Abstract
Submandibular acinar glands secrete numerous proteins such as digestive enzymes and defense proteins on the basis of the exocrine secretion mode. Exocytosis is a complex process, including a soluble NSF attachment protein receptor (SNARE)–mediated membrane fusion of vesicles and target membrane and the additional activation of cytoskeletal proteins. Relevant data are available predominantly for animal salivary glands, especially of the rat parotid acinar cells. The authors investigated the secretory molecular machinery of acinar (serous) cells in the human submandibular gland by immunohistochemistry and immunofluorescence and found diverse proteins associated with exocytosis for the first time. SNAP-23, syntaxin-2, syntaxin-4, and VAMP-2 were localized at the luminal plasma membrane; syntaxin-2 and septin-2 were expressed in vesicles in the cytoplasm. Double staining of syntaxin-2 and septin-2 revealed a colocalization on the same vesicles. Lactoferrin and α-amylase served as a marker for secretory vesicles and were labeled positively together with syntaxin-2 and septin-2 in double-staining procedures. Cytoskeletal components such as actin, myosin II, cofilin, and profilin are concentrated at the apical plasma membrane of acinar submandibular glands. These observations complement the understanding of the complex exocytosis mechanisms.
Keywords: salivary glands, secretory pathway, exocytosis, cytoskeleton, immunohistochemistry
The submandibular glands are located, on either side, between the mandible and the muscles that form the floor of the mouth. As a mixed gland, the serous acini are more numerous than the mucous acini. The intercalated ducts are relatively short; the striated ducts are longer. In humans, 70% of the salivary gland accounts for the submandibular glands (Bloom and Fawcett 1994). Salivary proteins are secreted by exocytosis (i.e., the fusion between secretory granule membranes and the apical plasma membrane of salivary acinar cells).
Soluble NSF attachment protein receptor (SNARE) proteins were first studied in neuronal cells and are involved in the exocytosis system, that is, in neurotransmission (Pfeffer 1996; Goda 1997). The SNARE hypothesis indicates that v-SNAREs are localized in vesicles and t-SNAREs at the target membrane (Söllner, Bennett, et al. 1993). A transport vesicle chooses its target for fusion when a soluble NSF-attachment protein (SNAP) receptor on the vesicle (v-SNARE) pairs with its cognate t-SNARE at the target membrane. SNARE proteins not only are found in synaptosomes but also have various homologues dealing with the common form of vesicular transport in many cells (Bennett et al. 1993; McMahon et al. 1993). Mammalian SNAREs comprise three conserved families: synaptobrevin/vesicle-associated membrane proteins (VAMPs), syntaxins, and SNAP-25 homologues. VAMPs are categorized as v-SNAREs, syntaxins, and SNAP-25 homologues as t-SNAREs. In neuronal cells, VAMP-2/synaptobrevin binds specifically to a heterodimeric complex of syntaxin-1 and SNAP-25 in the plasma membrane (Söllner, Whiteheart, et al. 1993). Syntaxin-3 and VAMP-2 are known to form an apical SNARE complex in stimulated lacrimal acini (Sou et al. 2005) and in gastric parietal cells (Ammar et al. 2002). In acinar cells from the pancreas gland, VAMP-2 was expressed at the apical region as a concentrated border around the acinar lumen (Braun et al. 1994). Oishi et al. (2006) demonstrated VAMP-2 in small cytoplasmic vesicles of a human parotid epithelial cell line using a VAMP2-GFP construct. Syntaxin-4, SNAP-23, and VAMP-8 regulate the exocytosis in mast cells (Paumet et al. 2000). In parotid and pancreatic acinar cells, several proteins that are expressed ubiquitously in non-neuronal cells have been detected—for example, NSF, α-SNAP, VAMP-2, syntaxin-4, and SNAP-23 (Braun et al. 1994; Ravichandran et al. 1996). However, when VAMP-2 was immunoprecipitated from lysates of parotid acinar cells, syntaxin-4 and SNAP-23 were not coprecipitated with VAMP-2 (Takuma et al. 2000). Imai et al. (2003) studied the intracellular localization of SNARE proteins by Western blotting and immunocytochemistry and found that in rat parotid acinar cells, syntaxin-2 and -3 were detected in the apical plasma membrane, and in addition, syntaxin-4 was localized in the basolateral membrane. Septins, proteins usually identified in processes of cytokinesis, may be involved in vesicle targeting or tethering (Kartmann and Roth 2001). Hsu et al. (1998) isolated a large septin complex, which helps tether vesicles to specialized regions of the plasma membrane.
Cytoskeletal proteins are essential for regulating cytoskeletal dynamics and participate in the process of exocytosis. Myosin II plays a role in the secretory processes of a variety of cells such as pancreatic acinar cells (Bhat and Thorn 2009), lacrimal acinar epithelial cells (Jerdeva et al. 2005), mast cells (Ludowyke et al. 2006), natural killer cells (Andzelm et al. 2007), and neurons (Mochida et al. 1994). Here, myosin II is necessary for the maintained opening of the fusion pore (Bhat and Thorn 2009). In pancreatic acinar cells, as in many other secretory cell types, a breakdown and reorganization of the actin cytoskeleton seem crucial for Ca2+-triggered exocytosis (Valentijn et al. 1999). In human parotid and submandibular glands, F-actin was localized underneath the luminal membrane to separate the secretory granules from the luminal membrane (Segawa et al. 1998). Cofilin, an actin-depolymerizing protein and one of the key components that control the turnover and branching of microfilaments, was supposed to be required in adrenal chromaffin cells to achieve the rapid reorganization of the cortical actin cytoskeleton that is necessary to allow the movement of yet undocked secretory granules to the plasma membrane (Birkenfeld et al. 2001). Profilin, a G-actin-binding protein, acts as a regulator of cytoskeletal dynamics and membrane transport (Birbach 2008). For profilin-2, which has been highly expressed in brain, a function in the regulation of exocytosis through the interaction with a special member of the glutamate receptor, the kainate receptor GluK2b (Mondin et al. 2010), could be demonstrated.
