin HY. Crosslinked chitosan: its physical properties and the effects of matrix stiffness on chondrocyte cell morphology and proliferation. J Biomed Mater Res A. 2005;75(3):742–53. doi: 10.1002/jbm.a.30489. [DOI] [PubMed] [Google Scholar]
- 41.Osol G. Mechanotransduction by vascular smooth muscle. J Vasc Res. 1995;32(5):275–92. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- 42.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 1999;17(10):979–83. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 43.Williams B. Mechanical influences on vascular smooth muscle cell function. J Hypertens. 1998;16(12 Pt 2):1921–9. doi: 10.1097/00004872-199816121-00011. [DOI] [PubMed] [Google Scholar]
- 44.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457(7233):1103–8. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz-Pinto DJ, Bulick AS, Hahn MS. Uncoupled investigation of scaffold modulus and mesh size on smooth muscle cell behavior. J Biomed Mater Res A. 2009;90(1):303–16. doi: 10.1002/jbm.a.32492. [DOI] [PubMed] [Google Scholar]
- 46.Collinsworth AM, Zhang S, Kraus WE, Truskey GA. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol. 2002;283(4):C1219–27. doi: 10.1152/ajpcell.00502.2001. [DOI] [PubMed] [Google Scholar]
- 47.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2006;2:1–9. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 48.Taylor Z, Miller K. Reassessment of brain elasticity for analysis of biomechanisms of hydrocephalus. J Biomech. 2004;37(8):1263–9. doi: 10.1016/j.jbiomech.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Jin W, Riley RM, Wolfinger RD, White KP, Passador-Gurgel G, Gibson G. The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat Genet. 2001;29(4):389–95. doi: 10.1038/ng766. [DOI] [PubMed] [Google Scholar]
- 50.Kendziorski C, Irizarry RA, Chen KS, Haag JD, Gould MN. On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci U S A. 2005;102(12):4252–7. doi: 10.1073/pnas.0500607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saban MR, Hellmich H, Nguyen NB, Winston J, Hammond TG, Saban R. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics. 2001;5(3):147–60. doi: 10.1152/physiolgenomics.2001.5.3.147. [DOI] [PubMed] [Google Scholar]
- 52.Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94(7):513–21. doi: 10.1093/jnci/94.7.513. [DOI] [PubMed] [Google Scholar]
- 53.Rutledge RG, Stewart D. A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotechnol. 2008;8:47. doi: 10.1186/1472-6750-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quackenbush J. Microarray data normalization and transformation. Nat Genet. 2002;32(Suppl):496–501. doi: 10.1038/ng1032. [DOI] [PubMed] [Google Scholar]
- 56.Bray I, Bryan K, Prenter S, Buckley PG, Foley NH, Murphy DM, Alcock L, Mestdagh P, Vandesompele J, Speleman F. Widespread dysregulation of MiRNAs by MYCN amplification and chromosomal imbalances in neuroblastoma: association of miRNA expression with survival. PLoS One. 2009;4(11):e7850. doi: 10.1371/journal.pone.0007850. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1(4):461–70. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi N, Chae BS, Zhang L, Kiick KL, Furst EM. Rheological Characterization of Polysaccharide-Poly(ethylene glycol) Star Copolymer Hydrogels. Biomacromolecules. 2005;6(4):1931–1940. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi N, Kiick KL. Polysaccharide-Poly(ethylene glycol) Star Copolymer as a Scaffold for the Production of Bioactive Hydrogels. Biomacromolecules. 2005;6(4):1921–1930. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL. Growth Factor Mediated Assembly of Cell Receptor-Responsive Hydrogels. J. Am. Chem. Soc. 2007;129(11):3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultz KM, Baldwin AD, Kiick KL, Furst EM. Rapid rheological screening to identify conditions of biomaterial hydrogelation. Soft Matter. 2009;5(4):740–742. doi: 10.1039/b818178k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasegawa T, Kimura A, Miyataka M, Inagaki M, Ishikawa K. Basic fibroblast growth factor increases regional myocardial blood flow and salvages myocardium in the infarct border zone in a rabbit model of acute myocardial infarction. Angiology. 1999;50(6):487–95. doi: 10.1177/000331979905000607. [DOI] [PubMed] [Google Scholar]
