Abstract
Heparan sulfate (HS) proteoglycans are commonly exploited by multiple viruses for initial attachment to host cells. Herpes simplex virus-1 (HSV-1) is unique because it can use HS for both attachment and penetration, provided specific binding sites for HSV-1 envelope glycoprotein gD are present. The interaction with gD is mediated by specific HS moieties or 3-O sulfated HS (3-OS HS), which are generated by all but one of the seven isoforms of 3-O sulfotransferases (3-OSTs). Here we demonstrate that several common experimental cell lines express unique sets of 3-OST isoforms. While the isoforms 3-OST-3, -5 and -6 were most commonly expressed, isoforms 3-OST-2 and -4 were undetectable in the cell lines examined. Since most cell lines expressed multiple 3-OST isoforms, we addressed the significance of 3-OS HS in HSV-1 entry by down-regulating 2-O-sulfation, a prerequisite for 3-OS HS formation, by knocking down 2-OST expression by RNA interference (RNAi). 2-OST knockdown was verified by reverse-transcriptase PCR and Western blot analysis, while 3-OS HS knockdown was verified by immunofluorescence. Cells showed a significant decrease in viral entry, suggesting an important role for 3-OS HS. Implicating 3-OS HS further, cells knocked-down for 2-OST expression also demonstrated decreased cell-cell fusion when cocultivated with effector cells transfected with HSV-1 glycoproteins. Our findings suggest that 3-OS HS may play an important role in HSV-1 entry into many different cell lines.
Introduction
Herpes simplex virus type-1 (HSV-1), a member of the alphaherpesvirus family, causes a variety of diseases ranging from cold sores or fever blisters on mucosal layers of the skin of the mouth and face to much more severe, life threatening illnesses, such as keratitis, encephalitis, and meningitis (Eisenstein et al., 2004; Whitley and Roizman, 2001). The ability of HSV-1 to infect a wide array of cell types leads to the diverse manifestations of HSV-1 infection. Heparan sulphate (HS) is a glycosaminoglycan ubiquitously expressed on the cell surface and extracellular matrix of almost all cell types as heparan sulfate proteoglycans (HSPGs) (Lindahl et al., 2008; Rosenberg et al., 1997). HSPGs are composed of HS polysaccharide side chains covalently linked to a protein core via a trisaccharide linker region. HS has been shown to play a critical role during HSV-1 infection. During HSV-1 entry, HSV-1 viral particles first attach to the host cell surface by binding to HS using HSV-1 glycoproteins gB or gC (Herold et al., 1991; Shieh et al., 1992; WuDunn and Spear, 1989). This enables HSV-1 glycoprotein gD to bind to one of its host cell receptors, triggering fusion of the HSV-1 viral envelope with the host cell plasma membrane, a process that also requires HSV-1 glycoproteins gB, gH, and gL. Fusion allows the HSV-1 viral capsid and tegument proteins to be released into the host cell cytoplasm (Spear, 2004). A similar fusion mechanism is used when HSV-1 enters cells through endocytosis and phagocytosis-like manners as well (Clement et al., 2006; Nicola et al., 2003).
There are three separate classes of known HSV-1 gD entry receptors (Spear, 2004). Nectin-1 and nectin-2 are members of the immunoglobulin superfamily (Geraghty et al., 1998; and Warner et al., 1998). Nectin-1 is the primary HSV-1 entry receptor in epithelial and neuronal cells (Campadelli-Fiume et al., 2000; Haarr et al., 2001). Herpes virus entry mediator (HVEM) belongs to the tumor necrosis factor receptor family and is used for viral entry into human T lymphocytes and trabecular meshwork cells (Montgomery et al., 1996; Tiwari et al., 2005a). Finally, 3-O-sulfated heparan sulfate (3-OS HS), which is a specifically modified form of HS generated by a family of enzymes known as 3-O sulfotransferases (3-OSTs) (Shukla et al., 1999). The 3-OST enzyme family consists of seven known isoforms (3-OST-1, -2, -3A, -3B, -4, -5, -6), which can recognize specific monosaccharide sequences around unique modification sites (Liu et al., 1999; Shukla et al., 1999; Shworak et al, 1999). All 3-OST isoforms, except 3-OST-1, have been shown to generate a form of 3-OS HS to which HSV-1 gD can bind to mediate viral entry (O’Donnell et al., 2006; Shukla et al., 1999; Tiwari et al., 2005b; Xia et al., 2002; Xu et al., 2005). 3-OS HS is a unique HSV-1 gD receptor due to the fact that it is a polysaccharide, containing specific sulfated motifs that specifically mediate HSV-1 entry, while nectin-1, nectin-2, and HVEM are all protein receptors.
HS polysaccharide chains are composed of repeating uronic acid (D-glucuronic acid or L-iduronic acid) and D-glucosamine disaccharide units (Lindahl et. 1998; Rosenberg et al., 1997). During HS biosynthesis, HS chains undergo extensive modifications, resulting in the generation of a variety of structurally diverse HS chains (Fig. 1). These modifications include N-deacetylation, N-sulfation, epimerization, and sulfation at the 2-OH, 6-OH, and 3-OH positions (Lindahl et al., 1998; Rosenberg et al., 1997). Interestingly, although both HSV-1 and HSV-2 use HS as an attachment receptor during viral entry, HSV-1 can bind to distinct modification sites on HS that HSV-2 is unable to, which could explain some of the differences in cell tropism exhibited by both viruses. For example, while N-sulfation and carboxyl groups are required for both HSV-1 and HSV-2 infectivity, only HSV-1 is able to bind the specific modification sites generated by 2-O, 6-O, and 3-O sulfations, which occur during 3-OS HS production (Herold et al., 1996). During HS biosynthesis, 2-O sulfation is accomplished by the 2-O-sulfotransferase (2-OST) enzyme, which is expressed as a highly conserved single isoform (Shworak et al., 1999). The 3-O sulfation of glucosamine residues, which generates HSV-1 gD binding sites on 3-OS HS, takes place only after 2-O-sulfation has occurred (Fig. 1).
Fig. 1.
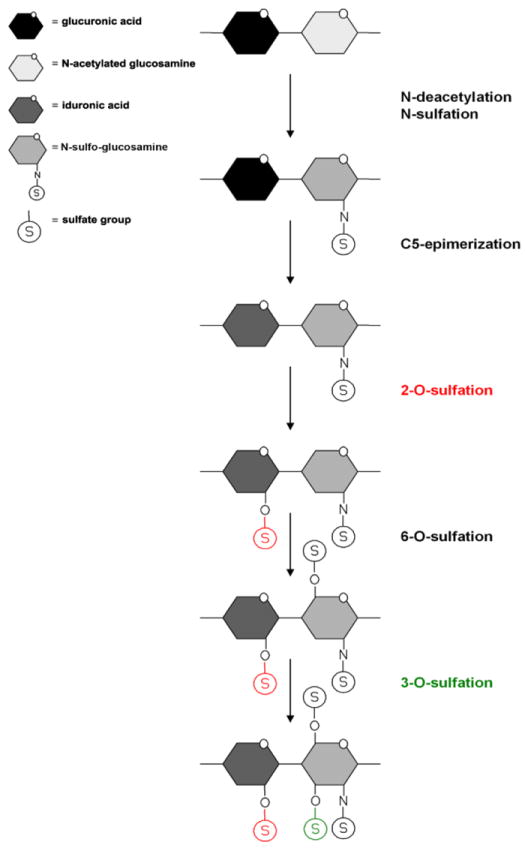
Heparan sulfate modifications. Heparan sulfate chains are initially synthesized as repeating disaccharide units of N-acetylated glucosamine and glucuronic acid. HS can then be modified by a series of enzymatic reactions, including N-deacetylation and N-sulfation of N-acetylated glucosamine converting it to N-sulfo-glucosamine, C5 epimerization of glucuronic acid to iduronic acid, and O-sulfation at the 2-OH, 6-OH, and 3-OH positions. First is 2-O-sulfation of iduronic acid and glucuronic acid, followed by 6-O-sulfation of N-acetylated glucosamine and N-sulfo-glucosamine units, and finally 3-O-sulfation of glucosamine residues. 2-O (red) and 3-O (green) sulfations are highlighted.