Most of the findings of the regulatory processes in salivary gland exocytosis were made either in animal tissue such as rat parotid acinar cells (Imai et al. 2003; Nashida et al. 2004) or with the help of human parotid epithelial cell lines (HSY cells) (Oishi et al. 2006). There is obviously no study showing the localization of SNARE proteins in tissues of the human submandibular gland by immunohistochemistry. The aim of our study was to elucidate the participation of actin, myosin II, cofilin, profilin, SNAP-23, syntaxin-2, syntaxin-4, VAMP-2, and septin-2 in the exocytosis mechanism in the human submandibular gland.
Materials and Methods
Tissue
Tissue of human submandibular glands was obtained from 20 persons, 9 males and 11 females, with an age range between 5 and 83 years. The patients underwent salivary gland surgery for different indications, and partially healthy tissue was adjacent to pathological tissue. Four percent phosphate-buffered formaldehyde was used for the fixation of the gland tissue. The study was performed according to the guidelines of the local ethics committee.
Immunohistochemical Staining
For immunohistochemical staining, 5-µm paraffin sections of formalin-fixed tissue samples were dewaxed and stained with antibodies to the following proteins: actin (Abcam; Cambridge, UK), myosin IIa (Sigma-Aldrich; Munich, Germany), cofilin (Abcam), profilin-1 (Novus Biologicals; Littleton, CO), SNAP-23 (Abcam), syntaxin-2 (Santa Cruz Biotechnology; Santa Cruz, CA), syntaxin-4 (Abcam), VAMP-2 (Abcam), and septin-2 (Santa Cruz Biotechnology). Sections were pretreated with microwave irradiation in citrate buffer at pH 6.0 for 15 min for all antibodies. The endogenous peroxidase activity was inhibited with 3% hydrogen peroxide for 10 min. Blocking of nonspecific binding was achieved by incubating the sections with 3% normal blood serum: goat serum for actin, myosin II, cofilin, profilin, SNAP-23, syntaxin-4, and VAMP-2 and rabbit serum for syntaxin-2 and septin-2. Sections were incubated with the primary antibodies for 1 hr at room temperature and overnight at 4°C. The secondary antibody (Vector Laboratories, Inc.; Burlingame, CA) was applied in a concentration of 1:200 for 45 min at room temperature. The detection of the antibodies was performed with avidin–biotin–horseradish peroxidase using the Vectastain-Kit (Vector Laboratories, Inc.). Diaminobenzidine was used as a chromogen. The sections were counterstained with hematoxylin. Controls, in which the primary antibody was replaced with buffer, were treated identically. Sections were analyzed with a Nikon Eclipse 80i microscope, and images were taken by a digital Nikon camera (Nikon; Duesseldorf, Germany). Labeling results were categorized as weak, medium, or strong staining.
Immunofluorescence Microscopy
In total, 4-µm sections of formalin-fixed tissues were dewaxed. For heat-induced epitope retrieval, sections were incubated in citrate buffer at pH 6.0 for 10 min. Nonspecific antibody-binding was blocked by 2% BSA (Sigma-Aldrich). Antibodies used were anti-septin-2 (Abcam), anti-syntaxin-2 (Santa Cruz Biotechnology), anti-lactoferrin (Abcam), and anti-α-amylase (Abcam). Sections were incubated for 1 hr at room temperature. As secondary fluorescence-labeled antibodies, we used a 1:300 dilution of Cy3-conjugated (red) or Alexa 488–conjugated (green) antibodies (Jackson Immuno Research; Suffolk, UK). DAPI was used for nuclear staining. Coverslips were mounted on glass slides with 90% glycerol (Sigma-Aldrich). Cells were viewed using an inverted fluorescence microscope (Zeiss Axiovert 200) or a confocal microscope (LSM510; Zeiss, Goettingen, Germany). Control sections were processed without treatment with primary antibodies.
In Table 1, the staining procedures for every antibody are summarized.
Table 1.
Primary Antibodies
| Antibody (Diaminobenzidine-Based Staining) | Host | Clone | Dilution | Blocking Serum 3% |
|---|---|---|---|---|
| Anti-actin | Mouse | ACTN05 (C4) | 1:100 | Goat |
| Anti–myosin II | Rabbit | Polyclonal | 1:2000 | Goat |
| Anti-cofilin | Rabbit | Polyclonal | 1:1000 | Goat |
| Anti-profilin | Rabbit | Polyclonal | 1:50 | Goat |
| Anti-SNAP-23 | Rabbit | Polyclonal | 1:100 | Goat |
| Anti-syntaxin-2 | Goat | Polyclonal | 1:100 | Rabbit |
| Anti-syntaxin-4 | Rabbit | Polyclonal | 1:300 | Goat |
| Anti-VAMP-2 | Rabbit | Polyclonal | 1:100 | Goat |
| Anti-septin-2 | Goat | Polyclonal | 1:80 | Rabbit |
| Anti-body (Immunofluorescence) | Host | Clone | Dilution | Blocking Serum 2% |
| Anti-septin-2 | Rabbit | Polyclonal | 1:250 | BSA |
| Anti-syntaxin-2 | Goat | Polyclonal | 1:50 | BSA |
| Anti-lactoferrin | Mouse | Monoclonal [2B8] | 1:1000 | BSA |
| Anti-α-amylase | Mouse | Monoclonal | 1:250 | BSA |
Results
Immunohistochemical Findings
Actin
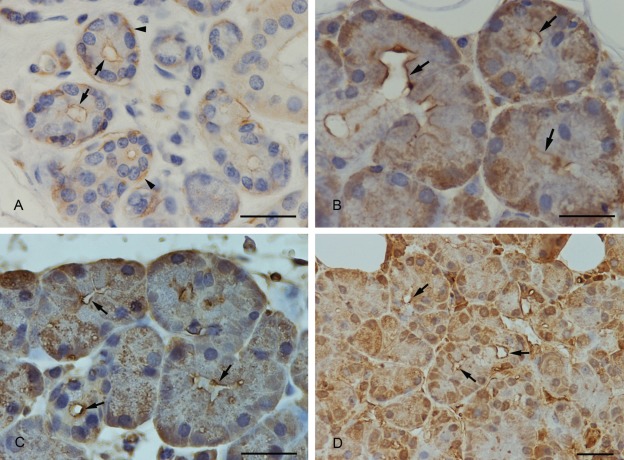
The luminal membrane of the submandibular acinar cells was stained with a weak to medium intensity. Myoepithelial cells at the base of the acinar cells stained strongly. Partially, lateral cell membranes of the acinar cells stained positively (Fig. 1A).
