Abstract
Exceptional survival results from complicated interplay between genetic and environmental factors. The effects of these factors on survival are mediated by the biological and physiological variables, which affect mortality risk. In this paper, we evaluated the role of blood glucose (BG) in exceptional survival using the Framingham Heart Study data for the main (FHS) and offspring (FHSO) cohorts. We found that: (i) the average cross-sectional age patterns of BG change over time; (ii) the values of BG level among the longest lived individuals in this study differ for different sub-cohorts; (iii) the longitudinal age patterns of BG differ from those of cross-sectional ones. We investigated mechanisms forming average age trajectories of BG in the FHS cohort. We found that the two curves: one, characterizing the average effects of allostatic adaptation, and another, minimizing mortality risk for any given age, play the central role in this process. We found that the average BG age trajectories for exceptional survivors are closer to the curve minimizing mortality risk than those of individuals having shorter life spans. We concluded that individuals whose age trajectories of BG are located around the curve minimizing chances of premature death at each given age have highest chances of reaching exceptional longevity.
Keywords: mortality risk, stochastic process model of aging, allostatic adaptation, age-specific physiological norm, blood glucose, Framingham Heart Study
1. Introduction
Exceptional survivors are individuals whose genetic construction adequately met the challenges of life resulting in life spans substantially exceeding those of less lucky members of the birth cohort. Exact definition of exceptional survivors differs from study to study and includes individuals survived to age 90 years, 100 years, or those who belong to the 10% percentile of longest lived individuals, etc. In many cases the definition is chosen to increase reliability of conclusions for a given sample size of the data. The biodemography of exceptional survival investigates biological and physiological variables capable of maximizing the survival probability. Although the exact list of determinants of exceptional survival remains unknown, a number of findings indicate an important role of physiological variables mediating effects of internal (e.g., genetic) and external (e.g., environmental) factors in this process. Note, that in order to reach exceptional longevity, an individual had to avoid premature death earlier in the life course. This requirement restricts the range of past values of biological and physiological variables: they have to be close to certain “optimal” values at any given age. That is why the determinants of exceptional survival are likely to be age trajectories rather than values of some indices. This observation is in concert with recent advances in statistical methodology of survival analyses where dynamic and stochastic aspects of aging related changes are explicitly taken into account.
Note that the attempts to take dynamic stochastic aspects of aging into account have been made in the middle of the last century (Strehler and Mildvan 1960; Sacher and Trucco 1962). However, only recently applications of such approaches to the analyses of data on aging related changes, linked with disease and mortality outcomes, became possible. This is because the description of the aging related deterioration in health and functioning has been performed together with development of statistical methods capable of analyzing data on measurements of such deterioration together with data on health and survival events. The details of the approach, which is called “degradation modeling,” are described in Bagdonavicius and Nikulin (2002). The parametric, semiparametric, and non-parametric versions of the approach have been developed and applied to available data (Bagdonavicius and Nikulin 2004; Bagdonavicius et al. 2006). The approach allows for analyzing non-proportional hazards models (Bagdonavicius and Nikuline 2005). Application of this approach to studying dementia and other disorders in health in the elderly are described by Nikulin et al. (2006). These approaches are well adapted to study the longitudinal data of different nature including bio-medical data on developing complex diseases in presence of the processes of aging (see also Sasco and Nikulin 2008 and references in it). The approach we use in this paper deals with the particular type of degradation model described in terms of stochastic differential equations and the quadratic hazard (Yashin et al. 2007). This approach is convenient for analyzing data on changes in physiological state with age. Here we implemented it to the analyses of the role of blood glucose (BG) in exceptional survival.
