Abstract
The glomerular basement membrane and its associated cells are critical elements in the renal ultrafiltration process. Traditionally the anionic charge associated with several carbohydrate moieties in the glomerular basement membrane are thought to form a charge selective barrier that restricts the transmembrane flux of anionic proteins across the glomerular basement membrane into the urinary space. The charge selective function, along with the size selective component of the basement membrane, serves to limit the efflux of plasma proteins from the capillary lumen. Heparan sulfate glycosaminoglycans are anionically charged carbohydrate structures attached to proteoglycan core proteins and have a role in establishing the charge selective function of the glomerular basement membrane. Although there are a large number of studies in the literature that support this concept, the results of several recent studies using molecular genetic approaches to minimize the anionic charge of the glomerular basement membrane would suggest that the role of heparan sulfate glycosaminoglycans in the glomerular capillary wall are still not yet entirely resolved, suggesting that this research area still requires new and novel exploration.
Keywords: heparan sulfate, proteoglycans, podocyte, glomerular, basement membrane, ultrafiltration
Introduction
In the middle of the last century, the developmental biologist, Clifford Grobstein (1916–1998), investigated key events involved in mediating epithelial/mesenchymal interactions that occurred during the course of organogenesis. His 1967 monograph Grobstein summarized the conclusions from 25+ years of research and put forth a series of statements that, retrospectively, provided a remarkably complete outline for future research directions in the area of cell-matrix interactions. Grobstein (1967, p. 291) wrote: “. . . materials can interact at interfaces between like tissues to produce new macromolecular complexes whose properties are jointly determined by the synthetic activities of the cells on the two sides. Once formed, the new complexes act back to alter the activities of the producing cells, possibly by provision of a new micro-environment which modifies the behavior of apposed cells. New developmental behaviors may be reciprocally induced and stabilized in this way.”
It was logical that Grobstein’s trainees would continue to sound the mantra of dynamic reciprocity that occurs between cells and their microenvironment. Merton Bernfield (1939–2002) extended Grobstein’s views in the development of the “Basement Membrane Remodeling Hypothesis” (Bernfield et al., 1984), which arose from studies by his group of molecular events surrounding glandular morphogenesis. Bernfield postulated a two compartment model in which the respective subunit structures of the basement membrane, i.e., the epithelium and its associated basal lamina, and reticular lamina with its associated cells (see below), played an essential role in maintaining/changing/accommodating epithelial morphology during development and differentiation. Over the last 45 years, the concept of dynamic reciprocity between cells and the pericellular microenvironment has continued to be investigated in most organ systems, with the basement membrane, its associated cells, and their cell-matrix receptors being the key players in maintaining tissue homeostasis.
Basement Membranes—The Biocomposite Material of Nature
Basement membranes are a polyprotein composite material existing at epithelial-mesenchymal interfaces and surround muscle, fat, and nerve cells (for reviews, see Yurchenco & O’Rear, 1993; Ray & Gately, 1996; Miner, 1999; Sanes, 2003; Sasaki et al., 2004; Iozzo, 2005; Kleinman & Martin, 2005; McCarthy, 2008; Kruegel & Miosge, 2010). Similar in organization to fiberglass or carbon fiber materials, rigid fibrillar elements are combined with resinous epoxies to yield composite materials, whose properties often supersede those of the individual elements themselves. Like composite materials, the overall properties of basement membranes are tailored/adapted to fit the functional demands of the tissues in which they exist. The classical understanding of basement membrane structure arises from morphological observations of electron microscopists, who subdivided basement membranes into two compartments, a basal lamina that immediately subtends the cell membrane and a reticular lamina that underlies the basal lamina (Inoue et al., 1983). In the majority of tissue basement membranes, the organization of the basal lamina was further subdivided into the lamina rara and lamina densa, the distinction based on differences of the electron density of these layers seen in numerous ultrastructural studies. It has been generally accepted that the basal lamina is the secretory product of epithelial/muscle/fat/nerve cells while the reticular lamina is thought to be the secretory product of fibroblasts that closely underlie the basement membrane. Although this pattern of organization has been universally accepted over the years, the development of alternative fixation and tissue processing approaches indicate that the distinctive ultrastructural morphology (i.e., lamina rara and lamina densa)could be a fixation artifact, demonstrated by the fact that tissues fixed by alternative means show basement membranes having a uniform staining density across their width (Chan et al., 1993; Chan & Inoue, 1994).
Type IV collagen (Kefalides, 1971; Clark et al., 1975) and fibronectin (Bray, 1978; Spiro, 1978; Stenman & Vaheri, 1978) were some of the first characterized basement membrane components, but it was not until the initial recognition and characterization of the Englebreth-Holm-Swarm tumor by Orkin et al. (1977) as a source of basement membrane-like material that allowed for subsequent isolation and purification of “biochemical quantities” of the initial set of constituent glycoproteins/proteoglycans, which comprise basement membranes-laminin, type IV collagen, heparan sulfate proteoglycan, and nidogen/entactin. These molecules were first proposed to form the molecular substructure of basal laminae (Timpl et al., 1979; Hassell et al., 1980; Kleinman et al., 1982), the term “matrisome” was first coined by Martin et al. (1984) to recognize this molecular grouping as being critical to basal lamina organization. Since that time, the number of component molecules or molecular isoforms thereof in basal laminae/basement membranes has increased significantly.
Bernfield’s explanation of the dynamic reciprocity that occurred between basement membranes and cells centered around observations that during tissue morphogenesis the basal lamina secreted by the overlying epithelial cells was modified (degraded in focal areas)by the underlying mesenchymal cells to accommodate changes in epithelial organization/growth (Banerjee et al., 1977). Glycosaminoglycans (GAG) were considered to be the linchpin molecules in the hypothesis, the key elements responsible for maintaining the stability and integrity of the basal lamina (Banerjee et al., 1977; Bernfield, 1977). Changes in epithelial organization were brought about by focal GAG degradation (Smith & Bernfield, 1982), and stabilization of epithelial organization brought about by focal collagen deposition (David & Bernfield, 1979, 1981). It is now accepted that GAGs are key elements in the process of branching morphogenesis (Malmstrom et al., 1980; Steer et al., 2004; Gorsi & Stringer, 2007; Pan et al., 2008; Shah et al., 2010, 2011; Reijmers et al., 2011), and subsequent studies by other investigators have since broadened the scope of events surrounding glandular morphogenesis to encompass multiple regulatory events mediated by cell-matrix receptors, matrix molecules, growth factors, and transcription factors (Metzger & Krasnow, 1999; Steer et al., 2004; Lu et al., 2006; Patel et al., 2006; Horowitz & Simons, 2008; Affolter et al., 2009; Costantini & Kopan, 2010; Hsu & Yamada, 2010).
Glycosaminoglycans and Proteoglycans
Glycosaminoglycans played a key role in the above hypothesis. However, with the exception of hyaluronan, GAGs exist in nature as carbohydrate structures covalently attached to proteins, the molecular family grouping known as proteoglycans. Proteoglycans are hypervariable/hyperfunctional molecules, the descriptors quite applicable to this diverse molecular family because the biological activities of these molecules are derived not only from motifs occurring in the core protein structure (e.g., primary, secondary, and tertiary structures)but also from motifs occurring in the copolymeric repeating structure of the GAGs. Currently there are 35+ core proteins that have been identified to serve as platforms for the assembly of glycosaminoglycan chains (Iozzo, 1997, 1998, 2005; Filmus & Selleck, 2001; Esko et al., 2009). The GAGs familiar to most cell biologists are heparan sulfate (HS) and chondroitin sulfate (CS), albeit a third family of glycosaminoglycans, known as keratan sulfate glycosaminoglycans, differs significantly from the former two families in the nature of copolymeric repeat structure and manner of linkage to their respective core proteins. Discussion of this rather unique and important family of proteoglycans/glycosaminoglycans is beyond the scope of this review article, but the functions of this molecular family have been surveyed by others in the field (Greiling, 1994; Funderburgh, 2000).
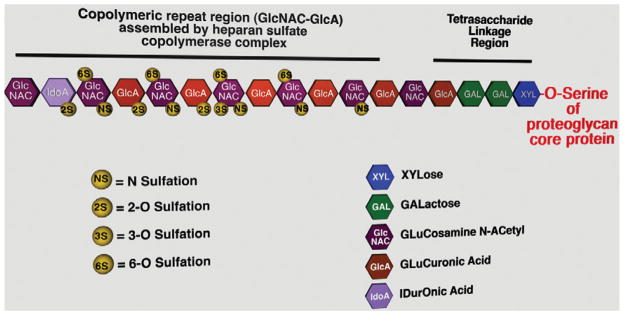
Heparans and chondroitins are linear disaccharide copolymeric repeating structures, the repetitive motif consisting of a hexosamine and uronic acid. Although the basic processes in the assembly of both GAG structures are essentially the same, the assembly process of HS-GAGs has been better characterized. Several thorough reviews of HS-GAG assembly can be found in the literature (Esko & Lindahl, 2001; Nakato & Kimata, 2002; Bishop et al., 2007; Esko et al., 2009), the following is a brief synopsis of the process (Fig. 1). Assembly of HS-GAG chains on proteoglycan core protein occurs via O-linkage to serine residues in a contextually-dependent manner (Zhang & Esko, 1994) and is initiated with the attachment of a xylose residue via an O-glycosidic linkage to a serine residue by xylosyltransferase (Gotting et al., 2000; Condac et al., 2007; Cuellar et al., 2007; Ponighaus et al., 2007). Subsequently, two galactose residues and a glucuronic acid residue are added, forming what is known as the tetrasaccharide linkage region, common to both HS and CS. Divergence between the two types of GAG chains occurs with the addition of the next residue, an N-acetyl glucosamine by the enzyme ExtL3 in the case of HS (Kim et al., 2001). Once the specific pentasaccharide has been assembled, then the HS copolymerase complex (Ext1/ Ext2) rapidly extends the GAG chain (Lind et al., 1998; McCormick et al., 2000). Select carbohydrate residues along the chain are subsequently modified as polymerization continues downstream via: (1) N-deacetylation/N-sulfation of N-acetyl glucosamine residues by NDSTs (Eriksson et al., 1994; Orellana et al., 1994; Dixon et al., 1995); (2) C5 epimerization reactions (which occur in both HS- and CS-GAGs) that convert glucuronic acid residues to iduronic acid residues (Crawford et al., 2001); (3) 3-O- and 6-O-sulfation of N-acetyl glucosamine residues by respective sulfotransferase enzymes (Shworak et al., 1997, 1999; Uchimura et al., 1998, 2000; Liu et al., 1999; Habuchi et al., 2000); and (4) 2-O-sulfation of uronic acid residues by 2-O-sulfotransferase (Rong et al., 2000, 2001). The modifications to the nascent GAG chains are highly orchestrated and sequential, leading to blocks of highly sulfated regions interspersed between regions of lower sulfation (Johnson et al., 2007; Sheng et al., 2011). Several studies have shown that deacetylation and sulfation of the amino group of N-acetylglucosamine is a key step in the modification of the HS, by N-deacetylase-sulfotransferases (NDSTs). Deletion of the NDST1 gene not only results in a significant decrease in N-sulfation of HS chains (Holmborn et al., 2004), but also a significant decrease in the amount of HS sulfation derived by the activity of the other sulfotransferases that appear to act downstream from NDST1 (Grobe et al., 2005). An additional level of complexity of the GAG assembly system is incurred by the existence of multiple isoforms for several of these enzyme families.
Figure 1.
A diagram of the basic organization of heparan sulfate glycosaminoglycans, indicating all of the carbohydrate elements and basic sulfation patterns that occur along a portion of the length of a glycosaminoglycan chain.
The importance of GAGs to overall systems biology can be appreciated by the wide variety of molecules with which they interact. The biological activities of the HS family are the best understood of the two GAG families listed above. A recent report (Ori et al., 2011) estimates at least 260 different gene products having the ability to interact with HS; the functional activities of those molecules encompass morphogens, cytokines and chemokines, growth factors, and other ECM components (Bernfield et al., 1999; Ori et al., 2011).
The Glomerular Basement Membrane—A Unique Basal Lamina
Although anticoagulant role of heparin may be the best recognized function for HS-GAGs among the medical and research communities (Rosenberg et al., 1997), the second most recognized function of HS-GAGs and their respective proteoglycan core proteins may be the role that these molecules play in renal ultrafiltration. The renal glomerulus is the central structure in the ultrafiltration process, the overall integrity of its capillary network and their respective walls being the critical elements in ultrafiltration. The glomerular capillary wall is composed of a luminal fenestrated (nondiaphragmatic) endothelial layer, a basement membrane, and an outer epithelial layer known as the visceral epithelium, the resident cells referred to as podocytes. The glomerular basement membrane (GBM) is unique among most basement membranes that have been characterized at the ultrastructural level in both its genesis during development and its organization in the adult animal (Farquhar, 1991). Early in glomerular development, both glomerular epithelial cells (podocytes), and endothelial cells each lie upon a distinct basement membrane—each with their own basal lamina and reticular lamina. As glomerular maturation proceeds, the reticular lamina region gradually disappears and the two basal lamina fuse to form a trilaminar basal lamina (lamina rara externa, lamina densa, lamina rara interna) interposed between the two cell types (Reeves et al., 1980; Abrahamson, 1985). Traditionally in mature glomeruli, GBM synthesis/secretion has been thought to be primarily from the podocytes (Abrahamson & Perry, 1986), but recent studies provide compelling evidence suggesting that the glomerular endothelial cells could contribute substantial amounts of laminin to the GBM (St John & Abrahamson, 2001; Abrahamson et al., 2007).
