Abstract
The mechanism by which thrombotic vessel occlusion occurs independently of plaque development or endothelial cell (EC) disruption remains unclear, largely because of an inability to visualize the formation of thrombus, especially at the single-platelet level in real time. Here we demonstrate that rapidly developing thrombi composed of discoid platelets can be induced in the mesenteric capillaries, arterioles, and large-sized arteries of living mice, enabling characterization of the kinetics of thrombosis initiation and the multicellular interrelationships during thrombus development. Platelet aggregation without EC disruption was triggered by reactive oxygen species (ROS) photochemically induced by moderate power laser irradiation. The inflammatory cytokines TNF-α and IL-1 could be key components of the EC response, acting through regulation of VWF mobilization to the cell surface. Thrombus formation was then initiated by the binding of platelet GPIbα to endothelial VWF in our model, and this effect was inhibited by the ROS scavenger N-acetylcysteine. Actin linker talin-dependent activation of alphaIIb-beta3 integrin or Rac1 in platelets was required for late-phase thrombus stability. Our novel imaging technology illustrates the molecular mechanism underlying inflammation-based thrombus formation by discoid platelets on undisrupted ECs and suggests control of ROS could be a useful therapeutic target for the prevention of thrombotic diseases.
Introduction
It is evident that inflammation plays a central role in the pathogenesis of cerebral and cardiovascular diseases, and the thrombotic response is an integral part of these inflammatory processes. Proinflammatory cytokines such as TNF-α and IL-1 promote coagulation with increased tissue factor expression,1 which in turn induces synthesis of pro–IL-1β in platelets.2 However, the cytokine-related, multicellular kinetics at the single-platelet level during the development of thrombus in vivo remain unclear because of the absence of a real-time visualization technique with sufficient resolution to assess single-platelet kinetics in living animals.
FeCl3-induced thrombus formation in carotid arteries is a frequently used model, but it takes minutes to completely occlude the affected vessel.3,4 Moreover, this method does not allow one to track single-platelet kinetics during the formation of thrombus. Jackson et al succeeded in following a single discoid platelet in a developing thrombus and suggested activated platelets mediate the formation of thrombus via shape changes under low shear conditions, whereas discoid platelets are incorporated into growing thrombi only under high shear conditions.5,6 However, the precise mechanisms are unknown. In addition, conventional thrombosis models appear to involve endothelial cell (EC) disruption and exposure of the subendothelial matrix.
However, it is now clinically evident that platelet aggregation can occur on undisrupted endothelium within atherosclerotic lesions,7 making it important for us to learn how thrombus formation occurs on undisrupted and structurally intact endothelium. We therefore developed a novel in vivo imaging method that enables clear visualization of single platelet kinetics within aggregations of discoid platelets in a thrombus developing on undisrupted endothelium. It also enables clarification of the respective contributions made by inflammatory cytokines, integrin activation, and actin reorganization to thrombus formation.
Reactive oxygen species (ROS) are thought to play major roles in the development of atherosclerosis and thrombosis.8 ROS cause EC dysfunction, contributing to thrombus formation in vivo,9 but the precise relationship between ROS and inflammatory cytokines during thrombosis is not yet clear. To address that issue, we recently developed an in vivo animal model of thrombosis,10 and although we suggested that moderately increased ROS levels induced by a photochemical reaction could quickly lead to vessel occlusion, the mechanism was not defined. Since then, we have optimized our technology, and here we show that our in vivo laser injury system induces rapid formation of thrombi composed of discoid platelets without apparent EC disruption.
VWF is a glycoprotein produced in ECs and megakaryocytes and is also present in the extracellular matrix, circulating plasma, and platelets.11 VWF contributes to the adhesion and aggregation of platelets in disrupted vessels and is critical for hemostasis and thrombosis. VWF is stored inside the Weibel-Palade bodies and undergoes exocytosis after stimulation of ECs by ROS, especially O2−.12,13 We visualized the mobilization of VWF to the EC surface in laser/ROS-stimulated vessels and demonstrated that the binding of platelet GPIbα to VWF on the endothelial surface is the first step in the formation of thrombus. Platelet attachment to undisrupted endothelium was followed by actin-dependent thrombus stabilization mediated by actin-linker talin-dependent activation of integrin14,15 and Rac1, a small Rho family GTPase.16,17 Herein we found that inflammatory cytokines contribute to the mobilization of VWF, leading to the platelet aggregations. Jackson et al's findings5,6 that shear gradient induced the discoid platelet aggregations might be related to our findings, but they did not claim the specific molecular mechanisms. Thus, it is tempting to speculate that shear gradient changes and inflammatory cytokine signaling coordinately contribute to the discoid platelet aggregations leading to the vessel occlusion.
Methods
An expanded methods section is available in supplemental Methods (see the Supplemental Materials link at the top of the article).
Cells, reagents, and mice
Male C57BL/6J mice, age-matched integrin mutant mice (L746A and Y747A knock-in mice), TNF-α knockout (KO), TNF-α–R1 KO, IL-1 α/β double KO, and IL-1RA KO mice were used.15,18–21 Platelet-specific Rac1 KO mice (Pf4-Rac1 KO) were generated by mating Rac1 flox/flox mice and Pf4-Cre transgenic mice that were kindly provided from Dr Radek Skoda (University Hospital Basel).22,23 To obtain chimeric mice, BM cells from wild-type, TNF-α KO, or TNF-R1 KO mice were intravenously transfused into lethally irradiated recipient mice. All animals and recombinant DNA experiments were approved by the Institutional Animal Care and Use Committee and strictly followed the guidelines for animal experiments of The University of Tokyo.
Intravital microscopy and formation of thrombus
To visually analyze thrombus formation in the microcirculation of the mesentery in living animals, we used in vivo laser/ROS injury with a visualization technique developed through the modification of conventional methods.10,24 Anesthetized mice with a small incision made so that the mesentery could be observed without being exteriorized were administrated FITC-dextran (molecular weight [MW] 150 kDa and 10 kDa) or Texas-Red–dextran (MW 70 kDa), and hematoporphyrin (1.8 mg/kg in capillaries and 5 mg/kg in arterioles and arteries) was injected to produce ROS on laser irradiation. Blood cell dynamics and thrombus formation were visualized during laser excitation, and ROS production (wave length 488 nm, 1.5 mW power at 100× objective lens). Sequential images were obtained with a spinning-disk confocal microscope (CSU-X1; Yokogawa) or a Nikon A1R System with a resonance scanning system.
Image analysis
To quantify platelet dynamics, sequential images (covering > 20 seconds) were obtained and analyzed as follows. To assess the initial adherence of the platelets, attachment was defined as continuous interaction (for > 2 seconds) with the endothelium by a platelet after laser-induced injury. It was quantified as the adherent platelet numbers per observed vascular length (usually 100 μm) per observation period). To quantify the development of thrombus, platelet numbers within thrombi were determined as the number of platelets tightly sticking to a vessel (> 2 seconds) or stabilized within the vessel (not moved by blood flow > 2 seconds) per observed vascular length (usually 100 μm) at 10 seconds and 20 seconds after laser injury.
FACS analysis of washed mouse platelets and HUVECs
Washed mouse platelets from wild-type C57BL/6J, TNF-α KO, and TNF-R1 KO mice and cultured HUVECs were stimulated with the use of hematoporphyrin (as a source of O2− by laser irradiation), H2O2, and/or N-acetylcysteine (NAC). Some HUVECs were also treated with si-RNA targeting TNF-receptor-1 (si-TNF-R1) or with control scrambled si-RNA (si-CTRL) 48 hours before the experiments.