Figure 1.
(A) Anti-actin immunostaining. Positive staining of the apical membrane of submandibular acinar cells (arrows) and of myoepithelial cells (arrowheads). (B) Anti–myosin II immunostaining. The luminal membrane showed a strong staining (arrows). (C) Anti-cofilin immunostaining. Cofilin was strongly expressed at the apical membrane (arrows). (D) Anti-profilin immunostaining. A strong positive staining of profilin was demonstrated at the apical luminal membrane (arrows) and in the nucleus. The whole cytoplasm was weaker stained. Scale bars: 25 µm.
Myosin II
Myosin II was expressed in the basal part of the acinar cells with a medium to strong intensity. The staining decreased in the direction of the lumen. The apical membrane, however, was strongly stained (Fig. 1B). In sections where the acinus was cut in a direction parallel to the secretory duct, the myosin II–positive contour of the apical plasma membrane was seen very clearly. When the lumen of the acinus was cut in a transversal direction, the apical membrane was strongly stained, indicating a concentrated border around the acinar lumen.
Cofilin
Cofilin staining was variable; cofilin was partially localized at the apical acinar plasma membrane (Fig. 1C), and to some extent, the apical part of the cells remained unstained within the same section. In addition, a weak overall staining of the whole-cell plasma with a stronger staining at the basal part of the cell was observed.
Profilin-1
In most cases, the apical membrane of acinar submandibular cells was stained strongly positive with the anti-profilin-1 antibody (Fig. 1D). Partially, within the same section, the luminal acinar membrane was less stained or unstained. In addition, the cytoplasm and the nucleus showed a medium intense immunostaining.
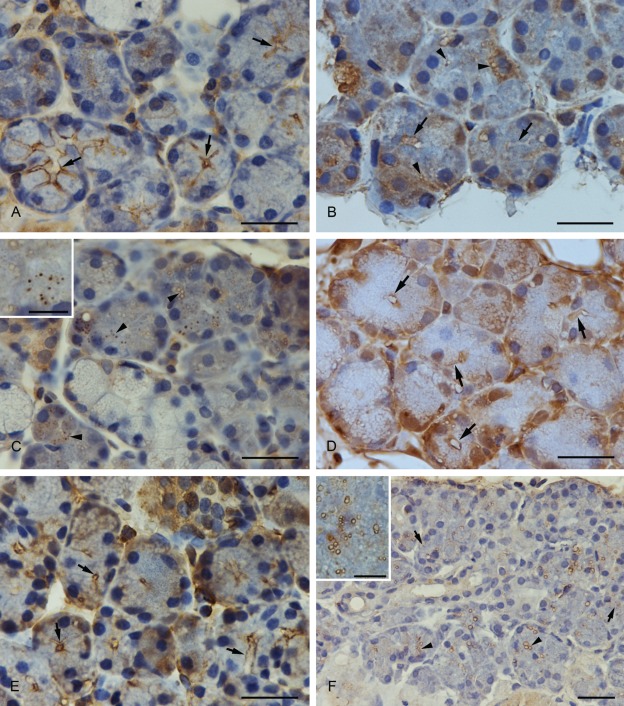
SNAP-23
SNAP-23 immunoreactivity showed a strong staining at the apical membrane of the acinar cells and partially at the upper part of the basolateral cell membrane (Fig. 2A).
Figure 2.
(A) Anti-SNAP-23 immunostaining. SNAP-23 was concentrated at the luminal and the basolateral membrane (arrows). (B) Anti-syntaxin-2 immunostaining. Syntaxin-2 showed a strong expression at the apical plasma membrane (arrows) and in smaller and larger vesicles (arrowheads). The basal part of the cell, especially the perinuclear compartment, was also strongly stained. (C) Anti-syntaxin-2 immunostaining. Larger vesicles with an unstained matrix and smaller completely stained vesicles showed a positive syntaxin-2 immunostaining. A higher magnification of the vesicles is demonstrated in the inset. (D) Anti-syntaxin-4 immunostaining. Syntaxin-4 was expressed at the apical and basolateral plasma membrane of submandibular acinar cells (arrows). (E) Anti-VAMP-2 immunostaining. VAMP-2 was localized at the apical region of the plasma membrane (arrows). (F) Anti-septin-2 immunostaining. Positively stained vesicles with an unstained matrix (arrowheads) and completely stained vesicles (arrows) were visible with the antibody to septin-2. A higher magnification of the vesicles is demonstrated in the inset. Scale bars: 25 µm; scale bars in insets: 6 µm.
Syntaxin-2
Syntaxin-2 was strongly expressed at the apical plasma membrane of the acinar cells and in smaller and larger vesicles in the cytoplasm (Fig. 2B,C). Although the larger vesicles revealed an unstained matrix, the smaller were stained as a whole. In the basal part of the cytoplasm, syntaxin-2 was partially concentrated.
Syntaxin-4
Syntaxin-4 was localized at the apical and basolateral plasma membrane (Fig. 2D). The staining intensity of the antibody was strong.
VAMP-2
A strong positive staining of the VAMP-2 immunoreactivity could be observed in the apical region of the acinar cell membrane (Fig. 2E). Positively stained vesicles in the cytoplasm could not be detected.
