Abstract
The secreted glycoprotein YKL-40 participates in cell differentiation, inflammation, and cancer progression. High YKL-40 expression is reported during early human development, but its functions are unknown. Six human embryonic stem cell (hESC) lines were cultured in an atmosphere of low or high oxygen tension, in culture medium with or without basic fibroblast growth factor, and on feeder layers comprising mouse embryonic fibroblasts or human foreskin fibroblasts to evaluate whether hESCs and their progeny produced YKL-40 and to characterize YKL-40 expression during differentiation. Secreted YKL-40 protein and YKL-40 mRNA expression were measured by enzyme-linked immunosorbent assay (ELISA) and quantitative RT-PCR. Serial-sectioned colonies were stained for YKL-40 protein and for pluripotent hESC (OCT4, NANOG) and germ layer (HNF-3β, PDX1, CD34, p63, nestin, PAX6) markers. Double-labeling showed YKL-40 expression in OCT4-positive hESCs, PAX6-positive neuroectodermal cells, and HNF-3β-positive endodermal cells. The differentiating progeny showed strong YKL-40 expression. Abrupt transition between YKL-40 and OCT4-positive hESCs and YKL-40-positive ecto- and neuroectodermal lineages was observed within the same epithelial-like layer. YKL-40-positive cells within deeper layers lacked contact with OCT4-positive cells. YKL-40 may be important in initial cell differentiation from hESCs toward ectoderm and neuroectoderm, with retained epithelial morphology, whereas later differentiation into endoderm and mesoderm involves a transition into the deeper layers of the colony.
Keywords: cell differentiation, development, hESC, histomorphology, YKL-40
The successful derivation of human embryonic stem cells (hESCs) in 1998 by Thomson and coworkers (Thomson et al. 1998) has led to promising insights into early human development. Further stem cell research is of paramount importance for developmental biology, generation of stem cell therapies, drug development, and regenerative medicine. Many factors influence the early differentiation as cells move from one stage of commitment to the next. Differentiation in vitro is affected by culture conditions such as the presence or the absence of feeder cells, certain growth factors, and oxygen tension in the culture medium (Ezashi et al. 2005; Eiselleova et al. 2008; Millman et al. 2009; Forristal et al. 2010). Recent studies have shown heterogeneity in hESC colonies from one culture dish and even within one colony showing cell populations simultaneously expressing markers of both pluripotency and germ layers even prior to formation of embryoid bodies, illustrating the complex network of signal transduction pathways involved (Itskovitz-Eldor et al. 2000; Reubinoff et al. 2000; Laursen et al. 2007; Hough et al. 2009; Brøchner et al. 2010).
During the past decade, a growing interest has evolved around the glycoprotein YKL-40 (Johansen et al. 2009; Lee et al. 2011), also known as chitinase 3–like 1 (CHI3L1) (Rehli et al. 1997) and as human cartilage glycoprotein–39 (HC-gp39) (Hakala et al. 1993). YKL-40 is a highly conserved protein (Bussink et al. 2007), a chi-lectin, and a member of mammalian chitinase-like proteins and family 18 glycosyl hydrolases with heparin-, chitin- and collagen-binding properties but without chitinase activity (Hakala et al. 1993; Shackelton et al. 1995; Rehli et al. 1997, 2003; Renkema et al. 1998; Bigg et al. 2006; Johansen 2006; Bussink et al. 2007). The gene encoding human YKL-40 (Rehli et al. 1997) is localized on chromosome 1q32.1, and the crystal structure of YKL-40 is known (Fusetti et al. 2003; Houston et al. 2003). The detailed functions of YKL-40 have, however, not yet been determined, but earlier studies suggested that YKL-40 was involved in angiogenesis (Malinda et al. 1999; Nishikawa and Millis 2003; Saidi et al. 2008). This has recently been confirmed in a study demonstrating that YKL-40 upregulates vascular endothelial growth factor expression in a glioblastoma cell line (Francescone et al. 2011). Interestingly, blockade of YKL-40 activity or expression decreases tumor growth, angiogenesis, and metastasis in xenografted SCID mice (Faibish et al. 2011; Francescone et al. 2011). Furthermore, studies suggest that YKL-40 plays a role in cell proliferation and differentiation (Shackelton et al. 1995; Rehli et al. 1997, 2003; Recklies et al. 2002; Johansen et al. 2007), the innate immune response (Dickey 2007), and inflammation and tissue remodeling (Baeten et al. 2000; Recklies et al. 2002, 2005; Ling and Recklies 2004; Johansen 2006; Chupp et al. 2007; Lee et al. 2011).