Several recent studies emphasized an importance of proper glucose metabolism and related metabolic, anthropometric, and endocrine characteristics in exceptional longevity (Paolisso et al. 2000; Barbieri et al. 2001). Comparing factors capable of contributing to longevity in individuals of different age with those of centenarians, Paolisso et al. (1996) found that a number of variables characterizing glucose metabolism in healthy centenarians are better than those observed in the 70-75 years old individuals, and resemble those observed in the 50-55 years old ones. The result looked surprising because earlier studies indicated that aging is associated with worsening of glucose metabolism (e.g., an increasing level of fasting blood glucose, insulin resistance, etc.) mainly due to an aging related decline in insulin mediated glucose uptake (Fink et al. 1983; Rowe et al. 1983). Barbieri et al. (2001) came to conclusion that connection between impairments in glucose metabolism and aging is mediated by the four main groups of processes, including: (i) anthropometric changes; (ii) changes in the diet and physical activity; (iii) neuron-hormonal variations; and (iv) a rise in oxidative stress. The authors emphasized an important role of progressive remodeling taking place in an aging organism which may partly compensate local aging related disturbances in functioning and thereby contribute to survival during the life course accompanying the aging process (Franceschi et al. 2000). It was, however, unclear whether an individual level of BG declined with age after ages 70-75 in healthy centenarians or this level followed a certain optimal age trajectory during the entire life course. In this paper, we investigate the role of blood glucose level in exceptional survival and emphasize the dynamic aspects of this problem using data collected in the Framingham Heart Study (FHS) and the Framingham Heart Study Offspring cohort (FHSO).
2. Data and Methods
FHS data
The Framingham Heart Study was set up more than 60 years ago to evaluate the relationships between potential risk factors of CHD, heart attack, stroke, or death in individuals, who had not yet developed these conditions (Dawber 1980). In the 1948, the study recruited 5209 non-institutionalized white subjects (2336 males and 2873 females, with 993 surviving participants as of February 28, 1999) between the ages of 28 and 62 in the town of Framingham, Massachusetts. A total of 5128 of 5209 these participants were described as free of “overt CHD.” Since then, the participants of the original cohort have been reexamined biennially for a physical examination, laboratory tests, a detailed medical history, and an extensive testing for cardiovascular risk factors including levels of blood glucose.
The FHSO dataset consists of a sample of 3,514 biological descendants of the Original Cohort, 1,576 of their spouses and 34 adopted offspring for a total sample of 5,124 subjects; 52% female (Kannel et al. 1979a). The FHSO subjects were enrolled in 1971-1975 using research protocols similar to those of the FHS so that comparisons of the results from the FHSO and the FHS could be made. As of February 1998, there were about 4,524 offspring surviving with only 20 lost to follow-up and 4 in whom survival status was unknown. In this paper, we considered the data on blood glucose using 19 exams (1-4, 6, 8-10, 13-23) of the FHS data and in five exams (1, 3-6) of the FHSO data.
The ideal approach to measuring blood glucose would have involved a glucose tolerance test and analysis of fasting glucose at each examination, but at the time the FHS study was planned, this did not appear practicable (Dawber 1980). Glucose tolerance testing was not systematically performed in the study and in the most of exams blood glucose measurements were casual (i.e., randomly fasting or non-fasting) (Kannel et al. 1979b; Port et al. 2006). The amount of glucose was determined in the subject’s whole blood, in milligrams per 100 milliliters. A recent study (Port et al. 2006) verified that the age- and sex-adjusted deciles of the fasting and random glucose distributions in examinations where fasting specimen was available are very close to the random ones (blood was drawn after many hours of examination), being slightly lower. Taking this into account, we equated measurements of random glucose with those of fasting glucose in the analyses of their age trajectories and used the notation “blood glucose” (BG) for all these measurements. According to the WHO criteria, the levels of BG exceeding 140mg/100 mL are associated with the Type 2 Diabetes Mellitus (T2DM).
Statistical analyses
The version of stochastic process model of aging (Yashin et al. 2007) was used in analyses of adaptive mechanisms forming the age trajectories of average BG. Detailed mathematical description of estimation procedures can be found in Yashin et al. (2007). Here we focus on the one-dimensional version of the model as applied to the data on BG in the FHS.
Description of individual BG trajectories
The model describes individual age trajectories of BG using stochastic differential equation
| (1) |
Here Yt is the value of a particular physiological index (i.e., BG in our case) at age t in an arbitrarily chosen individual, ft1 describes the effect of allostatic adaptation, which BG is forced to follow by homeostatic regulation. The strength of homeostatic regulation is characterized by the negative feedback coefficient, a(t). The coefficient B(t) characterizes the contribution of external disturbances described by a Wiener process, Wt. The random variable Y0 describes the variability of the initial value of the physiological index (BG), which is assumed to be independent of Wt. for each t ≥ 0 The introduction of ft1 and a(t) into the model facilitates the biological interpretation of the results of statistical analyses of longitudinal data. The discrete-time version of this model used in data analyses is described in Yashin et al. (2007).
