Abstract
Although metastasis is a major cause of death from breast cancer, our ability to predict which tumors will metastasize is limited (American Cancer Society 2010). Proper assessment of metastatic risk and elucidating the underlying mechanisms of metastasis will help personalize therapy and may provide insight into potential therapeutic targets. Traditionally, histologic grading, staging, hormone receptors, HER2/Neu, and proliferation assays have been the gold standard on which oncologists based their treatment decisions. However, all of these are indirect measures of metastatic risk. Recent insights from intravital imaging directly address questions of mechanism and have led to a new way of using histologic and cytologic material to assess metastatic risk. This review describes the tumor microenvironment model of invasion and intravasation, as well as an emerging histopathologic application based on this model. In particular, the authors describe a new immunohistochemical approach to the assessment of metastatic risk based on the density of intravasation microenvironment sites called the tumor microenvironment of metastasis. In addition, they describe an isoform assay for the actin regulatory protein Mena using fine needle aspiration samples and the details about how these 2 assays may be applied in clinical practice in a synergistic way to assess the risk of metastasis.
Keywords: breast cancer, metastasis, immunohistochemical markers, tumor microenvironment, fine needle aspiration (FNA) biopsy
Identification of Invasion Microenvironments
A traditional view of metastasis asserts that metastatic tumor cells arise from a process similar to natural selection in which multiple selective pressures result in tumor cells that are capable of migration and survival at distant sites. New technologies, however, including intravital imaging studies of mammary tumors in mice (Giavazzi et al. 1980; Mantovani et al. 1980; Milas et al. 1983; Wyckoff et al. 2004), expression profiling of whole human breast tumors (van de Vijver et al. 2002; Ramaswamy et al. 2003), and collection and profiling of the invasive subpopulation of tumor cells isolated from rat and mouse mammary tumors (Wang et al. 2004, 2005, 2007), indicate that metastatic ability is acquired at much earlier stages of tumor progression than that predicted by the Darwinian natural selection model. These results indicate that metastatic ability is encoded throughout the bulk of the primary tumor and involves transient changes in gene expression. In addition, they are consistent with the idea that oncogenic mutations in tumor cells in the primary tumor lead to microenvironments such as increased microvascular density (Leek and Harris 2002), inflammation (Condeelis and Pollard 2006), and hypoxia (Giraudo et al. 2004). These microenvironments can induce transient alterations in gene expression and alternative mRNA splicing in the tumor cells, which in turn drive dramatic but transient episodes of cell motility, migration, and intravasation (Condeelis et al. 2005; Wang et al. 2005, 2007).
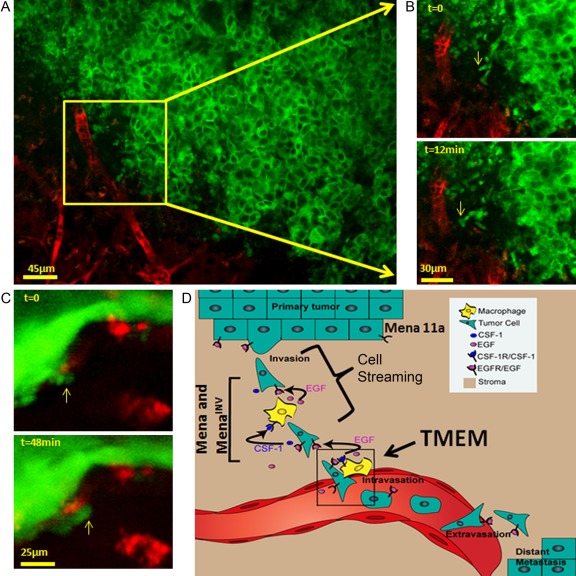
Multiphoton-based intravital imaging has highlighted the essential role of macrophages in these motility events (reviewed in Condeelis and Segall 2003; Condeelis and Pollard 2006; Yamaguchi et al. 2006; Kedrin et al. 2007). During invasion and dissemination, macrophages stream with tumor cells toward blood vessels where perivascular macrophages support intravasation. Both the invasion and intravasation microenvironments rely on an EGF/CSF-1 paracrine loop (Roussos et al. 2011a) (Fig. 1A, B, C).