Septin-2
A large number of positively marked vesicles with the anti-septin-2 antibody were found in the cytoplasm of acinar cells. Both large and small vesicles with an unstained matrix as well as smaller, completely stained vesicles showed a positive reaction with the anti-septin-2 antibody (Fig. 2F).
Immunofluorescence Double Staining
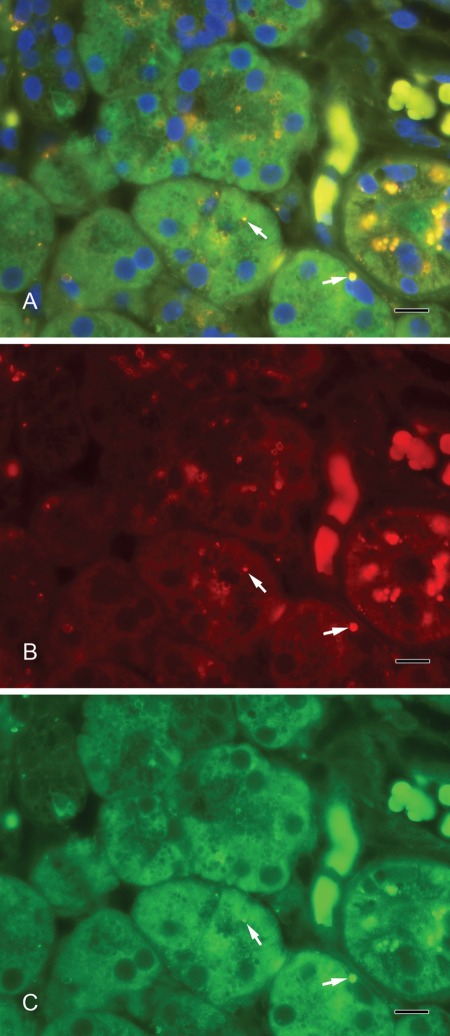
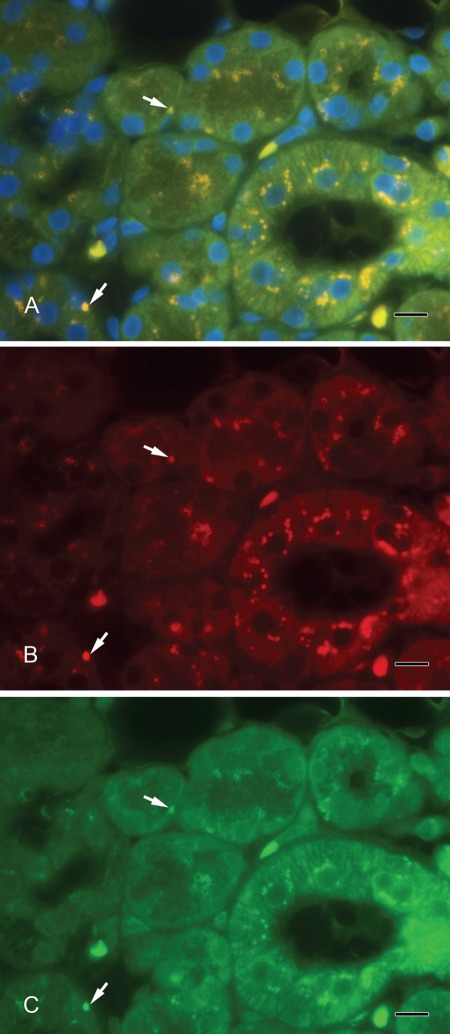
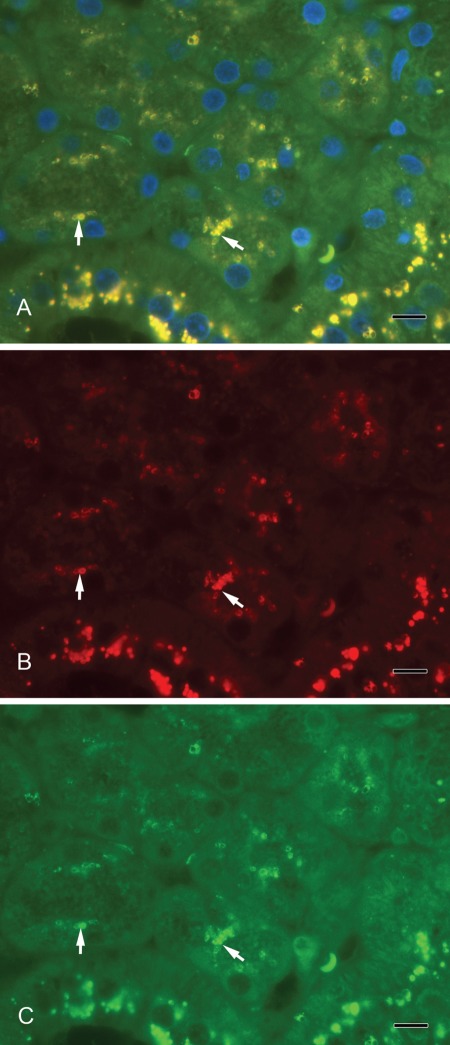
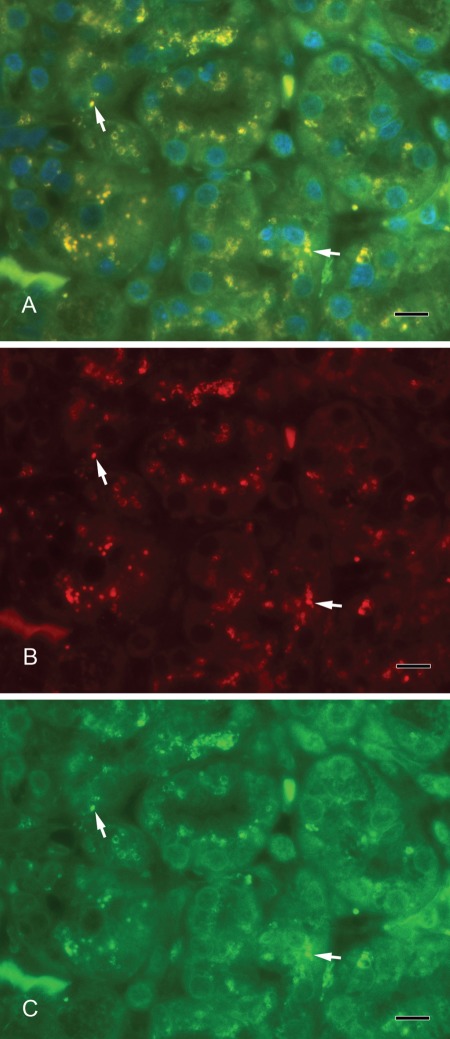
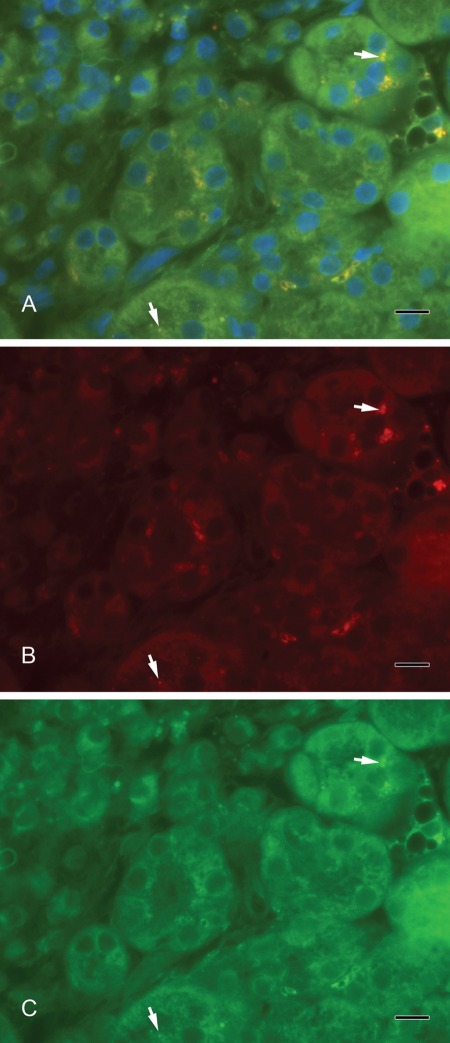
Septin-2 and amylase (Fig. 3A–C) as well as septin-2 and lactoferrin (Fig. 4A–C) colocalized in vesicles of the serous acini. Syntaxin-2 and amylase (Fig. 5A–C) as well as syntaxin-2 and lactoferrin (Fig. 6A–C) showed the same localization on secretory vesicles. Also, syntaxin-2 and septin-2 were expressed in the same vesicles (Fig. 7A–C).
Figure 3.
(A) Septin-2 colocalizes with amylase in secretory vesicles. Yellow vesicles indicate the overlay of septin-2 and amylase (arrows). (B) Septin-2 (red). (C) Amylase (green). Scale bar: 10 µm.
Figure 4.
(A) Colocalization of septin-2 and lactoferrin as a marker for secretory vesicles. Yellow vesicles indicate the overlay of septin-2 and lactoferrin (arrows). (B) Septin-2 (red). (C) Lactoferrin (green). Scale bar: 10 µm.
Figure 5.
(A) Syntaxin-2 colocalization with amylase. Yellow vesicles illustrate the overlay of syntaxin-2 and amylase (arrows). (B) Syntaxin-2 (red). (C) Amylase (green). Scale bar: 10 µm.
Figure 6.