We have recently demonstrated a strong YKL-40 expression in each of the three germ layers of human embryos. In early human fetal tissues, YKL-40 exists in two different isoforms, and YKL-40 expression is high in tissues characterized by rapid proliferation and marked differentiation, as well as in tissues undergoing morphogenetic changes (Johansen et al. 2007). The aim of the present study was to evaluate whether YKL-40 is produced by undifferentiated hESCs and by their progeny and to characterize the relative YKL-40 expression during the process of cell differentiation. We examined six different hESC lines and studied expression of both isoforms of YKL-40 from day 0 with entirely undifferentiated hESCs to day 28 with almost totally differentiated cells using different growth conditions over time—that is, low oxygen tension (7%) versus high oxygen tension (18%), absence or presence of basic fibroblast growth factor (bFGF), and feeder layers comprising either mouse embryonic fibroblasts (MEFs) or human foreskin fibroblasts (hFFs). We used real-time quantitative RT-PCR (QPCR) to evaluate YKL-40 expression. The secretion of YKL-40 protein into the culture medium was monitored by enzyme-linked immunosorbent assay (ELISA). A special paraffin embedding technique was applied, allowing for entire colony embedding, which made it possible to generate a survey of the topography for each specific colony by immunohistochemistry (IHC) using different markers on neighboring serial sections.
Materials and Methods
The hESC lines LRB01, LRB02, LRB03, LRB010, LRB016, and LRB017 used in the present study were established in our laboratory from surplus human embryos obtained from couples undergoing assisted reproduction after informed consent and cultured as previously described (Brøchner et al. 2010; Laursen et al. 2007). Validation of the derived hESC lines was performed by QPCR measurements of the six consensus markers (NANOG, POU5F1, GABRB3, TDGF1, GDF3, and DNMT3B) (Adewumi et al. 2007; Awan et al. 2010). All six markers were present simultaneously in morphological undifferentiated cells from all the used cell lines. In addition, the ability to differentiate into the three germ layers was monitored by the IHC expression of layer-specific antigens (e.g., p63, nestin, PAX6, and CD34 and HNF-3β) in all cell lines. The cell lines were found to be chromosomal normal shortly after derivation. The study was approved by the regional ethical committee of Copenhagen and Frederiksberg Municipalities (permission no. KF 01-188/03).
Derivation and Culture of hESC Lines
Embryos were grown to the blastocyst stage and the inner cell masses isolated by digesting the zona pellucida with 1 mg/ml pronase (Sigma no. P8811; Sigma, St. Louis, MO). The trophectodermal cells were then lysed by immunosurgery by incubation in rabbit anti-whole-serum antibody (Sigma no. H8765) diluted 1/3 in KnockOut Dulbecco’s modified Eagle’s medium (KO-DMEM; Invitrogen, Carlsbad, CA). After 30 min, the cells were washed three times in KO-DMEM and incubated in guinea pig serum (State Serum Institute; Copenhagen, Denmark) diluted 1/5 in KO-DMEM. Lysis of the trophectodermal cells was evaluated by microscopy. Alternatively, the inner cell mass was isolated by manual dissection using 27-G needles attached to 1-ml syringes. The resulting inner cell masses were plated out in culture dishes precoated with 0.1% gelatin solution and either mitotically inactivated MEFs or hFFs in hESC medium. Cells were maintained in an incubator at 37C with a humidified atmosphere consisting of either 6% CO2, 87% N2, and 7% O2 (i.e., low oxygen) or 18% O2 (i.e., high oxygen). The hESC culture medium consisted of KO-DMEM, 15% KnockOut serum replacement, 2 mM GlutaMAX, 1× non-essential amino acids, 50 IU/ml penicillin, 50 µg/ml streptomycin, and 0.1 mM β-mercaptoethanol (Invitrogen); 5 mg/ml human serum albumin (State Serum Institute); and either 4 ng/ml bFGF (“proliferation medium”) or no bFGF (“differentiation medium”) (RD Systems, Minneapolis, MN).