Statistics
We expressed the results as means ± SEM. The statistical significance of differences between 2 groups was assessed by the use of Student t tests. Differences between more than 2 groups were evaluated with ANOVA followed by posthoc Bonferroni tests. We examined correlations by using the Pearson correlation coefficient test. Values of P < .05 were considered significant.
Results
Photochemically generated ROS induce thrombi composed of discoid platelets without apparent EC disruption
To induce thrombus formation, we optimized a visualization system in which ROS are produced through a photochemical reaction within mesenteric capillaries.10 Our improved multicolor in vivo imaging technique enabled clear visualization of the real-time kinetics of leukocytes (Hoechst+), single platelets (anti-GPIbβ), and erythrocytes (fluorescent dextran; Figure 1A and supplemental Video 1). The conventional laser-induced thrombosis method uses a high-power laser to irradiate a limited area of endothelial surface, which disrupts the endothelium and exposes the collagen layer.3 In our model, by contrast, ROS (specifically O2−)25,26 are produced from hematoporphyrin by the use of moderate-power laser irradiation of the entire imaging field. By using this approach, we were able to induce thrombus formation on undisrupted endothelium (Figure 1B and supplemental Video 2). Thereafter, we sought to clarify how aggregates of discoid platelets form on the undisrupted endothelium in response to ROS.
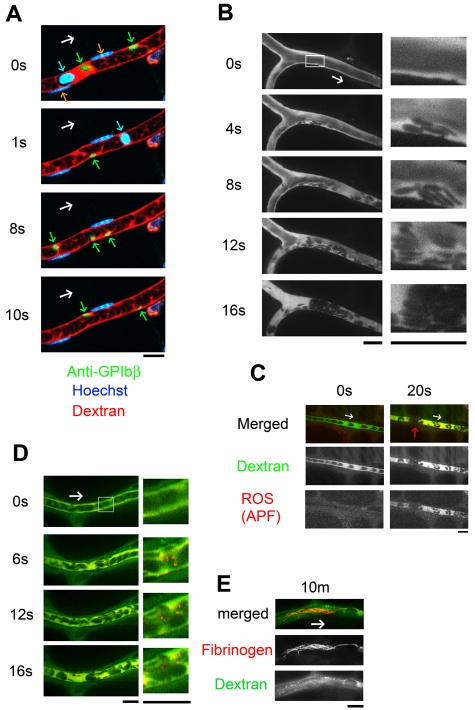
Figure 1.
Thrombus formed by discoid platelets visualized by in vivo imaging after laser/ROS-induced injury. (A) In vivo imaging enabling selective visualization of multiple cell types. Platelets, erythrocytes, and leukocyte/EC nuclei were visualized with an anti-GPIbβ antibody (green), fluorescent dextran (red), and Hoechst 33 342 (blue), respectively, in mesenteric capillaries of 12-week-old male control mice. Correspondingly colored arrows point to each cell type, and the white arrow indicates the direction of blood flow. (B) Discoid platelets contribute to thrombus formation after laser/ROS-induced injury. The microcirculation of the mesentery in living animals was visualized after laser/ROS-induced initiation of thrombus formation. (C) ROS production triggers thrombus formation. ROS production in the affected blood vessel after laser/ROS-induced injury was visualized by the administration of APF (red) with dextran (green). The red arrow indicates the developing thrombus. Original movies for panels A and B are available as supplemental Videos 1 and 2. (D-E) Distrbution of fibrinogen in a developing thrombus after short-term (D, 0-20 seconds) and long-term (E, 10 minutes) laser-induced injury. Injected fluorescent fibrinogen is shown in red, and dextran is in green. All scale bars are 10 μm.
After laser irradiation, platelets immediately started attaching to the vessel wall, after which they piled up, retarding blood flow, and the vessel lumen was eventually occluded by erythrocytes and/or leukocytes that formed a plug. The reproducibility of this system was confirmed by the finding that 90.5% ± 2.4% (n = 50 vessels from 5 animals) of the blood vessels tested were occluded. Two of the most striking findings were that the platelets retained a discoid shape within the developing thrombi and that the platelets attached to the apparently undisrupted endothelial wall, not to the exposed extracellular matrix. Laser/ROS-induced injury did not cause apparent disruption in the endothelium (supplemental Figure 1). We were also able to induce thrombus formation in small mesenteric arterioles (diameters, 30.5 ± 2.4 μm; n = 20) with almost 100% reproducibility by increasing the concentration of hematoporphyrin and the duration of laser irradiation (1 minute). Single platelets also could be visualized in the arterioles (Figure 2A).
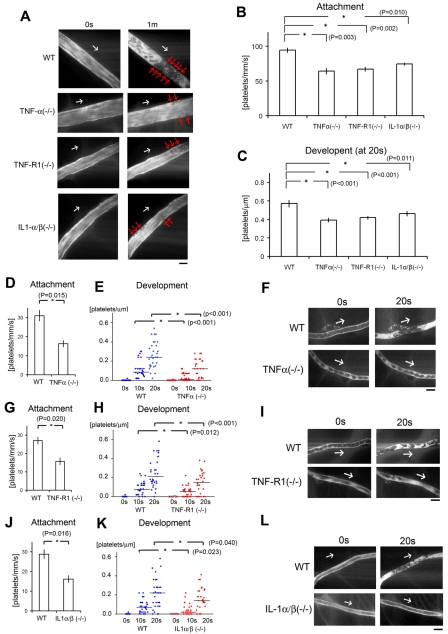
Figure 2.
Inflammatory cytokines are key components for discoid platelet attachment and thrombus development in our laser/ROS-induced injury model. (A) In vivo imaging of thrombus formation in small-sized mesenteric arterioles of 12-week-old male TNF-α KO, TNF-R1 KO, and IL-1-α/β double KO mice and wild-type (WT) controls. The red arrows show the developing thrombi, and white arrows show the direction of blood flow. Numbers of platelets attached to the vessel wall 10 seconds after laser/ROS-induced injury (B) and numbers of platelets within the developing thrombus 20 seconds after injury (C) in each mouse type. The initial attachment of platelets to ECs after laser/ROS-induced injury was significantly diminished in all inflammatory cytokine KO mice, as was thrombus development. (D-L) Numbers of platelets attached to the vessel wall 10 seconds after laser/ROS-induced injury (D,G,J) and numbers of platelets within the developing thrombus 0 seconds, 10 seconds, and 20 seconds after injury (E,H,K) in mesenteric capillaries of 12-week-old male TNF-α (D-E) and TNF-R1 (G-H) KO mice, IL-1-α/β double KO mice (J-K), and their respective WT littermates. (F,I,L) In vivo imaging of thrombus formation within capillaries of TNF-α KΟ, TNF-R1 KO, and IL-1-α/β double KO mice and WT mice. The white arrows show the direction of blood flow. Both “attachment” and “development” were diminished in all inflammatory cytokine KO mice. Horizontal lines denote median values (E,H,K). (n = 30 vessels from 5 animals for each group). All scale bars are 10 μm.