Previous studies on HSV-1 gD receptors have primarily focused on nectin-1 and HVEM, as both have been shown to mediate HSV-2 infection as well (Taylor et al., 2007). This study focuses on the less often studied, HSV-1 specific receptor, 3-OS HS. Our study demonstrates cell type specific expression of 3-OST isoforms, the importance of the 2-OST enzyme in the 3-OS HS biosynthetic pathway, and demonstrates the significance of 3-OS HS during HSV-1 binding, entry, and cell-cell fusion in a variety of cell types. Our study also identifies differences in the dependence on 3-OS HS as an HSV-1 entry receptor by Vero, HeLa, and retinal pigment epithelial (RPE) cells.
Results
3-O sulfotransferase expression in various cell types
Though the majority of 3-OST isoforms generate HSV-1 specific gD receptors, very limited information is available on their expression in various experimental cell lines commonly used for studying HSV-1 infection. Previous studies looking at 3-OST tissue distribution indicate 3-OSTs are expressed in a tissue specific manner. Isoforms 3-OST-2 and 3-OST-4 are predominantly expressed in the human brain, 3-OST-5 is primarily expressed in human skeletal muscle tissue, while the remaining 3-OST isoforms demonstrate a wider tissue distribution (Lawrence et al., 2007; Shworak et al., 1999; Xia et al., 2002; Yabe et al., 2005). To better understand the importance 3-OS HS may play during HSV-1 entry, 3-OST expression was investigated in various experimental cell lines using reverse-transcriptase PCR (RT-PCR) to identify the range of cell lines expressing these enzymes. Most 3-OST isoforms were targeted, including 3-OST-2, 3-OST-3A, 3-OST-3B, 3-OST-4, 3-OST-5, and 3-OST-6. The 3-OST-1 isoform was not included since it fails to generate an HSV-1 gD receptor (Shukla et al., 1999). CHO-K1 cells transfected with expression constructs for individual isoforms served as positive controls.
Experimental cell lines analyzed in this study included mouse neural P19N, HeLa, Vero, and retinal pigment epithelial (RPE) cells. Each of these cell lines are commonly used for studying HSV-1 infection and provide excellent in vitro models for studying viral entry and replication. RT-PCR results showed each cell line expressed a distinct set of 3-OST isoform mRNAs (Fig. 2). RPE cells expressed 3-OST-3A, -3B, -5, and -6; HeLa cells 3-OST-3B, -5, and -6; and Vero cells expressed 3-OST-3A, -6, and possibly a very low expression level of 3-OST-3B. Interestingly, expression of 3-OST-2 and 3-OST-4 was absent in each cell line examined (data not shown). This result is not totally unexpected since expression of both isoforms is highly restricted to the human brain (Shworak et al, 1999). Among the various cell lines examined, only P19N cells were found to not express any of the 3-OST isoforms. While is quite possible P19N cells do not express any of the known 3-OST isoforms, it is also possible RT-PCR failed to detect any signals due to low expression of 3-OSTs in P19N cells.
Fig. 2.
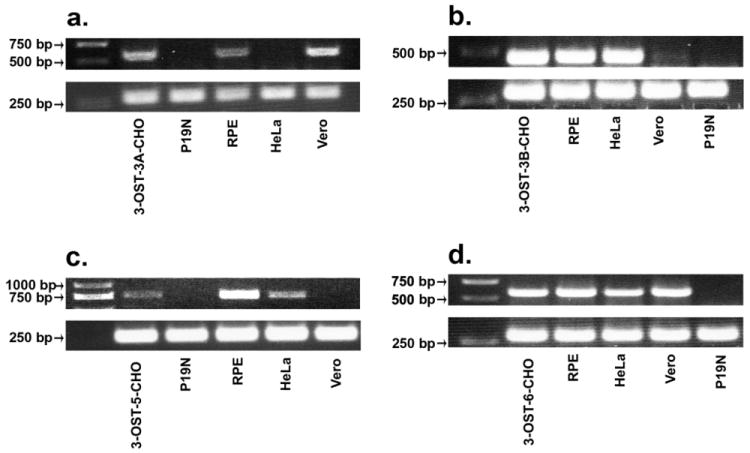
3-OST isoform expression in various cell lines. RT-PCR detection of isoforms 3-OST-3A (a), 3-OST-3B (b), 3-OST-5 (c), and 3-OST-6 (d) was performed in Vero, RPE, HeLa, and P19N cells. CHO-K1 cells transfected with 3-OST isoforms (-3A, -3B, -5, -6) served as a positive control. The cDNAs were produced from total RNA isolated from cells. Superscript II reverse transcriptase was used for RT-PCR. PCR products were separated by electrophoresis on an agarose gel and stained with ethidium bromide. Expected PCR product sizes were 604 bps (3-OST-3A), 442 bps (3-OST-3B), 777 bps (3-OST-5), and 570 bps (3-OST-6). β-actin mRNA (bottom panels) was used as a control with an expected PCR product size of 285 bps.
Effect of 2-O sulfotransferase downregulation on receptor expression
Since the majority of cell lines examined expressed one or more 3-OST isoforms, the significance of 3-OS HS on HSV-1 infection was examined by selectively knocking down the expression of enzymes required for the generation of 3-OS HS using siRNA (Carthew and Sontheimer, 2009). Since cells that expressed 3-OSTs expressed multiple isoforms, it makes it difficult to use siRNA specific for 3-OSTs because downregulation of one 3-OST isoform may not lead to a noticeable decrease in 3-OS HS expression due to the expression of the remaining 3-OST isoforms still capable of generating 3-OS HS. Also siRNA treatment against each isoform may prove to be too stressful for the cells. Instead, the 2-OST enzyme was targeted instead. 2-O-sulfation is a prerequisite for 3-O-sulfation of HS and the generation of 3-OS HS. Thus, targeting of the individual 2-OST enzyme can prevent 3-O-sulfation and the generation of 3-OS HS (Shukla et al., 1999). Downregulation of the single 2-OST isoform prevents all 3-OST isoforms from generating 3-OS HS without altering 3-OST expression (Sugahara and Kitagawa, 2002).
Cells were treated 2-OST specific siRNA (Sigma) to downregulate 2-OST expression. Initially, 2-OST downregulation was verified after 2-OST siRNA treatment using RT-PCR. For RT-PCR, Vero, HeLa, RPE, and CHO-K1 cells were tested. CHO-K1 cells were transfected with nectin-1, HVEM, or the 3-OST-3B isoform. CHO-K1 cells transfected with 3-OST-3B are able to generate HSV-1 binding 3-OS HS and express it on the cell surface. RT-PCR demonstrated that mRNA specific for 2-OST was downregulated in each cell line after treatment with 2-OST siRNA compared to cells that were mock treated or transfected with a negative control scrambled siRNA (Fig. 3). No downregulation was seen in cells transfected with scrambled siRNA. CHO-K1 cells transfected with the various gD receptors, which will serve as controls for subsequent HSV-1 entry experiments, also demonstrated a downregulation in 2-OST expression (Fig. 3b).
Fig. 3.