The GBM “matrisome” is unique from many other basement membranes. The GBM and the mesangial matrix are actually two distinct extracellular matrices in close proximity to one another, yet each of these matrices has its own complement of molecular constituents (Fig. 2). Laminin 521 is the predominant laminin heterotrimeric species of the glomerular basement membrane, whereas laminin 111 is found in the directly adjacent mesangial matrix (Miner, 2005). The type IV collagen heterotrimer in the glomerular basement membrane consists of α3, α4, and α5 type IV chains; the mesangial matrix contains the α1 and α2 type IV heterotrimer (Hudson et al., 1989; Khoshnoodi et al., 2008). Nidogens are ubiquitously distributed in both GBM and mesangial matrix (Katz et al., 1991; Miosge et al., 2000). The basement membrane proteoglycan complement of the two basal laminae differ; agrin (Groffen et al., 1998; Raats et al., 1998; Burgess et al., 2000), perlecan (Kasinath et al., 1996; Groffen et al., 1997, 1999), and type XVIII collagen (Halfter et al., 1998) (minor component) are found in the GBM, whereas the mesangial matrix contains a chondroitin sulfate proteoglycan (McCarthy et al., 1989; McCarthy & Couchman, 1990), type XVIII collagen (Halfter et al., 1998), and perlecan (Thomas et al., 1995).
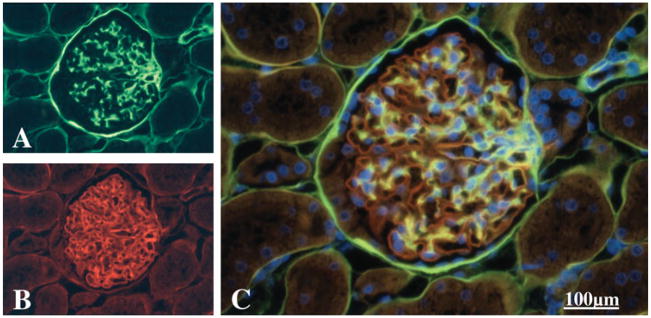
Figure 2.
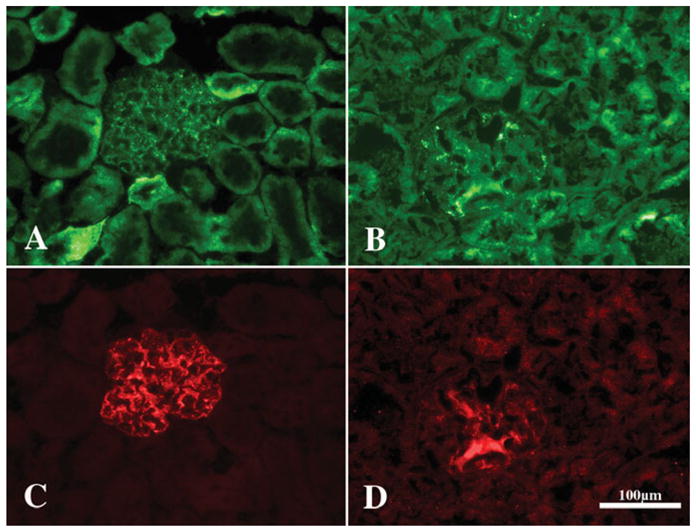
A panel of micrographs of a paraffin section of a rat glomerulus (C, triple label) that had been immuno-stained with a polyclonal antiserum directed against a basement membrane HSPG core protein (B, red) and a monoclonal antibody against a basement membrane chondroitin sulfate proteoglycan (A, green) and a Hoechst 33242 nuclear counterstain as described in McCarthy and Couchman (1990). The glomerular basement membrane is almost exclusively stained for the heparan sulfate proteoglycan whereas the yellow color indicates where the two proteoglycans colocalize, which is the mesangial matrix. Bowman’s capsule stains more intensely for the chondoitin sulfate proteoglycan. Final magnification, 400×; scale bar = 100 μm.
Besides serving a basic structural function as the glomerular capillary wall extracellular matrix, the GBM serves as one key component of the glomerular ultrafiltration barrier; the other components of the barrier are the glomerular capillary endothelium (and its associated glycocalyx)(Hjalmarsson et al., 2004; Singh et al., 2007; Satchell & Braet, 2009; Friden et al., 2011) and the pedicels/slit diaphragms of the glomerular capillary epithelial cells (podocytes) (Rodewald & Karnovsky, 1974; Fujigaki et al., 1996; Holzman et al., 1999; Saleem et al., 2002). It is generally accepted that the constituent molecules of the GBM confer both size and charge selectivity to its respective barrier function, the charge selectivity function having been traditionally assigned to the HS-GAGs and other polyanions present in the GBM (Kanwar & Farquhar, 1979; Kanwar et al., 1980). Data from early studies exploring GBM permeability provide compelling evidence in support this concept, demonstrating that anionic charge does establish an electrostatic barrier that hinders/ constrains the flux of polycationic solutes across the GBM (Chang et al., 1975; Rennke et al., 1975; Brenner et al., 1977, 1978; Deen et al., 1977; Rennke & Venkatachalam, 1977). In extrapolating that conceptual function to explain pathophysiologic events seen in disease, it was logical to surmise that the loss of GBM anionic charge would develop in diseases/ disease models in which proteinuria occurs (Ala-Houhala & Pasternack, 1987; Okada et al., 1989; Wada et al., 1990; Gambaro et al., 1992; Morano et al., 1993; Fox et al., 1994; Morano et al., 1994; Goode et al., 1995; van den Born et al., 1995; Raats et al., 2000; Sakagami et al., 2004; Takahashi et al., 2006). Although the GBM has both size and charge selectivity, the molecular weight cutoff for the barrier is still a highly contested issue in the field of renal function (Singh et al., 2011).
Manipulating the Anionic Charge of Glomerular Basement Membrane Heparan Sulfate
Several approaches have been taken over the years have been done to experimentally perturb the anionic charge associated with GBM HS-GAG chains. The seminal studies in this area by Kanwar and Farquhar (Kanwar et al., 1980) used enzymatic removal of HS-GAG via in situ treatment with heparanase to show that permeability of the GBM to neutral ferritin was enhanced by this method. A subsequent approach opted to interfere with the GBM-associated anionic charge via binding of cationic moieties (Kanwar & Rosenzweig, 1982), resulting in the accumulation of the neutral ferritin probe and albumin to the point that the permeability of the GBM to inulin was significantly reduced. The results led to the conclusion that one function of the HS-GAG anionic charge was to limit the access of plasma proteins to the GBM, thus preventing the accumulation of these molecules in the GBM and avoiding clogging of the filtration medium (Kanwar & Rosenzweig, 1982). A latter approach using a polyclonal antiserum raised against the core protein of a 130 kD GBM proteoglycan (Miettinen et al., 1986) to target the core protein of a GBM proteoglycan. This method did not directly bind HS-GAGS, but rather targeted the “platform” to which HS-GAGs are attached. The findings also showed irregular alterations in GBM thickness and a moderate increase in albuminuria in a rodent model. A subsequent study by another group took yet another iterative approach to show a rapid (2 h post-injection), dose-dependent increase in proteinuria after intravenous injection of a monoclonal antibody (JM-403) targeting an epitope present within the HS-GAG chains (van den Born et al., 1992). A recent report, investigating the biology of mammalian heparanase in tissues, globally overexpressed human heparanase in a constitutive manner (Zcharia et al., 2004). Although HS-GAG was not entirely eliminated from tissue extracts, the net size of the HS-GAG chains was significantly reduced (from 35 kDA to 12 kDA) (Zcharia et al., 2004). Although multiple subsystems (e.g., hair growth, glandular morphogenesis) were affected in these animals, with respect to glomerular alterations, broadening and flattening of the podocyte pedicels (i.e., foot process effacement) was seen in this model. Concomitant with the structural changes, a twofold increase in urinary protein levels was noted.
The investigation of developmental importance of HS-GAGs in lower organisms (Toyoda et al., 2000; Hwang et al., 2003a, 2003b; Morio et al., 2003) led to the move toward using mammalian molecular genetic approaches to specifically target the ability of cells to assemble or modify HS-GAG. Since HS-GAGs interact with a wide range of morphogens and growth factors (see reviews listed above), as one would surmise experiments utilizing global gene knockout of specific enzymes involved in the polymerization of HS-GAG chains often led to cessation of the developmental programming and thus eventual embryonic lethality (Lin et al., 2000; Stickens et al., 2005). Global deletion of some, but not all, of the enzymes involved in postassembly modification of glycosaminoglycan chains often permitted full length gestation of murine fetuses but often led to peri-/post-natal lethality due to compromise of one or more organ systems (Bullock et al., 1998; Habuchi et al., 2007). With respect to the kidney, the deletion of either 2-OST (Bullock et al., 1998) or the C5 epimerase enzymes (Li et al., 2003)led to the development of renal agenesis, the deletion of 2-OST affecting the branching morphogenesis of the ureteric bud potentially via alteration in Wnt signaling (Shah et al., 2010).
To circumvent the developmental consequences involved in the global knockout approach, alternative strategies to eliminate HS-GAG from tissues have been employed by several laboratories. Elimination of exon 3 from the perlecan gene (Hspg2Δ3/Δ3 mutation) leads to the global expression of perlecan lacking domain 1 (Rossi et al., 2003; Morita et al., 2005), which encodes the three originally described sites for the attachment of glycosaminoglycan chains to the perlecan core protein (Noonan et al., 1991; Kallunki & Tryggvason, 1992; Murdoch et al., 1992). Mice having this mutation appear phenotypically normal at birth and live a normal life span. With regard to the kidney, this particular mutation appears not to have had a significant effect on normal renal function (Rossi et al., 2003); the morphology of the various components of the glomerular filtration barrier also appeared normal. There were no apparent differences in anionic charge density/distribution within the GBM nor were the levels of expression of perlecan, agrin, or type XVIII collagen different from that seen in controls (Morita et al., 2005). Although the anionic sites were shown to be mainly derived from HS-GAG, immunostaining with monoclonal antibodies directed against CS-GAG also showed enhanced deposition that particular GAG in the GBM in the Hspg2Δ3/Δ3 mutant mouse. Although the amino terminal GAG acceptor site had been deleted in this model, the possibility remains that alternative acceptor sites, on domain V of perlecan (Tapanadechopone et al., 1999), could be used in this system in a compensatory manner. However, when a physiological stressor, i.e., albumin overload, was applied to this particular model (Morita et al., 2005), microalbuminuria did occur.
The implementation of Cre-Lox technology has recently allowed glycobiologists to bypass the severe developmental deficits associated with the former global knockout approaches, in many cases facilitating the study of tissue-specific effects of the deletion of HS-GAG assembly (Inatani et al., 2003; Matsumoto et al., 2007, 2010; Chen et al., 2008; Iwao et al., 2009, 2010; Jones et al., 2010; Mundy et al., 2011; Ogata-Iwao et al., 2011) or modification enzymes (Grobe et al., 2005; Fuster et al., 2007; MacArthur et al., 2007; Garner et al., 2008, 2011; Zuberi et al., 2009; Adhikari et al., 2010; Crawford et al., 2010; Stanford et al., 2010) in vivo.
In the case of the renal glomerulus, experimentation in this area has been facilitated by the development of transgenic mice expressing Cre-recombinase in a constitutive (Eremina et al., 2002; Moeller et al., 2003) or inducible (Shigehara et al., 2003; Belteki et al., 2005; Juhila et al., 2006) tissue-specific manner in glomerular podocytes. Although the pattern of Cre-recombinase expression was shown to occur in other tissues outside of the kidney in the Neph-Cre mouse (Eremina et al., 2002), the 2.5-P-Cre construct of Moeller et al. (2003) appears to have limited the expression of Cre-recombinase to glomerular podocytes. Expression of Cre-recombinase from the 2.5P-Cre transgene begins in glomerular podocytes at the capillary loop stage of development as the podocytes begin to differentiate and is maintained in glomerular podocytes throughout the life of the animal (Moeller et al., 2003). Using podocyte specific expression of Cre-recombinase, studies have investigated the role of cell matrix receptors (Pozzi et al., 2008; Jarad et al., 2011), signaling pathways (El-Aouni et al., 2006; Brukamp et al., 2007; Heikkila et al., 2010; Steenhard et al., 2010), cytoskeleton (Mollet et al., 2009; Garg et al., 2010), microRNAs (Harvey et al., 2008; Ho et al., 2008), growth factors (Eremina et al., 2006; Kazama et al., 2008; Dai et al., 2010; Nakagawa et al., 2011), podocyte depletion (Jia et al., 2008), and lineage tracing/fate mapping (Asano et al., 2005; Appel et al., 2009)
Three recent reports (Harvey et al., 2007; Chen et al., 2008; Goldberg et al., 2009) incorporated the use of the 2.5P-Cre expressing mouse to explore the contribution of HS-GAGs to GBM anionic charge density. In the first two models, GBM anionic charge density was down-regulated via targeted deletion [exons 6–36 of the agrin core protein (Harvey et al., 2007)] or, in a subsequent extension of the study, by incorporating an additional mutation in the agrin deletion model via breeding with the Hspg2Δ3/Δ3 (perlecan) mutant mouse (Goldberg et al., 2009). Podocyte-specific deletion of exons 6–36 of agrin removes the region of the core protein containing the two known GAG acceptor sites on the agrin core protein. Although this manipulation potentially generated a “miniagrin” protein, the protein was incapable of serving as a substrate for the addition of GAG chains (Harvey et al., 2007). Immunostaining tissue sections from mutant animals with C-terminal antibodies against the agrin core protein were negative, as was immunostaining with antibody JM-403, which recognizes an epitope present on HS-GAG chains, thus demonstrating the deletion of full length agrin core protein and that HS-GAG substitution on the “miniagrin” did not occur. Although intact agrin secretion was entirely deleted, there was no difference in either the staining intensity (mainly mesangial staining) or distribution of perlecan core protein in glomeruli from control and mutant animals. Despite the loss of GBM-agrin, the glomeruli from mutant animals showed no ostensible glomerular damage at the light microscopy level. Ultrastructural studies of polyethylenimine (PEI) labeled specimens from mutant animals demonstrated a significant decrease in anionic charge density in the GBM in the lamina rara externa and lamina rara interna. Also at the ultrastructural level, no podocyte pedicel (foot process) effacement was noted; functionally there was no significant difference in albuminuria in the mutant animals compared to control animals. The study did note, however, an age-related increase in the presence of “bumps” of GBM protruding from the lamina rara externa toward the pedicel foot processes.