(A) Colocalization of syntaxin-2 and lactoferrin. Yellow vesicles demonstrate the overlay of syntaxin-2 and lactoferrin (arrows). (B) Syntaxin-2 (red). (C) Lactoferrin (green). Scale bar: 10 µm.
Figure 7.
(A) Syntaxin-2 colocalizes with septin-2. Yellow vesicles indicate the overlay of syntaxin-2 and septin-2 (arrows). (B) Syntaxin-2 (red). (C) Septin-2 (green). Scale bar: 10 µm.
The staining results do not correlate with age or sex of the patients.
Discussion
The human submandibular gland as a typical exocytotic gland produces a great amount of proteins that are part of the oral saliva. Secreted proteins, including mucins, lysozyme, lactoferrin, amylase, statherin, histatin, and many more, play a role in the maintenance of a healthy oral cavity and are part of the digestive process (Kouznetsova et al. 2010). There are proteins that are constitutively released without external stimuli, such as amylase (Busch et al. 2002), kallikrein (Garrett et al. 1996), IgA, and other proteins (Proctor et al. 2003). Regulated exocytosis uses the SNARE-mediated exocytotic pathway (Wang et al. 2007). We investigated the secretory molecular machinery of acinar (serous) cells in the human submandibular gland and looked at proteins that are part of a SNARE-mediated membrane fusion of vesicles and target membrane, as well as cytoskeletal components that are necessary for these processes. This study identifies for the first time the localization of a considerable number of SNAREs and cytoskeletal proteins in the human submandibular gland.
