Abstract
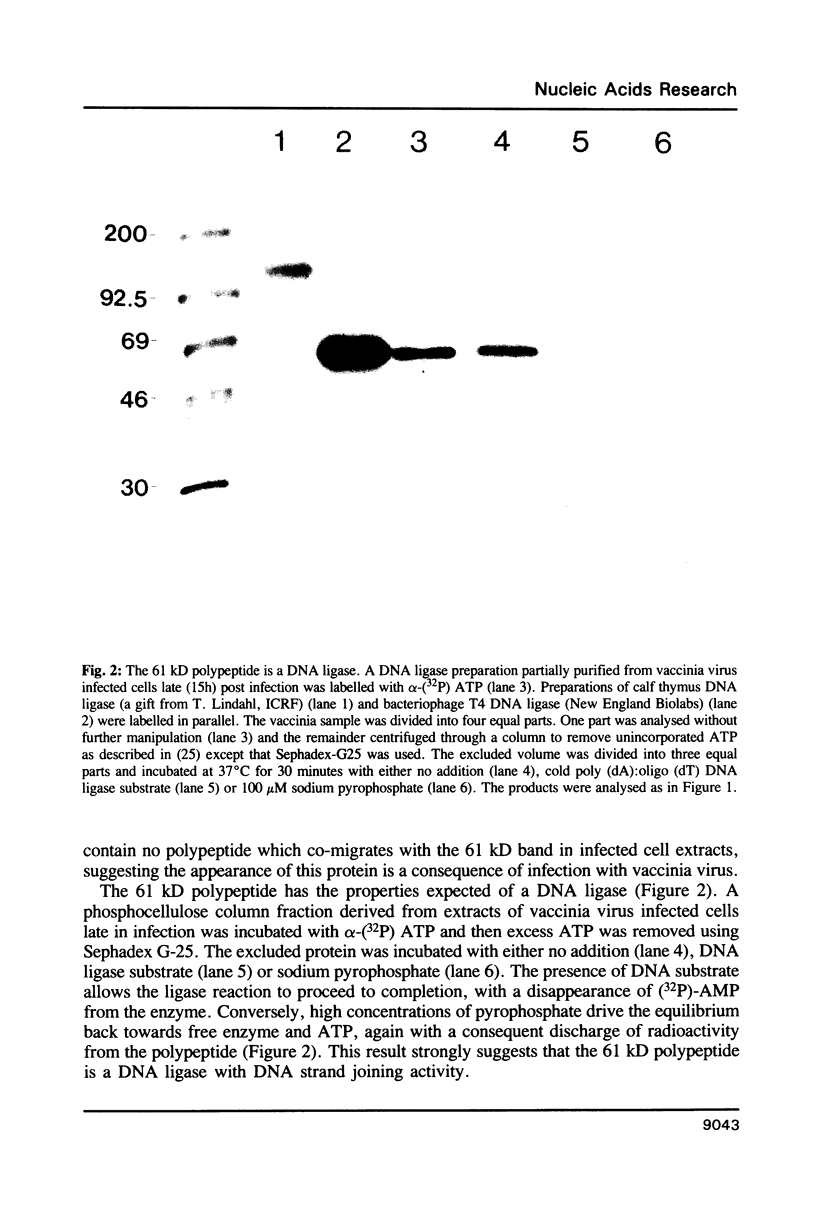
Vaccinia virus gene SalF 15R potentially encodes a polypeptide of 63 kD which shares 30% amino acid identity with S. pombe and S. cerevisiae DNA ligases. DNA ligase proteins can be identified by incubation with alpha-(32P)ATP, resulting in the formation of a covalent DNA ligase-AMP adduct, an intermediate in the enzyme reaction. A novel radio-labelled polypeptide of approximately 61 kD appears in extracts from vaccinia virus infected cells after incubation with alpha-(32P)ATP. This protein is present throughout infection and is a DNA ligase as the radioactivity is discharged in the presence of either DNA substrate or pyrophosphate. DNA ligase assays show an increase in enzyme activity in cell extracts after vaccinia virus infection. A rabbit antiserum, raised against a bacterial fusion protein of beta-galactosidase and a portion of SalF 15R, immune-precipitates polypeptides of 61 and 54 kD from extracts of vaccinia virus-infected cells. This antiserum also immune-precipitates the novel DNA ligase-AMP adduct, thus proving that the observed DNA ligase is encoded by SalF 15R.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. E., Willis A. E., Goldsmith I., Lindahl T. Different substrate specificities of the two DNA ligases of mammalian cells. J Biol Chem. 1986 Jul 15;261(20):9079–9082. [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Banks G. R., Barker D. G. DNA ligase-AMP adducts: identification of yeast DNA ligase polypeptides. Biochim Biophys Acta. 1985 Dec 18;826(4):180–185. doi: 10.1016/0167-4781(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Bloom D. C., Massung R., Savage L., Morrison D. K., Moyer R. W. Recruitment to the cytoplasm of a cellular lamin-like protein from the nucleus during a poxvirus infection. Virology. 1989 Mar;169(1):115–126. doi: 10.1016/0042-6822(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F., German J., Ray J. H. Altered DNA ligase I activity in Bloom's syndrome cells. Nature. 1987 Jan 22;325(6102):357–359. doi: 10.1038/325357a0. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Jones E. V., Moss B. Homology between DNA polymerases of poxviruses, herpesviruses, and adenoviruses: nucleotide sequence of the vaccinia virus DNA polymerase gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3659–3663. doi: 10.1073/pnas.83.11.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M., Flores L., Holowczak J. A. Model for vaccinia virus DNA replication. Virology. 1977 Dec;83(2):467–473. doi: 10.1016/0042-6822(77)90197-0. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Mapping and identification of the vaccinia virus thymidine kinase gene. J Virol. 1982 Aug;43(2):403–409. doi: 10.1128/jvi.43.2.403-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakritz N., Foglesong P. D., Reddy M., Baum S., Hurwitz J., Bauer W. R. A vaccinia virus DNase preparation which cross-links superhelical DNA. J Virol. 1985 Mar;53(3):935–943. doi: 10.1128/jvi.53.3.935-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Moyer R. W. Detection of a subunit of cellular Pol II within highly purified preparations of RNA polymerase isolated from rabbit poxvirus virions. Cell. 1986 Feb 28;44(4):587–596. doi: 10.1016/0092-8674(86)90268-0. [DOI] [PubMed] [Google Scholar]