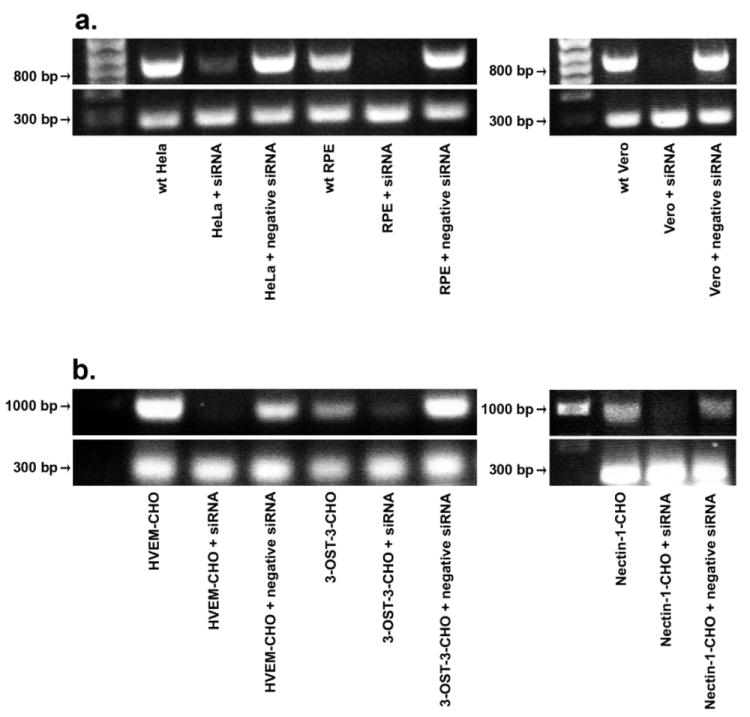
RT-PCR to verify reduced 2-OST gene expression. RT-PCR analysis of 2-OST expression was performed with HeLa, RPE, and Vero cells (a), and CHO-K1 cells expressing 3-OST-3B, HVEM, or nectin-1 (b). Cells were mock treated (wt) or transfected with scrambled siRNA (+ negative siRNA) or 2-OST siRNA (+ siRNA). About 48 h after siRNA transfection, total RNA was isolated from each cell line. Superscript II reverse transcriptase was used for RT-PCR. PCR amplification of cDNA was done using specific 2-OST primers. Expected PCR product sizes were 792 bps (2-OST) and 285 bps (β-actin) (bottom panels).
Downregulation of 2-OST was also seen at the protein level using western blot analysis. Using CHO-K1 cells as a representative cell line, treatment with 2-OST siRNA resulted in a significant decrease in 2-OST protein expression, further confirming the specific downregulation of 2-OST expression after 2-OST siRNA treatment (Fig. 4). HeLa, Vero, and RPE cells also showed a significant decrease in 2-OST protein expression after siRNA treatment (data not shown). Protein bands were quantified using NIH ImageJ v1.41 to better demonstrate the downregulation of 2-OST expression observed after siRNA treatment. 2-OST protein expression was quantified by calculating the relative intensity of each 2-OST protein band in relation to the corresponding band intensity of its β-actin expression.
Fig. 4.
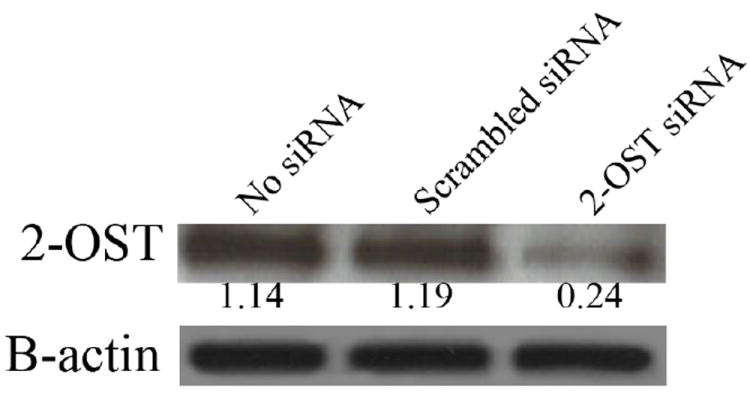
Western Blot analysis of 2-OST protein expression after siRNA down regulation. 2-OST protein expression was measured in a sample of CHO-K1 cells treated with 2-OST siRNA, scrambled siRNA, or mock treated cells (no siRNA). Protein expression was measured 48 h after siRNA transfection. β-actin protein expression was measured as a loading control. Protein bands were quantified using NIH ImageJ v1.41.
Though the previous experiments showed 2-OST expression was downregulated, it was still required to determine if 3-OS HS expression was also reduced. Immunofluorescence was performed to verify that siRNA knockdown of 2-OST did, in fact, reduce 3-OS HS surface expression. HeLa cells that were mock treated, transfected with scrambled siRNA, or transfected with 2-OST specific siRNA were incubated with the antibody HS4C3, which specifically targets 3-OS HS (Ten Dam et al., 2006). Incubation with HS4C3 was done at 4 °C, and after, cells were fixed but not permeablized, before the FITC-conjugated secondary antibody was added, to specifically label cell surface 3-OS HS. FITC labeled cells were examined by confocal microscopy to compare 3-OS HS expression. A significant reduction in cell surface 3-OS HS was observed in HeLa cells transfected with 2-OST specific siRNA compared to those mock treated or transfected with scrambled siRNA (Fig. 5). Significantly reduced 3-OS HS expression was also observed in Vero, RPE, and 3-OST-3B expressing CHO-K1 cells (data not shown). Though some intracellular 3-OS HS labeling was seen, the majority of 3-OS HS staining occurred at the cell membrane. Some minor 3-OS HS expression was seen in 2-OST siRNA treated cells, which is most likely due to the fact that 2-OST expression was not completely eliminated by siRNA knockdown, so it would be expected to see some minor 3-OS HS expression. However, the results suggest downregulation of 2-OST subsequently leads to a significant decrease in 3-OS HS expression.
Fig. 5.
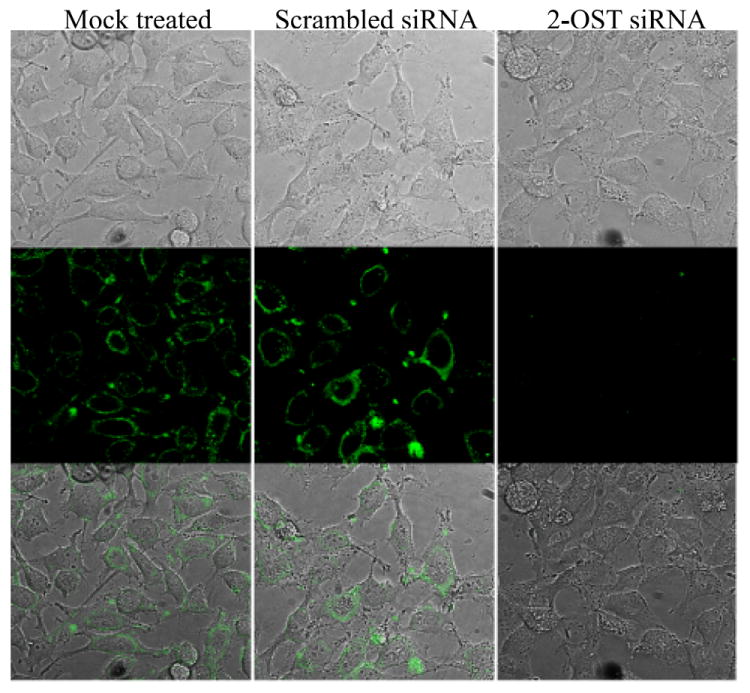
Use of immunofluorescence to confirm reduced 3-OS HS expression. HeLa cells were incubated with the antibody HS4C3 for 1 h at 4 °C. Cells were then fixed for 20 min, and incubated with FITC conjugated anti-mouse IgG to label 3-OS HS surface expression. 3-OS HS surface expression was compared in HeLa cells that were mock treated (left panels) or transfected with scrambled siRNA (middle panels) or 2-OST siRNA (right panels). Imaging was performed using confocal microscopy at a 60x oil objective.