Description of conditional mortality rate
Individual trajectories describing BG level must be stopped at random time characterizing life span of an individual. The probability distribution of this stopping time is characterized by conditional mortality rate given the value of the BG. This mortality rate is represented by the quadratic form:
| (2) |
The term μ0(t) is a function of age. It shows how the total mortality rate would change if the respective physiological index Yt. followed the optimal trajectory ft. The function ft is associated with the notion of the age-dependent BG “norm” in the model. The positive function Q(t) shows how the steepness of the parabola representing the quadratic hazard changes with age.
The model outlined above takes into account the fact that available longitudinal data (including the FHS data) do not contain records characterizing when and how external disturbances affected individuals during their life course. So, the direct estimation of external effects is not possible. The studies of how persistent external unfavorable conditions get “under the skin” of affected person increasing his/her susceptibility to diseases and death (Sterling and Eyer 1988; McEwen 2000) resulted in understanding that many such conditions affect set-points of physiological homeostasis changing physiological balance from the “normal”, ft , to “abnormal”, ft1, state. These effects, represented in equation (1), were estimated from the FHS data on BG using the statistical estimation procedure by Yashin et al. (2007), thereby providing indirect evaluation of the effects of external disturbances without measuring them.
3. Results
Age structures of the FHS-FHSO population
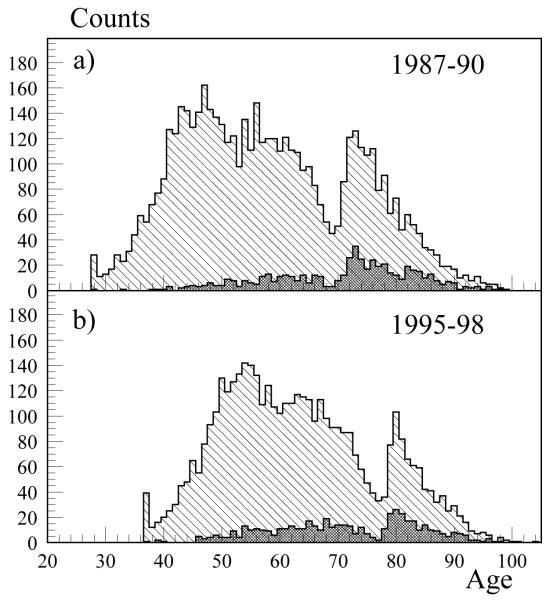
The participants of the FHS-FHSO comprise a population of individuals with its own age structure. This population evolves over time following demographic laws, which allow us to consider and investigate both cross-sectional and longitudinal age patterns of various physiological, demographic and health characteristics. At each given time point (corresponding to the years of exams), the FHS-FHSO population can be represented by its age structure. The population health can be represented by the age structure of individuals having a selected health problem. For example, serious impairments in blood glucose homeostasis result in the T2DM and may occur at different ages. The age structures of the FHS-FHSO population as well as population of individuals having T2DM (dark grey columns) are shown in Fig. 1 for two time points: 1987-1990 and 1995-1998. Together with other age groups, this diagram represents exceptional survivors with their health problems. Such representation is convenient because it visualizes the population health structure and shows how it changes with age and over time. To address the question to what extent the level of BG could be considered as a factor contributing to exceptional survival, one has to consider the diagram showing an average level of BG for individuals of different age and compare whether its level for exceptional survivors differs from those of other age categories.
Fig. 1.