To better define the cell population within the thrombi, we repeated the thrombosis protocol in the presence of a fluorescent anti-GPIbβ antibody to selectively identify platelets. We observed that platelets comprised almost 90% of the cells within the thrombi in both the capillaries and arterioles (supplemental Figure 2), that is these thrombi were composed mainly of discoid platelets and were largely devoid of other cell types, such as leukocytes and/or erythrocytes. This finding is in stark contrast to FeCl3-induced thrombi, which are composed of several other cell types.4 In our model, moreover, vessel occlusion was quickly induced, within 20-30 seconds, which is quite different from earlier thrombosis models, which required at least several minutes for occlusion.3,4
To confirm that the observed discoid platelet aggregation occurred on undisrupted ECs, we traced the endothelial layer by using fluorescent IB4 isolectin and determined that the endothelium on which the thrombi developed was structurally intact (supplemental Figure 1); there was no extravasation of fluorescent dye (70-140 kDa fluorescent dextran; Figure 1B). By contrast, when we used small dextran (10 kDa), administration of recombinant TNF-α or IFN-γ led to visible extravasation of the fluorescent dyes, as previously reported, possibly reflecting the ECs' inflammation status (supplemental Figure 3).24 Under normal conditions, however, we observed none of these phenomena, which indicates that the machinery involved in our model is quite different from that involved in earlier models that use high-power laser injury, often associated with EC disruption, exposure of the extracellular matrix, and increased vascular permeability.27–29 How then is the formation of thrombus initiated in our model if exposed extracellular matrix is not involved? By using a fluorescent ROS indicator ([APF; 3′-(p-aminophenyl]) fluorescein), we confirmed that the trigger for platelet aggregation was ROS production after laser irradiation (specifically O2−) within all of the vessels examined (Figure 1C). In addition, we confirmed that an antioxidant and ROS scavenger, NAC, completely blocked the evoked platelet aggregation in vivo (supplemental Figure 4), suggesting the potential clinical usefulness of targeting ROS in the treatment of cardiovascular diseases.
Our visualization system also enabled characterization of the fibrinogen kinetics. When the injury was created by laser, patchy fibrinogen binding was observed after 20 seconds (Figure 1D). Then, after 10 minutes, we observed the solidification of the developing thrombus with fibrinogen/fibrin network formation, possibly achieved via the coagulation cascade (Figure 1E).
Proinflammatory TNF-α and IL-1 signaling in ECs contributes to the formation of thrombus
It has been suggested that inflammation and ROS play major roles in the pathogenesis of vascular and thrombotic diseases,1,30 but a direct contribution of proinflammatory cytokines and ROS to thrombus formation has not been demonstrated. It is known, however, that under pathophysiologic conditions platelets can stick to an endothelial monolayer that is not disrupted or inflamed, without exposure of the extracellular matrix.7,31,32 Normal resting endothelium has a nonadhesive surface, but activated and inflamed ECs treated with cytokines are known to promote the adherence of circulating blood platelets.
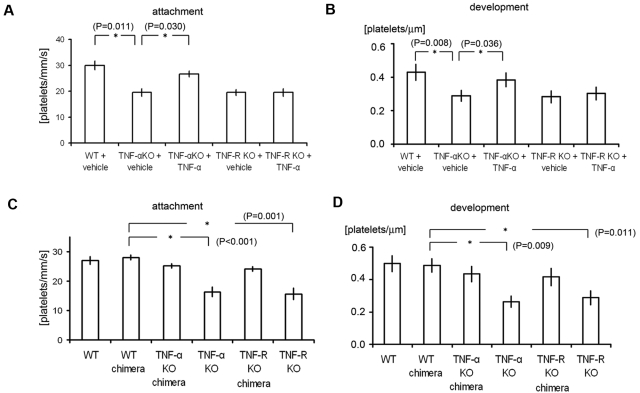
Therefore, to determine the contribution made by proinflammatory cytokine signaling to our thrombosis model, we first assessed the formation of thrombus by laser/ROS in KO mice deficient in the proinflammatory cytokine TNF-α or IL-1 (Figure 2). We found that initial adhesion of platelets to the vessel wall and subsequent thrombus growth were both markedly impaired in TNF-α KO mice (Figure 2D-F). We also observed similarly impaired thrombus formation in small arterioles (Figure 2B-C). When we calculated the initial platelet “attachment” and thrombus “development,” we found that the impairment of thrombus formation in TNF-α and TNF-R1 KO mice was observed similarly all in small-sized capillaries (Figure 2), small-sized arterioles, and large-sized (femoral) arteries (supplemental Figure 5), indicating the usefulness as an evaluation method of thrombus formation in vivo in various sized vessels, and we can apply this thrombus formation technique also in femoral artery levels. We also confirmed the expression of TNF-R1 on the ECs of the mesenteric capillaries (supplemental Figure 6). Administration of rTNF-α restored impaired thrombus formation in TNF-α–deficient mice but not in TNF-R1–deficient mice, indicating that TNF-α is a key mediator possibly involved in ROS-induced thrombosis (Figure 3A-B).
Figure 3.
Analysis of TNF-α chimeric mice and the effects of TNF-α administration revealed the importance of inflammatory cytokine signaling in ECs in our thrombosis model. (A-B) Quantification of platelet attachment and thrombus development after laser/ROS-induced injury in 12-week-old male TNF-α and TNF-R1 KO mice and their wild-type (WT) mice treated with vehicle (PBS) or TNF-α (100 ng/kg; n = 30 vessels from 5 animals for each group). Recombinant TNF-α restored impaired thrombus formation in TNF-α–deficient mice but not in TNF-R1–deficient mice. (C-D) Platelet attachment and thrombus development after laser/ROS injury assessed in WT, WT-chimeric, TNF-α KO-chimeric, TNF-α KO, TNF-R1 KO-chimeric, and TNF-R1 KO mice. None of the chimeric mice showed impaired thrombus formation, in contrast to the total TNF-α KO and TNF-R1 KO mice, indicating that cell types other than blood cells contribute to thrombus formation in our model. Asterisks indicate statistical significance (P < .05).
We next examined how TNF-α/TNF-R1 signaling associated with ROS signaling can mediate the rapid formation of thrombus. To distinguish the role of TNF-α/TNF-R1 signaling in ECs from that in blood cells, we transplanted BM from TNF-α KO or TNF-R1 KO mice to generate chimeric recipient mice. The endothelium remained intact in the chimeras, but their blood cells were derived entirely from the transplanted BM. This approach previously enabled us to determine which cell type contributed to the impaired thrombus formation observed in the recipient mice (Figure 3C-D).10 Notably, these chimeras exhibited no significant impairment of thrombus formation, which suggests TNF-α/TNF-R1 signaling in ECs, not blood cells, is critical for thrombus formation on undisrupted endothelium.
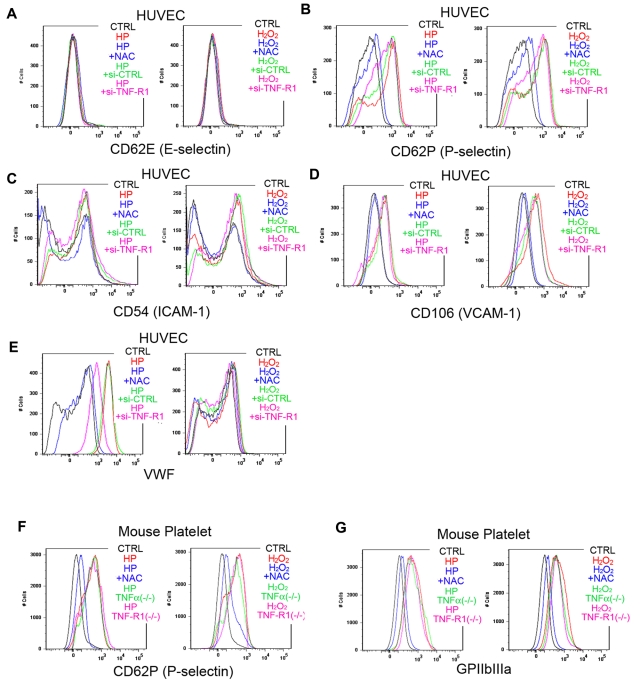
To further clarify how ROS trigger platelet aggregation, we performed a FACS analysis of ROS-treated ECs (Figure 4A-E). We found that laser-treated hematoporphyrin (O2−) markedly increased cell-surface expression of VWF in ECs and that this effect was completely blocked by the administration of NAC. This finding is consistent with an earlier report that O2−, but not H2O2, can directly induce VWF secretion from cultured ECs.13 In HUVECs with si-RNA–mediated TNF-α knockdown, VWF mobilization was markedly reduced, indicating that TNF-α in ECs plays a key role in mobilizing VWF from the Weibel-Palade bodies to the cell surface on stimulation by ROS, mainly O2−. Moreover, the response to ROS stimulation was not diminished in platelets obtained from TNF-α KO or TNF-R1 KO mice, indicating that EC cells, not blood cells, are indispensible for the response to ROS/laser injury in our model (Figure 4F-G). In addition, we confirmed that ROS increase endothelial P-selectin expression without affecting E-selectin expression (Figure 4A-B).