Together, these results demonstrated that 2-OST siRNA treatment led to a significant knockdown of 2-OST expression at both the mRNA and protein levels, and this also led to a significant decrease in 3-OS HS expression. These controls were necessary to verify the efficacy of 2-OST siRNA treatment on 3-OS HS downregulation before investigating the effects of 3-OS HS on HSV-1 entry and fusion.
Effect of 3-OS HS downregulation on HSV-1 entry
HeLa, Vero, and RPE cell lines all expressed distinct sets of 3-OST isoforms, suggesting 3-OS HS may play a crucial role as an HSV-1 entry receptor in these cell lines. After verifying knockdown of 2-OST expression, and subsequent downregulation in 3-OS HS expression, we next examined the effect of reduced 3-OS HS expression on HSV-1 entry. To demonstrate that any effects on viral entry due to 2-OST knockdown were specific to 3-OS HS, and not another gD receptor, viral entry was also measured in CHO-K1 cells transfected with nectin-1 or HVEM, without any 3-OS HS expression, which is naturally absent in CHO-K1 cells. To also demonstrate specificity to 3-OS HS, HSV-1 entry was measured in P19N cells which were shown to not express any 3-OST isoforms, thus, siRNA treatment should have no effect on entry in P19N cells. In addition, herpes simplex virus type-2 (HSV-2) entry was measured in HeLa, Vero, and 3-OST-3B expressing CHO-K1 cells (Supplemental Fig. 1). HSV-2 cannot use 3-OS HS as a receptor, so HSV-2 entry should not be affected by a downregulation of 3-OS HS expression.
Previously described HSV-1 entry assays were used to compare viral entry in 2-OST siRNA treated cells with those transfected with scrambled siRNA or mock treated. Cells were infected with a recombinant β-galactosidase expressing HSV-1 (KOS) gL86 reporter virus (Shukla et al., 1999). A significant decrease in HSV-1 entry was observed in cells treated with 2-OST siRNA compared to those treated with scrambled siRNA or mock treated (Fig. 6). RPE, HeLa, and Vero cells all showed a similar decrease in viral entry of around 45-50%. CHO-K1 cells expressing 3-OST-3B showed the most significant decrease in viral entry after 2-OST siRNA treatment, which was expected since 3-OS HS is the only known HSV-1 gD receptor expressed by these cells. The negative control was infected CHO-K1 cells transfected with control pcDNA3.1 plasmid. These cells do not express a gD receptor and are resistant to HSV-1 entry.
Fig. 6.
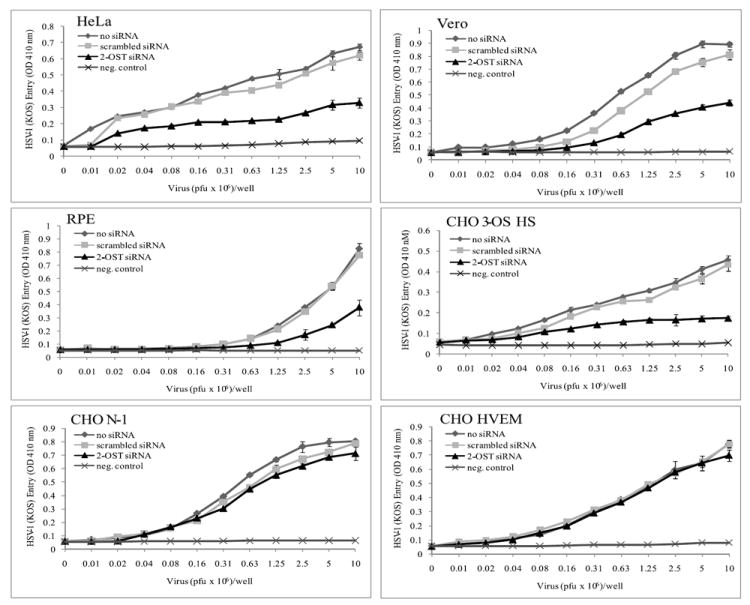
HSV-1 entry is dependent on 2-OST and 3-OS HS expression. HSV-1 entry was analyzed in HeLa, Vero, RPE, and CHO-K1 cells expressing 3-OST-3B, nectin-1, or HVEM. Cells were mock treated (no siRNA) or transfected with scrambled siRNA or 2-OST siRNA. Cells were replated in a 96-well culture dishes and inoculated with β-galactosidase-expressing recombinant HSV-1(KOS) gL86 for 6 h. The soluble substrate ONPG was added, and enzymatic activity was measured with a microplate reader at 410 nm.
As expected, CHO-K1 cells expressing nectin-1 or HVEM that were treated with 2-OST siRNA did not demonstrate a similar decrease in viral entry, however, there did appear to be a minor decrease in CHO-K1 nectin-1 expressing cells. Similar levels of HSV-2 entry were seen in HeLa and Vero cells no matter if the cells were treated with siRNA or not (Supplemental Fig. 1). This suggests siRNA treatment was not having any bystander effects. CHO-K1 cells expressing 3-OST-3B also showed no differences in viral entry after 2-OST siRNA treatment and showed levels of entry similar to the negative control, which was expected, because these cells do not express any HSV-2 gD receptors and cannot mediate HSV-2 entry. P19N cells also showed no changes in HSV-1 entry when treated with 2-OST siRNA, which was also expected because they do not express any 3-OST isoforms (Supplemental Fig. 1). Together, these results highlight the specific effect of 3-OS HS on HSV-1 entry, and the results suggest the reduction seen in viral entry was specifically due to reduced 3-OS HS expression. This supports the notion that decreased 2-OST expression can significantly decrease HSV-1 entry in cell lines that can use 3-OS HS as an entry receptor.
Effect of 2-OST downregulation on HSV-1 binding
Since HS is involved in virus attachment, the decreased entry seen in the previous figure could be in part due to reduced binding of HSV-1 to the host cell surface. To determine if downregulation of 2-OST expression did in fact have a significant effect on HSV-1 binding, a viral binding assay was performed, comparing HSV-1 binding to cells in the presence and absence of 2-OST siRNA. According to the results, there was some decrease in HSV-1 binding seen for each cell line in cells that were treated with 2-OST siRNA compared to those mock treated or treated with scrambled siRNA (Fig. 7). When compared with corresponding scrambled siRNA controls, the decrease in attachment ranged from about 8-16% in the 2-OST siRNA transfected cells. The viral binding assay was performed at 4 °C, which allows for viral attachment but not viral entry, using HSV-1 (K26GFP) virus, which expresses the fluorescent GFP protein. CHO-K1 cells expressing nectin-1 also showed a decrease in HSV-1 binding, which could explain why a minor decrease was seen in HSV-1 entry in these cells after 2-OST siRNA treatment even though this cell line does not express 3-OS HS. Though it appears decreased HSV-1 binding was partially responsible for reduced HSV-1 entry, the results still suggest 3-OS HS is playing a critical role during the penetration step of HSV-1 entry as well.
Fig. 7.