Age structures of the FHS-FHSO populations in (a) 1987-1990 and (b) 1995-1998 (light grey columns) and populations of individuals having T2DM (dark grey columns) in these periods
Cross-sectional age trajectories of BG
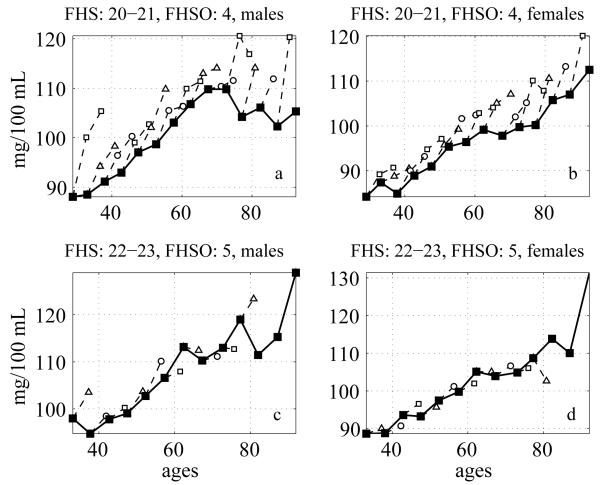
The respective cross-sectional age patterns of average level of BG are shown in Fig. 2 (solid lines). Note that since the dates of exams in the FHS and in the FHSO do not coincide, each point on these graphs represents the average of values measured in two neighboring FHS exams (approximately corresponding to the respective FHSO exam). One can see from this figure that the patterns depend on the dates when respective cross-sectional measurements have been performed. The pattern shown in the upper left panel indicates that the level of BG for males increases up to age 70, then declines and remains about the same at ages 80-95. It is interesting that the level of BG in the oldest old males is smaller than that measured in 70-year old individuals and is similar to that of 55-60-year old individuals. The conclusion, which could be made from the analysis of this graph, is similar to that given by Barbieri et al. (2001) who found that the glucose metabolism in healthy centenarians was better than that among the old individuals and similar to that of individuals in adult ages. In the upper left panel for males and in the upper right panel for females the BG level increases over time for each sub-cohort comprising cross-sectional data. Similar graph, constructed from the BG measurements in males performed four years later is shown in the left lower panel. One can see that the BG level for the oldest old became higher than in the old ages, so the analogy with the Barbieri et al. (2001) result is no longer valid. Females’ patterns of the BG can be approximated by straight lines in both cases (right upper and lower panels).
Fig. 2.
Cross-sectional average trajectories of BG (solid lines) in different FHS and FHSO exams: a) males, FHS exams: 20-21, FHSO exam: 4; b) females, FHS exams: 20-21, FHSO exam: 4; c) males, FHS exams: 22-23, FHSO exam: 5; d) females, FHS exams: 22-23, FHSO exam: 5. Respective values for individuals from different age groups in the subsequent exams are shown by the dashed lines
The longitudinal trends are shown in the same figures for the sub-cohorts represented by different age groups in cross-sectional data (dashed lines). The presence of such trends indicates that cohort changes do not always follow cross-sectional age patterns of physiological indices. One can see from these graphs that exceptional survivors have different average values of the BG at different time points. Indeed, Fig. 2 displays cross-sectional age patterns of BG measured at different time points. Top panels represent BG values measured in the FHS exams 20-21(conducted in years 1986-1992) and the FHSO exam 4 (years 1987-1990). Bottom panels represent BG values measured in the FHS exams 22-23 (years 1990-1996) and the FHSO exam 5 (years 1991-1995). Let us consider individuals having age 90 years and over. Respective BG levels for these age groups are shown by the right end points at each of the four panels represented in Fig. 2. Individuals from this age group at the top panels represent earlier birth cohort than individuals of the same age group at the bottom panels. The average level of BG for 90+ old males at the top panel is about 105 mg/100mL. The average level of BG for males from the same age group at the bottom panel is 130 mg/100mL. Similar difference can be observed for females. The top panels represent BG levels measured at earlier time point than the bottom panels. Therefore the groups of 90+ males and females, considered as exceptional survivors in this paper, have different values of BG at different time points.
Longitudinal average age trajectories of BG
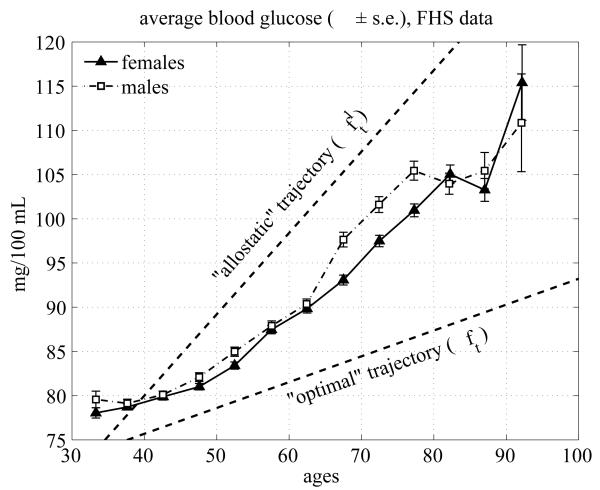
The average age trajectories of BG observed longitudinally in the pooled sample of the FHS participants are shown in Fig. 3 for males and females. One can see from this figure that males and females have similar age trajectories with slightly higher values for males. Both curves almost monotonically increase with age until about 75-80 years for males and 80-85 years for females. After these ages, the BG level slightly declines and then increases after the ages of 80-85 for males and 85-90 years for females.