- Moyer R. W. The role of the host cell nucleus in vaccinia virus morphogenesis. Virus Res. 1987 Sep;8(3):173–191. doi: 10.1016/0168-1702(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., O'Shea M. T. The mode of replication of vaccinia virus DNA. Virology. 1978 Jan;84(1):1–8. doi: 10.1016/0042-6822(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Reddy M. K., Bauer W. R. Activation of the vaccinia virus nicking-joining enzyme by trypsinization. J Biol Chem. 1989 Jan 5;264(1):443–449. [PubMed] [Google Scholar]
- Rodriguez J. F., Kahn J. S., Esteban M. Molecular cloning, encoding sequence, and expression of vaccinia virus nucleic acid-dependent nucleoside triphosphatase gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9566–9570. doi: 10.1073/pnas.83.24.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer R., Traktman P. Vaccinia virus encapsidates a novel topoisomerase with the properties of a eucaryotic type I enzyme. J Biol Chem. 1987 Jul 5;262(19):9309–9315. [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh M., Roseman N., Davis R., Mathews C. Vaccinia virus-encoded ribonucleotide reductase: sequence conservation of the gene for the small subunit and its amplification in hydroxyurea-resistant mutants. J Virol. 1988 Feb;62(2):519–527. doi: 10.1128/jvi.62.2.519-527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S. Properties of DNA ligase from uninfected and virus-infected HeLa cells. Nucleic Acids Res. 1976 Aug;3(8):2155–2167. doi: 10.1093/nar/3.8.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Smith G. L. Expression and characterization of herpes simplex virus type 1 (HSV-1) glycoprotein G (gG) by recombinant vaccinia virus: neutralization of HSV-1 infectivity with anti-gG antibody. J Gen Virol. 1987 Oct;68(Pt 10):2587–2598. doi: 10.1099/0022-1317-68-10-2587. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. DNA ligases of eukaryotes. FEBS Lett. 1976 Aug 1;67(1):1–8. doi: 10.1016/0014-5793(76)80858-7. [DOI] [PubMed] [Google Scholar]
- Tengelsen L. A., Slabaugh M. B., Bibler J. K., Hruby D. E. Nucleotide sequence and molecular genetic analysis of the large subunit of ribonucleotide reductase encoded by vaccinia virus. Virology. 1988 May;164(1):121–131. doi: 10.1016/0042-6822(88)90627-7. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A. E., Lindahl T. DNA ligase I deficiency in Bloom's syndrome. Nature. 1987 Jan 22;325(6102):355–357. doi: 10.1038/325355a0. [DOI] [PubMed] [Google Scholar]