Figure 4.
FACS analysis showing ROS-induced increases in VWF on HUVECs, and in P-selectin and GPIIbIIIa on mouse platelets. (A-E) Adhesion molecules, activation markers, and cell-surface VWF were analyzed by flow cytometry in cultured HUVECs. Some cells were treated with hematoporphyrin (1mM, HP, as a source of O2−) for 20 minutes and then stimulated with laser irradiation to induce ROS production. Other cells were treated with 1mM H2O2 and/or 100μM NAC for 20 minutes before the incubation with antibodies. Some HUVECs were treated with si-RNA targeting TNF-R1 (si-TNF-R1) or with control scrambled si-RNA (si-CTRL) 48 hours before the experiments. Black lines denote vehicle-treated control cells (CTRL); red lines, H2O2 or hematoporphyrin treatment; blue lines, ROS source plus NAC; green lines, si-CTRL and ROS; and purple lines, si-TNF-R1 and ROS. Note that O2− markedly increased cell surface expression of VWF on HUVECs, which was completely blocked by NAC, and diminished in si-TNF-R1 cells. Expression of P-selectin in ECs was also increased by ROS, without affecting E-selectin expression. ICAM-1 expression was moderately increased by O2−. (F-G) Washed platelets from 12-week-old male wild-type C57BL/6J, TNF-α KO and TNF-R1 KO mice were collected and analyzed after ROS stimulation as in the HUVEC experiments. Black lines denote vehicle-treated control cells from wild-type mice (CTRL); red lines, H2O2 or hematoporphyrin treatment; blue lines, ROS source plus NAC; green lines, ROS plus TNF-α KO platelets; and purple lines, ROS plus TNF-R1 KO platelets. Note that expression of P-selectin, and GPIIbIIIa was increased by ROS treatment, and that these effects were inhibited by NAC.
We also examined expression of the EC activation markers ICAM-1 and VCAM-1 after ROS stimulation of HUVECs to determine whether the impaired thrombus formation seen in TNF-α/TNF-R1 KO mice was simply caused by a lower degree of cell activation. Although hematoporphyrin (as a source of O2−) treatment for 20 minutes increased ICAM-1 and VCAM-1 expression slightly, si-RNA–mediated TNF-α or TNF-R1 knockdown had no affect (Figure 4C-D). In addition, short-term treatment (20 seconds, our stimulation time for thrombus formation) did not elicit these changes (data not shown). These results rule out the possibility that TNF-α deficiency reduced EC activation after treatment with ROS (O2−), leading to impaired thrombus formation in the KO mice. Considering that our thrombus model achieved very quick vessel occlusion in capillaries, EC and platelet activation after ROS treatment does not mainly contribute to our very quick thrombus formation in our new models.
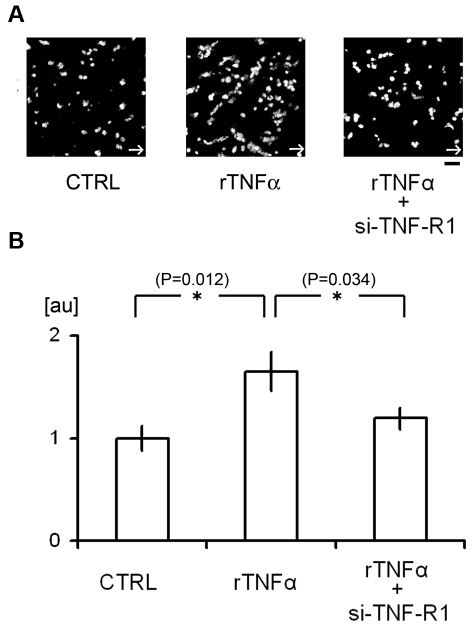
Our in vivo results could be reproduced in vitro in microfluidic devices. We used a flow chamber system to assess in more detail how TNF-α/TNF-R1 signaling contributes to platelet attachment to cultured HUVECs (Figure 5). We found that administration of rTNF-α increased the attachment of platelets to HUVECs under high shear flow conditions (200 dynes/cm2), where platelet aggregation is dependent on the binding of endothelial VWF to platelet GPIbα.33,34 si-RNA mediated knockdown of TNF-R1 on HUVECs reduced platelet attachment, again confirming that TNF-α/TNF-R1 signaling within ECs regulates platelet attachment to the endothelial surface under high shear conditions, which is consistent with our in vivo findings.
Figure 5.
Flow chamber analysis of the contribution of TNF-α signaling to platelet attachment to HUVECs. (A-B) Attachment of human platelets to cultured HUVECs. Some cells were treated with si-TNF-R1 48 hours before the experiments; CTRL denotes HUVECs treated with control scrambled si-RNA. Some cells were also treated with human rTNF-α (24 hours, 10 ng/mL). After perfusing tetramethylrhodamine ethyl ester–stained platelets with Tyrode buffer for 5 minutes at 200 dynes/cm2, adherent platelets were visualized (A). White arrows denote the direction of flow. The numbers of adherent platelets were quantified and normalized to the number obtained using CTRL cells (B). AU denotes arbitrary units. (n = 15 experiments for each group). Asterisks indicate statistical significance (P < .05). Scale bar is 10 μm.
The results so far suggest that ROS (O2−)–dependent VWF mobilization is likely modulated via the TNF-α/TNF-R1 axis in ECs. To further test this idea, we analyzed endothelial VWF expression in vivo. Administration of Alexa 647-conjugated anti-VWF antibodies enabled visualization of the EC surface expression of VWF in vivo (Figure 6). Intravital staining revealed that after laser/ROS-induced injury, VWF appears on the surface of ECs within the affected capillaries and arterioles, which is consistent with our FACS results in ROS-treated HUVECs and with the idea that elevation of ROS levels induces the binding of VWF to GPIbα (Figures 4 and 6). Moreover, VWF intravital staining also indicated that mobilization of VWF after laser/ROS-induced injury was diminished in both TNFα KO and TNF-R1 KO mice, again confirming that TNF-α/TNF-R1 signaling participates in the rapid display of VWF on the EC surface (Figure 6 and supplemental Figure 7).
Figure 6.