Fig. 3.
Average age trajectories of BG in the FHS (pooled data from all exams) for females and males; dashed lines denote the allostatic curve (upper line, ft1) and the optimal curve (lower line, ft) approximated by linear functions and estimated using the model by Yashin et al. (2007)
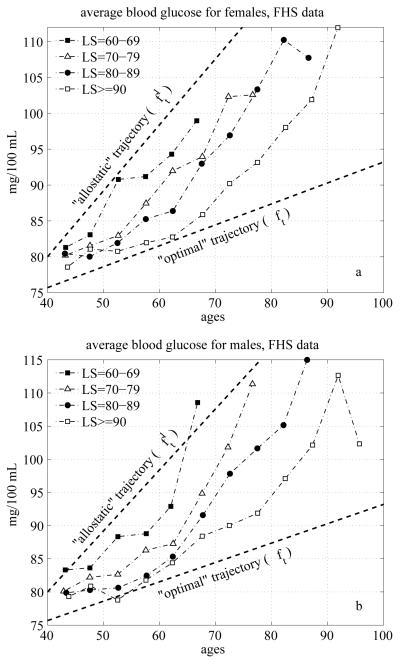
It is important to note that the BG trajectories shown in Fig. 3 do not represent the average biological changes in the BG concentration. This is because compositional changes due to the mortality selection may affect the dynamic properties of these curves. Since the BG level is an established risk factor, individuals whose BG levels are too high (i.e., substantially deviate from the “normal” level) have increased chances of dying first. Hence, these individuals drop out of the averaging procedure thereby affecting the average BG age trajectories. The longitudinal patterns shown in Fig. 3 also reflect the effects of biological adaptation of the BG level to changes induced by persistent external disturbances and aging-related changes developing in a human body. These changes can be captured using the extended version of stochastic process model of human mortality and aging adjusted to analyses of longitudinal data (Yashin et al. 2007). This model includes two functions (shown by dashed lines in Fig. 3) which play a central role in regulating dynamic properties of the average longitudinal BG trajectories. These functions are approximated by straight lines in our analyses. The upper line ft1 characterizes the tendency to increase the BG level due to effect of allostatic adaptation to persistent external disturbances. This tendency results in impaired glucose metabolism and T2DM. The lower line ft describes the optimal trajectory corresponding to the minimal value of mortality risk for each given age. The average age trajectories of BG level for two genders, shown between these lines, resulted from the action of these two regulating mechanisms. Before describing the mechanisms involved in regulation of average BG levels it is useful to compare average BG trajectories in the group of exceptional survivors with those of individuals from the groups with shorter life spans. BG changes for long lived and short lived individuals. If the deviations from the optimal function (normal curve) ft towards the allostatic curve ft1 increase mortality risk, one can expect that the average BG trajectories constructed for exceptional survivors (long lived individuals) will tend to be located closer to the optimal curve ft than those constructed for the short lived individuals. To empirically test this conjecture, we constructed average BG trajectories for individuals died at different ages. Fig. 4 shows average age trajectories of BG for the exceptional survivors (ES) and short lived males and females.
Fig. 4.
Average age trajectories of BG for exceptional survivors (life span (LS) exceeding 90 years (“LS>=90”)) and short lived (those with LS=60-69, 70-79 and 80-89) females (a) and males (b); dashed lines denote the allostatic curve (upper line, ft1) and the optimal curve (lower line, ft) approximated by linear functions and estimated using the model by Yashin et al. (2007)
The short lived individuals are represented by the groups of those who died between the ages of 60 and 69 (denoted by “LS=60-69”), 70 and 79 (“LS=70-79”), and 80 and 89 years (“LS=80-89”). The group of ES includes those whose age at death exceeded 90 years (“LS>=90”). Note that until the age 90 the average trajectories for the group of ES describe average biological changes in BG among these individuals.