Figure 1.
Observation of tumor cell behavior in mammary tumors using multiphoton imaging. (A) Using multiphoton imaging, the migration of breast tumor cells can be seen deep inside the mammary tumor of a living mouse. (B) Green tumor cells respond to signals from blood vessel–associated macrophages by crawling (yellow arrows) toward blood vessels (red). Tumor cells then use the blood vessels to escape from the primary tumor and seed metastatic tumors at distant sites. Images from Roussos et al. (2011a). (C) Green tumor cells enter blood vessels where perivascular macrophages (red) are located. Images from Wyckoff et al. (2007). (D) Cartoon summary of tumor cells streaming and TMEM assembly. Tumor cell migration is believed to involve the tumor microenvironment–initiated decreased expression of the epithelial isoform of Mena, Mena11a, and increased expression of MenaINV, which promotes discohesion of the tumor and paracrine-dependent tumor cell migration called cell streaming (bracket), as well as TMEM assembly leading to intravasation (box). TMEM, tumor microenvironment of metastasis.
Using a needle filled with EGF or CSF-1, the motile subpopulation of tumor cells in the primary tumor can be collected and then characterized by gene expression profiling (Wang et al. 2004, 2007). Collectively, the constellation of genes identified by this profiling is called the invasion signature, and it characterizes the migrating and disseminating tumor cells of mammary tumors. These cells are motile, chemotactic to EGF receptor ligands, nonapoptotic, and chemotherapy resistant. Key among the motility genes is Mena, an actin binding protein that regulates pathways involved in EGF-stimulated actin polymerization, chemotaxis, and cell migration (Gertler and Condeelis 2011; Roussos et al. 2011b).
Mena Modulates Breast Cancer Cell Invasion and Intravasation
Mena, a member of the Ena/VASP protein family, regulates cell motility by controlling the assembly of actin networks (Krause et al. 2003). Mena expression is upregulated in rat, mouse, and human mammary tumors grown in SCID mice (Wang et al. 2004, 2007; Goswami et al. 2009) and in primary tumors of human breast cancer patients (Di Modugno et al. 2004, 2006). Mena is required for tumor progression, as Mena null mice with PyMT transgene–induced mammary tumors exhibit delayed metastatic progression and decreased hematogenous dissemination of tumor cells (Roussos et al. 2010), although the PyMT tumors are typically extremely aggressive (Lin et al. 2003). Mena contains 4 alternatively included exons not present in other Ena/VASP proteins: “+,” “++,” “INV” (formerly “+++”), and 11a (Gertler et al. 1996; Di Modugno 2007; Philippar et al. 2008). Menaclassic (which contains only the constitutive exons) and MenaINV are both upregulated in the invasive subpopulation of tumor cells isolated from rat, mouse, and human mammary tumors (grown in SCID mice), whereas Mena 11a, abundant in primary tumors, is downregulated in the same invasive tumor cells (Goswami et al. 2009) (Fig. 1D). Tumors formed by MenaINV-expressing carcinoma cells show no differences in primary tumor growth, but such tumors exhibit a discohesive morphology and are significantly more metastatic than are tumors arising from cells expressing the other Mena isoforms (Philippar et al. 2008; Roussos et al. 2011a). MenaINV-expressing tumor cells are more invasive and motile, form streams with macrophages, and intravasate far more efficiently (Roussos et al. 2011c). The steps in metastasis following intravasation are not affected by MenaINV expression (Roussos et al. 2011a). At the mechanistic level, MenaINV potentiates chemotactic and invasive responses of tumor cells to EGF: MenaINV-expressing tumor cells respond to 25- to 50-fold lower EGF concentrations than do cells expressing Menaclassic (Philippar et al. 2008; Roussos et al. 2011a). MenaINV expression allows tumor cells to respond to otherwise undetectable EGF levels, enhancing paracrine signaling with macrophages, ultimately leading to a “streaming” phenotype that allows tumor cells to reach blood vessels and intravasate more efficiently than do cells lacking MenaINV (Fig. 1D) (Roussos et al. 2011a).