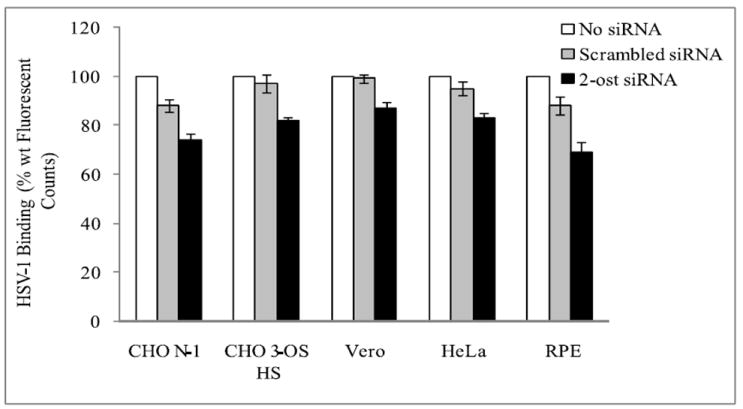
2-OST downregulation affects HSV-1 binding. HSV-1 binding to the cell surface was measured in Vero, HeLa, RPE, and CHO-K1 cells expressing either 3-OST-3B or nectin-1 that were mock treated (no siRNA) or transfected with either scrambled siRNA or 2-OST siRNA. About 48 h after siRNA transfection, cells were infected with HSV-1 (K26GFP) (10 M.O.I.) for 1 h at 4 °C. Fluorescence readings were taken using a GENios Pro fluorescence reader.
The effect of 2-OST expression on HSV-1 mediated cell-cell fusion
After establishing the importance of 3-OS HS in HSV-1 entry, it was next examined if 3-OS HS played a critical role in HSV-1 mediated cell-cell fusion, which is one of the ways by which HSV-1 spreads within a host. Interestingly, while expression of gD receptors, such as 3-OS HS, are required for cell-cell fusion, unmodified HS is not required, as it is during the attachment step of HSV-1 entry (Pertel et al., 2001). Similar cells lines used for studying HSV-1 entry were also used to determine the relative effects of 2-OST knockdown on cell-cell fusion. A standard luciferase reporter gene based cell-cell fusion assay was performed to quantify HSV-1 cell-cell fusion. Each cell line was split into two populations: target and effector cells. Target cells were transfected with a plasmid expressing the luciferase reporter gene under the control of the T7 promoter. CHO-K1 target cells were additionally transfected with nectin-1, HVEM, or 3-OST-3B. It was not required to transfect the other cell lines with a gD receptor since they naturally express them. Target cells were also mock treated or transfected with scrambled siRNA or 2-OST specific siRNA. Effector cells were transfected with plasmids expressing HSV-1 glycoproteins gB, gD, gH and gL and a plasmid expressing T7 RNA polymerase.
According to the results, most cell lines showed a significant decrease in cell-cell fusion when target cells were treated with 2-OST siRNA compared to those mock treated or treated with scrambled siRNA (Fig. 8). HeLa, RPE, and CHO-K1 cells expressing 3-OST-3B all showed a similar decrease in cell-cell fusion. HeLa and RPE cells, which express other gD receptors, stilled showed a decrease in cell-cell fusion of around 55% compared to cells treated with scrambled siRNA, highlighting the importance of 3-OS HS during this process. Interestingly, this same decrease was not observed in Vero cells, which may indicate, in the case of Vero cells, HSV-1 gD receptors other than 3-OS HS play a primary role in HSV-1 cell-cell fusion. Since Vero cells showed a similar decrease in HSV-1 entry compared to the other cell lines tested, the results also suggest that 3-OS HS may have additionally functions during HSV-1 entry, at least in the case of Vero cells.
Fig. 8.
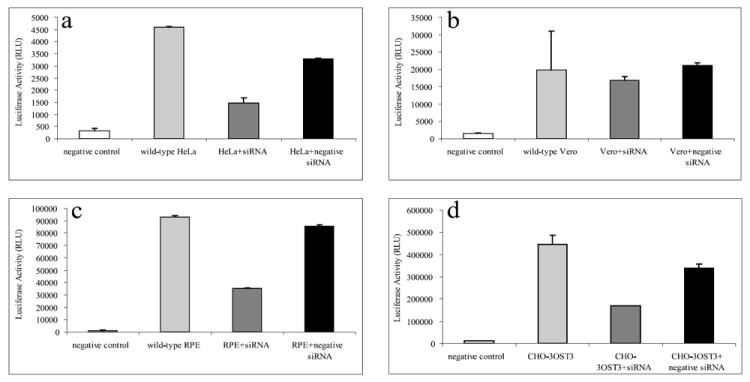
Cell-cell fusion is differentially affected by 2-OST downregulation in various cell lines. Target cells for Vero (a), HeLa (b), RPE (c), and CHO-K1 cells expressing 3-OST-3B (d) were mock treated (wild type), treated with scrambled siRNA (+negative siRNA), or treated with 2-OST siRNA (+siRNA). About 48 h after siRNA transfection, target and effector cells were mixed together in a 1:1 ratio and luciferase activity was measured after 24 h.
P19N cells, which were shown not to express any 3-OST isoforms, did not show a decrease in cell-cell fusion when treated with 2-OST siRNA (Supplemental Fig. 2). In addition, effector cells transfected with the HSV-2 glycoproteins necessary to induce cell-cell fusion (gB-2, gD-2, gH-2, gL-2) also did not show a decrease in cell-cell fusion when treated with 2-OST siRNA (Supplemental Fig. 2). CHO-K1 cells expressing 3-OST-3B showed levels of fusion similar to the negative control because these cells do not permit HSV-2 mediated cell-cell fusion. Together, these results reinforce the idea that it is specifically the downregulation of 3-OS HS that is causing the decrease in cell-cell fusion. Cells not expressing 3-OS HS or cells infected with a virus that does not use 3-OS HS as a receptor showed no effect on entry or cell-cell fusion when 3-OS HS was downregulated.
Discussion
HSV-1 entry and cell-cell fusion are complex processes requiring participation from multiple viral glycoproteins and cell receptors. This study provides further insight into the functions of the lesser understood gD receptor, 3-OS HS. For the first time, we demonstrate most experimental cell lines tested express mRNAs for multiple 3-OSTs raising a strong possibility that their final product, 3-OS HS, is also commonly expressed. While tissue specific expression of 3-OSTs has been shown in the past (Lawrence et al., 2007; Shworak et al., 1998; Yabe et al., 2005), little information on cell type specific expression of 3-OSTs is known. Our results not only demonstrate these cell lines express mRNAs for 3-OSTs, but also suggest an interesting possibility that each cell line expresses a unique, signature set of 3-OSTs. This possibility may be crucial for understanding the normal physiological functions of 3-OSTs. Further studies must be done to understand why 3-OSTs demonstrate cell-type specific combinations.
This study also demonstrates, for the first time, the broader significance of 3-OS HS in HSV-1 entry by showing a significant decrease in viral entry after downregulation of 3-OS HS that was specific for cells expressing 3-OSTs. Even cell lines such as HeLa, Vero, and RPE, which express multiple HSV-1 gD receptors, all showed a significant decrease in HSV-1 entry when treated with 2-OST siRNA. This suggests that 3-OS HS can still play a role in entry even when other gD receptors are present.
However, it was interesting that such a significant reduction in entry occurred in these cells (~50%) considering they express other gD receptors. It seems highly unlikely that 3-OS HS would constitute 50% of the HSV-1 gD receptors present on the cell surface considering a previous study has found that the treatment of HeLa cells with anti-nectin-1 can block HSV-1 entry (Cocchi et al., 1998). If 3-OS HS did compose 50% of the gD receptors, then how come antibodies against nectin-1 and HVEM can block entry by more than 50% ? One answer may lie in the use of assays. Our assay (down regulation by siRNA) is different than antibody blocking, which does not affect protein expression. Antibody blocking may only affect the entry process while reduction in protein expression may affect entry and any post entry events combined. It is possible that 3-OS HS is also important for additional yet unknown entry events and/or early post entry events as well, which may be a reason why we saw significant decrease in the reporter β-galactosidase activity, which in essence is dependent on immediate early transcription from the viral genome (Montgomery et al., 1996). The combined effect, however, must be specific to cell types and HSV-1 since P19N and HSV-2 did not show any such effects (Supplementary Fig. 1). Another possible explanation could be that 3-OS HS actually has a novel function as a co-receptor for nectin-1 and HVEM. Studies have shown that heparan sulfate proteoglycans can function as co-receptors for a variety of cell surface molecules (Mythreye and Blobe, 2009). Thus, it is possible that nectin-1 and/or HVEM work in concert with 3-OS HS for efficient mediation of entry and downregulating either co-receptor may have relatively profound, but possibly independent, effects on entry. A recent study (Satoh et al., 2009) suggested that CHO-K1 cells, which were used for initial identification of HVEM and nectin-1 (Montgomery et al., 1996, Spear, 2004) may naturally express low levels of 3-OST-3. The only exception here is P19N cells, it is possible that a functional analog of 3-OS HS can take over its functions during entry.