One can see from these figures that for all selected sub-cohorts of short lived individuals the average age trajectories of BG are located closer to ft1 than that for the exceptional survivors (ES individuals) for both genders. The BG trajectories of exceptional survivors are located closer to the optimal curve ft, for both genders as predicted. These observations confirm interpretation of curves ft1 and ft as set-point functions in dynamic regulation of average values of BG given in the previous section. It is important to note that the consumption of sugar dramatically increased during the last century, so different birth cohorts experienced different exposure to nutritional sugar. Therefore in Fig. 4 we show age trajectories of BG for individuals taken from the same birth cohorts (due to a limited sample size of the FHS data, we combined different birth cohorts and used those aged 40-60 years at the time of the first FHS exam) and show that those who have shorter life spans have higher age pattern of BG.
Mechanisms regulating average values of BG in the cohort
It is shown by Yashin et al. (2007) that trajectories of mean values (m) of physiological indices (Y) described by equations (1) and (2) satisfy the following ordinary differential equation:
| (3) |
Here the coefficient γ(t) is a positive function of age (Yashin et al. 2007) and all other coefficients are specified in the descriptions of equations (1) and (2). The coefficient a(t) is negative by the definition. Thus, equation (3) describes two negative feedback mechanisms regulating the age trajectory of m(t). One of them, characterized by the feedback loop coefficient a(t), deals with homeostatic adaptation to the values of slow-time allostatic response ft1 to persistent external disturbances (e.g., stresses of life). The role of this mechanism is to keep the value of m(t) around the function ft1. This function estimated for the values of BG in the FHS data is represented by the upper straight line in Fig. 3 and Fig. 4. The second mechanism is represented by the negative feedback loop describing the effect of mortality selection on m(t) (the average age trajectory of BG). This mechanism is characterized by the feedback coefficient -2γ(t)Q(t). Its task is to keep the value of m(t) around the function ft, the optimal age trajectory of BG (i.e., the function of age which minimizes mortality risk at a given age). This function estimated from the FHS data on BG is shown by the lower straight line in Fig. 3 and Fig. 4.
4. Discussion
Studying biodemography of exceptional survival involves evaluation of connection between biological and physiological indices and mortality risk. The results of our analyses indicate that exceptional survival requires dynamic investments eliminating tendency of physiological state to substantially deviate from its “optimal” age pattern due to persistent external disturbances. At each given age one has to keep the values of physiological indices around certain optimal values. These values are not fixed for the entire life course but change with individual’s age. Respective “optimal” curve can be evaluated from longitudinal data.
The analyses emphasized an importance of two dynamic mechanisms forming age trajectories of average physiological indices. One is biological. It deals with the negative feedback loop, which tends to keep the trajectory of each index (the BG in our case) around respective allostatic curve ft1. This curve summarizes average effects of persistent external disturbances on physiological state making gradual changes in homeostatic set-point functions in equation (1). The second mechanism represents effects of mortality selection. It is described by negative feedback loop which tends to keep the average trajectory around the optimal curve ft. The resulting curve m(t) is a compromised solution. It is located between two functions ft and ft1 which in our analyses are approximated by straight lines (see Fig. 3). In the absence of such disturbances ft1 coincides with ft, so homeostatic regulation in equation (1) keeps an individual trajectory of physiological index around the optimal curve ft. In this case, the average trajectory m(t) of physiological index is also kept around ft.
The approach used in this paper for evaluating the role of BG in exceptional survival can be implemented for studying other physiological and biological indices and their effects on survival. The approach can be extended for considering multidimensional case when several mutually dependent physiological indices dynamically affect morbidity and mortality risks. This will require a model describing changes in multidimensional physiological space and will involve more intensive computations.
The limitations of this approach deal with the fact that it evaluates average effects of allostatic adaptation and average optimal (normal) curves. It would be useful to investigate individual dynamic effects of allostatic adaptation and individual optimal age trajectories of physiological indices. Such extension, however, would involve more data and require development of more sophisticated dynamic models describing numerous aging related changes in their mutual connection and their effects on mortality risk.
Acknowledgements
The research reported in this paper was supported by the National Institute on Aging grants R01AG027019, R01AG028259, and 5P01AG008761. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. The Framingham Heart Study (FHS) is conducted and supported by the NHLBI in collaboration with the FHS Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the FHS or the NHLBI.