Detection of Tumor Cell Intravasation Sites Called TMEM: Tumor Microenvironment of Metastasis in Humans
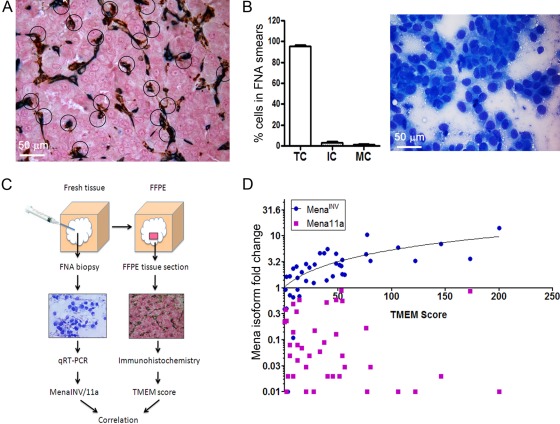
Given these in vivo and in vitro observations, we asked how pathologists might apply these insights to routine surgical pathology and cytology samples of human breast cancers. Because intravasation requires an interaction between a migrating intravasation-competent tumor cell, an endothelial cell, and a perivascular macrophage, we developed a triple immunostain with 3 different chromogens to enable simultaneous visualization and identification of each cell type. The antibodies used were CD31 (endothelial cell), CD68 (macrophage), and pan Mena (intravasation competent tumor cell) (Robinson et al. 2009). The TMEM was defined as the direct apposition of an endothelial cell, a perivascular macrophage, and a Mena-expressing tumor cell. Images of a 2.5-mm2 area of tumor, with high cellularity and vascularity, were taken at ×400, and the number of TMEMs per image was quantitated and summed over the area (Fig. 1D and 2A).
Figure 2.
The methods and correlation of TMEM and FNA assays. (A) TMEM scoring area. A section from a breast tumor from a patient with metastatic disease stained for TMEM (circled). (B) FNA smear analysis. Left, cellular composition in FNA samples. Right, Diff-Quick–stained FNA smear. (C) Workflow of sampling tumor tissue for TMEM scoring and Mena isoform expression. Fresh tumor is first sampled by FNA. Following the FNA procedure, the entire lesion is formalin-fixed and paraffin-embedded (FFPE). The FNA tissue sample is assessed for adequacy and analyzed by qRT-PCR for Mena isoform expression. A representative section of the FFPE tumor is chosen and triple immunostained for TMEM scoring. Both analyses are performed on the same tumor (Roussos et al. 2011c). (D) The correlation of Mena isoform expression in FNA samples and TMEM count from 40 patients with breast cancer. There is a strong correlation between MenaINV fold change and TMEM score (Spearman’s correlation coefficient = 0.78, p = 10–6) and no correlation between Mena11a and TMEM score (Spearman’s correlation coefficient = −0.26, p=0.13) (Roussos et al. 2011c). FNA, fine needle aspiration; IC, inflammatory cell; MC, mesenchymal cell; TC, tumor cell.
In a retrospective study of 30 matched pairs of patients with invasive ductal carcinoma of the breast—matched for tumor size, differentiation, lymph node status, lymphovascular invasion, hormone receptor, and HER2/Neu status (and differing only in metastatic outcome)—there was a significant difference in TMEM count between the metastatic and nonmetastatic cohorts (median=105 vs. 50, respectively; p=0.00006). Specifically, for an increase in the TMEM count of 10, the risk of metastasis nearly doubled (odds ratio=1.9, 95% confidence interval, 1.1–3.4). The ability of TMEM density to predict systemic spread of carcinoma cells was independent of other currently used prognosticators (Robinson et al. 2009).