Colonies of outgrowing hESCs were passaged on either fresh MEFs or hFFs once every 7 to 10 days. Areas with just a single confluent layer of cells that displayed the typical appearance of undifferentiated hESCs as determined under the inverted microscope were selected for passage. Then, 1-ml syringes attached with 27-G needles were used to cut out clumps of cells containing approximately 100 to 200 cells. Four to eight clumps of cells were normally passaged from one culture dish to a new dish. The day of transfer was designated “the starting point” or “day 0” for that particular culture.
Analysis of YKL-40 mRNA Expression
Ten culture dishes from each cell line were cultured in parallel. At the desired time points, individual colonies were carefully divided into approximately equally sized halves; half of the colony was transferred to RNAlater for mRNA analysis, whereas the other half of the colony was paraffin embedded to be used for IHC analysis (see the next section). Total RNA was isolated using TRIzol Reagent (Invitrogen), and cDNA was synthesized with SuperScript II (RNase H-) reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. QPCR analysis was performed using an ABI 7500 Fast real-time PCR system and with a LightCycler FastStart DNA MasterPLUS SYBR GreenI kit (Roche, Hvidovre, Denmark). The expression level was determined by the comparative threshold cycle (CT) method using beta-actin (B-ACT) as an endogenous reference gene to calculate a normalized target gene value. All samples were run in duplicates. The identity of the PCR products was confirmed by DNA sequencing. The primer sequences are listed in Table 1.
Table 1.
Primer Sequences and Details of Primary Antibodies Used
| Primer Sequence |
Gene |
||||
|---|---|---|---|---|---|
| TGAGAGGGAAGCGCAGAT, TGAGAGGGAAGCGCAGAT | YKL-40 (long isoform) | ||||
| AGAGACAAACAGCATTTTACC, GGAGGAAGTCACAGATAGTGT | YKL-40 (short isoform) | ||||
| TTGCTGCAGAAGTGGGTGGA, GAGCCCAGAGTGGTGACGGA | OCT4/POU5F1 | ||||
| AAGTGTGACGTTGACATCCG, GATCCACATCTGCTGGAAGG |
B-ACT |
||||
| Bright-field Light Microscopy | |||||
| Antigen |
Manufacturer |
Code No. |
Antibody Species |
Pretreatment |
Dilution |
| YKL-40 | Kindly provided by Paul A. Price, University of California, San Diego, USA | 201.F9 | Mouse, IgG2bk | ÷ | Different |
| OCT4 | Abcam | Ab 19857 | Rabbit | TEG | 1:1500 |
| OCT 3/4 | Santa Cruz | Sc-8629 | Goat | ÷ | 1:100 |
| NANOG | R&D | AF 1997 | Goat | Citrate pH 6 | 1:50 |
| HNF-3β | Santa Cruz | Sc-6554 | Goat | TEG | 1:150 |
| PDX1 |
Kindly provided by Ole D. Madsen, Hagedorn Research Institute, Gentofte, Denmark |
÷ |
Goat |
TEG |
1:3000 |
| CD34 |
DakoCytomation |
M 7165 |
Mouse, IgG1 |
÷ |
1:25 |
| p63 |
Oncogene Research Products |
OP 132 |
Mouse, IgG2a |
TEG |
1:50 |
| nestin |
Chemicon |
MAB 5326 |
Mouse, IgG1 |
÷ |
1:300 |
| PAX6 |
Chemicon |
AB 5409 |
Rabbit |
÷ |
1:10000 |
| Fluorescence Microscopy | |||||
| Antigen |
Manufacturer |
Code No. |
Antibody Species |
Pretreatment |
Dilution |
| YKL-40 |
Kindly provided by Paul A. Price, University of California, San Diego, USA |
201.F9 |
Mouse, IgG2bk |
÷ |
1:100 1:250 |
| OCT4a | Abcam | Ab19857 | Rabbit | ÷ | 1:50 |
| OCT4 |
Abcam |
Ab19857 |
Rabbit |
TEG 10 min |
1:100 |
| HNF-3β |
Santa Cruz |
Sc-6554 |
Goat |
TEG 16 min |
1:50 |
| PAX6 |
Chemicon |
AB 5409 |
Rabbit |
÷ |
1:500 |
Note: ÷ indicates no pretreatment.
OCT4 as used in initial studies.