It is also possible that interfering with 3-OS HS may perturb cellular signaling mechanisms required for efficient virus entry and/or post entry events. HS functions in a variety of signaling pathways that regulate processes such as actin cytoskeleton reorganization, apoptosis, NF-κB activation, and the inflammatory response, all of which are involved in HSV-1 infection (Campo et al., 2009; Carr and Tomanek, 2006; Gregory et al., 2004; Lindahl et al., 1998; Nguyen and Blaho, 2007; O’Donnell and Shukla, 2009). While significant information is known about the role of HS in intracellular signaling, little is known about the specific contribution of 3-OS HS in these signaling pathways. Newer information suggest that 3-OS HS functions during Notch signaling (Kamimura et al., 2004), in a ligand dependent manner, and additional pathways may also be regulated by 3-OS HS (Kobayashi et al., 2007). 3-OS HS knockout mice showed postnatal lethality and intrauterine growth retardation suggesting 3-OS HS functions in other signaling pathways (Kamimura et al., 2004). 3-OS HS may also be involved in the proliferation of various cancer cells (Miyamoto et al., 2003). So it is tempting to speculate that initial interactions with nectin-1 and/or HVEM are needed to mobilize the fusion machinery while a parallel interaction of gD with 3-OS HS can in addition activate signaling pathways required for efficient viral entry. At this point, we also cannot discount the possibility that 3-OS HS may serve as a co-receptor for an unknown signaling receptor, which is required for the activation of signaling pathways required for entry. The ability of HS to act as co-receptor to bring it into contact with other signaling receptors is another feature of HS (Mythreye and Blobe, 2009). Further work may provide evidence of an unknown function of 3-OS HS during intracellular cell signaling.
This still does not explain why CHO-K1 cells expressing 3-OST-3B do not show complete inhibition of entry or cell-cell fusion when treated with 2-OST siRNA considering 3-OS HS was the only gD receptor expressed on these cells. Western blot analysis showed siRNA treatment did not completely inhibit 2-OST expression in CHO-K1 cells, suggesting there is still some 3-OS HS expression, which is one possible explanation as to why entry and cell-cell fusion were not completely inhibited. CHO-K1 cells transfected with 3-OST-3B over express the enzyme, and likely overexpress 3-OS HS, possibly making it more difficult to completely inhibit 3-OS HS expression in these cells.
This study also sheds light on the potential benefit of blocking 2-OST expression for inhibiting HSV-1 infection. While it is known that a 2-O-sulfated glucosamine is a prerequisite for 3-O sulfation, the direct impact of 2-O-sulfation on HSV-1 infection was previously unknown. We demonstrate for the first time that reduced 2-O-sulfation, due to 2-OST knockdown, can inhibit HSV-1 entry and cell-cell fusion in various cell lines. This observation identifies a target for inhibiting 3-OS HS mediated HSV-1 infection. Further studies can help elucidate whether blocking of 2-OST activity, or earlier steps in the HS biosynthetic pathway, can lead to the development of more effective microbicides. Based on this study and other previous works (Herold et al., 1996, Shukla et al., 1999), it is quite possible targeting 2-OST may inhibit HSV-1 attachment, penetration, and spread via cell-cell fusion.
Though each cell line expressing 3-OST isoforms showed decreased HSV-1 entry, the effect of 3-OS HS downregulation on cell-cell fusion was not as defined. Our study shows various cell lines are differentially affected by downregulation of 2-OST, and reduced 3-OS HS expression during HSV-1 cell-cell fusion. Unlike the other cell types studied that showed a significant decrease in cell-cell when treated with 2-OST siRNA, Vero cells treated with 2-OST siRNA showed only a minor decrease in cell-cell fusion, appearing to be the least dependent on 3-OS HS for cell-cell fusion. The controls used for this study suggest this decrease in cell-cell fusion may be a direct result of reduced 3-OS HS expression.
Further studies must be done to determine why Vero cells showed no decrease in cell-cell fusion. If 3-OS HS does function in intracellular signaling that are critical for viral entry, it is possible 3-OS HS also functions in signaling pathways activated during cell-cell fusion. While HeLa, RPE, and CHO-K1 cells may depend on 3-OS HS mediated signaling for cell-cell fusion, Vero cells may not rely on 3-OS HS for the induction of these pathway(s), possibly because Vero cells express another receptor that can perform a similar function. The fact that Vero cells expressed the least amount of 3-OST isoforms could be why these cells do not rely on 3-OH HS as much for cell-cell fusion. Unlike the other cells lines tested, Vero cells did not express 3-OST-5, and if it does express 3-OST-3B, it was still significantly less than the other cell lines. 3-OSTs have the ability to generate their own unique 3-OS HS, with its own specific set of functions. This difference in 3-OST expression between Vero cells and the rest of the cell lines tested could be contributing significantly to the differences seen between these cells during cell-cell fusion.
RPE cells expressed the widest range of 3-OST isoforms, and therefore, likely express relatively high levels of 3-OS HS. This may be a primary reason why 2-OST knockdown had such a strong effect on entry and cell-cell fusion. Ocular HSV-1 infection is a worldwide epidemic resulting in diseases such as stromal keratitis, epithelial keratitis, conjunctivitis, acute retinal necrosis, and possibly blindness (Liesegang, 2001; Uchio et al, 2000). Evidence of cell-cell fusion is particularly significant because HSV-1 infection of retinal cells can spread towards the cornea and conjunctiva, inducing the most common and dangerous manifestations of HSV-1 ocular disease. Due to lack of a curative treatment for HSV-1, prevention of HSV-1 ocular spread is of great significance. This study helps show the potential significance of 3-OS HS in preventing HSV-1 entry and spread during ocular infection. This can be studied further in animal models in vivo. Further in vivo studies will be required to develop a more accurate understanding of the HSV-1 infectious process and to assess the benefit of blocking 2-OST or 3-OS HS in animal models.
While is not completely clear why the downregulation of 2-OST, and subsequent reduction in 3-OS HS, has such a significant impact on HSV-1 entry and cell-cell fusion, this study has helped provide the groundwork for some very intriguing, and possibly very crucial, novel functions 3-OS HS and 2-OST may be playing during HSV-1 infection. Further studies not only on the specific role of 3-OS HS in HSV-1 infection, but on the physiological role of 3-OS HS in general, will be critical for furthering our understanding of 3-OS HS during HSV-1 infection.