References
- Bagdonavicius V, Bikelis A, Kazakevicius V, Nikulin M. Non-parametric estimation in degradation-renewal-failure models. In: Nikulin M, Commenges D, Huber C, editors. Probability, Statistics and Modelling in Public Health. Springer; New York: 2006. pp. 23–36. [Google Scholar]
- Bagdonavicius V, Nikulin M. Accelerated Life Models. Chapman and Hall/CRC; Boca Raton: 2002. [Google Scholar]
- Bagdonavicius V, Nikulin M. Semiparametric Analysis of Degradation and Failure Time Data with Covariates. In: Nikulin MS, Balakrishnan N, Mesbah M, Limnios N, editors. Parametric and Semiparametric Models with Applications to Reliability, Survival Analysis, and Quality of Life. Birkhauser; Boston: 2004. pp. 41–65. [Google Scholar]
- Bagdonavicius V, Nikuline M. Analysis of survival data with non-proportional hazards and crossings of survival functions. In: Edler L, Kitsos C, editors. Recent Advances in Quantitative Methods in Cancer and Human Health Risk Assessment. John Wiley and Sons; New York: 2005. pp. 197–210. [Google Scholar]
- Barbieri M, Rizzo MR, Manzella D, Paolisso G. Age-related insulin resistance: is it an obligatory finding? The lesson from healthy centenarians. Diabetes Metab Res Rev. 2001;17:19–26. doi: 10.1002/dmrr.178. [DOI] [PubMed] [Google Scholar]
- Dawber TR. The Framingham Study: The Epidemiology of Atherosclerotic Disease. Harvard University Press; Cambridge, MA: 1980. [Google Scholar]
- Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71:1523–1535. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, De Benedictis C. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol. 2000;35:879–896. doi: 10.1016/s0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979a;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Gordon T, Castelli WP. Obesity, lipids, and glucose intolerance. The Framingham Study. Am J Clin Nutr. 1979b;32:1238–1245. doi: 10.1093/ajcn/32.6.1238. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Nikulin M, Barberger-Gateau P, Bagdonavicius V. Accelerated degradation model and its applications to statistical analysis of the role played by dementia and sex in the loss of functional autonomy in elderly patients. Advances in Gerontology. 2006;19:44–54. [PubMed] [Google Scholar]
- Paolisso G, Barbieri M, Bonafe M, Franceschi C. Metabolic age modelling: the lesson from centenarians. Eur J Clin Invest. 2000;30:888–894. doi: 10.1046/j.1365-2362.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Gambardella A, Ammendola S, Damore A, Balbi V, Varricchio M, Donofrio F. Glucose tolerance and insulin action in healthy centenarians. Am J Physiol Endocrinol Metabol. 1996;270:E890–E894. doi: 10.1152/ajpendo.1996.270.5.E890. [DOI] [PubMed] [Google Scholar]
- Port SC, Boyle NG, Hsueh WA, Quinones MJ, Jennrich RI, Goodarzi MO. The predictive role of blood glucose for mortality in subjects with cardiovascular disease. Am J Epidemiol. 2006;163:342–351. doi: 10.1093/aje/kwj027. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Minaker KL, Pallotta JA, Flier JS. Characterization of the insulin resistance of aging. J Clin Invest. 1983;71:1581–1587. doi: 10.1172/JCI110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher GA, Trucco E. The stochastic theory of mortality. Ann N Y Acad Sci. 1962;96:985–1007. doi: 10.1111/j.1749-6632.1962.tb54116.x. [DOI] [PubMed] [Google Scholar]
- Sasco AJ, Nikulin MS. Flexible regression models for dynamic prediction of survival in elderly in presence of cancer or other chronic diseases. Advances in Gerontology. 2008;21:41–48. [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A New Paradigm to Explain Arousal Pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. John Wiley & Sons; New York: 1988. pp. 629–649. [Google Scholar]
- Strehler BL, Mildvan AS. General theory of mortality and aging. Science. 1960;132:14–21. doi: 10.1126/science.132.3418.14. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Akushevich I, Kulminski A, Akushevich L, Ukraintseva SV. Stochastic model for analysis of longitudinal data on aging and mortality. Math Biosci. 2007;208:538–551. doi: 10.1016/j.mbs.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]