The subsequent study by the same group explored potential changes on glomerular architecture and function in a double mutant mouse in which the Hspg2Δ3/Δ3 mutant was overlayed on the background of the podocyte–specific agrin mutant mouse (Goldberg et al., 2009). Although the possibility existed that overlaying Hspg2Δ3/Δ3 mutant phenotype onto the agrin-deletion mutant might have at least an additive effect of minimizing the overall contribution to anionic charge density from two basement membrane proteoglycan core proteins, the study reported no differences in glomerular morphology (with the exception of GBM “bumps”) or function between control or double mutant animals. Consistent with previous studies (Morita et al., 2005), no significant differences in anionic charge density between control and Hspg2Δ3/Δ3 mutant mice were observed, but the anionic charge density was significantly depleted in the agrin mutant and agrin-Hspg2Δ3/Δ3 double mutant mice when compared to control or Hspg2Δ3/Δ3 mutant mice. However, there was no significant difference in anionic charge density in the lamina rara externa seen between the agrin mutant and agrin-Hspg2Δ3/Δ3 mutant mice. A minor but statistically significant decrease in anionic charge density was seen in the lamina rara interna in both the Hspg2Δ3/Δ3 mutant and agrin-Hspg2Δ3/Δ3 mutant mice when compared to that seen in the control animals and in the agrin mutant animals. Overall, the data from both studies strongly suggested that the loss of GBM anionic charge contributed by HS-GAGs associated with perlecan and agrin had minimal effect on the overall biology or function of the glomerular filtration barrier.
The Same Story but from a Different Point of View
Yet a third alternative approach to modulate the GBM anionic charge associated with HS-GAG used the 2.5P-Cre mouse (Moeller et al., 2003) to delete the floxed Ext1 allele (Inatani et al., 2003) in a podocyte-specific manner (Chen et al., 2008). Ext1 (McCormick et al., 1998) is one of the subunits of the HS copolymerase complex (Lind et al., 1998) responsible for extending the HS copolymeric repeat once the initial pentasaccharide structure has been assembled on the proteoglycan core protein. Loss of Ext1 protein component leads to loss of copolymerase activity and cessation of HS-GAG assembly (McCormick et al., 2000). As mentioned above, the embryos of animals homozygous for deletion of the Ext1 allele fail to gastrulate; HS-GAG synthesis heterozygotic animals for Ext1 deletion falls to less than 50% seen in wild-type cells (Lin et al., 2000). Animals homozygous for deletion of Ext2, which encodes the other subunit of the HS copolymerase complex, also lack HS-GAG and fail to gastrulate (Stickens et al., 2005).
The phenotype of the PEXTKO mouse (podocyte-specific Ext1 knockout) (Chen et al., 2008) corroborate some results outlined in the above studies (Harvey et al., 2007; Goldberg et al., 2009). At the same time, data from this model once again serve to refocus attention on the role that HS-GAGs play in the ultrafiltration process, but the function of HS-GAG may be in a different context (see below). Rather than serving as a passive filtration medium, the data suggested that HS-GAGs have direct affects on podocyte behavior. Similar to what was seen in the previous three mutant mice for HS-GAG insufficiency, in routine tissue sections (H&E) there was no immediate ostensible difference in glomerular morphology. However glomerular hypertrophy was found in tissue sections from PEXTKO animals when compared to controls. Immunohistochemistry of tissue sections from PEXTKO animals showed no change in GBM or mesangium-associated perlecan core protein staining but enhanced GBM staining for agrin core protein. As seen in the agrin mutant and agrin-Hspg2Δ3/Δ3 mutant mice, there was a significant depletion of GBM anionic charge density as measured by PEI staining, with significant losses of anionic charge in both the lamina rara externa and interna. The loss of HS-GAG was also confirmed by the lack of GBM immunostaining in glomeruli from PEXTKO animals with the phage display antibody, HS4C3 (Smits et al., 2006; Ten Dam et al., 2006), which binds to 3-O-sulfated motifs present on HS-GAG chains.
The PEXTKO mouse, however, does differ from the former models for GBM HS-GAG insufficiency in several respects (Fig. 3). Although “bumps” of GBM material that protruded from the GBM as described in the agrin mutant mouse (Harvey et al., 2007) were also seen in the PEXTKO mouse, the severity of basement membrane distortion was greater and appeared earlier (1 month postnatal) in the PEXTKO model. Although overt albuminuria/proteinuria did not develop in this model, a modest (but not statistically significant) increase in urinary albumin was seen at 2 and 8 months of age (Chen et al., 2008) that continued to gradually increase over the life of the animal (Chen & McCarthy, unpublished observations). Concomitant with the increase in urinary albumin excretion, age-related changes in proximal tubule cells, i.e., the presence of cytoplasmic vacuoles, were seen in H&E sections beginning at 6 months of age. Recent ongoing studies in the laboratory have identified the vacuoles as being lysosomal in nature (Fig. 4). Lastly, and perhaps most importantly, podocyte pedicel (foot process) effacement and microvillous transformation were seen in podocytes from PEXTKO animals at all ages examined (Fig. 3).
Figure 3.
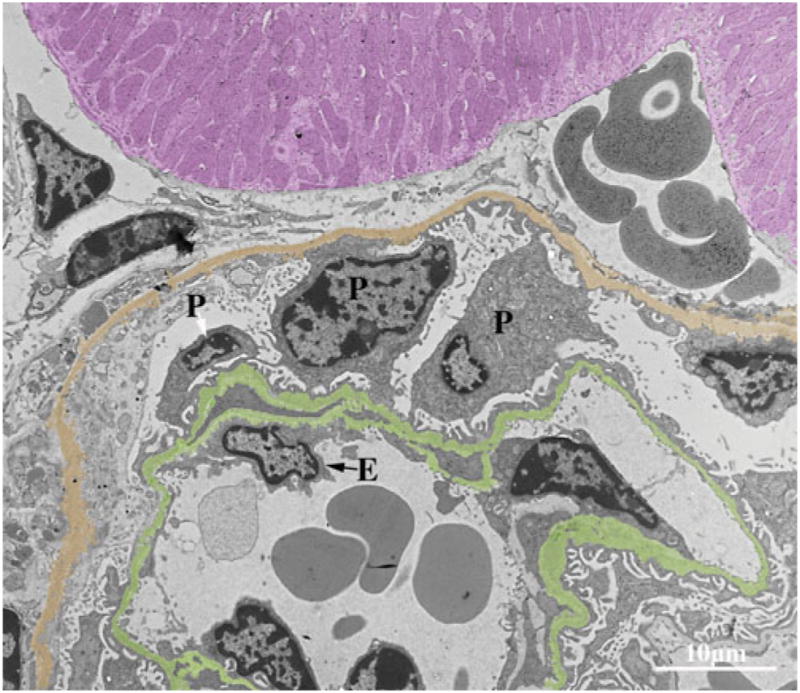
An electron micrograph of a glomerular capillary from an adult PEXTKO mouse. The glomerular basement membrane is pseuodocolored green, Bowman’s capsule gold, and an adjacent proximal tubule purple. The cell bodies of three podocytes (P) extend into Bowman’s space, two of the podocytes show abnormal connections between the glomerular basement membrane and Bowman’s capsule; in some areas (black arrows), the podocytes appear to be forming pedicels on the capsule itself. The pedicels of all three podocytes show some degree of effacement at the point of contact with the glomerular basement membrane. All three podocytes exhibit microvillous transformation. The glomerular basement membrane is irregular in outline, and numerous “bumps” of basement membrane material that extend from the outer border can be found throughout its entire length. The glomerular endothelial cell (E) also shows some degree of abnormality; in some areas there appears to be lost of fenestrae. Final magnification, 2,950×; scale bar = 10 μm.
Figure 4.
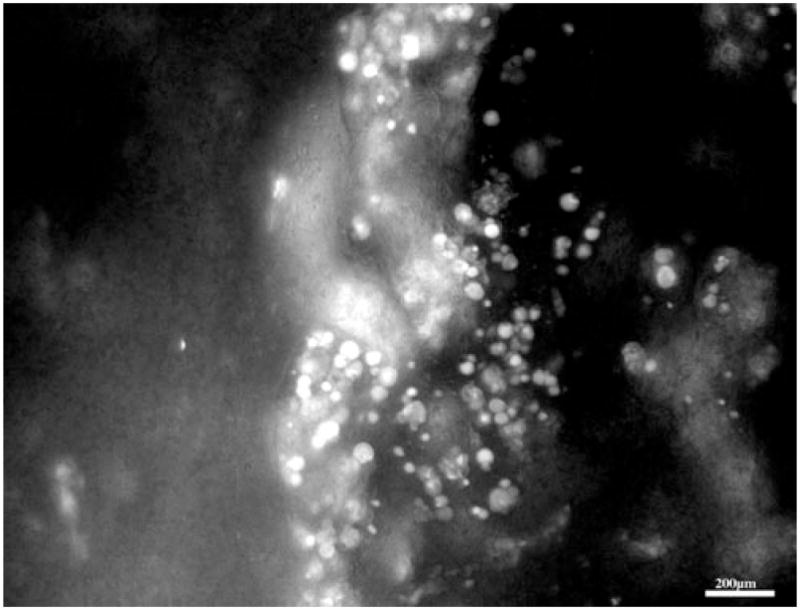
A fluorescence micrograph of a 500 μm thick slice of renal cortex from an adult PEXTKO animal. The cortical slice was incubated for 30 min in Lysotracker™ (Invitrogen, Carlsbad, CA, USA) prior to whole mounting on a microscope slide. An image stack (2 μm steps) was acquired and the image stack processed by digital deconvolution and shown as an extended focus image (I-Vision™, Biovision, Exton, PA, USA). The micrograph shows two proximal convoluted tubules whose epithelial cells show large, rounded vacuoles that are positive for Lysotracker™. As described in Chen et al. (2008), in H&E stained paraffin sections of adult PEXTKO mice, these vacuoles appear as empty structures that almost fill the entire cell body of the epithelial tubule cells. Final magnification, 200×; scale bar = 200 μm.
The Function of Gbm Heparan Sulfate Glycosaminoglycans: Possibly a Matter of Location … Location … Location
The latter changes in podocyte morphology discussed above in the PEXTKO mouse were not reported in the Hspg2Δ3/Δ3 mutant mouse (Morita et al., 2005), the agrin mutant mouse (Harvey et al., 2007), or the agrin-Hspg2Δ3/Δ3 mutant mouse (Goldberg et al., 2009). The difference from these models, with respect to the observed effects on the development of podocyte pedicel (foot process) effacement, indicated basement membrane proteoglycan HS-GAGs were not the mediators of some aspects of the podocyte defect in the PEXTKO model. This led to the proposal that the functional deficit seen in the PEXTKO podocytes was mediated by HS-GAGs, but attached to a different proteoglycan species other than basement membrane proteoglycans.
Immortalized HS-GAG+ and HS-GAG null podocytes were developed to test the hypothesis, the latter generated by in vitro deletion of EXT1 (Chen et al., 2010). Initial studies focusing on cell-matrix adhesion, cell spreading, and cell migration showed that HS-GAG-null podocytes adhered, spread, and migrated less efficiently on a fibronectin substratum compared to HS-GAG+ podocytes (Chen et al., 2010), providing the initial evidence that HS-GAGs attached to one or more cell surface proteoglycans were involved in mediating podocyte-matrix interactions.
Although there are several species of cell surface proteoglycans, members of the syndecan family of cell surface proteoglycans are known to participate in cell-matrix interactions and signaling (for reviews, see David & Bernfield, 1998; Woods & Couchman, 1998, 2001; Bernfield et al., 1999; Zimmermann & David, 1999; Park et al., 2000; Couchman et al., 2001; Rapraeger, 2001; Woods, 2001; Humphries et al., 2005; Tkachenko et al., 2005; Morgan et al., 2007). Syndecans are single pass transmembrane proteins, the ectodomain of the protein capable of bearing one or more GAG chains. Syndecan-4 is ubiquitously expressed in most tissues and has been shown to be present in the glomerulus (Yung et al., 2001) and in glomerular podocytes (Bjornson Granqvist et al., 2006). Despite the fact that syndecan-4 functions as an adhesion co-receptor alongside integrins (Oh et al., 1997a, 1997b; Couchman & Woods, 1999; Saoncella et al., 1999; Woods et al., 2000; Simons & Horowitz, 2001; Woods & Couchman, 2001; Tkachenko et al., 2005; Bass et al., 2007), the renal phenotype is apparently normal in syndecan-4 knockout mice not subjected to physiologic stressors (Ishiguro et al., 2001; Wilcox-Adelman et al., 2002; Cevikbas et al., 2008).
Using the distribution of syndecan-4 staining as a readout, syndecan-4 immunostaining studies of HS-GAG+ and HS-GAG null podocytes showed differences in the size and pattern of syndecan-4 aggregates at the cell surface (Chen et al., 2010). In HS-GAG+ podocytes, syndecan-4 staining appeared as large aggregates at the point of cell-matrix contact; in the HS-GAG null podocytes, the syndecan-4 staining was associated with numerous small clusters around the circumference of the cells. Accordingly, differences in the actin cytoskeleton were seen, the HS-GAG+ cells having prominent stress fibers whereas the HS-GAG null cells had cortical actin distribution (Fig. 5). This latter observation is consistent with previous reports where syndecan-4 engagement to matrix ligands was hindered due to lack of HS-GAG substitution (Saoncella et al., 1999; Gopal et al., 2010).
Figure 5.