Analysis of Secreted YKL-40 by ELISA
The colonies were grown in a culture chamber containing an atmosphere of either a low (7%) or high (18%) oxygen tension for up to 28 days. The culture medium without or with bFGF (4 ng/ml) was changed three times per week and the used medium stored at −80C until YKL-40 analysis. YKL-40 concentrations in conditioned medium were determined by a commercial two-site, sandwich-type ELISA (Quidel Corporation, Santa Clara, CA) using streptavidin-coated microplate wells, a biotinylated-Fab monoclonal capture antibody, and an alkaline phosphatase–labeled polyclonal detection antibody (Harvey et al. 1998). The sensitivity of the ELISA is 5 µg/l. The intra- and interassay coefficients of variations are ≤5% and ≤6%.
Immunohistochemical Analysis of hESCs Grown for 0 to 42 Days
Colonies of the six hESC lines were cultured under conditions as described above for 0, 4, 7, 10, 17, 21, 28, 32, and 42 days. To obtain a fully differentiated stage, cell line LRB01 was followed until day 42. Following the final culture medium change, colonies were paraffin embedded employing cells from culture dishes used for either QPCR or ELISA and others that were used only for IHC.
To preserve the two-dimensional structure of flattened colonies and the three-dimensional structure of the more differentiated—and thus bulging—colonies, individual samples were fixed and embedded using two different strategies according to their appearance. Flat colonies were fixed in Bouin’s fixative in situ in the culture dish. After 1 to 2 hr of fixation, the fixative was replaced with 70% ethanol, and 24 to 48 hr later, the 70% ethanol was replaced with 90% ethanol. Following overnight dehydration in 90% ethanol, the samples were exposed to 99% ethanol for 12 hr. Then the colonies were gently dissected free from the bottom using a Cell Scraper (Nunc, Roskilde, Denmark) starting from the periphery. Colonies were then lifted carefully from the bottom of the culture dish to the small metal embedding mold into which xylene was pipetted. After 1 hr of exposure to xylene, paraffin was gently added to the embedding mold. The more differentiated bulging colonies were, prior to fixation, carefully dissected free and lifted from the bottom of the culture dish using hypodermic needles. Individual colonies were placed between two cover-glasses and fixed in Bouin’s fixative for 1 to 2 hr followed by dehydration in graded alcohols. Finally, the colonies were cleared for 1 hr in xylene and embedded in paraffin (Brøchner et al. 2010). Specimens from both embedding techniques were then cut in 3- to 5-µm-thick serial horizontal sections, strictly in parallel to the bottom of the cultures.
The mouse monoclonal YKL-40 antibody was generated toward human YKL-40 purified from serum-free, conditioned medium from monolayer cultures of the YKL-40-producing human osteosarcoma cell line MG63 (Johansen et al. 1992) and subsequently purified with high-pressure liquid chromatography. Later analysis demonstrated that this monoclonal mouse IgG2bk binds the epitope GAWRGTTGHHS corresponding to amino acids 210 to 220 of the human YKL-40 protein.
The YKL-40 antibody was used in different concentrations of 1, 2, 3.5, or 7.5 µg/ml. Positive controls included parallel staining of sections with neutrophils, macrophages, mast cells, endothelial cells, arthritic chondrocytes, synovial cells, muscle cells, different types of cancer cells, embryonic carcinoma, and fetal cells that have previously been demonstrated to show YKL-40 immunoreactivity using this antibody (Johansen et al. 2007; Schultz and Johansen 2010). Negative controls consisted of sections incubated with mouse IgG1, IgG2a, IgG2b, and irrelevant goat or rabbit antibodies or sections where primary or secondary antibodies were omitted. The staining specificity of YKL-40 was previously tested by preincubation of the antibody with purified human YKL-40 for 2 hr at room temperature to block YKL-40 binding sites and reveal possible nonspecific staining of the sections (see Fig. 1 in Johansen et al. 2007).
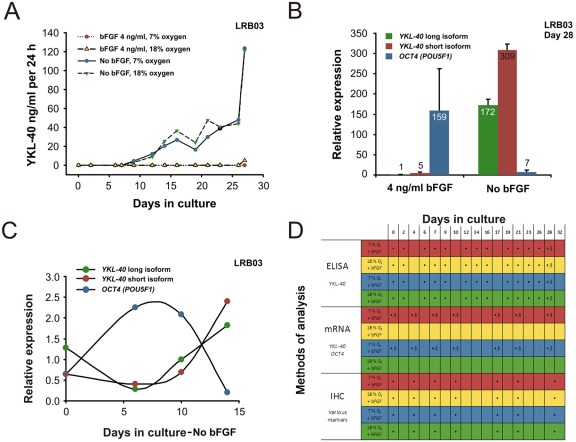
Figure 1.