Cytological Assays Correlated with TMEM
Fine needle aspiration (FNA) is a technique well suited for the collection of discohesive tumor cells from primary breast tumors. FNA biopsy not only collects an almost pure cancer cell population but also produces samples enriched for discohesive, potentially invasive cancer cells (Fig. 2B). Because MenaINV-expressing cells have poorly formed cell-cell junctions as demonstrated by intravital imaging and immunohistochemical analysis of E-cadherin and β-catenin, they are easily pulled into the FNA needle by capillary action (Roussos et al. 2011c). In addition, FNA is a very simple, inexpensive method of tissue collection that can be performed without anesthesia, during the patient’s first visit. Thus, tissue samples collected by FNA may be used early in the diagnostic and prognostic process before treatment decisions have been made. In a study involving 40 human invasive ductal breast cancers, we have shown that FNA yielded sufficient tumor cells to allow determination of Mena isoform levels by qRT-PCR. The discohesive cells obtained showed high levels of MenaINV (Roussos et al. 2011c).
Total Mena levels, and in particular MenaINV levels, are correlated with TMEM assembly and breast cancer cell intravasation and hematogenous metastatic spread in mice (Philippar et al. 2008, 2010, 2011a), suggesting that tumors with high MenaINV levels will have high TMEM scores. Consistent with this hypothesis, MenaINV expression levels correlated with TMEM scores in invasive ductal carcinomas in 40 patients, whereas the expression levels of Mena11a did not (Roussos et al. 2011c) (Fig. 2C, D). This suggests that MenaINV expression is also implicated in TMEM assembly and cancer cell intravasation in humans. These results suggest that both MenaINV expression status in FNA samples and TMEM have related prognostic value because they are related to the same mechanism of tumor cell dissemination. Because no differences in Mena isoform mRNA expression were found between tumors of different grade, size, lymph node status, ER, PR, and HER2/Neu expression (Roussos et al. 2011c), both TMEM score and MenaINV mRNA expression reflect linked mechanisms of tumor invasion/progression independent of most currently used clinical and pathological parameters.
Conclusions
New techniques using correlated histologic and cytologic methods are being developed to assess metastatic risk in breast cancer patients at the time of presentation or early in the course of management. TMEM is a triple immunostain that allows quantitation of a microanatomic structure of intravasation that can be performed on excisional biopsies (after formalin fixation and paraffin embedding) and some needle core biopsies. Determination of Mena isoform levels on FNA-based material can give similar prognostic information based on the discohesion of the motile cells at an early stage of presentation. The novelty of these tests, as compared with other prognostic indicators to date, is that they are based on direct observation of cancer cell intravasation mechanism in vivo. Population studies are currently underway to determine how best to personalize the prognosis and treatment of breast cancer patients based on these new methods.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- American Cancer Society Breast cancer facts & figures 2009-2010 Atlanta: American Cancer Society; 2010 [Google Scholar]
- Condeelis J, Pollard JW. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 124:263–266 [DOI] [PubMed] [Google Scholar]
- Condeelis J, Segall JE. 2003. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 3:921–930 [DOI] [PubMed] [Google Scholar]
- Condeelis J, Singer RH, Segall JE. 2005. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol. 21:695–718 [DOI] [PubMed] [Google Scholar]
- Di Modugno F, Bronzi G, Scanlan MJ, Del Bello D, Cascioli S, Venturo I, Botti C, Nicotra MR, Mottolese M, Natali PG, Santoni A, Jager E, Nistico P. 2004. Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer. 109:909–918 [DOI] [PubMed] [Google Scholar]
- Di Modugno F, DeMonte L, Balsamo M, Bronzi G, Nicotra MR, Alessio M, Jager E, Condeelis JS, Santoni A, Natali PG, Nistico P. 2007. Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines. Cancer Res. 67:2657–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Modugno F, Mottolese M, Di Benedetto A, Conidi A, Novelli F, Perracchio L, Venturo I, Botti C, Jager E, Santoni A, Natali PG, Nistico P. 2006. The cytoskeleton regulatory protein hMena (ENAH) is overexpressed in human benign breast lesions with high risk of transformation and human epidermal growth factor receptor–2-positive/hormonal receptor-negative tumors. Clin Cancer Res. 12:1470–1478 [DOI] [PubMed] [Google Scholar]