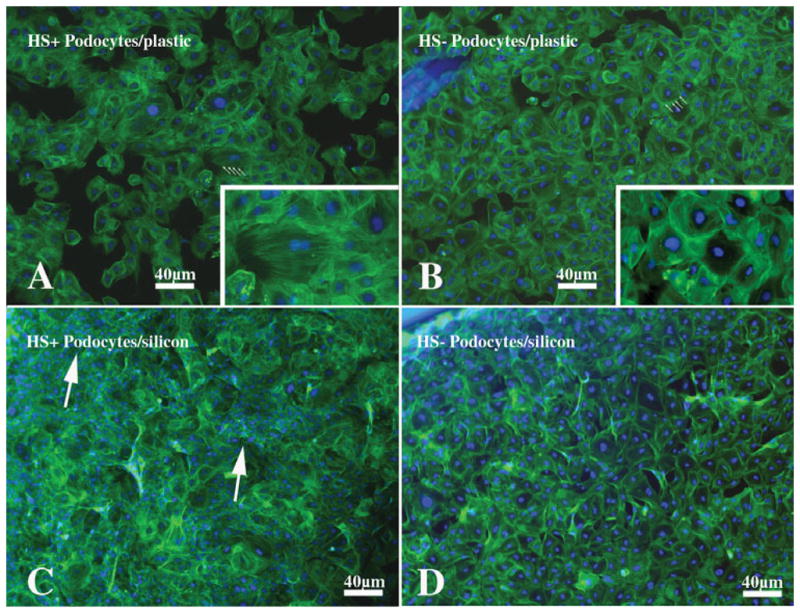
A series of fluorescence micrographs of HS-GAG+ podocytes (A, C) or HS-GAG null podocytes (B, D) that were grown on tissue culture plastic (A, B) or a derivatized silicon substratum (Young’s modulus approximately 1.0 kPa) to which fibronectin had been coupled prior to cell plating. The cells were stained with Alexa-488 phalloidin to demonstrate actin fibers and counterstained with Hoechst 33242 nuclear counterstain (Invitrogen). The HS-GAG+ cells (A) grown on plastic are well spread and show prominent stress fibers (arrows) that span the length of the cells. The HS-GAG null cells (B) show prominent bands of actin that travel around the perimeter of the cell (cortical actin pattern). The insets in panels A and B are enlargements of the cells indicated with arrows in their respective figures that better show the distinct cytoskeletal organization of these cells. In some areas on the flexible silicon substratum, the HS-GAG+ podocytes (C) appear to contract the surface bringing the cell bodies and nuclei closer together, as shown by the areas of nuclear (blue) piling (arrows). The HS-GAG null podocytes (D) cultured on the same substratum still have a morphology similar to that shown for the HS-GAG null podocytes in panel B and still maintain a cortical actin cystoskeletal organization. The cells do not appear to contract the surface to any significant extent. Final magnification, 40 ×; scale bar = 40 μm.
In tissue sections from control animals, syndecan-4 antibodies stained the perimeter of glomerular capillaries (Fig. 6A), the staining colocalizing with synaptopodin (Fig. 6C), an actin binding protein found in podocytes. In the glomeruli from PEXTKO animals, the pattern of syndecan-4 staining was altered; the regular pattern of staining around the perimeter of the glomerular capillaries lost (Fig. 6B). The staining for synaptopodin (Fig. 6D) was also altered in a similar fashion.
Figure 6.
A series of fluorescence micrographs of glomeruli from control (A, C) or PEXTKO (B, D) mice immuno-stained with monoclonal antibodies against syndecan-4 (A, B; Becton Dickinson-Pharmigen, Sparks, MD, USA) or a polyclonal antiserum against synaptopodin (C, D; Synaptic Systems, Goettingen, Germany), an actin binding protein present in podocytes. These micrographs are similar to those reported in Chen et al. (2010). In control glomeruli syndecan-4 staining can be found outlining the perimeter of the glomerular capillaries as well as being present on mesangial cells within the mesangial matrix. Both syndecan-4 (A) and synaptopodin (C) colocalize in the glomerular capillary walls in control animals. In the PEXTKO animal, the regular linear pattern of syndecan-4 (B) is lost from the perimeter of the capillaries. The pattern of synaptopodin expression is also lost in a similar fashion (D), suggesting a potential interaction between the two proteins. Moreover the intensity of synaptopodin expression in PEXTKO glomeruli is decreased because the exposure time for panel C was 408 ms and for panel D was 1.025 s. Final magnification, 400×; scale bar = 100 μm.
The function of the syndecan family members as mediators of cellular behavior and cell-matrix interactions has been well described in the literature (see above reviews). When one explores the renal literature over the past three decades, with regard to the study GBM biology and function, the primary focus of the entire renal field has centered on the role of basement membrane proteoglycans. Yet, when one revisits the same body of literature in the context of what is known about syndecan biology in their role as modulators of cell-matrix adhesion, there are data present in earlier studies that are consistent with the premise that cell surface proteoglycans could play an important role in podocyte biology. Early studies in perfused kidneys that neutralized anionic charge using the polycation, protamine, demonstrated broadening and flattening of podocyte pedicels (foot processes), which was reversible by the adminstration of heparin (Seiler et al., 1975). Subsequent studies demonstrated that these changes were associated with altered cell surface anionic charge, the findings remarkably similar in morphology to puromycin aminonucleoside nephrosis (PAN) (Seiler et al., 1977; Andrews, 1978). Kanwar and Farquhar (1979), in their seminal paper in this field, may have first demonstrated the existence of cell surface proteoglycans extending from the basal surface of the podocyte pedicels (foot processes) in ruthenium red stained tissue sections. However, since their work preceded the first reports of cell surface-associated HS-GAGs (Woods et al., 1984, 1985; David & Van den Berghe, 1985; Jalkanen et al., 1985; Koda et al., 1985; Rapraeger & Bernfield, 1985; Rapraeger et al., 1985), the anionic charge at that time was assumed to be associated with basement membrane proteoglycans. Whiteside and co-workers (1993) later demonstrated the existence of the similarly appearing fibers extending from the pedicel base into the GBM and showed that these fibers are lost in the PAN model, along with concomitant collapse of the podocyte cytoskeleton in these processes. Interestingly in this same report, the authors speculated that syndecans might be associated with the fibers connecting the basal surface of the pedicels to the GBM. Rada and Carlson (1991) showed that in the PAN model there was no significant loss of GBM HSPG (agrin), but podocyte pedicel effacement still occurred in the model, demonstrating that pedicel effacement does not coincide with the loss of basement membrane proteoglycans.
The role of cell surface proteoglycans in serving as adhesion co-receptors in podocytes needs further exploration. Reports in the literature investigating this area show that expression of syndecan-4 in glomeruli in renal disease can be variable and dependent on the underlying disease pathophysiology. Bjornson Granqvist et al. (2006) demonstrated in the PAN model that the mRNAs encoding Ext1 and NDST1 were significantly decreased compared to control but no significant changes in the level of expression for the mRNAs or protein for syndecan-1 or -4. In patients with IgA nephropathy, a proliferative disease, a sixfold level of expression syndecan-4 mRNA over that seen in specimens from patients with thin membrane disease, was found; an ostensible increase in immunostaining in glomeruli from patients with IgA nephropathy was also seen (Yung et al., 2001). In the KK/Ta mouse model of type 2 diabetes mellitus, a 26-fold increase in the mRNA expression of syndecan-4 was seen in kidneys from diabetic animals with albuminuria compared to controls. In the unilateral nephrectomized (UNX-a model of mild glomerular hyperfiltration) syndecan-4 knockout (Sdc4−/−)mice, mesangial expansion and nodular accumulation of mesangial matrix material were seen, but podocyte pedicel effacement was not reported (Cevikbas et al., 2008). A compensatory up-regulation of glomerular syndecan-2 expression was seen in the UNX-Sdc4−/− mice; a similar response was not seen in the glomeruli from UNX–wild-type mice (Cevikbas et al., 2008).
Although it is appealing to conclude that syndecan-4 is the pivotal proteoglycan in podocyte cell-matrix interactions, the data from the PEXTKO mouse model cannot conclusively or exclusively assign this role to syndecan-4. Podocytes express at least three species of cell surface proteoglycans (two syndecans and one glypican) (Bjornson Granqvist et al., 2006; Cevikbas et al., 2008), all of which could facilitate some degree of cell-matrix interactions with the GBM via engagement of their HS-GAGs with heparan-binding domains present in those members of the GBM “matrisome” family. Since all of these proteoglycan core proteins are capable of bearing HS-GAG attachment, it is highly likely that a combinatorial approach—i.e., generating mice having podocytes, specific deletions of several of these proteoglycan core proteins—will have to be done to obtain a similar phenotype to the PEXTKO mouse.
“From Bench to Inside”—Future Directions and Conclusion
In light of the discussion of the renal phenotypes of the mutant mice described in the above narrative, one would logically beg the question “so what is happening with regard to ultrafiltration in the mouse models?” Of the large, encapsulated organs within the abdominal cavity, the kidney is relatively easy to surgically exteriorize and immobilize for specialized microscopy-based imaging. Over the past five years, several renal biology research groups have taken advantage of this minimally invasive surgical approach and, using fluorescently labeled probes and multiphoton (two-photon) microscopy, have begun to ask specific questions about renal cell behavior and renal function in rodent models (Dunn et al., 2003; Peti-Peterdi, 2005; Molitoris & Sandoval, 2006, 2007; Peti-Peterdi et al., 2009; Russo et al., 2009; Tanner, 2009; Peti-Peterdi & Sipos, 2010). The majority of the studies using this approach have been done in the Munich-Wistar rat (or subspecies thereof). In these animals, many of the outer cortical glomeruli reside in close proximity to the superficial cortex directly adjacent to the subcapsular zone in the kidney. In the previous 40 years, these animals were the “workhorse” models that were used to initiate/facilitate the classical renal micropuncture studies (Blantz et al., 1975, 1976; Blantz & Wilson, 1976; Schor et al., 1981; Frommer et al., 1982, 1984; Anderson et al., 1986), which provided much of the baseline information with regard to the nephron physiology. The superficial placement of these glomeruli is within the penetration range of an 800 nm wavelength laser, allowing reliable imaging to a depth of 150 μm in kidney, the depth of resolution being limited by the degree of light scattering and spherical aberration encountered due to the heterogeneity of the renal parenchyma (Young et al., 2011a, 2011b).
Although the Munich-Wistar rat model is the current gold standard for renal multiphoton imaging, the number of rat mutant lines having significant renal phenotypic/functional changes is extremely modest when compared to the number of available mutant mouse lines with specific renal functional deficits. Ironically, the application of the multiphoton approach to renal imaging in the murine system has met with limited success (Camirand et al., 2011), due primarily to the fact that there is no murine model having superficial glomeruli of similar placement and numbers to those seen in the Munich-Wistar rat. The overall paucity of glomeruli in close proximity to the outermost cortical regions presently relegates in vivo imaging of murine glomerular processes more toward the realm of serendipity rather than routine as in the Munich-Wistar rat model. Thus, at present, there is “mismatch” in melding two very powerful technologies—there are many mutant mice having significant glomerular phenotypes, but the technology to easily image in a high-resolution, minimally invasive fashion is not within easy grasp. In the very short term, systematic mapping of glomerular position within the regions of murine renal cortex from the currently available murine strains would allow for investigators using the multi-photon approach to increase their probability for imaging superficial (< 150 μm) glomeruli. Over the longer term, it will become important to identify and phenotypically characterize nonmutant murine strains whose kidneys show enriched/enhanced distribution of surface glomeruli. Although this may not be an easy task to begin with, in the context of renal research the development of such a murine strain may be even more complicated by the fact that the renal response to diseases such as diabetes mellitus is well known to be strain-dependent (Breyer et al., 2005).
In line with multiphoton microscopy, there is the need for the continued development of fluorescent probes that will permit identification/localization of glycoconjugates in vivo along the length of the nephron. Although there are fluorochrome-labeled lectins currently available that are capable of targeting a wide variety of glycoconjugates, there are relatively few labeled probes having specificity for HS or CS GAGs or sulfation motifs along the GAG chain. Granted there are several commercially-available monoclonal antibodies that recognize HS or CS that could be potentially conjugated with fluorochromes, but the cost of obtaining enough antibody for use in large-scale in vivo studies is prohibitive, placing these types of studies outside of the budgets of most investigators. Glycosaminoglycan-specific antibodies generated using phage-display technology (van Kuppevelt et al., 1998, 2001; Jenniskens et al., 2000; Dennissen et al., 2002; Smits et al., 2006; Thompson et al., 2007) could be one potential alternative large-scale source of fluoroconjugates for in vivo multiphoton imaging studies.
In terms of glomerular biology, a second key element that needs development is a murine system that expresses Cre-recombinase specifically in glomerular mesangial cells. The process of “mesangial expansion,” i.e., the gradual enlargement of mesangial volume relative to the volume of the glomerular tuft, has long been associated with the progression of glomerulosclerosis (Mauer et al., 1984; Steffes & Mauer, 1984; Osterby, 1986; Steffes et al., 1989; Lane et al., 1990), especially in human diabetic nephropathy and animal models of diabetic nephropathy. Currently two of three resident glomerular cells are capable of being targeted using tissue-specific Cre-recombinase drivers, but there have been no reports of the development of a mesangial cell-specific Cre-recombinase expression construct. One could propose that it would be feasible to use a Cre-recombinase expression construct driven by the smooth muscle α-actin promoter because it is well known that mesangial cells express this protein in culture (Elger et al., 1993; Kitamura et al., 1996; Stephenson et al., 1998). However, in vivo, normal mesangial cells usually do not make this actin isoform until they become activated as a result of pathophysiological processes (Couser & Johnson, 1994). That and the consideration of the potential for Cre-mediated excision occurring in other smooth muscle beds throughout the organism severely limit this approach to a subset of relatively few genes. One potential candidate construct that remains to be thoroughly explored in the realm of renal biology is the Tcf21-driven inducible Cre line recently reported by Acharya and co-investigators (Acharya et al., 2011). The power of this particular model is that it is driven by a tamoxifen-inducible Cre-expression system that potentially, in the right context, is capable of mesangial-specific deletion of floxed alleles.