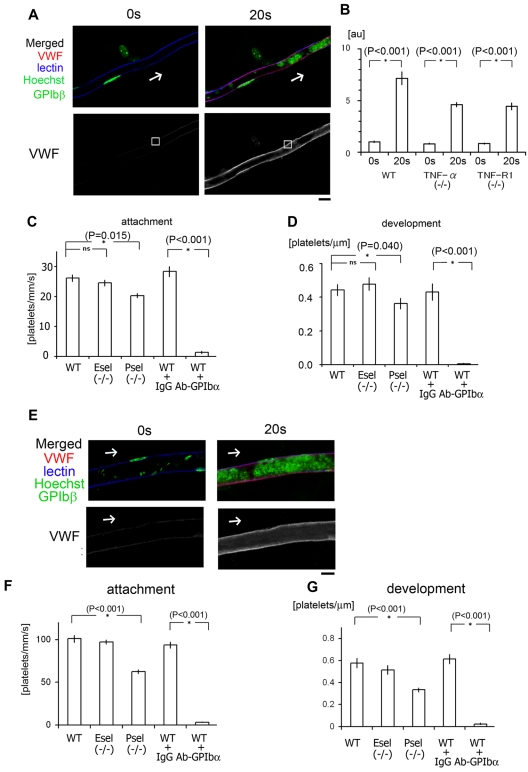
Binding of endothelial VWF to platelet GPIb plays a pivotal role in our laser/ROS-induced thrombosis model in both capillaries and arterioles. (A) In vivo imaging of VWF mobilization to the EC surface of small capillaries. Fluorescent anti-VWF antibody (red) was administrated to wild-type mice and visualized before and after laser/ROS-induced injury. VWF is expressed on the EC surface within the affected vessel after laser/ROS-induced injury. The cells were also costained with lectin (blue, vasculature), anti-GPIbβ (green, platelets), and Hoechst (green, nucleus). (B) Relative change in anti-VWF signals after laser/ROS-induced thrombus formation in wild type (WT), TNF-α KO, and TNF-R1 KO mice. Anti-VWF signal intensity was determined in the EC surface regions (white boxes in panel A) before (0 seconds) and 20 seconds after laser/ROS-induced injury. Note that VWF expression on endothelium after laser/ROS-induced injury was significantly reduced in both KO mice. AU denotes arbitrary units (n = 15 vessels from 5 animals). Representative original VWF images are shown in supplemental Figure 7. Asterisks indicate statistical significance (P < .05). (C) Numbers of platelets attached to vessel walls 10 seconds after laser/ROS-induced injury in WT, P-selectin KO (P-sel−/−), and E-selectin KO (E-sel−/−) mice, as well as WT mice treated with control IgG (WT + IgG), or neutralizing anti-GPIbα antibody (WT + Ab-GPIbα). Numbers of attached cells were markedly reduced in anti-GPIbα–treated WT mice (n = 30 vessels from 5 animals in each group). (D) Calculated numbers of platelets in developing thrombi 20 seconds after laser/ROS-induced injury (n = 30 vessels from 5 animals for each group). (E) Visualization of VWF mobilization to the EC surface in small arterioles, performed as in A. (F-G) Quantification of initial platelet attachment and thrombus development after laser injury in small arterioles (n = 30 vessels from 5 animals in each group). Asterisks indicate statistical significance (P < .05). The scale bar is 10 μm.
It is well recognized that platelets are important mediators involved in vascular inflammation, thrombosis, hemostasis, and atherogenesis,7,35 and that inflammation is characterized by interactions between leukocytes, ECs, and platelets.36 For example, activated platelets are known to produce IL-1α and -β.2,37,38 Thus, determining the degree to which inflammatory cytokines are involved in platelet aggregation on ECs would further clarify the molecular link between thrombosis and inflammation.
This prompted us to examine thrombosis in IL-1 α/β double KO mice (Figure 2J-L). We found that the initial adhesion of platelets to ECs after laser/ROS-induced injury was diminished in the double KO mice, as was thrombus growth, which indicates IL-1 signaling is also involved in ROS-mediated thrombus formation in our model. Conversely, we confirmed that both the initial attachment of platelets to the vessel wall and subsequent thrombus development are enhanced after laser/ROS-induced injury in IL-1 receptor antagonist KO mice (supplemental Figure 8). Thus, inflammatory processes associated with IL-1 production likely contribute to ROS-initiated thrombus formation.35
Platelet activation agents induce formation of thrombi containing leukocytes and deformed platelets
To examine the formation of thrombi via mechanisms not involving ROS, we assessed the effect of promoting platelet aggregation in mice administered thrombin (supplemental Figure 9). Thrombin directly induces integrin-mediated inside-out signaling, leading to irreversible platelet aggregation.39 After we administered thrombin, we observed formation of thrombi with a fibrinogen network (possibly fibrin) in occluded vessels. This effect was similar to the one seen in the long-term after laser/ROS-induced thrombus formation (Figure 1E). In contrast to the laser injury model, however, anti-GPIbβ staining showed that only 49.4% ± 7.0% of the cells (n = 30 occluded vessels) making up the thrombin-induced thrombi were platelets; the others were erythrocytes,al though leukocytes (14.5% ± 1.2%, n = 30 occluded vessels from 5 animals) were also present, suggesting platelet-leukocyte interactions play a major role in thrombin-induced thrombosis. However, the most remarkable finding was that the platelets within the thrombi had lost their discoid shape. Thus, the thrombosis that occurs in our model is quite different from that induced by platelet activating agents from the viewpoint of the cellular kinetics, and we consider our method to be a more useful tool with which to analyze single-platelet kinetics in the absence of other cell types, and to elucidate the effect of gene manipulation on single platelet function.
We also examined the effect of thrombin inhibitor (hirudin), and in vivo imaging showed thrombin inhibitor reduced the thrombus development in late phase (20 seconds after laser injuries), but not the initial platelet attachment during first 10 seconds, indicating that thrombin contribute to the stabilization of developing thrombus in our models (supplemental Figure 10).
Integrin activation and thrombus stability
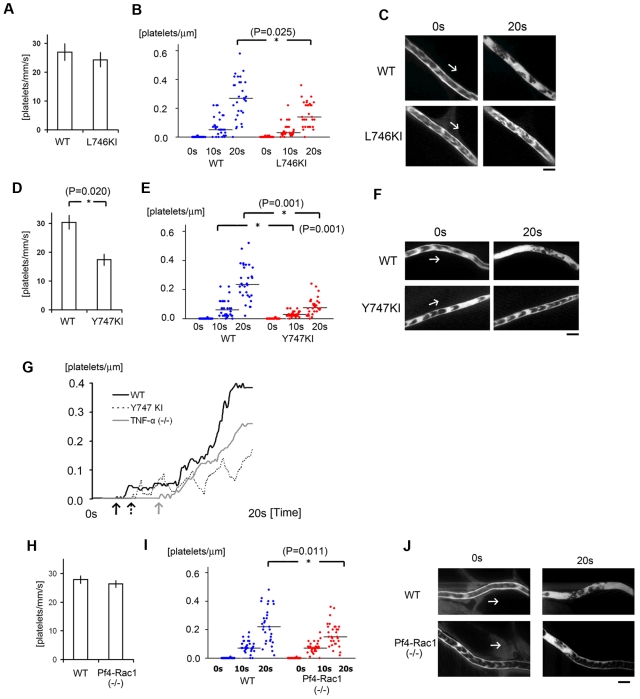
Initial platelet aggregation reportedly depends on a structural change in integrin αIIbβ3 (GPIIbIIIa) mediated by “inside-out” signaling through talin-binding at Leu746 and Tyr747 of the β3 integrin subunit.14,15 In addition, evidence suggests the level of Tyr747 phosphorylation linked with the tyrosine phosphorylation of Fyn and Lnk may contribute to the stability of the developing thrombus in this model.10 To test whether a selective deficiency on Tyr747 phosphorylation (Y747A knock-in [KI] mice) would influence laser/ROS-induced thrombus formation in vivo compared with a Leu746 deficiency (L746A KI mice), we repeated the thrombosis protocol by using these integrin mutant mice, in which talin-dependent integrin activation was eliminated (Figure 7).15 After laser exposure, initial attachment to the vessel wall was unchanged in L746A KI mice (Figure 7A), but thrombus growth during the late phase was diminished (Figure 7B-C). However, both initial platelet attachment and subsequent thrombus growth were severely diminished in Y747A KI mice (Figure 7D-F), suggesting Tyr747 but not Leu746 is involved in initial platelet attachment leading to thrombus formation.
Figure 7.