Materials and Methods
Cells, Viruses, siRNA, and Antibodies
Wild type Chinese hamster ovarian (CHO-K1) and Vero cells were provided by P.G. Spear (Northwestern University). CHO-K1 cells were grown in Ham’s F-12 medium (Gibco/BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) and streptomycin/penicillin (P/S) (Gibco/BRL). Vero cells were grown in DMEM (Gibco/BRL) supplemented with 5% FBS and P/S. RPE cells provided by B.Y. Yue (University of Illinois at Chicago) were grown in DMEM (Gibco/BRL) supplemented with 10% FBS, 5% fetal calf serum (FCS), and P/S. HeLa cells obtained from B.P. Prabhakar (University of Illinois at Chicago) were grown in DMEM supplemented with 10% FBS and P/S. P19N cells (ATCC; CRL-1825) were cultured as previously described (Kavouras et al., 2006). The β-galactosidase expressing recombinant HSV-1(KOS) gL86 virus was provided by P. G. Spear (Northwestern University). The siRNA against human 2-OST was obtained from Sigma (Product # SASI_Hs01_00214049, SASI_Hs01_00214052). HS4C3 antibody, which targets 3-OS HS, was obtained from Toin H. van Kuppevelt (Radboud University, Nijmegen, Netherlands) (Ten Dam et al, 2006). Experiments using HS4C3 antibody were performed in a 0.5 M NaCl solution to increase the specificity of antibody to 3-OS HS.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total mRNA was isolated from the various cell lines tested using a Qiagen RNeasy kit (Qiagen Co., Valencia, CA). Superscript II reverse transcriptase (Invitrogen) was used for RT, and the following 3-OST isoform primers were used for PCR amplification of cDNA: 5’-CCGGAAGTTCTTGCTGATGC-3’ and 5’-CCGCGTGACGAAGTAACTGG-3’ (3-OST-3A); 5’-CCACTGGCTTCAGGCAAGGA-3’ and 5’-TGGACAGCGTCTGCGTGTAG (3-OST-3B); 5’-AGGCGTGGCTGAGACAGAAG-3’ and 5’-CTGGCAGAGGTTCCGTGATG-3’ (3-OST-5); 5’-CTCATCGTTGGCGTGAAGAA-3’ and 5’-GGTGGCGTTGAAGTAGAAGT -3’ (3-OST-6). RT-PCR analysis was performed as described elsewhere (Tiwari et al., 2006). Expected PCR product sizes were 604 bps (3-OST-3A), 442 bps (3-OST-3B), 777 bps (3-OST-5), and 570 bps (3-OST-6).
A similar method was used to determine 2-OST gene knockdown approximately 48 after 2-OST siRNA transfection. 2-OST primers used for PCR amplification of cDNAs were: 5’-CCAAGTTGCAGCTGCTGGCGGTGGT-3’ and 5’-CCCTGAAAAACCGGGGCAATGCTGCCTCCA-3’. For PCR amplification of β-actin, which was used as a control, the primers 5’-TCATGAAGTGTGACGTTGACATCCGT-3’ and 5’-CTTAGAAGCATTTGCGGTGCACGATG-3’ were used. Expected PCR product sizes were 792 bps (2-OST) and 285 bps (β-actin).
Western Blot Analysis
Cells mock treated, treated with scrambled siRNA, or treated with 2-OST siRNA were lysed with cell lysis buffer supplemented with protease inhibitor approximately 48 h after siRNA transfection. Cell lysate was centrifuged at 12,000 × g for 15 min at 4 °C. Proteins were resolved on SDS-PAGE gels and transferred to nitrocellulose membranes. After blocking at room temp for 2 h in Tris buffered saline (TBS) with 3% bovine serum albumin (BSA), anti-2-OST antibody (1:500) (Abgent, Catalog # AP7648a) was incubated overnight at 4 °C. After multiple washings, horse radish peroxidase (HRP) conjugated anti–rabbit IgG (Jackson Immuno-Research Laboratories) was added for 1 h at room temperature. After another round of multiple washings, immunoreactive bands were developed with Super Signal West Femto Maximum Sensitivity substrate (Pierce Biotechnology) and imaged on KODAK Biomax MR film. β-actin protein expression was measured as a loading control.
Cell Imaging
HeLa cells were mock treated, transfected with scrambled siRNA, or transfected with 2-OST siRNA, and after 48 h, cells were replated in 35 mm glass bottom dishes (MatTek) and incubated with HS4C3, an antibody specifically targeting 3-OS HS, for 1 h at 4 °C. During replating, cells were disassociated from the cell surface using Cell Disassociation Buffer Enzyme-Free Hank’s based (Gibco). After cells were replated into glass bottom dishes, the cells were incubated at 37 °C for about 3-4 hours to allow the cells to adhere to bottom of the dishes before the HS4C3 antibody was added. Cells were washed with phosphate buffer saline (PBS), fixed for 20 min but not permeablized, and washed again with PBS. Next, FITC-conjugated anti-mouse IgG (Sigma) was added to cells for 1 h. Cells were washed again and fresh media was added. FITC labeling was observed at the 60x NA 1.40 oil objective on a confocal microscope (Leica DMIRE2) equipped with a camera (Leica TCSSP2). Images were captured using Leica Confocal Software v. 261.
HSV-1 Viral Entry Assays
Entry assays were performed as previously described (Shukla et al, 1999). Briefly, cell lines were mock treated, transfected with scrambled siRNA or 2-OST specific siRNA using LipofectAMINE 2000 (Invitrogen). CHO-K1 cells were previously transfected with 1 μg of 3-OST-3B, nectin-1, or HVEM also using LipofectAMINE 2000. After approximately 48 h, cells were disassociated from the culture dishes using Cell Disassociation Buffer Enzyme-Free Hank’s based (Gibco) and replated into 96-well culture dishes. After the cells were replated, the cells were incubated at 37 °C for about 3-4 hours to allow the cells to adhere to bottom of the dishes before virus was added. After the cells were adhered, cells were infected with HSV-1(KOS) gL86 in a twofold serial dilution for 6 h at 37 °C. After 6 h, cells were washed with PBS and the soluble substrate o-nitrophenyl-β-d-galactopyranoside (ONPG) was added. Enzymatic activity was measured at 410 nm using a micro-plate reader (Spectra Max 190, Molecular Devices, Sunnydale, CA USA). Cells infected with the β-galactosidase expressing recombinant HSV-2 (333) gJ- virus strain was used as a control. A similar protocol was followed as described above.
Viral Binding Assay
A previously described virus binding assay was used (Scanlan et al., 2005). Cells that were mock treated, transfected with scrambled siRNA, or transfected with 2-OST specific siRNA were replated into 96 well dishes about 48 h after siRNA transfection using Cell Disassociation Buffer Enzyme-Free Hank’s based (Gibco). After incubating the cells at 37 °C for about 3-4 hours to allow them to adhere to bottom the 96 well dishes, the cells were washed once with PBS before addition of virus. Cells were infected with HSV-1 (K26GFP), diluted in 50 μl of Opti-MEM (Gibco), at an M.O.I. of 10 for 1 h at 4 °C. Cells treated with Opti-MEM alone served as a negative control. After, cells were gently washed with PBS. Fluorescence readings were taken using a GENios Pro fluorescence reader.
Cell-cell fusion assays
Cell-cell fusion assays were performed as previously described (Pertel et al., 2001). For each cell line, cells were split into two populations. “Target” cells were cotransfected with the luciferase reporter gene (0.5 μg) and either mock treated, transfected with scrambled siRNA, or transfected with 2-OST siRNA. CHO-K1 cells were additionally transfected with 1 μg of 3-OST-3B. “Effector” cells were transfected with plasmids expressing HSV-1 glycoproteins required for cell-cell fusion (gD, gB, gH, gL) (0.5 μg) and T7 RNA polymerase (0.5 μg). After 48 h, target and effector cells were mixed together in a 1:1 ratio and replated into 24-well culture dishes. Luciferase activity was measured 24 h later. As a negative control, target cells were mixed with effector cells lacking HSV-1 gD. For a control, fusion was also measured when effector cells were transfected with 0.5 μg of the four glycoproteins necessary to mediate HSV-2 cell-cell fusion: gB-2, gD-2, gH-2, and gL-2.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Patricia Spear (Northwestern University), Dr. Prashant Desai (Johns Hopkins University) and Dr. Toin van Kuppevelt (Nijmegen Centre for Molecular Life Sciences) for reagents. The authors would also like to thank Myung-Jin Oh for help with cell culture. This work was supported by National Institutes of Health grant RO1 AI057860 to D. Shukla and a core grant EY01792.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher D. O’Donnell, Email: codonn3@uic.edu.