The glycobiology field has begun to move at an ever-accelerating pace from the classical approaches of molecular characterization studies that laid the groundwork for the field during the last century to now asking highly specific questions with regard to how these molecules participate in systems physiology. This transition has been facilitated in part by the rapidly-increasing inventory of available rodent models having genetic mutations in the alleles encoding glycosaminoglycan assembly/modification enzymes and proteoglycan core proteins. However these, along the growing number of mutant rodent models that use Cre-Lox technology (both constitutive or inducible systems) to delete targeted alleles in a tissue specific manner, are only one set of elements in the process of discerning the in vivo functions of GAGs/proteoglycans. To accomplish the latter task, there are key elements extrinisic to the genetic approaches that now need to be melded into the investigative mixture—i.e., the development of non- or minimally invasive methods of detecting functional changes in tissues between wild-type and mutant mice and a repetoire of biomarkers/tracers that permit measurement of such differences.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01DK077860-01A2 (K.J.M.).
References
- Abrahamson DR. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1985;100:1988–2000. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson DR, Perry EW. Evidence for splicing new basement membrane into old during glomerular development in newborn rat kidneys. J Cell Biol. 1986;103:2489–2498. doi: 10.1083/jcb.103.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson DR, St John PL, Isom K, Robert B, Miner JH. Partial rescue of glomerular laminin alpha5 mutations by wild-type endothelia produce hybrid glomeruli. J Am Soc Nephrol. 2007;18(8):2285–2293. doi: 10.1681/ASN.2007020207. [DOI] [PubMed] [Google Scholar]
- Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD. Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis. 2011;49 (11):870–877. doi: 10.1002/dvg.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari N, Basi DL, Townsend D, Rusch M, Mariash A, Mullegama S, Watson A, Larson J, Tan S, Lerman B, Esko JD, Selleck SB, Hall JL. Heparan sulfate Ndst1 regulates vascular smooth muscle cell proliferation, vessel size and vascular remodeling. J Mol Cell Cardiol. 2010;49 (2):287–293. doi: 10.1016/j.yjmcc.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol. 2009;10 (12):831–842. doi: 10.1038/nrm2797. [DOI] [PubMed] [Google Scholar]
- Ala-Houhala I, Pasternack A. Fractional dextran and protein clearances in glomerulonephritis and in diabetic nephropathy. Clin Sci (Lond) 1987;72(3):289–296. doi: 10.1042/cs0720289. [DOI] [PubMed] [Google Scholar]
- Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77(6):1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PM. Scanning electron microscopy of the kidney glomerular epithelium after treatment with polycations in situ and in vitro. Am J Anat. 1978;153(2):291–303. doi: 10.1002/aja.1001530208. [DOI] [PubMed] [Google Scholar]
- Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20(2):333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T. Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol. 2005;16(8):2257–2262. doi: 10.1681/ASN.2004121134. [DOI] [PubMed] [Google Scholar]
- Banerjee SD, Cohn RH, Bernfield MR. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass MD, Morgan MR, Humphries MJ. Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation. Soft Matter. 2007;3(3):372–376. doi: 10.1039/b614610d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33(5):2765–2775. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M. The basal lamina in epithelial-mesenchymal morphogenetic interactions. Ups J Med Sci. 1977;82(2):111–112. doi: 10.3109/03009737709179091. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Banerjee SD, Koda JE, Rapraeger AC. Remodeling of the basement membrane as a mechanism of morphogenetic tissue interaction. In: Trelstad RL, editor. The Role of Extracellular Matrix in Development. New York: Alan R. Liss; 1984. pp. 545–572. [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446(7139):1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Bjornson Granqvist A, Ebefors K, Saleem MA, Mathieson PW, Haraldsson B, Nystrom JS. Podocyte proteoglycan synthesis is involved in the development of nephrotic syndrome. Am J Physiol Renal Physiol. 2006;291(4):F722–F730. doi: 10.1152/ajprenal.00433.2005. [DOI] [PubMed] [Google Scholar]
- Blantz RC, Konnen KS, Tucker BJ. Glomerular filtration response to elevated ureteral pressure in both the hydropenic and the plasma-expanded rat. Circ Res. 1975;37(6):819–829. doi: 10.1161/01.res.37.6.819. [DOI] [PubMed] [Google Scholar]
- Blantz RC, Konnen KS, Tucker BJ. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz RC, Wilson CB. Acute effects of antiglomerular basement membrane antibody on the process of glomerular filtration in the rat. J Clin Invest. 1976;58(4):899–911. doi: 10.1172/JCI108543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray BA. Cold-insoluble globulin (fibronectin)in connective tissues of adult human lung and in trophoblast basement membrane. J Clin Invest. 1978;62(4):745–752. doi: 10.1172/JCI109185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner BM, Bohrer MP, Baylis C, Deen WM. Determinants of glomerular permselectivity: Insights derived from observations in vivo. Kidney Int. 1977;12(4):229–237. doi: 10.1038/ki.1977.107. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Hostetter TH, Humes HD. Glomerular permselectivity: Barrier function based on discrimination of molecular size and charge. Am J Physiol. 1978;234(6):F455–F460. doi: 10.1152/ajprenal.1978.234.6.F455. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Bottinger E, Brosius FC, Coffman TM, Fogo A, Harris RC, Heilig CW, Sharma K. Diabetic nephropathy: Of mice and men. Adv Chronic Kidney Dis. 2005;12(2):128–145. doi: 10.1053/j.ackd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Brukamp K, Jim B, Moeller MJ, Haase VH. Hypoxia and podocyte-specific Vhlh deletion confer risk of glomerular disease. Am J Physiol Renal Physiol. 2007;293(4):F1397–1407. doi: 10.1152/ajprenal.00133.2007. [DOI] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12(12):1894–1906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini: Differential expression, localization, and function. J Cell Biol. 2000;151(1):41–52. doi: 10.1083/jcb.151.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camirand G, Li Q, Demetris AJ, Watkins SC, Shlomchik WD, Rothstein DM, Lakkis FG. Multiphoton intravital microscopy of the transplanted mouse kidney. Am J Transplant. 2011;11(10):2067–2074. doi: 10.1111/j.1600-6143.2011.03671.x. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Schaefer L, Uhlig P, Robenek H, Theilmeier G, Echtermeyer F, Bruckner P. Unilateral nephrectomy leads to up-regulation of syndecan-2- and TGF-beta-mediated glomerulosclerosis in syndecan-4 deficient male mice. Matrix Biol. 2008;27(1):42–52. doi: 10.1016/j.matbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Chan FL, Inoue S. Lamina lucida of basement membrane: An artefact. Microsc Res Tech. 1994;28(1):48–59. doi: 10.1002/jemt.1070280106. [DOI] [PubMed] [Google Scholar]
- Chan FL, Inoue S, Leblond CP. The basement membranes of cryofixed or aldehyde-fixed, freeze-substituted tissues are composed of a lamina densa and do not contain a lamina lucida. Cell Tissue Res. 1993;273(1):41–52. doi: 10.1007/BF00304610. [DOI] [PubMed] [Google Scholar]
- Chang RL, Deen WM, Robertson CR, Brenner BM. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 1975;8(4):212–218. doi: 10.1038/ki.1975.104. [DOI] [PubMed] [Google Scholar]
- Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int. 2008;74(3):289–299. doi: 10.1038/ki.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wassenhove-McCarthy D, Yamaguchi Y, Holzman L, van Kuppevelt TH, Orr AW, Funk S, Woods A, McCarthy K. Podocytes require the engagement of cell surface heparan sulfate proteoglycans for adhesion to extra-cellular matrices. Kidney Int. 2010;78(11):1088–1099. doi: 10.1038/ki.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CC, Minor RR, Koszalka TR, Brent RL, Kefalides NA. The embryonic rat parietal yolk sac. Changes in the morphology and composition of its basement membrane during development. Dev Biol. 1975;46:243–261. doi: 10.1016/0012-1606(75)90103-7. [DOI] [PubMed] [Google Scholar]
- Condac E, Silasi-Mansat R, Kosanke S, Schoeb T, Towner R, Lupu F, Cummings RD, Hinsdale ME. Poly-cystic disease caused by deficiency in xylosyltransferase 2, an initiating enzyme of glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 2007;104(22):9416–9421. doi: 10.1073/pnas.0700908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18(5):698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR, Chen L, Woods A. Syndecans and cell adhesion. Int Rev Cytol. 2001;207:113–150. doi: 10.1016/s0074-7696(01)07004-8. [DOI] [PubMed] [Google Scholar]
- Couchman JR, Woods A. Syndecan-4 and integrins: Combinatorial signaling in cell adhesion. J Cell Sci. 1999;112(Pt 20):3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]
- Couser WG, Johnson RJ. Mechanisms of progressive renal disease in glomerulonephritis. Am J Kidney Dis. 1994;23(2):193–198. doi: 10.1016/s0272-6386(12)80971-1. [DOI] [PubMed] [Google Scholar]
- Crawford BE, Garner OB, Bishop JR, Zhang DY, Bush KT, Nigam SK, Esko JD. Loss of the heparan sulfate sulfotransferase, Ndst1, in mammary epithelial cells selectively blocks lobuloalveolar development in mice. PLoS One. 2010;5(5):e10691. doi: 10.1371/journal.pone.0010691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford BE, Olson SK, Esko JD, Pinhal MA. Cloning, Golgi localization, and enzyme activity of the full-length heparin/heparan sulfate-glucuronic acid C5-epimerase. J Biol Chem. 2001;276(24):21538–21543. doi: 10.1074/jbc.M100880200. [DOI] [PubMed] [Google Scholar]
- Cuellar K, Chuong H, Hubbell SM, Hinsdale ME. Biosynthesis of chondroitin and heparan sulfate in chinese hamster ovary cells depends on xylosyltransferase II. J Biol Chem. 2007;282(8):5195–5200. doi: 10.1074/jbc.M611048200. [DOI] [PubMed] [Google Scholar]
- Dai C, Saleem MA, Holzman LB, Mathieson P, Liu Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 2010;77(11):962–973. doi: 10.1038/ki.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Bernfield MR. Collagen reduces glycosami-noglycan degradation by cultured mammary epithelial cells: Possible mechanism for basal lamina formation. Proc Natl Acad Sci USA. 1979;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Bernfield M. Type I collagen reduces the degradation of basal lamina proteoglycan by mammary epithelial cells. J Cell Biol. 1981;91(1):281–286. doi: 10.1083/jcb.91.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Bernfield M. The emerging roles of cell surface heparan sulfate proteoglycans. Matrix Biol. 1998;17(7):461–463. doi: 10.1016/s0945-053x(98)90092-0. [DOI] [PubMed] [Google Scholar]
- David G, Van den Berghe H. Heparan sulfate-chondroitin sulfate hybrid proteoglycan of the cell surface and basement membrane of mouse mammary epithelial cells. J Biol Chem. 1985;260(20):11067–11074. [PubMed] [Google Scholar]
- Deen WM, Bohrer MP, Robertson CR, Brenner BM. Determinants of the transglomerular passage of macromolecules. Fed Proc. 1977;36(12):2614–2618. [PubMed] [Google Scholar]
- Dennissen MA, Jenniskens GJ, Pieffers M, Versteeg EM, Petitou M, Veerkamp JH, van Kuppevelt TH. Large, tissue-regulated domain diversity of heparan sulfates demonstrated by phage display antibodies. J Biol Chem. 2002;277(13):10982–10986. doi: 10.1074/jbc.M104852200. [DOI] [PubMed] [Google Scholar]
- Dixon J, Loftus SK, Gladwin AJ, Scambler PJ, Wasmuth JJ, Dixon MJ. Cloning of the human heparan sulfate-N-deacetylase/N-sulfotransferase gene from the Treacher Collins syndrome candidate region at 5q32-q33.1. Genomics. 1995;26 (2):239–244. doi: 10.1016/0888-7543(95)80206-2. [DOI] [PubMed] [Google Scholar]
- Dunn KW, Sandoval RM, Molitoris BA. Intravital imaging of the kidney using multiparameter multiphoton microscopy. Nephron Exp Nephrol. 2003;94(1):e7–11. doi: 10.1159/000070813. [DOI] [PubMed] [Google Scholar]
- El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006;17(5):1334–1344. doi: 10.1681/ASN.2005090921. [DOI] [PubMed] [Google Scholar]
- Elger M, Drenckhahn D, Nobiling R, Mundel P, Kriz W. Cultured rat mesangial cells contain smooth muscle a-actin not found in vivo. Am J Path. 1993;142(2):497–509. [PMC free article] [PubMed] [Google Scholar]
- Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol. 2006;17(3):724–735. doi: 10.1681/ASN.2005080810. [DOI] [PubMed] [Google Scholar]
- Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE. Glomerular-specific gene excision in vivo. J Am Soc Nephrol. 2002;13(3):788–793. doi: 10.1681/ASN.V133788. [DOI] [PubMed] [Google Scholar]
- Eriksson I, Sandback D, Ek B, Lindahl U, Kjellen L. cDNA cloning and sequencing of mouse mastocytoma glucosaminyl N-deacetylase/N-sulfotransferase, an enzyme involved in the biosynthesis of heparin. J Biol Chem. 1994;269(14):10438–10443. [PubMed] [Google Scholar]
- Esko JD, Kimata K, Lindahl U. Proteoglycans and sulfated glycosaminoglycans. In: Varki A, editor. Essentials of Glycobiology. 2. New York: Cold Spring Harbor Press; 2009. pp. 229–249. [PubMed] [Google Scholar]
- Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108(2):169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MG. The glomerular basement membrane: A selective macromolecular filter. In: Hay ED, editor. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1991. pp. 365–418. [Google Scholar]
- Filmus J, Selleck SB. Glypicans: Proteoglycans with a surprise. J Clin Invest. 2001;108(4):497–501. doi: 10.1172/JCI13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, Quin JD, O’Reilly DS, Boulton-Jones JM. Glomerular charge selectivity in primary glomerulopathies. Clin Sci (Lond) 1994;87(4):421–425. doi: 10.1042/cs0870421. [DOI] [PubMed] [Google Scholar]