- 63.Hughes GC, Biswas SS, Yin B, Coleman RE, DeGrado TR, Landolfo CK, Lowe JE, Annex BH, Landolfo KP. Therapeutic angiogenesis in chronically ischemic porcine myocardium: comparative effects of bFGF and VEGF. Ann Thorac Surg. 2004;77(3):812–8. doi: 10.1016/j.athoracsur.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 64.Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N, Schatz RA, Asahara T, Isner JM, Kuntz RE. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105(17):2012–8. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 65.Okumura M, Okuda T, Nakamura T, Yajima M. Acceleration of wound healing in diabetic mice by basic fibroblast growth factor. Biol Pharm Bull. 1996;19(4):530–5. doi: 10.1248/bpb.19.530. [DOI] [PubMed] [Google Scholar]
- 66.Ruel M, Laham RJ, Parker JA, Post MJ, Ware JA, Simons M, Sellke FW. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J Thorac Cardiovasc Surg. 2002;124(1):28–34. doi: 10.1067/mtc.2002.121974. [DOI] [PubMed] [Google Scholar]
- 67.Wilcke I, Lohmeyer JA, Liu S, Condurache A, Kruger S, Mailander P, Machens HG. VEGF(165) and bFGF protein-based therapy in a slow release system to improve angiogenesis in a bioartificial dermal substitute in vitro and in vivo. Langenbecks Arch Surg. 2007;392(3):305–14. doi: 10.1007/s00423-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 68.Nuttelman CR, Mortisen DJ, Henry SM, Anseth KS. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res. 2001;57(2):217–23. doi: 10.1002/1097-4636(200111)57:2<217::aid-jbm1161>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 69.McDonald JA, Kelley DG. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. Journal of Biological Chemistry. 1980;255(18):8848–8858. [PubMed] [Google Scholar]
- 70.Wagner DD, Hynes RO. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. Journal of Biological Chemistry. 1979;254(14):6746–6754. [PubMed] [Google Scholar]
- 71.Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35(3):348–55. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miqin Z, Desai T, Ferrari M. Proteins and cells on PEG immobilized silicon surfaces. Biomaterials. 1998;19(10):953–960. doi: 10.1016/s0142-9612(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 73.Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12(3):601–13. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 74.Ji Y, Ghosh K, Li B, Sokolov JC, Clark RA, Rafailovich MH. Dual-syringe reactive electrospinning of cross-linked hyaluronic acid hydrogel nanofibers for tissue engineering applications. Macromol Biosci. 2006;6(10):811–7. doi: 10.1002/mabi.200600132. [DOI] [PubMed] [Google Scholar]
- 75.Kurihara H, Nagamune T. Cell adhesion ability of artificial extracellular matrix proteins containing a long repetitive Arg-Gly-Asp sequence. J Biosci Bioeng. 2005;100(1):82–7. doi: 10.1263/jbb.100.82. [DOI] [PubMed] [Google Scholar]
- 76.Yan H, Saiani A, Gough JE, Miller AF. Thermoreversible protein hydrogel as cell scaffold. Biomacromolecules. 2006;7(10):2776–82. doi: 10.1021/bm0605560. [DOI] [PubMed] [Google Scholar]
- 77.Broderick EP, O’Halloran DM, Rochev YA, Griffin M, Collighan RJ, Pandit AS. Enzymatic stabilization of gelatin-based scaffolds. J Biomed Mater Res B Appl Biomater. 2005;72(1):37–42. doi: 10.1002/jbm.b.30119. [DOI] [PubMed] [Google Scholar]
- 78.Linnes MP, Ratner BD, Giachelli CM. A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials. 2007;28(35):5298–306. doi: 10.1016/j.biomaterials.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sieminski AL, Was AS, Kim G, Gong H, Kamm RD. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem Biophys. 2007;49(2):73–83. doi: 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- 80.Jung JP, Jones JL, Cronier SA, Collier JH. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials. 2008;29(13):2143–2151. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaudhuri S, Vyas K, Kapasi P, Komar AA, Dinman JD, Barik S, Mazumder B. Human ribosomal protein L13a is dispensable for canonical ribosome function but indispensable for efficient rRNA methylation. Rna. 2007;13(12):2224–37. doi: 10.1261/rna.694007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazumder B, Sampath P, Seshadri V, Maitra RK, DiCorleto PE, Fox PL. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell. 2003;115(2):187–98. doi: 10.1016/s0092-8674(03)00773-6. [DOI] [PubMed] [Google Scholar]