αIIbβ3 integrin signaling and actin cytoskeletal remodeling are required for stabilization of developing thrombi in vivo. (A) Numbers of platelets attached to the vessel wall 10 seconds after laser/ROS-induced injury in L746A KI mice and their wild-type (WT) littermates (n = 30 vessels from 5 animals in each group). (B) Calculated numbers of platelets within developing thrombi 0 seconds, 10 seconds, and 20 seconds after laser/ROS-induced injury. (C) Representative in vivo imaging after laser injury (n = 30 vessels from 5 animals in each group). Scale bar is 10 μm. Asterisks indicate statistical significance (P < .05). (D-F) Thrombus formation in Y747A KI mice and their WT littermates. The experimental protocols were the same as in panels A-C. Note that thrombus formation is more severely impaired in Y747A KI mice than L746A KI mice. (G) Representative curve showing the numbers of platelets in a developing thrombus after laser/ROS-induced injury in wild-type (WT, black line), TNF-α KO (gray line), and Y747A KI (dotted line) mice. Arrows in each line show the time of initial platelet attachment to the endothelium. (H-J) Impaired thrombus formation in Pf4-Rac1 KO mice. Platelet attachment (H) and thrombus development (I) after laser/ROS-induced injury was quantified, and in vivo images are shown (J). Horizontal lines denote median values (B,E,I).
To evaluate the kinetics of thrombus growth and stability in more detail, we compared the changes in platelet number within developing thrombi in wild-type and Y747A mice (Figure 7G). In wild-type mice, the number of platelets within thrombi increased irreversibly. In Y747A KI mice, by contrast, platelet numbers within thrombi increased transiently but subsequently declined, reflecting the instability of the thrombi. It can be seen in Figure 7G, for example, that thrombi in Y747A KI mice were unstable and were washed away by the blood flow. From these findings, we conclude that talin-dependent integrin activation is required for thrombus stability and that phosphorylation at Tyr747 within the talin-binding NPXY motif of integrin is crucial.
Similar calculation of platelet numbers in developing thrombi showed that the platelet kinetics in TNF-α KO mice differed from both wild-type and Y747A KI mice (Figure 7G). Most notably, the initial attachment of platelets to the endothelium was delayed, which caused there to be a time lag before platelet numbers began to increase within thrombi. In addition, the subsequent accumulation of platelets was more gradual in TNF-α KO mice than in wild-type mice. The delayed initiation of thrombus formation in TNF-α KO mice may be caused by impaired adhesion of platelets to the undisrupted ECs, again suggesting TNF-α/TNF-R1 signaling contributes to VWF mobilization after ROS/laser injury.
To clarify the contribution made by platelet adhesion molecules in our thrombosis model, we again used flow cytometry to examine the effects of ROS stimulation on the expression of adhesion molecules on mouse platelets (Figure 4). We found that the levels of P-selectin and GPIIbIIIa expression on platelets were all increased by ROS treatment, and these effects were inhibited by NAC. However, the increase in the expression of these platelet activation markers was relatively mild compared with levels seen with platelet activation agonists.40 Other markers of platelet functionality and activation were unaffected by ROS treatment. Again, the rapidity with which thrombi formed in our model (within 20 seconds) make it unlikely that ROS-induced EC or platelet activation is a primary contributor to thrombus formation in our new model.
Actin cytoskeleton associated with lamellipodia formation is also required for thrombus stability
Because thrombus stability appeared to require a mechanical link between β3 integrin and the actin cytoskeleton, we next examined the role of actin cytoskeletal reorganization in our thrombosis model. Rac1 is the major mediator of lamellipodia formation in platelets in vitro.16,17 In addition, Rac1 is reportedly required for thrombus stabilization in conventional in vivo thrombosis models entailing thrombin-stimulated actin reorganization,16 and in thrombin-stimulated granule secretion.41 When we assessed thrombus formation in platelet-specific Rac1 KO mice (platelet factor 4 promoter-dependent deletion of Rac1: Pf4-Rac1 KO), we found that only the late-phase thrombus stabilization was impaired, but the effect of Pf4-Rac1 KO was modest in comparison to Y747A KI (Figure 7D-F, and 7H-J). This finding indicates that both thrombin-PAR signaling-mediated integrin activation and Rac1 activation may be involved in thrombus stabilization, but not in initial platelet adhesion, and further confirm that tyrosine phosphorylation at Y747 of β3 integrin is likely required for stable adhesion of thrombi to the endothelium.
Binding of endothelial VWF to platelet GPIbα contributes to the initiation of discoid platelet aggregation
We next asked how ROS acting via the TNF-α/TNF-R1 axis cause ECs to trap circulating platelets, leading to discoid platelet aggregation in our models? We suspected that increased exposure of endothelial VWF might contribute to the binding of VWF to platelet GPIbα. To test that idea, we administered a neutralizing anti-GPIbα antibody, which completely blocked both platelet adhesion and thrombus development, which confirmed that that VWF-GPIb binding is a critical step in the rapid onset of discoid platelet aggregation in both capillaries and arterioles after ROS/laser injuries (Figure 6).
We also assessed the contributions of P-selectin and E-selectin to thrombus formation using respective KO mice. We found that P-selectin deficiency impaired both initial platelet adhesion and thrombus development, but the effect was relatively minor, compared with GPIbα inhibition. E-selectin deficiency did not alter thrombus formation (Figure 6).
From the results summarized in Figure 7G, which show that TNF-α deficiency delayed adhesion of platelets to ECs, we suggest that TNF-α/TNF-R1 signaling maintains and/or amplifies ROS signaling, leading to mobilization of VWF to the EC surface. Indeed, the absence of TNF-α/TNF-R1 signaling led to weak and delayed discoid platelet aggregation (Figures 2 and 7G). We therefore conclude that the rapid onset of discoid platelet aggregation on undisrupted ECs is mediated by proinflammatory cytokine signaling, even when the endothelium is not disrupted, and that subsequent thrombus stability is mediated via integrins and actin linkers. Finally, our in vivo imaging is not only useful for analyzing platelet kinetics, it could also provide novel clues to improved strategies for the treatment of thrombotic diseases.
Discussion
Severe vascular injuries cause de-endothelialization and exposure of the subendothelial matrix. However, it also has been reported that thrombosis can occur on the undisrupted endothelium.7 It is therefore crucially important to determine how platelets and ECs interact to form thrombi in the absence of apparent disruption of the vessel lumen. Our in vivo imaging system, which enables characterization of single-platelet kinetics during thrombus formation, revealed that (1) discoid platelet aggregation occurs rapidly on undisrupted endothelium after photochemical O2− production among mesenteric capillaries and arterioles and femoral arteries (Figure 1 and supplemental Figure 5); (2) the thrombi formed are composed almost entirely of discoid platelets (Figure 1 and supplemental Figure 2); (3) the proinflammatory TNF-α/TNF-R1 axis within ECs is a key regulator of this rapid attachment of platelets to the endothelium, possibly acting via stimulation of VWF display on the endothelium in response to ROS (Figure 2 and 7G); (4) the early phase of platelet attachment to undisrupted endothelium requires specific integrin signaling via Tyr747 but not Leu746 in the β3 cytoplasmic domain (Figure 7); (5) talin-dependent activation of αIIbβ3 integrin is indispensable for thrombus stability (Figure 7)15; (6) Rac1, which is essential for the actin reorganization needed for lamellipodial changes in vitro and thrombin-induced granule secretion, plays a major role in thrombus stabilization (Figure 7); (7) expression of P-selectin, but not E-selectin, is increased by ROS and contributes to platelet cohesion and subsequent thrombus development (Figure 6); and (8) the ROS scavenger NAC potently inhibits discoid platelet aggregation-based thrombus formation in our model, and also normalizes platelet activation after ROS treatment in vitro (Figure 4 and supplemental Figure 4).