- Friden V, Oveland E, Tenstad O, Ebefors K, Nystrom J, Nilsson UA, Haraldsson B. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 2011;79(12):1322–1330. doi: 10.1038/ki.2011.58. [DOI] [PubMed] [Google Scholar]
- Frommer JP, Laski ME, Wesson DE, Kurtzman NA. Internephron heterogeneity for carbonic anhydrase-independent bicarbonate reabsorption in the rat. J Clin Invest. 1984;73 (4):1034–1045. doi: 10.1172/JCI111288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer JP, Sheth AU, Senekjian HO, Babino H, Weinman EJ. Free-flow micropuncture study of renal urate transport in the Munich-Wistar rat. Miner Electrolyte Metab. 1982;7(6):324–330. [PubMed] [Google Scholar]
- Fujigaki Y, Morioka T, Matsui K, Kawachi H, Orikasa M, Oite T, Shimizu F, Batsford SR, Vogt A. Structural continuity of filtration slit (slit diaphragm)to plasma membrane of podocyte. Kidney Int. 1996;50(1):54–62. doi: 10.1038/ki.1996.286. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology. 2000;10(10):951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- Fuster MM, Wang L, Castagnola J, Sikora L, Reddi K, Lee PH, Radek KA, Schuksz M, Bishop JR, Gallo RL, Sriramarao P, Esko JD. Genetic alteration of endothelial heparan sulfate selectively inhibits tumor angiogenesis. J Cell Biol. 2007;177(3):539–549. doi: 10.1083/jcb.200610086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambaro G, Cavazzana AO, Luzi P, Piccoli A, Borsatti A, Crepaldi G, Marchi E, Venturini AP, Baggio B. Glycosaminoglycans prevent morphological renal alterations and albuminuria in diabetic rats. Kidney Int. 1992;42(2):285–291. doi: 10.1038/ki.1992.288. [DOI] [PubMed] [Google Scholar]
- Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, Mizuno K, Gurniak C, Witke W, Holzman LB. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem. 2010;285(29):22676–22688. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner OB, Bush KT, Nigam KB, Yamaguchi Y, Xu D, Esko JD, Nigam SK. Stage-dependent regulation of mammary ductal branching by heparan sulfate and HGF-cMet signaling. Dev Biol. 2011;355:394–403. doi: 10.1016/j.ydbio.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner OB, Yamaguchi Y, Esko JD, Videm V. Small changes in lymphocyte development and activation in mice through tissue-specific alteration of heparan sulphate. Immunology. 2008;125(3):420–429. doi: 10.1111/j.1365-2567.2008.02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant. 2009;24(7):2044–2051. doi: 10.1093/ndt/gfn758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode NP, Shires M, Crellin DM, Aparicio SR, Davison AM. Alterations of glomerular basement membrane charge and structure in diabetic nephropathy. Diabetologia. 1995;38(12):1455–1465. doi: 10.1007/BF00400607. [DOI] [PubMed] [Google Scholar]
- Gopal S, Bober A, Whiteford JR, Multhaupt HA, Yoneda A, Couchman JR. Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. J Biol Chem. 2010;285(19):14247–14258. doi: 10.1074/jbc.M109.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsi B, Stringer SE. Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 2007;17(4):173–177. doi: 10.1016/j.tcb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Gotting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K. Molecular cloning and expression of human UDP-d-Xylose:proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol. 2000;304(4):517–528. doi: 10.1006/jmbi.2000.4261. [DOI] [PubMed] [Google Scholar]
- Greiling H. Structure and biological functions of keratan sulfate proteoglycans. EXS. 1994;70:101–122. doi: 10.1007/978-3-0348-7545-5_7. [DOI] [PubMed] [Google Scholar]
- Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD. Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development. 2005;132(16):3777–3786. doi: 10.1242/dev.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967;26:279–299. [PubMed] [Google Scholar]
- Groffen AJ, Hop FW, Tryggvason K, Dijkman H, Assmann KJ, Veerkamp JH, Monnens LA, Van den Heuvel LP. Evidence for the existence of multiple heparan sulfate proteoglycans in the human glomerular basement membrane and mesangial matrix. Eur J Biochem. 1997;247(1):175–182. doi: 10.1111/j.1432-1033.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, Assman KJ, Monnens L, Veerkamp JH, van den Heuvel LP. Agrin is a major heparan sulfate proteoglycan in the glomerular basement membrane. J Histochem Cytochem. 1998;46:19–27. doi: 10.1177/002215549804600104. [DOI] [PubMed] [Google Scholar]
- Groffen AJ, Veerkamp JH, Monnens LA, van den Heuvel LP. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant. 1999;14(9):2119–2129. doi: 10.1093/ndt/14.9.2119. [DOI] [PubMed] [Google Scholar]
- Habuchi H, Nagai N, Sugaya N, Atsumi F, Stevens RL, Kimata K. Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem. 2007;282(21):15578–15588. doi: 10.1074/jbc.M607434200. [DOI] [PubMed] [Google Scholar]
- Habuchi H, Tanaka M, Habuchi O, Yoshida K, Suzuki H, Ban K, Kimata K. The occurrence of three isoforms of heparan sulfate 6-O-sulfotransferase having different specificities for hexuronic acid adjacent to the targeted N-sulfoglucosamine. J Biol Chem. 2000;275(4):2859–2868. doi: 10.1074/jbc.275.4.2859. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem. 1998;273(39):25404–25412. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19 (11):2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SV, Jarad G, Cunningham J, Rops AL, Van Der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Path. 2007;171(1):139–152. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Robey PG, Barrach HJ, Wilczek J, Rennard SI, Martin GR. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci USA. 1980;77:4504–4508. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila E, Juhila J, Lassila M, Messing M, Perala N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H. beta-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant. 2010;25(8):2437–2446. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson C, Johansson BR, Haraldsson B. Electron microscopic evaluation of the endothelial surface layer of glomerular capillaries. Microvasc Res. 2004;67(1):9–17. doi: 10.1016/j.mvr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA. Podocyte-specific loss of functional micro-RNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol. 2008;19(11):2069–2075. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmborn K, Ledin J, Smeds E, Eriksson I, Kusche-Gullberg M, Kjellen L. Heparan sulfate synthesized by mouse embryonic stem cells deficient in NDST1 and NDST2 is 6-O-sulfated but contains no N-sulfate groups. J Biol Chem. 2004;279(41):42355–42358. doi: 10.1074/jbc.C400373200. [DOI] [PubMed] [Google Scholar]
- Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56(4):1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Simons M. Branching morphogenesis. Circ Res. 2008;103(8):784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- Hsu JC, Yamada KM. Salivary gland branching morphogenesis—Recent progress and future opportunities. Int J Oral Sci. 2010;2(3):117–126. doi: 10.4248/IJOS10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BG, Wieslander J, Wisdom BJ, Noelken ME. Biology of disease. Goodpasture syndrome: Molecular architecture and function of basement membrane antigen. Lab Invest. 1989;61:256–269. [PubMed] [Google Scholar]
- Humphries MJ, Mostafavi-Pour Z, Morgan MR, Deakin NO, Messent AJ, Bass MD. Integrin-syndecan cooperation governs the assembly of signalling complexes during cell spreading. Novartis Found Symp. 2005;269:178–188. discussion 188–192, 223–230. [PubMed] [Google Scholar]
- Hwang HY, Olson SK, Brown JR, Esko JD, Horvitz HR. The Caenorhabditis elegans genes sqv-2 and sqv-6, which are required for vulval morphogenesis, encode glycosaminoglycan galactosyltransferase II and xylosyltransferase. J Biol Chem. 2003a;278(14):11735–11738. doi: 10.1074/jbc.C200518200. [DOI] [PubMed] [Google Scholar]
- Hwang HY, Olson SK, Esko JD, Horvitz HR. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature. 2003b;423(6938):439–443. doi: 10.1038/nature01634. [DOI] [PubMed] [Google Scholar]
- Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- Inoue S, LeBlond CP, Laurie GW. Ultrastructure of Reichert’s membrane, a multilayered basement membrane in the parietal wall of the rat yolk sac. J Cell Biol. 1983;97:1524–1537. doi: 10.1083/jcb.97.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. CR Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: From molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Basement membrane proteoglycans: From cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6(8):646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Matsuo S, Kusugami K, Saito H, Muramatsu T. Syndecan-4 deficiency increases susceptibility to kappa-carrageenan-induced renal damage. Lab Invest. 2001;81(4):509–516. doi: 10.1038/labinvest.3780259. [DOI] [PubMed] [Google Scholar]
- Iwao K, Inatani M, Matsumoto Y, Ogata-Iwao M, Takihara Y, Irie F, Yamaguchi Y, Okinami S, Tanihara H. Heparan sulfate deficiency leads to Peters anomaly in mice by disturbing neural crest TGF-beta2 signaling. J Clin Invest. 2009;119(7):1997–2008. doi: 10.1172/JCI38519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao K, Inatani M, Ogata-Iwao M, Yamaguchi Y, Okinami S, Tanihara H. Heparan sulfate deficiency in periocular mesenchyme causes microphthalmia and ciliary body dysgenesis. Exp Eye Res. 2010;90(1):81–88. doi: 10.1016/j.exer.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Jalkanen M, Nguyen H, Rapraeger A, Kurn N, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells: Localization on the cell surface with a monoclonal antibody. J Cell Biol. 1985;101(3):976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol Renal Physiol. 2011;300(3):F811–F820. doi: 10.1152/ajprenal.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenniskens GJ, Oosterhof A, Brandwijk R, Veerkamp JH, van Kuppevelt TH. Heparan sulfate heterogeneity in skeletal muscle basal lamina: Demonstration by phage display-derived antibodies. J Neurosci. 2000;20(11):4099–4111. doi: 10.1523/JNEUROSCI.20-11-04099.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, McDill BW, Sankarapandian B, Wu S, Liapis H, Holzman LB, Capecchi MR, Miner JH, Chen F. Ablation of developing podocytes disrupts cellular interactions and nephrogenesis both inside and outside the glomerulus. Am J Physiol Renal Physiol. 2008;295(6):F1790–F1798. doi: 10.1152/ajprenal.90519.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CE, Crawford BE, Stavridis M, Ten Dam G, Wat AL, Rushton G, Ward CM, Wilson V, van Kuppevelt TH, Esko JD, Smith A, Gallagher JT, Merry CL. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells. 2007;25(8):1913–1923. doi: 10.1634/stemcells.2006-0445. [DOI] [PubMed] [Google Scholar]
- Jones KB, Piombo V, Searby C, Kurriger G, Yang B, Grabellus F, Roughley PJ, Morcuende JA, Buckwalter JA, Capecchi MR, Vortkamp A, Sheffield VC. A mouse model of osteochondromagenesis from clonal inactivation of Ext1 in chondrocytes. Proc Natl Acad Sci USA. 2010;107(5):2054–2059. doi: 10.1073/pnas.0910875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhila J, Roozendaal R, Lassila M, Verbeek SJ, Holthofer H. Podocyte cell-specific expression of doxycycline inducible Cre recombinase in mice. J Am Soc Nephrol. 2006;17 (3):648–654. doi: 10.1681/ASN.2005050547. [DOI] [PubMed] [Google Scholar]
- Kallunki P, Tryggvason K. Human basement membrane heparan sulfate proteoglycan core protein: A 467-kD protein containing multiple domains resembling elements of the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Cell Biol. 1992;116:559–571. doi: 10.1083/jcb.116.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar YS, Farquhar MG. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the lamina rarae by cationic probes. J Cell Biol. 1979;81:137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar YS, Linker A, Farquhar MG. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980;86:688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar YS, Rosenzweig LJ. Clogging of the glomerular basement membrane. J Cell Biol. 1982;93:489–494. doi: 10.1083/jcb.93.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinath BS, Grellier P, Choudhury GG, Abboud SL. Regulation of basement membrane heparan sulfate proteoglycan, perlecan, gene expression in glomerular epithelial cells by high glucose medium. J Cell Physiol. 1996;167(1):131–136. doi: 10.1002/(SICI)1097-4652(199604)167:1<131::AID-JCP15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Katz A, Fish AJ, Kleppel MM, Hagen SG, Michael AF, Butkowski RJ. Renal entactin (nidogen): Isolation, characterization and tissue distribution. Kidney Int. 1991;40(4):643–652. doi: 10.1038/ki.1991.256. [DOI] [PubMed] [Google Scholar]
- Kazama I, Mahoney Z, Miner JH, Graf D, Economides AN, Kreidberg JA. Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol. 2008;19(11):2181–2191. doi: 10.1681/ASN.2007111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides NA. Isolation of a collagen from basement membranes containing three identical—Chains. Biochem Biophys Res Commun. 1971;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Techn. 2008;71(5):357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BT, Kitagawa H, Tamura J, Saito T, Kusche-Gullberg M, Lindahl U, Sugahara K. Human tumor suppressor EXT gene family members EXTL1 and EXTL3 encode alpha 1,4-N-acetylglucosaminyltransferases that likely are involved in heparan sulfate/heparin biosynthesis. Proc Natl Acad Sci USA. 2001;98(13):7176–7181. doi: 10.1073/pnas.131188498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M, Mitarai T, Nagasawa R, Maruyama N. Differentiated phenotype of glomerular mesangial cells in nodular culture. Am J Physiol. 1996;270(4 Pt 2):F614–F622. doi: 10.1152/ajprenal.1996.270.4.F614. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15 (5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Koda JE, Rapraeger A, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985;260(13):8157–8162. [PubMed] [Google Scholar]
- Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010;67(17):2879–2895. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PH, Steffes MW, Mauer SM. Renal histologic changes in diabetes mellitus. Semin Nephrol. 1990;10:254–259. [PubMed] [Google Scholar]
- Li JP, Gong F, Hagner-McWhirter A, Forsberg E, Abrink M, Kisilevsky R, Zhang X, Lindahl U. Targeted disruption of a murine glucuronyl C5-epimerase gene results in heparan sulfate lacking L-iduronic acid and in neonatal lethality. J Biol Chem. 2003;278(31):28363–28366. doi: 10.1074/jbc.C300219200. [DOI] [PubMed] [Google Scholar]
- Lin X, Wei G, Shi Z, Dryer L, Esko JD, Wells DE, Matzuk MM. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 2000;224(2):299–311. doi: 10.1006/dbio.2000.9798. [DOI] [PubMed] [Google Scholar]
- Lind T, Tufaro F, McCormick C, Lindahl U, Lidholt K. The putative tumor suppressors EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J Biol Chem. 1998;273(41):26265–26268. doi: 10.1074/jbc.273.41.26265. [DOI] [PubMed] [Google Scholar]
- Liu J, Shriver Z, Blaiklock P, Yoshida K, Sasisekharan R, Rosenberg RD. Heparan sulfate D-glucosaminyl 3-O-sulfotransferase-3A sulfates N-unsubstituted glucosamine residues. J Biol Chem. 1999;274(53):38155–38162. doi: 10.1074/jbc.274.53.38155. [DOI] [PubMed] [Google Scholar]
- Lu P, Sternlicht MD, Werb Z. Comparative mechanisms of branching morphogenesis in diverse systems. J Mammary Gland Biol Neoplasia. 2006;11(3–4):213–228. doi: 10.1007/sl0911-006-9027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur JM, Bishop JR, Stanford KI, Wang L, Bensadoun A, Witztum JL, Esko JD. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J Clin Invest. 2007;117(1):153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom A, Roden L, Feingold DS, Jacobsson I, Backstrom G, Lindahl U. Biosynthesis of heparin. Partial purification of the uronosyl C-5 epimerase. J Biol Chem. 1980;255(9):3878–3883. [PubMed] [Google Scholar]
- Martin GR, Kleinman HK, Terranova VP, Ledbetter S, Hassell JR. The regulation of basement membrane formation and cell-matrix interactions by defined supramolecular complexes. Ciba Found Symp. 1984;108:197–212. doi: 10.1002/9780470720899.ch13. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Irie F, Inatani M, Tessier-Lavigne M, Yamaguchi Y. Netrin-1/DCC signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. J Neurosci. 2007;27(16):4342–4350. doi: 10.1523/JNEUROSCI.0700-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Matsumoto K, Irie F, Fukushi J, Stallcup WB, Yamaguchi Y. Conditional ablation of the heparan sulfate-synthesizing enzyme Ext1 leads to dysregulation of bone morphogenic protein signaling and severe skeletal defects. J Biol Chem. 2010;285(25):19227–19234. doi: 10.1074/jbc.M110.105338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer SM, Steffes MW, Ellis EN, Sutherland DER, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KJ, editor. Microscopy Research and Technique. New York: Wiley Subscription Services, Inc; 2008. Basement Membranes: From the Matrisome to Beyond. [DOI] [PubMed] [Google Scholar]
- McCarthy KJ, Accavitti MA, Couchman JR. Immunological characterization of a basement membrane-specific chondroitin sulfate proteoglycan. J Cell Biol. 1989;109:3187–3198. doi: 10.1083/jcb.109.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KJ, Couchman JR. Basement membrane chondroitin sulfate proteoglycans: Localization in adult rat tissues. J Histochem Cytochem. 1990;38:1479–1486. doi: 10.1177/38.10.2401786. [DOI] [PubMed] [Google Scholar]
- McCormick C, Duncan G, Goutsos KT, Tufaro F. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci USA. 2000;97(2):668–673. doi: 10.1073/pnas.97.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Leduc Y, Martindale D, Mattison K, Esford LE, Dyer AP, Tufaro F. The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nat Genet. 1998;19(2):158–161. doi: 10.1038/514. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284(5420):1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Miettinen A, Stow JL, Mentone S, Farquhar MG. Antibodies to basement membrane heparan sulfate proteoglycans bind to the lamina rarae of the glomerular basement membrane (GBM) and induce subepithelial basement membrane thickening. J Exp Med. 1986;163:1064–1084. doi: 10.1084/jem.163.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH. Renal basement membrane components. Kidney Int. 1999;56(6):2016–2024. doi: 10.1046/j.1523-1755.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- Miner JH. Building the glomerulus: A matricentric view. J Am Soc Nephrol. 2005;16(4):857–861. doi: 10.1681/ASN.2004121139. [DOI] [PubMed] [Google Scholar]
- Miosge N, Kother F, Heinemann S, Kohfeldt E, Herken R, Timpl R. Ultrastructural colocalization of nidogen-1 and nidogen-2 with laminin-1 in murine kidney basement membranes. Histochem Cell Biol. 2000;113(2):115–124. doi: 10.1007/s004180050014. [DOI] [PubMed] [Google Scholar]
- Moeller M, Sanden S, Soofi A, Wiggins R, Holzman L. Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis. 2003;35:39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Sandoval RM. Pharmacophotonics: Utilizing multi-photon microscopy to quantify drug delivery and intracellular trafficking in the kidney. Adv Drug Deliv Rev. 2006;58 (7):809–823. doi: 10.1016/j.addr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Sandoval RM. Quantifying dynamic kidney processes utilizing multi-photon microscopy. Contrib Nephrol. 2007;156:227–235. doi: 10.1159/000102088. [DOI] [PubMed] [Google Scholar]
- Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, Gubler MC, Antignac C, Esquivel EL. Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol. 2009;20 (10):2181–2189. doi: 10.1681/ASN.2009040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano S, D’Erme M, Sensi M, De Rossi MG, Medici F, Galliccia F, Andreani D, Di Mario U. Characteristics of proteinuria in experimental diabetes mellitus. Biochem Med Metab Biol. 1994;53(2):92–97. doi: 10.1006/bmmb.1994.1063. [DOI] [PubMed] [Google Scholar]
- Morano S, Pietravalle P, De Rossi MG, Mariani G, Cristina G, Medici F, Sensi M, Andreani D, Di Mario U. A charge selectivity impairment in protein permselectivity is present in type 2 diabetes. Acta Diabetol. 1993;30 (3):138–142. doi: 10.1007/BF00572857. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8(12):957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morio H, Honda Y, Toyoda H, Nakajima M, Kurosawa H, Shirasawa T. EXT gene family member rib-2 is essential for embryonic development and heparan sulfate biosynthesis in Caenorhabditis elegans. Biochem Biophys Res Commun. 2003;301(2):317–323. doi: 10.1016/s0006-291x(02)03031-0. [DOI] [PubMed] [Google Scholar]
- Morita H, Yoshimura A, Inui K, Ideura T, Watanabe H, Wang L, Soininen R, Tryggvason K. Heparan sulfate of perlecan is involved in glomerular filtration. J Am Soc Nephrol. 2005;16:1703–1710. doi: 10.1681/ASN.2004050387. [DOI] [PubMed] [Google Scholar]
- Mundy C, Yasuda T, Kinumatsu T, Yamaguchi Y, Iwamoto M, Enomoto-Iwamoto M, Koyama E, Pacifici M. Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol. 2011;351(1):70–81. doi: 10.1016/j.ydbio.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozzo RV. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/Perlecan) J Biol Chem. 1992;267:8544–8557. [PubMed] [Google Scholar]
- Nakagawa T, Izumino K, Ishii Y, Oya T, Hamashima T, Jie S, Ishizawa S, Tomoda F, Fujimori T, Nabeshima Y, Inoue H, Sasahara M. Roles of PDGF receptor-beta in the structure and function of postnatal kidney glomerulus. Nephrol Dial Transplant. 2011;26(2):458–468. doi: 10.1093/ndt/gfq468. [DOI] [PubMed] [Google Scholar]
- Nakato H, Kimata K. Heparan sulfate fine structure and specificity of proteoglycan functions. Biochim Biophys Acta. 2002;1573(3):312–318. doi: 10.1016/s0304-4165(02)00398-7. [DOI] [PubMed] [Google Scholar]
- Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, Yamada Y, Hassell JR. The complete sequence of Perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266:22939–22947. [PubMed] [Google Scholar]
- Ogata-Iwao M, Inatani M, Iwao K, Takihara Y, Nakaishi-Fukuchi Y, Irie F, Sato S, Furukawa T, Yamaguchi Y, Tanihara H. Heparan sulfate regulates intraretinal axon pathfinding by retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011;52(9):6671–6679. doi: 10.1167/iovs.11-7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ES, Woods A, Couchman JR. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C. J Biol Chem. 1997a;272(18):11805–11811. doi: 10.1074/jbc.272.18.11805. [DOI] [PubMed] [Google Scholar]
- Oh ES, Woods A, Couchman JR. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J Biol Chem. 1997b;272(13):8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- Okada K, Kawakami K, Yano I, Funai M, Kagami S, Kuroda Y, Oite T. Ultrastructural alterations of glomerular anionic sites in IgA nephropathy. Clin Nephrol. 1989;31 (2):96–102. [PubMed] [Google Scholar]
- Orellana A, Hirschberg CB, Wei Z, Swiedler SJ, Ishihara M. Molecular cloning and expression of a glycosaminoglycan N-acetylglucosaminyl N-deacetylase/N-sulfotransferase from a heparin-producing cell line. J Biol Chem. 1994;269(3):2270–2276. [PubMed] [Google Scholar]
- Ori A, Wilkinson MC, Fernig DG. A systems biology approach for the investigation of the heparin/heparan sulfate interactome. J Biol Chem. 2011;286(22):19892–198904. doi: 10.1074/jbc.M111.228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145(1):204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterby R. Structural changes in the diabetic kidney. Clin Endocrin Met. 1986;15:733–751. [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K, Feng GS, Zhang X. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development. 2008;135(2):301–310. doi: 10.1242/dev.014829. [DOI] [PubMed] [Google Scholar]
- Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: Selective regulators of ligand-receptor encounters. J Biol Chem. 2000;275(39):29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74(7):349–364. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Peti-Peterdi J. Multiphoton imaging of renal tissues in vitro. Am J Physiol Renal Physiol. 2005;288(6):F1079–F1083. doi: 10.1152/ajprenal.00385.2004. [DOI] [PubMed] [Google Scholar]
- Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21(11):1835–1841. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti-Peterdi J, Toma I, Sipos A, Vargas SL. Multi-photon imaging of renal regulatory mechanisms. Physiol (Bethesda) 2009;24:88–96. doi: 10.1152/physiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponighaus C, Ambrosius M, Casanova JC, Prante C, Kuhn J, Esko JD, Kleesiek K, Gotting C. Human xylosyltransferase II is involved in the biosynthesis of the uniform tetrasaccharide linkage region in chondroitin sulfate and heparan sulfate proteoglycans. J Biol Chem. 2007;282(8):5201–5206. doi: 10.1074/jbc.M611665200. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316(2):288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raats CJ, Bakker MA, Hoch W, Tamboer WP, Groffen AJ, van den Heuvel LP, Berden JH, van den Born J. Differential expression of agrin in renal basement membranes as revealed by domain-specific antibodies. J Biol Chem. 1998;273(28):17832–17838. doi: 10.1074/jbc.273.28.17832. [DOI] [PubMed] [Google Scholar]
- Raats CJ, Van Den Born J, Berden JH. Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria. Kidney Int. 2000;57(2):385–400. doi: 10.1046/j.1523-1755.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- Rada JA, Carlson EC. Anionic site and immunogold quantitation of heparan sulfate proteoglycans in glomerular basement membranes of puromycin aminonucleoside nephrotic rats. Anatomical Record. 1991;231:35–47. doi: 10.1002/ar.1092310106. [DOI] [PubMed] [Google Scholar]
- Rapraeger A, Bernfield M. Cell surface proteoglycan of mammary epithelial cells. Protease releases a heparan sulfate-rich ectodomain from a putative membrane-anchored domain. J Biol Chem. 1985;260(7):4103–4109. [PubMed] [Google Scholar]
- Rapraeger A, Jalkanen M, Endo E, Koda J, Bernfield M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J Biol Chem. 1985;260(20):11046–11052. [PubMed] [Google Scholar]
- Rapraeger AC. Molecular interactions of syndecans during development. Semin Cell Dev Biol. 2001;12(2):107–116. doi: 10.1006/scdb.2000.0239. [DOI] [PubMed] [Google Scholar]
- Ray MC, Gately LE., 3rd Basement membrane zone. Clin Dermatol. 1996;14(4):321–330. doi: 10.1016/0738-081x(96)00061-2. [DOI] [PubMed] [Google Scholar]
- Reeves WH, Kanwar YS, Farquhar MG. Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J Cell Biol. 1980;85 (3):735–753. doi: 10.1083/jcb.85.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers RM, Groen RW, Kuil A, Weijer K, Kimberley FC, Medema JP, van Kuppevelt TH, Li JP, Spaargaren M, Pals ST. Disruption of heparan sulfate proteo-glycan conformation perturbs B-cell maturation and APRIL-mediated plasma cell survival. Blood. 2011;117(23):6162–6171. doi: 10.1182/blood-2010-12-325522. [DOI] [PubMed] [Google Scholar]
- Rennke HG, Cotran RS, Venkatachalam MA. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennke HG, Venkatachalam MA. Glomerular permeability: In vivo tracer studies with polyanionic and polycationic ferritins. Kidney Int. 1977;11(1):44–53. doi: 10.1038/ki.1977.6. [DOI] [PubMed] [Google Scholar]
- Rodewald R, Karnovsky M. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974;60:423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong J, Habuchi H, Kimata K, Lindahl U, Kusche-Gullberg M. Expression of heparan sulphate L-iduronyl 2-O-sulphotransferase in human kidney 293 cells results in increased D-glucuronyl 2-O-sulphation. Biochem J. 2000;346:463–468. [PMC free article] [PubMed] [Google Scholar]