- 83.Goldie LC, Nix MK, Hirschi KK. Embryonic vasculogenesis and hematopoietic specification. Organogenesis. 2008;4(4):257–63. doi: 10.4161/org.4.4.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volpert OV, Lawler J, Bouck NP. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastases via thrombospondin-1. Proc Natl Acad Sci U S A. 1998;95(11):6343–8. doi: 10.1073/pnas.95.11.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119(12):3637–51. doi: 10.1172/JCI38308. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez-Navarro H, Vinue A, Vila-Caballer M, Fortuno A, Beloqui O, Zalba G, Burks D, Diez J, Andres V. Molecular mechanisms of atherosclerosis in metabolic syndrome: role of reduced IRS2-dependent signaling. Arterioscler Thromb Vasc Biol. 2008;28(12):2187–94. doi: 10.1161/ATVBAHA.108.175299. [DOI] [PubMed] [Google Scholar]
- 87.Petrovic N, Bhagwat SV, Ratzan WJ, Ostrowski MC, Shapiro LH. CD13/APN transcription is induced by RAS/MAPK-mediated phosphorylation of Ets-2 in activated endothelial cells. J Biol Chem. 2003;278(49):49358–68. doi: 10.1074/jbc.M308071200. [DOI] [PubMed] [Google Scholar]
- 88.Sakurai D, Tsuchiya N, Yamaguchi A, Okaji Y, Tsuno NH, Kobata T, Takahashi K, Tokunaga K. Crucial role of inhibitor of DNA binding/differentiation in the vascular endothelial growth factor-induced activation and angiogenic processes of human endothelial cells. J Immunol. 2004;173(9):5801–9. doi: 10.4049/jimmunol.173.9.5801. [DOI] [PubMed] [Google Scholar]
- 89.Breen EC. VEGF in biological control. J Cell Biochem. 2007;102(6):1358–67. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- 90.Wang D, Sai J, Carter G, Sachpatzidis A, Lolis E, Richmond A. PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry. 2002;41(22):7100–7. doi: 10.1021/bi025902m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16(4):472–9. doi: 10.1111/j.1524-475X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 92.Qian S, Somlo G, Zhou B, Zhu L, Mi S, Mo X, Cheung EM, Qiu W, Lin RJ, Rossi J. Ribozyme cleavage leads to decreased expression of fibroblast growth factor receptor 3 in human multiple myeloma cells, which is associated with apoptosis and downregulation of vascular endothelial growth factor. Oligonucleotides. 2005;15(1):1–11. doi: 10.1089/oli.2005.15.1. others. [DOI] [PubMed] [Google Scholar]
- 93.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1(27):re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102(6):637–52. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 95.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13(7):301–7. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 96.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, Zhu W, Wu D, Xu A. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28(5):835–40. doi: 10.1161/ATVBAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 98.Bornstein P. Thrombospondins function as regulators of angiogenesis. J Cell Commun Signal. 2009 doi: 10.1007/s12079-009-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HY, Kang YJ, Song IH, Choi HC, Kim HS. Upregulation of interleukin-8/CXCL8 in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens Res. 2008;31(3):515–23. doi: 10.1291/hypres.31.515. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, Castresana MR, Newman WH. NF-kappaB is required for TNF-alpha-directed smooth muscle cell migration. FEBS Lett. 2001;508(3):360–4. doi: 10.1016/s0014-5793(01)03109-x. [DOI] [PubMed] [Google Scholar]
- 101.Weber DS. A novel mechanism of vascular smooth muscle cell regulation by Notch: platelet-derived growth factor receptor-beta expression? Circ Res. 2008;102(12):1448–50. doi: 10.1161/CIRCRESAHA.108.179044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.