Actin and Actin-Binding Proteins in the Process of Exocytosis
Studies indicate that the actin cytoskeleton, which is localized under the plasma membrane, prevents secretory granules from reaching their exocytotic destination (Burgoyne and Cheek 1987; Aunis and Bader 1988). In most models, the actin cytoskeleton is disassembled upon stimulation and rearranged, thereby allowing secretory granules to reach the site of exocytosis (Cheek and Burgoyne 1986; Perrin et al. 1992). In contrast, investigations by Williams (1977), Muallem et al. (1995), and others have shown that disruption of the actin cytoskeleton in exocrine glands inhibits stimulated secretion. Moreover, the actin cytoskeleton may play a positive role in secretion from exocrine glands—for example, by regulating an early step in the formation of retrieval vesicles and/or movement of membrane back into the cell (Valentijn et al. 1999). The variable staining intensity of the luminal border with the actin antibody in our studies reflects this phenomenon, too. The localization of myosin II in the acinar cells of the submandibular gland is the same as in pancreatic acinar cells, predominantly but not exclusively the apical cell domain, especially a narrow band along the lumen (Bhat and Thorn 2009). Cofilin is an actin-depolymerizing protein that has been dephosphorylated by various secretory stimuli in parotid acini (Takuma et al. 1996). Cofilin is involved in many membrane-modulating activities, such as cell growth, motility, and secretion; for instance, cofilin is activated upon Ca2+-regulated noradrenalin secretion from adrenal chromaffin cells (Birkenfeld et al. 2001). In our investigation, cofilin was found concentrated at the apical part of the membrane, indicating its role as a part of the actin cortical network where a breakdown and reorganization of the actin cytoskeleton are necessary for exocytosis. The fact that cofilin could not be demonstrated at the apical membrane in every acinus might indicate the regional dynamic processes in secretory cell types reflected by the breakdown and reorganization of the actin cytoskeleton. In recent studies, profilin has been attributed with a role in synaptic vesicle exocytosis in the brain (Pilo Boyl et al. 2007). Mondin et al. (2010) found out that the specific interaction of profilin-2 (PfnIIa) to a diproline motif in a kainate receptor (GluK2b), a member of glutamate receptors, leads to the control of exocytosis of this receptor. The ubiquitously expressed G-actin-binding protein profilin-1, a key molecule for regulating actin dynamics in all cell types, was strongly expressed at the apical membrane of submandibular glands in our study. We speculate that profilin might be involved in exocytotic events similar to presynaptic exocytotic processes in synapses of the brain.
SNARE Proteins Play a Central Role in Exocytosis
SNAP-23
It has been reported that SNAP-23 is necessary for both apical and basolateral transport in polarized cells such as hepatocytes, kidney cells (Low et al. 1998), and rat parotid acinar cells (Takuma et al. 2000). We demonstrated the expression of SNAP-23 at exactly these positions in the human submandibular acinar cells.
VAMP-2
VAMP-2 was located at the apical membrane of the submandibular acinar cells, but we could not detect the protein in vesicles in the cytoplasm, although it is called a vesicle-associated protein. This is in accordance with other investigations in acinar cells of the rat exocrine pancreas. Braun et al. (1994) demonstrated the localization of VAMP protein as a border around the acinar lumen by immunofluorescence microscopy in frozen pancreas sections. However, the identification of a VAMP-like protein in the zymogen granule membrane fraction was proved biochemically. It was speculated that the detrimental effects of aldehyde fixation make it difficult to detect VAMP within other membrane compartments. Takuma et al. (2000) investigated the lysate of parotid acinar cells and found VAMP-2 in the crude secretory-granule fraction by immunoprecipitation. Fujita-Yoshigaki et al. (1999) reported that an unknown protein x makes it difficult to detect VAMP-2 on the secretory granules of the parotid acinar cells. Moreover, syntaxin-3 was detected on the granule membrane fraction of rat parotid acinar cells by Western blotting but was not detectable by immunocytochemistry. Imai et al. (2003) speculated that the reason for that phenomenon is similar to that described by Fujita-Yoshigaki et al. (1999).
Syntaxins
Syntaxin-2 and -4 have been classified as t-SNARE proteins (Braun et al. 1994; Ravichandran et al. 1996); syntaxin-2 also has been found in intracellular vesicular structures in normal rat kidney (NRK) cells (Band and Kuismanen 2005). We corroborate this finding with our results. We could find syntaxin-2 in the apical plasma membrane of the submandibular acinar cells but also in granules in the cytoplasm. Syntaxin-2 was expressed in the limiting membrane of larger granules with an unstained matrix and in smaller granules with an overall staining. In electron microscope images, typical serous granules of human salivary glands display a complex internal substructure as a result of the presence of components of various electron densities, reflecting a different chemical composition (Tandler and Erlandson 1972; Tandler and Phillips 1993). On the electron microscope level, secretory granules of the submandibular gland are generally surrounded by a limiting membrane, showing an oval corpuscle of medium density in the matrix. In addition, small secretory granules exist in the Golgi area (Sato et al. 1966). Presumably, the different sizes of the granules represent different maturation stages. In our observations, smaller granules might be granules released by the Golgi apparatus, and the larger granules are obviously mature granules. Both express syntaxin-2 and are therefore destined for the exocytotic pathway. Antibodies to septin-2 react only with the limiting membrane of the larger vesicles and not with the smaller granules, pointing to a role in the latter process of exocytosis.
Septin-2
Septins participate in diverse aspects of cell biology. Originally, septins were characterized as proteins involved in cytokinesis in yeast (Haarer and Pringle 1987). In the meantime, the identification of numerous septin family members with functions, for example, in cell cycle control (Barral et al. 2000) and septin homologues in postmitotic cells (Longtine et al. 1996) has suggested additional roles for these proteins. In vesicle transport and exocytosis, septins interact with components of the so-called exocyst complex (Hsu et al. 1998) and with syntaxin (Beites et al. 1999). We could determine the localization of septin-2 to vesicles in the cytoplasm near the luminal outline, which suggests a clear role in exocytosis. Furthermore, the localization of syntaxin-2 in vesicles also concludes an interactive role between septin and syntaxin. Whether the interaction is the same as for the septin CDCrek-1, which binds syntaxin and inhibits exocytosis, has to be elucidated in further studies. The syntaxin localized at the plasma membrane might also be a target for septin in the regulation of exocytosis.