- Rong J, Habuchi H, Kimata K, Lindahl U, Kusche-Gullberg M. Substrate specificity of the heparan sulfate hexuronic acid 2-O-sulfotransferase. Biochemistry. 2001;40(18):5548–5555. doi: 10.1021/bi002926p. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99(9):2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BJ, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22(2):236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20(3):489–494. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami Y, Nakajima M, Takagawa K, Ueda T, Akazawa H, Maruhashi Y, Shimoyama H, Kamitsuji H, Yoshioka A. Analysis of glomerular anionic charge status in children with IgA nephropathy using confocal laser scanning microscopy. Nephron Clin Pract. 2004;96(3):c96–c104. doi: 10.1159/000076747. [DOI] [PubMed] [Google Scholar]
- Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW. Co-localization of nephrin, podocin, and the actin cytoskeleton: Evidence for a role in podocyte foot process formation. Am J Pathol. 2002;161(4):1459–1466. doi: 10.1016/S0002-9440(10)64421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278(15):12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96(6):2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Fassler R, Hohenester E. Laminin: The crux of basement membrane assembly. J Cell Biol. 2004;164(7):959–963. doi: 10.1083/jcb.200401058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell SC, Braet F. Glomerular endothelial cell fenestrations: An integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296(5):F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor N, Ichikawa I, Brenner BM. Mechanisms of action of various hormones and vasoactive substances on glomerular ultrafiltration in the rat. Kidney Int. 1981;20(4):442–451. doi: 10.1038/ki.1981.160. [DOI] [PubMed] [Google Scholar]
- Seiler MW, Rennke HG, Venkatachalam MA, Cotran RS. Pathogenesis of polycation-induced alterations (fusion) of glomerular epithelium. Lab Invest. 1977;36(1):48–61. [PubMed] [Google Scholar]
- Seiler MW, Venkatachalam MA, Cotran RS. Glomerular epithelium: Structural alterations induced by polycations. Science. 1975;189(4200):390–393. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- Shah MM, Sakurai H, Gallegos TF, Sweeney DE, Bush KT, Esko JD, Nigam SK. Growth factor-dependent branching of the ureteric bud is modulated by selective 6-O sulfation of heparan sulfate. Dev Biol. 2011;356(1):19–27. doi: 10.1016/j.ydbio.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Sakurai H, Sweeney DE, Gallegos TF, Bush KT, Esko JD, Nigam SK. Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching. Dev Biol. 2010;339(2):354–365. doi: 10.1016/j.ydbio.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Liu R, Xu Y, Liu J. The dominating role of N-deacetylase/N-sulfotransferase 1 in forming domain structures in heparan sulfate. J Biol Chem. 2011;286(22):19768–19776. doi: 10.1074/jbc.M111.224311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14(8):1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- Shworak NW, Liu J, Fritze LM, Schwartz JJ, Zhang L, Logeart D, Rosenberg RD. Molecular cloning and expression of mouse and human cDNAs encoding heparan sulfate D-glucosaminyl 3-O-sulfotransferase. J Biol Chem. 1997;272(44):28008–28019. doi: 10.1074/jbc.272.44.28008. [DOI] [PubMed] [Google Scholar]
- Shworak NW, Liu J, Petros LM, Zhang L, Kobayashi M, Copeland NG, Jenkins NA, Rosenberg RD. Multiple isoforms of heparan sulfate D-glucosaminyl 3-O-sulfotransferase. Isolation, characterization, and expression of human cdnas and identification of distinct genomic loci. J Biol Chem. 1999;274(8):5170–5184. doi: 10.1074/jbc.274.8.5170. [DOI] [PubMed] [Google Scholar]
- Simons M, Horowitz A. Syndecan-4-mediated signalling. Cell Signal. 2001;13(12):855–862. doi: 10.1016/s0898-6568(01)00190-5. [DOI] [PubMed] [Google Scholar]
- Singh A, Friden V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, Haraldsson B, Mathieson PW, Satchell SC. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol. 2011;300(1):F40–F48. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glyco-calyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18(11):2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- Smith RL, Bernfield M. Mesenchyme cells degrade epithelial basal lamina glycosaminoglycan. Dev Biol. 1982;94(2):378–390. doi: 10.1016/0012-1606(82)90355-4. [DOI] [PubMed] [Google Scholar]
- Smits NC, Lensen JF, Wijnhoven TJ, Ten Dam GB, Jenniskens GJ, van Kuppevelt TH. Phage display-derived human antibodies against specific glycosaminoglycan epitopes. Methods Enzymol. 2006;416:61–87. doi: 10.1016/S0076-6879(06)16005-X. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Nature of the glycoprotein components of basement membranes. Ann NY Acad Sci. 1978;312:106–121. doi: 10.1111/j.1749-6632.1978.tb16796.x. [DOI] [PubMed] [Google Scholar]
- St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60(3):1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Wang L, Castagnola J, Song D, Bishop JR, Brown JR, Lawrence R, Bai X, Habuchi H, Tanaka M, Cardoso WV, Kimata K, Esko JD. Heparan sulfate 2-O-sulfotransferase is required for triglyceride-rich lipoprotein clearance. J Biol Chem. 2010;285(1):286–294. doi: 10.1074/jbc.M109.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhard BM, Isom K, Stroganova L, St John PL, Zelenchuk A, Freeburg PB, Holzman LB, Abrahamson DR. Deletion of von Hippel-Lindau in glomerular podocytes results in glomerular basement membrane thickening, ectopic subepithelial deposition of collagen {alpha}1{alpha}2{alpha}1(IV), expression of neuroglobin, and proteinuria. Am J Pathol. 2010;177(1):84–96. doi: 10.2353/ajpath.2010.090767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, Schwesinger C, Bai X, Esko JD, Nigam SK. Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol. 2004;272(2):310–327. doi: 10.1016/j.ydbio.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Steffes MW, Mauer SM. Diabetic glomerulopathy in man and experimental animal models. Int Rev Exp Path. 1984;26:147–175. [PubMed] [Google Scholar]
- Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–1081. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- Stenman S, Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LA, Haney LB, Hussaini IM, Karns LR, Glass WF., 2nd Reguation of smooth muscle a-actin expression and hypertrophy in cultured mesangial cells. Kidney Int. 1998;54:1175–1187. doi: 10.1046/j.1523-1755.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Stickens D, Zak BM, Rougier N, Esko JD, Werb Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005;132(22):5055–5068. doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Watanabe S, Wada N, Murakami H, Funaki S, Yan K, Kondo Y, Harada K, Nagata M. Charge selective function in childhood glomerular diseases. Pediatr Res. 2006;59(2):336–340. doi: 10.1203/01.pdr.0000196733.47083.81. [DOI] [PubMed] [Google Scholar]
- Tanner GA. Glomerular sieving coefficient of serum albumin in the rat: A two-photon microscopy study. Am J Physiol Renal Physiol. 2009;296(6):F1258–F1265. doi: 10.1152/ajprenal.90638.2008. [DOI] [PubMed] [Google Scholar]
- Tapanadechopone P, Hassell JR, Rigatti B, Couchman JR. Localization of glycosaminoglycan substitution sites on domain V of mouse perlecan. Biochem Biophys Res Commun. 1999;265(3):680–690. doi: 10.1006/bbrc.1999.1714. [DOI] [PubMed] [Google Scholar]
- Ten Dam GB, Kurup S, van de Westerlo EM, Versteeg EM, Lindahl U, Spillmann D, van Kuppevelt TH. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J Biol Chem. 2006;281(8):4654–4662. doi: 10.1074/jbc.M506357200. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Shewring L, McCarthy KJ, Couchman JR, Mason RM, Davies M. Rat mesangial cells in vitro synthesize a spectrum of proteoglycan species including those of the basement membrane and interstitium. Kidney Int. 1995;48(4):1278–1289. doi: 10.1038/ki.1995.412. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Connell MG, Fernig DG, Ten Dam GB, van Kuppevelt TH, Turnbull JE, Jesudason EC, Losty PD. Novel ’phage display antibodies identify distinct heparan sulfate domains in developing mammalian lung. Pediatr Surg Int. 2007;23:411–417. doi: 10.1007/s00383-006-1864-8. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohde M, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin: A glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Tkachenko E, Rhodes JM, Simons M. Syndecans: New kids on the signaling block. Circ Res. 2005;96(5):488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Selleck SB. Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J Biol Chem. 2000;275(4):2269–2275. doi: 10.1074/jbc.275.4.2269. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Fasakhany F, Kadomatsu K, Matsukawa T, Yamakawa T, Kurosawa N, Muramatsu T. Diversity of N-acetylglucosamine-6-O-sulfotransferases: Molecular cloning of a novel enzyme with different distribution and specificities. Biochem Biophys Res Commun. 2000;274(2):291–296. doi: 10.1006/bbrc.2000.3141. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Muramatsu H, Kadomatsu K, Fan QW, Kurosawa N, Mitsuoka C, Kannagi R, Habuchi O, Muramatsu T. Molecular cloning and characterization of an N-acetylglucosamine-6-O-sulfotransferase. J Biol Chem. 1998;273(35):22577–22583. doi: 10.1074/jbc.273.35.22577. [DOI] [PubMed] [Google Scholar]
- van den Born J, Van Den Heuvel LPWJ, Bakker MAH, Veerkamp JH, Assmann KJM, Berden JHM. A monoclonal antibody against GBM heparan sulfate induces an acute selective proteinuria in rats. Kidney Int. 1992;411:115–123. doi: 10.1038/ki.1992.15. [DOI] [PubMed] [Google Scholar]
- van den Born J, van Kraats AA, Bakker MA, Assmann KJ, Dijkman HB, van der Laak JA, Berden JH. Reduction of heparan sulphate-associated anionic sites in the glomerular basement membrane of rats with streptozotocin-induced diabetic nephropathy. Diabetologia. 1995;38(10):1169–1175. doi: 10.1007/BF00422365. [DOI] [PubMed] [Google Scholar]
- van Kuppevelt TH, Dennissen MA, van Venrooij WJ, Hoet RM, Veerkamp JH. Generation and application of type-specific anti-heparan sulfate antibodies using phage display technology. Further evidence for heparan sulfate heterogeneity in the kidney. J Biol Chem. 1998;273(21):12960–12966. doi: 10.1074/jbc.273.21.12960. [DOI] [PubMed] [Google Scholar]
- van Kuppevelt TH, Jenniskens GJ, Veerkamp JH, ten Dam GB, Dennissen MA. Phage display technology to obtain antiheparan sulfate antibodies. Methods Mol Biol. 2001;171:519–534. doi: 10.1385/1-59259-209-0:519. [DOI] [PubMed] [Google Scholar]
- Wada N, Ueda Y, Iidaka K, Inage Z, Kikkawa Y, Kitagawa T. Portions of basement membrane with decreased negative charge in various glomerulonephritis. Clin Nephrol. 1990;34(1):9–16. [PubMed] [Google Scholar]
- Whiteside CI, Cameron R, Munk S, Levy J. Podocytic cytoskeletal disaggregation and basement-membrane detachment in puromycin aminonucleoside nephrosis. Am J Pathol. 1993;142(5):1641–1653. [PMC free article] [PubMed] [Google Scholar]
- Wilcox-Adelman SA, Denhez F, Iwabuchi T, Saoncella S, Calautti E, Goetinck PF. Syndecan-4: Dispensable or indispensable? Glycoconj J. 2002;19(4–5):305–313. doi: 10.1023/A:1025304602057. [DOI] [PubMed] [Google Scholar]
- Woods A. Syndecans: Transmembrane modulators of adhesion and matrix assembly. J Clin Invest. 2001;107(8):935–941. doi: 10.1172/JCI12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecans: Synergistic activators of cell adhesion. Trends Cell Biol. 1998;8(5):189–192. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol. 2001;13(5):578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR, Hook M. Heparan sulfate proteoglycans of rat embryo fibroblasts. A hydrophobic form may link cytoskeleton and matrix components. J Biol Chem. 1985;260(19):10872–10879. [PubMed] [Google Scholar]
- Woods A, Hook M, Kjellen L, Smith CG, Rees DA. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol. 1984;99(5):1743–1753. doi: 10.1083/jcb.99.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Longley RL, Tumova S, Couchman JR. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys. 2000;374(1):66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- Young PA, Clendenon SG, Byars JM, Decca RS, Dunn KW. The effects of spherical aberration on multiphoton fluorescence excitation microscopy. J Microsc. 2011a;242(2):157–165. doi: 10.1111/j.1365-2818.2010.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PA, Clendenon SG, Byars JM, Dunn KW. The effects of refractive index heterogeneity within kidney tissue on multiphoton fluorescence excitation microscopy. J Microsc. 2011b;242(2):148–156. doi: 10.1111/j.1365-2818.2010.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung S, Woods A, Chan TM, Davies M, Williams JD, Couchman JR. Syndecan-4 up-regulation in proliferative renal disease is related to microfilament organization. FASEB J. 2001;15(9):1631–1633. doi: 10.1096/fj.00-0794fje. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, O’Rear J. Supramolecular organization of basement membranes. In: Rohrbach DH, Timpl R, editors. Molecular and Cellular Aspects of Basement Membranes. San Diego CA: Academic Press; 1993. pp. 19–47. [Google Scholar]
- Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, Weinstein T, Li JP, Lindahl U, Vlodavsky I. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 2004;18(2):252–263. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]
- Zhang L, Esko JD. Amino acid determinants that drive heparan sulfate assembly in a proteoglycan. J Biol Chem. 1994;269(30):19295–19299. [PubMed] [Google Scholar]
- Zimmermann P, David G. The syndecans, tuners of transmembrane signaling. FASEB J. 1999;13(Suppl):S91–S100. doi: 10.1096/fasebj.13.9001.s91. [DOI] [PubMed] [Google Scholar]
- Zuberi RI, Ge XN, Jiang S, Bahaie NS, Kang BN, Hosseinkhani RM, Frenzel EM, Fuster MM, Esko JD, Rao SP, Sriramarao P. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J Immunol. 2009;183(6):3971–3979. doi: 10.4049/jimmunol.0901604. [DOI] [PMC free article] [PubMed] [Google Scholar]