Maria Kovacs, Email: marcsika101@yahoo.com.
Jihan Akhtar, Email: jakhta2@uic.edu.
Tibor Valyi-Nagy, Email: tiborv@uic.edu.
Deepak Shukla, Email: dshukla@uic.edu.
References
- Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Sama D, Calatroni A. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J Cell Biochem. 2009;106:83–92. doi: 10.1002/jcb.21981. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Tomanek L. Herpes simplex virus and the chemokines that mediate the inflammation. Curr Top Microbiol Immunol. 2006;303:47–65. doi: 10.1007/978-3-540-33397-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174:1009–21. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein LE, Calio AJ, Cunha BA. Herpes simplex (HSV-1) aseptic meningitis. Heart Lung. 2004;33:196–197. doi: 10.1016/j.hrtlng.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Gregory D, Hargett D, Holmes D, Money E, Bachenheimer SL. Efficient replication by herpes simplex virus type 1 involves activation of the IkappaB kinase-IkappaB-p65 pathway. J Virol. 2004;78:3582–13590. doi: 10.1128/JVI.78.24.13582-13590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarr L, Shukla D, Rodahl R, Dal Canto MC, Spear PG. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology. 2001;287:301–309. doi: 10.1006/viro.2001.1041. [DOI] [PubMed] [Google Scholar]
- Herold BC, WuDunn D, Soltys N, Spear PG. Glycoprotein C of herpes simplex virus type 1 plays a principle role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold BC, Gerber SI, Belval BJ, Siston AM, Shulman N. Differences in the Susceptibility of Herpes Simplex Virus Types 1 and 2 to Modified Heparin Compounds Suggest Serotype Differences in Viral Entry. J Virol. 1996;70:3461–3469. doi: 10.1128/jvi.70.6.3461-3469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K, Rhodes JM, Ueda R, McNeely M, Shukla D, Kimata K, Spear PG, Shworak NW, Nakato H. Regulation of Notch signaling by Drosophila heparan sulfate 3-O sulfotransferase. J Cell Biol. 2004;166:1069–79. doi: 10.1083/jcb.200403077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavouras JH, Prandovszky E, Valyi-Nagy K, Kovacs SK, Tiwari V, Kovacs M, Shukla D, Valyi-Nagy T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J Neurovirol. 2007;13:416–425. doi: 10.1080/13550280701460573. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Habuchi H, Tamura K, Ide H, Kimata K. Essential role of heparan sulfate 2-O-sulfotransferase in chick limb bud patterning and development. J Biol Chem. 2007;282:19589–97. doi: 10.1074/jbc.M610707200. [DOI] [PubMed] [Google Scholar]
- Lawrence R, Yabe T, Hajmohammadi S, Rhodes J, McNeely M, Liu J, Lamperti ED, Toselli PA, Lech M, Spear PG, Rosenberg RD, Shworak NW. The principal neuronal gD-type 3-O-sulfotransferases and their products in central and peripheral nervous system tissues. Matrix Biol. 2007;26:442–455. doi: 10.1016/j.matbio.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Kusche-Gullberg M, Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- Liu J, Shworak NW, Sinaÿ P, Schwartz JJ, Zhang L, Fritze LM, Rosenberg RD. Expression of heparan sulphate D-glucosaminyl 3-O-sulphotransferase isoforms reveals novel substrate specificities. J Biol Chem. 1999;274:5185–5192. doi: 10.1074/jbc.274.8.5185. [DOI] [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Nguyen ML, Blaho JA. Apoptosis during herpes simplex virus infection. Adv Virus Res. 2007;69:67–97. doi: 10.1016/S0065-3527(06)69002-7. [DOI] [PubMed] [Google Scholar]
- Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77:5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell CD, Tiwari V, Oh MJ, Shukla D. A role for heparin sulfate 3-O-sulfotransferase isoform 2 in herpes simplex virus type 1 entry and spread. Virology. 2006;346:452–459. doi: 10.1016/j.virol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Pertel P, Fridberg A, Parish M, Spear PG. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH–gL requires a gD receptor but not necessarily heparan sulfate. Virology. 2001;279:313–324. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Showrak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system: specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99:2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan PM, Tiwari V, Bommireddy S, Shukla D. Spinoculation of heparan sulfate deficient cells enhances HSV-1 entry, but does not abolish the need for essential glycoproteins in viral fusion. J Virol Methods. 2005;128:104–112. doi: 10.1016/j.jviromet.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Shieh MT, WuDunn D, Montgomery RI, Esko JD, Spear PG. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Shworak NW, Liu J, Petros LM, Schwartz JJ, Zhang L, Kobayashi M, Copeland NG, Jenkins NA, Rosenberg RD. Multiple isoforms of heparan sulfate d-glucosaminyl 3-O-sulfotransferase. Isolation, characterization, and expression of human cDNAs and identification of distinct genomic loci. J Biol Chem. 1999;274:5170–5184. doi: 10.1074/jbc.274.8.5170. [DOI] [PubMed] [Google Scholar]
- Spear PG. Herpes Simplex Virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Kitagawa H. Heparin and Heparan Sulfate biosynthesis. IUBMBLife. 2002;54:163–175. doi: 10.1080/15216540214928. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Dam GB, Kurup S, van de Westerlo EM, Versteeg EM, Lindahl U, Spillmann D, van Kuppevelt TH. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J Biol Chem. 2006;281:4654–4662. doi: 10.1074/jbc.M506357200. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Clement C, Scanlan PM, Tue BYJT, Shukla D. A role for HVEM as the receptor for herpes simplex virus-1 entry into primary human trabecular meshwork cells. J Virol. 2005a;79:13173–13179. doi: 10.1128/JVI.79.20.13173-13179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, O’Donnell CD, Oh MJ, Valyi-Nagy T, Shukla D. A role for 3-O sulfotransferase isoform 2 in herpes simplex virus type 1 entry and spread. Virology. 2005b;346:452–459. doi: 10.1016/j.virol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Clement Cl, Xu D, Valyi-Nagy T, Yue BY, Liu J, Shukla D. A role for 3-O-sulfated heparin sulfate as the receptor for herpes simplex virus type 1 entry into primary human corneal fibroblasts. J Virol. 2006;80:8970–8980. doi: 10.1128/JVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Oh M-J, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J. 2008;275:5272–85. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchio E, Takeuchi S, Itoh N, Matsuura N, Ohno S, Aoki K. Clinical and epidemiological features of acute follicular conjunctivitis with specia reference to that caused by herpes simplex virus type 1. Br J Ophthalmol. 2000;84:968–972. doi: 10.1136/bjo.84.9.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–89. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Chen J, Tiwari V, Ju W, Li JP, Malmström A, Shukla D, Liu J. Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus type1. J Biol Chem. 2002;277:37912–37919. doi: 10.1074/jbc.M204209200. [DOI] [PubMed] [Google Scholar]
- Xu D, Tiwari V, Xia G, Clement C, Shukla D, Liu J. Characterization of heparan sulphate 3-O-sulphotransferase isoform 6 and its role in assisting the entry of herpes simplex virus type 1. Biochem J. 2005;385:451–459. doi: 10.1042/BJ20040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Hata T, He J, Maeda N. Developmental and regional expression of heparan sulfate sulfotransferase genes in the mouse brain. Glycobiology. 2005;15:982–993. doi: 10.1093/glycob/cwi090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