Localization of SNARE Proteins on Secretory Vesicles by Double Staining
We used α-amylase as a marker for secretory vesicles that was localized in serous acini. Lactoferrin is another well-investigated marker, especially for the acini in submandibular glands (Korsud and Brandtzaeg 1982). In contrast, lysozyme has been present predominantly in mucous acini (Kouznetsova et al. 2010) and in intercalated ducts of the serous duct system (Korsud and Brandtzaeg 1982). In electron microscope studies by Marchetti et al. (2000), lysozyme and amylase were found in pale granules in acinar cells of the mouse submandibular gland. The colocalization in the immunofluorescence of syntaxin-2 and lactoferrin as well as of syntaxin-2 and α-amylase showed that syntaxin-2 is localized on secretory vesicles. Furthermore, we showed an overlay of the fluorescence signal in a double staining of septin-2 and lactoferrin as well as of septin-2 and α-amylase, pointing to a localization of septin-2 on exocytotic vesicles. Both septin-2 and syntaxin-2 are present on the same secretory vesicles and involved in the exocrine secretory mechanisms.
Acknowledgments
We are grateful to Kaori Ochi for excellent technical assistance.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors received no financial support for the research and/or authorship of this article.
References
- Andzelm MM, Chen X, Krewski K, Orange JL, Strominger JL. 2007. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 204:2285–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar DA, Zhou R, Forte JG, Yao X. 2002. Syntaxin 3 is required for cAMP-induced acid secretion: streptolysin O-permeabilized gastric gland model. Am J Physiol Gastrointest Liver Physiol. 282:G23–G33 [DOI] [PubMed] [Google Scholar]
- Aunis D, Bader MF. 1988. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 139:253–266 [DOI] [PubMed] [Google Scholar]
- Band AM, Kuismanen E. 2005. Localization of plasma membrane t-SNAREs syntaxin 2 and 3 in intracellular compartments. BMC Cell Biol. 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 5:841–851 [DOI] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. 1999. The septin CDCrek-1binds syntaxin and inhibits exocytosis. Nat Neurosci. 2:434–439 [DOI] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraräs JE, Elferink A, Peterson K, Fleming AM, Hazuka CD, Scheller RH. 1993. The syntaxin family of vesicular transport receptors. Cell. 74:863–873 [DOI] [PubMed] [Google Scholar]
- Bhat P, Thorn P. 2009. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol Biol Cell. 20:1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbach A. 2008. Profilin, a multi-modal regulator of neuronal plasticity. BioEssays. 30:994–1002 [DOI] [PubMed] [Google Scholar]
- Birkenfeld J, Kartmann B, Betz H, Roth D. 2001. Cofilin activation during Ca2+-triggered secretion from adrenal chromaffin cells. Biochem Biophys Res Commun. 286:493–498 [DOI] [PubMed] [Google Scholar]
- Bloom W, Fawcett DW. 1994. A textbook of histology. New York: Chapman & Hall [Google Scholar]
- Braun JEA, Fritz BA, Wong SME, Lowe AW. 1994. Identification of a vesicle-associated membrane protein (VAMP)–like membrane protein in zymogen granules of the rat exocrine pancreas. J Biol Chem. 269:5328–5335 [PubMed] [Google Scholar]
- Burgoyne RD, Cheek TR. 1987. Reorganisation of peripheral actin filaments as a prelude to exocytosis. Biosci Rep. 7:281–288 [DOI] [PubMed] [Google Scholar]
- Busch L, Sterin-Borda L, Borda E. 2002. Differences in the regulatory mechanism of amylase release by rat parotid and submandibular glands. Arch Oral Biol. 47:717–722 [DOI] [PubMed] [Google Scholar]
- Cheek TR, Burgoyne RD. 1986. Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 207:110–114 [DOI] [PubMed] [Google Scholar]
- Fujita-Yoshigaki J, Dohke Y, Hara-Yokoyama M, Furuyama S, Sugiya H. 1999. Presence of a complex containing vesicle-associated membrane protein 2 in rat parotid acinar cells and its disassembly upon activation of cAMP-dependent protein kinase. J Biol Chem. 27:23642–23646 [DOI] [PubMed] [Google Scholar]
- Garrett JR, Zhang XS, Proctor GB, Anderson LC, Shori DK. 1996. Apical secretion rat submandibular tissue kallikrein continues in the absence of external stimulation: evidence for a constitutive secretory pathway. Acta Physiol Scand. 157:299–304 [DOI] [PubMed] [Google Scholar]
- Goda Y. 1997. SNAREs and regulated vesicle exocytosis. Proc Natl Acad Sci U S A. 94:769–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer BK, Pringle JR. 1987. Immunofluorescence localization of the Saccharomyces cerevisiae cell cycle: localization of CDC3 gene product and the timing of events at the budding site. J Cell Biol. 7:3678–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. 1998. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 20:1111–1122 [DOI] [PubMed] [Google Scholar]
- Imai A, Nashida T, Yoshie S, Shimomura H. 2003. Intracellular localisation of SNARE proteins in rat parotid acinar cells: SNARE complexes on the apical plasma membrane. Arch Oral Biol. 48:597–604 [DOI] [PubMed] [Google Scholar]
- Jerdeva GV, Wu K, Yarber FA, Rhodes CJ, Kalman D, Schechter JE, Hamm-Alvarez SF. 2005. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 118:4797–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartmann B, Roth D. 2001. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci. 114:839–844 [DOI] [PubMed] [Google Scholar]
- Korsud FR, Brandtzaeg P. 1982. Characterization of epithelial elements in human major salivary glands by functional markers: localization of amylase, lactoferrin, lysozyme, secretory component, and secretory immunoglobulins by paired immunofluorescence staining. J Histochem Cytochem. 30:657–666 [DOI] [PubMed] [Google Scholar]