With our system, we can visualize and quantitatively analyze single platelet kinetics in vessels ranging from capillaries to arterioles. Consequently, this method can be very effective for analyzing disease models targeting both small vessels (eg, disseminated intravascular coagulation) and larger arteries (eg, acute coronary syndrome closely related to thrombosis and artery occlusive diseases). Single-platelet analysis enabled us to separately examine the initial platelet attachment to vessels, thrombus growth, and stabilization of the developing thrombus, which are all dependent on different underlying molecular mechanisms. In addition, visualization of fluorescent molecules, such as fibrinogen and VWF, could be used to analyze the molecular kinetics in vivo, which would be useful for analyzing the cellular kinetics of thrombus formation.
In our model, thrombi were composed mainly of discoid platelets, without other cell types. By increasing the laser power and limiting the stimulation field to increase the ROS levels, we could induce platelet deformation like that seem after administration of platelet activation drugs such as thrombin (supplemental Figure 9 and 11), but the emergence of deformed platelets is a rare phenomenon. We therefore used moderate laser power to induce thrombus formation and mainly analyzed the kinetics of undeformed discoid platelets. This approach kept EC damage to a minimum and enabled us to address the clinical questions: How do platelets initially attach to vessel walls in living animals, and what are the first steps in the pathogenesis of cardiovascular diseases, especially those involving the occurrence of thrombotic events within vessels in the absence of plaque or erosion?7
Inflammation leads to an imbalance between the procoagulant and anticoagulant properties of ECs, which can lead to local stimulation of the coagulation cascade. TNF-α, the first proinflammatory cytokine released at sites of infection, is a potent inducer of immune defense mechanisms and a mediator of leukocyte recruitment. The authors of previous studies showed that TNF-α promotes coagulation by inhibiting synthesis of protein C and stimulating synthesis of tissue factor.36 Here we observed impaired thrombus formation in TNF-α KO mice, and the results obtained with chimeric mice indicate that TNF-α/TNF-R1 signaling within ECs is a key positive regulator of thrombus formation. This is consistent with earlier results that ECs stimulated with inflammatory cytokines exhibit increased platelet adhesion.42,43 We observed that ECs treated with TNF-α showed increased platelet attachment under flow conditions in vitro (Figure 5). Conversely, mobilization of VWF to EC surfaces after laser/ROS-induced injury was reduced by TNF-α deficiency both in vivo and in vitro (Figure 6 and supplemental Figure 7), although the level of impairment of thrombus formation in TNF-α KO mice was not dramatic (Figure 2).
Considering that we were able to induce thrombus formation within seconds in capillaries after laser injuries, inflammatory cytokines within ECs, even at low levels, may determine sensitivities to ROS signaling and the levels of adhesion molecule expression. In fact, VWF mobilization to the EC surface is, at least in part, mediated by TNF-α/TNFR1 signaling (Figure 6 and supplemental Figure 7). In addition, IL-1, which is present at relatively high concentrations in platelet granules, also appears to be involved in thrombus formation in our model. IL-1β has also been identified as a key mediator of platelet-induced activation of ECs,44 and its production has been observed in activated platelets2 (Figure 2). These findings, together with our observation that IL-1α and -β, play key roles in the initial attachment of discoid platelets to the vessel wall and in subsequent thrombus formation suggest a vicious cycle is activated in which thrombotic events stimulate pro-thrombotic inflammatory responses in both ECs and platelets.
ROS are key mediators of the signaling that underlies the vascular inflammation leading to atherogenesis.9 Exogenous ROS reduce the threshold for platelet activation in response to agonists and induce spontaneous platelet aggregation through unknown mechanisms.8 In addition, NAD(P)H-oxidase-dependent platelet ROS production enhances the recruitment of platelets to developing thrombi. It has been assumed that under pathophysiologic conditions up-regulation of O2− production by hyperactivated platelets serves as the physiologic basis for prolonged thrombus formation.9 In addition, the oxidant stress linked to ROS production promotes dysregulation of ECs. All known risk factors for atherothrombosis promote ROS production,8,45 and it is thus tempting to speculate that ROS and proinflammatory cytokines act in concert within the “inflamed” vasculature, although atherosclerotic lesions have not been directly visualized in human subjects. In the present study, we demonstrated that ROS rapidly induce the onset of thrombus formation on undisrupted endothelium independently of platelet agonists. In addition, platelet activation via talin-dependent αIIbβ3 integrin signaling appears to be critical for late phase thrombus stability in our model (Figure 7 and supplemental Figure 12).46 Haling et al reported that talin-dependent integrin activation associated with actin cytoskeletal changes is required for fibrin clot retraction and is essential for thrombus stability.47 Our in vivo findings are consistent with those earlier results.
We also found that the administration of NAC reduced levels of both GPIIbIIIa, as well as P-selectin expression in platelets, and inhibited platelet aggregation in vivo (Figure 4 and supplemental Figure 4). Although NAC is reported to be a potent inhibitor to plasma VWF multimers,48 it may also act directly as a ROS scavenger, suggesting ROS scavenging might be a useful therapeutic strategy for inhibiting thrombus formation in vivo. Sarratt et al49 reported that GPVI and integrin α2 play critical roles during platelet aggregation on collagen under flow conditions.
The present findings do not enable us conclude as to the precise molecular mechanism by which ROS acted via TNF-α signaling and VWF mobilization in ECs to induce thrombus formation. Whereas GPIbα blockade and NAC treatment almost completely blocked ROS/laser-induced thrombus formation in vivo (Figure 2 and supplemental Figure 4), TNF-α, TNF-R1, IL-1, and Rac-1 deficiency and functional integrin mutation all had only moderate effects on thrombus formation, though Y747A integrin mutation had a more marked effect. It may be that inflammatory cytokine and integrin signals act cooperatively with actin reorganization to mediate thrombus formation in our model. Alternatively, one or more as yet unknown factors might also influence VWF mobilization. Although elucidating this mechanism could be important when considering possible future therapeutic interventions, we considered that this is beyond the scope of this technical report.
In summary, by using our imaging system, we have clarified the mechanism by which thrombi are rapidly formed by discoid platelets on undisputed endothelium. Specifically, in vivo imaging along with kinetic studies showed the importance of endothelial TNF-α and IL-1 signaling for initiating GPIb binding to VWF. The initial platelet aggregation subsequently leads to irreversible integrin- and actin-dependent thrombus development.
Jackson et al's previous findings show that shear gradient induced the discoid platelet aggregations,5,6 and here in we clarified that inflammatory cytokine signaling in ECs play pivotal role in discoid platelet aggregations in vivo. Although, they did not claim the specific molecular mechanisms, but it is tempting to speculate that shear gradient changes and inflammatory cytokine signaling coordinately contribute to the discoid platelet aggregations leading to the vessel occlusion.
Supplementary Material
Acknowledgments
The authors thank Michikio Tajima, Chiho Yoshinaga, Xiao Yingda, Hiroko Tsukui, Yuji Yamazaki, and Yumiko Ishii for their excellent technical help. They also thank Dr Mark H. Ginsberg (University of California, San Diego) for his great help with integrin mutant mice, and the kind gift of Pf4-Cre mice from Dr Radek Skoda (University Hospital Basel).