- Gertler F, Condeelis J. 2011. Metastasis: tumor cells becoming MENAcing. Trends Cell Biol. 21:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. 1996. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 87:227–239 [DOI] [PubMed] [Google Scholar]
- Giavazzi R, Alessandri G, Spreafico F, Garattini S, Mantovani A. 1980. Metastasizing capacity of tumour cells from spontaneous metastases of transplanted murine tumours. Br J Cancer. 42:462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo E, Inoue M, Hanahan D. 2004. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 114:623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Philippar U, Sun D, Patsialou A, Avraham J, Wang W, Di Modugno F, Nistico P, Gertler FB, Condeelis JS. 2009. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis. 26:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE. 2007. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 12:143–152 [DOI] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. 2003. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 19:541–564 [DOI] [PubMed] [Google Scholar]
- Leek RD, Harris AL. 2002. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 7:177–189 [DOI] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. 2003. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 163:2113–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Giavazzi R, Polentarutti N, Spreafico F, Garattini S. 1980. Divergent effects of macrophage toxins on growth of primary tumors and lung metastases in mice. Int J Cancer. 25:617–620 [DOI] [PubMed] [Google Scholar]
- Milas L, Peters LJ, Ito H. 1983. Spontaneous metastasis: random or selective? Clin Exp Metastasis. 1:309–315 [DOI] [PubMed] [Google Scholar]
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, Gertler FB. 2008. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 15:813–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR. 2003. A molecular signature of metastasis in primary solid tumors. Nat Genet. 33:49–54 [DOI] [PubMed] [Google Scholar]
- Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, Jones JG. 2009. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 15:2433–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, Lauffenburger DA, Bresnick AR, Gertler FB, Condeelis JS. 2011a. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 124:2120–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Condeelis JS, Patsialou A. 2011b. Chemotaxis in cancer. Nat Rev Cancer. 11:573–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Goswami S, Balsamo M, Wang Y, Stobezki R, Adler E, Robinson BD, Jones JG, Gertler FB, Condeelis JS, Oktay MH. 2011c. Mena invasive (Mena(INV)) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clin Exp Metastasis. 28:515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Wang Y, Wyckoff JB, Sellers RS, Wang W, Li J, Pollard JW, Gertler FB, Condeelis JS. 2010. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle-T transgenic mouse mammary tumors. Breast Cancer Res. 12:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. 2002. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 347:1999–2009 [DOI] [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. 2004. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 64:8585–8594 [DOI] [PubMed] [Google Scholar]
- Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. 2005. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 15:138–145 [DOI] [PubMed] [Google Scholar]
- Wang W, Wyckoff JB, Goswami S, Wang Y, Sidani M, Segall JE, Condeelis JS. 2007. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 67:3505–3511 [DOI] [PubMed] [Google Scholar]
- Wyckoff J, Segall J, Condeelis J. 2004. Single cell imaging in animal tumors in vivo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratories Press [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. 2007. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67:2649–2656 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Pixley F, Condeelis J. 2006. Invadopodia and podosomes in tumor invasion. Eur J Cell Biol. 85:213–218 [DOI] [PubMed] [Google Scholar]