- Kouznetsova I, Gerlach KL, Zahl C, Hoffmann W. 2010. Expression analysis of human salivary glands by laser microdissection: differences between submandibular and labial glands. Cell Physiol Biochem. 26:375–382 [DOI] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, DeVirgilio C, Pringle JR. 1996. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 8:106–119 [DOI] [PubMed] [Google Scholar]
- Low SH, Roche PA, Anderson HA, van Ijzendoorn SC, Zhang M, Mostov KE, Weimbs T. 1998. Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells. J Biol Chem. 273:3422–3430 [DOI] [PubMed] [Google Scholar]
- Ludowyke RI, Elgundi Z, Kranenburg T, Stehn JR, Schmitz-Pfeiffer C, Hughes WE, Biden TJ. 2006. Phosphorylation of nonmuscle myosin heavy chain IIA on Ser 19 17 is mediated by protein kinase CβII and coincides with the onset of stimulated degranulation of RBL-2H3 mast cells. J Immun. 177:1492–1499 [DOI] [PubMed] [Google Scholar]
- Marchetti L, Gabrielli MG, Materazzi G, Menghi G. 2000. Cellular compartmentation of lysozyme and alpha-amylase in the mouse salivary glands: Immunogold approaches at light and electron microscopy level. Histol Histopathol. 15:337–346 [DOI] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Südhof TC. 1993. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 364:346–349 [DOI] [PubMed] [Google Scholar]
- Mochida S, Kobayashi H, Matsuda Y, Yuda Y, Muramoto K, Nonomura Y. 1994. Myosin II is involved in transmitter release at synapses formed between rat sympathetic neurons in culture. Neuron. 13:1131–1142 [DOI] [PubMed] [Google Scholar]
- Mondin M, Carta M, Normand E, Mulle C, Coussen F. 2010. Profilin II regulates the exocytosis of kainate glutamate receptors. J Biol Chem. 285:40060–40071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. 1995. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 128:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashida T, Yoshie S, Imai A, Shimomura H. 2004. Presence of cytoskeleton proteins in parotid glands and their roles during secretion. Arch Oral Biol. 49:975–982 [DOI] [PubMed] [Google Scholar]
- Oishi Y, Arakawa T, Tanimura A, Itakura M, Takahashi M, Tajima Y, Mizoguchi I, Takuma T. 2006. Role of VAMP-2, VAMP-7, and VAMP-8 in constitutive exocytosis from HSY cells. Histochem Cell Biol. 125:273–281 [DOI] [PubMed] [Google Scholar]
- Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. 2000. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 164:5850–5857 [DOI] [PubMed] [Google Scholar]
- Perrin D, Möller K, Hanke K, Söling HD. 1992. cAMP and Ca2+-mediated secretion in parotid acinar cells is associated with reversible changes in the organization of the cytoskeleton. J Cell Biol. 116:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. 1996. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Dev Biol. 12:441–461 [DOI] [PubMed] [Google Scholar]
- Pilo Boyl P, Di Nardo A, Mulle C, Sassoè-Pognetto M, Panzanelli P, Mele A, Kneussel M, Constantini V, Perlas E, Massimi M, et al. 2007. Profilin2 contributes to synaptic vesicle exocytosis, neuronal excitability, and novelty-seeking behaviour. EMBO J. 26:2991–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH, Segawa A, Garrett JR, Ebersole L. 2003. Constitutive secretion of immunoglobulin A and other proteins into lumina of unstimulated submandibular glands in anaesthetised rats. Exp Physiol. 88(1):7–12 [DOI] [PubMed] [Google Scholar]
- Ravichandran V, Chawla A, Roche PA. 1996. Identification of novel syntaxoin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 271:3300–3303 [DOI] [PubMed] [Google Scholar]
- Sato M, Noguchi T, Yokoyama M, Yotsumoto M. 1966. On the secretory granules of the serous cell in the human submandibular gland. J Electron Microsc. 15:1–14 [PubMed] [Google Scholar]
- Segawa A, Riva A, Loffredo F, Congiu T, Yamashina S, Testa Riva F. 1998. Cytoskeletal regulation of human salivary secretion studied by high resolution electron microscopy and confocal laser microscopy. Eur J Morphol. 36(Suppl):41–45 [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. 1993. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 75:409–418 [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature. 362:318–324 [DOI] [PubMed] [Google Scholar]
- Sou E, Yarber FA, Rose CM, Chiu CTW, Mircheff AK, Karvar S, Hamm-Alvarez SF. 2005. Characterization of soluble NSF attachment protein receptors (SNARES) in rabbit lacrimal gland acinar cells. Ocul Surf. 3(Suppl):S115 [Google Scholar]
- Takuma T, Arakawa T, Tajima Y. 2000. Interaction of SNARE proteins in rat parotid acinar cells. Arch Oral Biol. 45:369–375 [DOI] [PubMed] [Google Scholar]
- Takuma T, Ichida T, Yokoyama N, Tamura S, Obinata T. 1996. Dephosphorylation of cofilin in parotid acinar cells. J Biochem. 120:35–41 [DOI] [PubMed] [Google Scholar]
- Tandler B, Erlandson RA. 1972. Ultrastructure of the human submaxillary gland: IV. Serous granules. Am J Anat. 135:419–433 [DOI] [PubMed] [Google Scholar]
- Tandler B, Phillips CJ. 1993. Structure of serous cells in salivary glands. Microsc Re Tech. 26:32–48 [DOI] [PubMed] [Google Scholar]
- Valentijn KM, Gumkowski FD, Jamieson JD. 1999. The subapical actin cytoskeleton regulates secretion and membrane retrieval in pancreatic acinar cells. J Cell Sci. 112:81–96 [DOI] [PubMed] [Google Scholar]
- Wang CC, Shi H, Guo K, Ng CP, Li J, Gan BQ, Chien Liew H, Leinonen J, Rajaniemi H, Zhou ZH, et al. 2007. VAMP8/endobrevin as a general vesicular SNARE for regulated exocytosis of the exocrine system. Mol Biol Cell. 18:1056–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA. 1977. Effects of cytochalasin B on pancreatic acinar cell structure and secretion. Cell Tiss Res. 179:453–466 [DOI] [PubMed] [Google Scholar]