This study was supported by the Funding Program for Next Generation World-Leading Researchers (S.N.), the Japan Society for the Promotion of Science (JSPS) through its “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program, R.N.); research fellowships from a Grant-in-Aid for Scientific Research on Innovative Areas “Fluorescence Live imaging” (no. 22113008) from The Ministry of Education, Culture, Sports, Science, and Technology, Japan (S.N.); Grants-in-Aid for Scientific Research (I.M., K.E., and R.N., respectively); grants for Translational Systems Biology and Medicine Initiative (S.N., R.N., T.K.); the global COE program from the Ministry of Education, Culture, Sports, Science and Technology of Japan (R.N., and T.K.); Banyu Life Science Foundation International (S.N.); and a research grant from the National Institute of Biomedical Innovation (R.N.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.N., M.N., S.K., Y.I., N.T., J.O., M.O., A.K., B.P., T.U., T.K., S. Sato, and A.A. performed experiments; S.N., I.M., and K.E. analyzed results and made the figures; and S.N., I.M., B,P., H.Y., S. Sugiura, T.K., H.N., K.E., and R.N. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare that no competing financial interests.
Correspondence: Satoshi Nishimura, MD, PhD, Department of Cardiovascular Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; e-mail: snishi-tky@umin.ac.jp; and Koji Eto, MD, PhD, Clinical Application Section, Center for iPS Cell Research and Application, Kyoto University, 53 Shogoin-Kawaharamachi, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: kojieto@cira.kyoto-u.ac.jp.
References
- 1.Neumann FJ, Ott I, Marx N, et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol. 1997;17(12):3399–3405. doi: 10.1161/01.atv.17.12.3399. [DOI] [PubMed] [Google Scholar]
- 2.Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154(3):485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furie B, Furie BC. In vivo thrombus formation. J Thromb Haemost. 2007;5(suppl):112–117. doi: 10.1111/j.1538-7836.2007.02482.x. [DOI] [PubMed] [Google Scholar]
- 4.Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60(4):269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- 5.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109(12):5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 6.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15(6):665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 7.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krötz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol. 2004;24(11):1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 9.Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3(2):254–267. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 10.Takizawa H, Nishimura S, Takayama N, et al. Lnk regulates integrin alphaIIbbeta3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J Clin Invest. 2010;120(1):179–190. doi: 10.1172/JCI39503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120(suppl 1):S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaux G, Abbitt KB, Collinson LM, Haberichter SL, Norman KE, Cutler DF. The physiological function of von Willebrand's factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev Cell. 2006;10(2):223–232. doi: 10.1016/j.devcel.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Vischer UM, Jornot L, Wollheim CB, Theler JM. Reactive oxygen intermediates induce regulated secretion of von Willebrand factor from cultured human vascular endothelial cells. Blood. 1995;85(11):3164–3172. [PubMed] [Google Scholar]
- 14.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302(5642):103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 15.Petrich BG, Fogelstrand P, Partridge AW, et al. The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117(8):2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarty OJ, Larson MK, Auger JM, et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280(47):39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eto K, Nishikii H, Ogaeri T, et al. The WAVE2/Abi1 complex differentially regulates megakaryocyte development and spreading: implications for platelet biogenesis and spreading machinery. Blood. 2007;110(10):3637–3647. doi: 10.1182/blood-2007-04-085860. [DOI] [PubMed] [Google Scholar]
- 18.Horai R, Asano M, Sudo K, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187(9):1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi T, Takata M, Ikeda A, Momotani E, Sekikawa K. Failure of germinal center formation and impairment of response to endotoxin in tumor necrosis factor alpha-deficient mice. Lab Invest. 1997;77(6):647–658. [PubMed] [Google Scholar]
- 20.Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 21.Zhao BQ, Ikeda Y, Ihara H, et al. Essential role of endogenous tissue plasminogen activator through matrix metalloproteinase 9 induction and expression on heparin-produced cerebral hemorrhage after cerebral ischemia in mice. Blood. 2004;103(7):2610–2616. doi: 10.1182/blood-2003-03-0835. [DOI] [PubMed] [Google Scholar]
- 22.Kassai H, Terashima T, Fukaya M, et al. Rac1 in cortical projection neurons is selectively required for midline crossing of commissural axonal formation. Eur J Neurosci. 2008;28(2):257–267. doi: 10.1111/j.1460-9568.2008.06343.x. [DOI] [PubMed] [Google Scholar]
- 23.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura S, Manabe I, Nagasaki M, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118(2):710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noda H, Ohya-Nishiguchi H. H. K. Mechanism for the production and reaction of active-oxygen species from photoexcited hematoporphyrin absorbed on a biological heterogeneous system. Bull Chem Soc Jpn. 1997;70:405–412. [Google Scholar]
- 26.Tanielian C, Heinrich G. Effect of aggregation on the hematoporphyrin-sensitized production of singlet molecular oxygen. Photochem Photobiol. 1995;62(2):131–135. [Google Scholar]
- 27.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115(12):3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92(3):486–494. [PubMed] [Google Scholar]
- 29.Saniabadi AR, Umemura K, Matsumoto N, Sakuma S, Nakashima M. Vessel wall injury and arterial thrombosis induced by a photochemical reaction. Thromb Haemost. 1995;73(5):868–872. [PubMed] [Google Scholar]
- 30.Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115(1):3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- 31.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med. 1998;187(3):329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heindl B, Zahler S, Welsch U, Becker BF. Disparate effects of adhesion and degranulation of platelets on myocardial and coronary function in postischaemic hearts. Cardiovasc Res. 1998;38(2):383–394. doi: 10.1016/s0008-6363(98)00032-7. [DOI] [PubMed] [Google Scholar]
- 33.Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109(2):566–576. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda Y, Handa M, Kawano K, et al. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J Clin Invest. 1991;87(4):1234–1240. doi: 10.1172/JCI115124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 36.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23(12):2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 37.Hawrylowicz CM, Santoro SA, Platt FM, Unanue ER. Activated platelets express IL-1 activity. J Immunol. 1989;143(12):4015–4018. [PubMed] [Google Scholar]
- 38.Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1{alpha} drives cerebrovascular inflammation. Blood. 2010;115(17):3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- 39.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104(6):1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 40.Michelson AD, Barnard MR. Thrombin-induced changes in platelet membrane glycoproteins Ib, IX, and IIb-IIIa complex. Blood. 1987;70(5):1673–1678. [PubMed] [Google Scholar]
- 41.Pandey D, Goyal P, Dwivedi S, Siess W. Unraveling a novel Rac1-mediated signaling pathway that regulates cofilin dephosphorylation and secretion in thrombin-stimulated platelets. Blood. 2009;114(2):415–424. doi: 10.1182/blood-2008-10-183582. [DOI] [PubMed] [Google Scholar]
- 42.Babinska A, Kedees MH, Athar H, et al. F11-receptor (F11R/JAM) mediates platelet adhesion to endothelial cells: role in inflammatory thrombosis. Thromb Haemost. 2002;88(5):843–850. [PubMed] [Google Scholar]
- 43.Frenette PS, Moyna C, Hartwell DW, Lowe JB, Hynes RO, Wagner DD. Platelet-endothelial interactions in inflamed mesenteric venules. Blood. 1998;91(4):1318–1324. [PubMed] [Google Scholar]
- 44.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 2004;61(3):498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 45.Loscalzo J. Oxidant stress: a key determinant of atherothrombosis. Biochem Soc Trans. 2003;31(Pt 5):1059–1061. doi: 10.1042/bst0311059. [DOI] [PubMed] [Google Scholar]
- 46.Petrich BG, Marchese P, Ruggeri ZM, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204(13):3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haling JR, Monkley SJ, Critchley DR, Petrich BG. Talin-dependent integrin activation is required for fibrin clot retraction by platelets. Blood. 2011;117(5):1719–1722. doi: 10.1182/blood-2010-09-305433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Reheman A, Gushiken FC, et al. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J Clin Invest. 2011;121(2):593–603. doi: 10.1172/JCI41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer DA, Kahn ML. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106(4):1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.