Cell line LRB03 grown for up to 32 days. ELISA measurements of YKL-40 protein secretion into the culture medium (A) and mRNA analysis of YKL-40 and OCT4 expression in culture by quantitative RT-PCR (QPCR) (B, C). (A) Concentration of YKL-40 secreted into the culture medium during 28 days of culture, determined by ELISA. Oxygen tension alterations show no difference in amount of YKL-40 measured, whereas absence of basic fibroblast growth factor (bFGF) leads to earlier secretion of YKL-40 into the medium already after 10 days in culture. (B) Relative expression of mRNA for OCT4 and YKL-40 short and long isoforms in relation to culture medium composition with absence (“differentiation medium”) or presence (“proliferation medium”) of 4 ng/ml bFGF, after 28 days of culture. Upregulation of YKL-40 mRNA expression in the human embryonic stem cells (hESCs) cultured without bFGF was 172-fold and 62-fold for the long and short isoforms, respectively, whereas OCT4 was only high when grown with bFGF added. (C) Measurements of OCT4 and both isoforms of YKL-40 by QPCR from culture days 0 to 15 grown without bFGF. YKL-40 isoforms follow each other, leaving no significant change in expression throughout culture age. At culture day 0, OCT4 and both isoforms of YKL-40 mRNA are found in low levels. Shortly thereafter, OCT4 expression rises with a maximum toward day 7 as YKL-40 diminishes. From here on, OCT4 and YKL-40 expression is inversed, which correlates well with the findings of less undifferentiated cells and more differentiated germ layer marker-positive cells. (D) Overview of the different culture conditions employed. Conducted experiments with immunohistochemistry analysis in direct relation to either ELISA or QPCR measurements are shown. •Analysis performed. §Colony terminated. Same color code in (A) and (D) facilitates comparison.
Bright-field light microscopy
For bright-field light microscopy, prior to staining, nonspecific binding was inhibited by incubation for 30 min with blocking buffer (ChemMate antibody diluent S2022; DakoCytomation, Glostrup, Denmark) at room temperature. The sections were incubated overnight at 4C, washed with Tris-buffered saline (TBS), and incubated for 30 min with a peroxidase-labeled polymer conjugated to goat anti-mouse immunoglobulins (EnVision+ System/HRP K4007; DakoCytomation) and then washed with TBS and incubated for 10 min with 3,3′-diaminobenzidine chromogen solution. Positive staining was recognized as a brown color. The sections were counterstained with Mayer’s hematoxylin and dehydrated in graded alcohols followed by xylene and coverslipped with DPX mounting media. Neighboring sections were stained with antibodies against different stem cell markers and differentiation markers to study regional distribution and co-localization of the markers. The antibodies against OCT4, OCT3/4, NANOG, HNF-3β, PDX1, CD34, p63, nestin, and PAX6 are described in detail in Table 1. For detecting mouse and rabbit primary antibodies, the REAL EnVision Detection System, Peroxidase/DAB+, Rabbit/Mouse was used (K5007; DakoCytomation). For detecting goat primary antibodies, Amersham Biosciences RPN (1025V diluted 1:20 in 10% donkey serum/TBS; Amersham Biosciences, Piscataway, NJ) and StreptABComplex/HRP (K0377; DakoCytomation) were used. A Zeiss Axiophot microscope (Zeiss, Jena, Germany) was used for bright-field light microscopy.
Fluorescence microscopy: double staining for YKL-40 and OCT4 as used in initial studies
Prior to staining, nonspecific binding was inhibited by incubation for 30 min with 10% goat serum (04-009-1A; Biological Industries, Kibbutz Beit-Haemek, Israel), followed by 0.3% hydrogen peroxide for 15 min. After overnight incubation with a mixture of YKL-40 and OCT4 diluted in 10% goat serum at 4C, the sections were incubated for 30 min with labeled polymer–HRP anti-mouse (EnVision+ System/HRP K4007; DakoCytomation) followed by Tyramid Signal Amplification (Sa-Fluorescein, NEL 741 B; PerkinElmer, Waltham, MA) and then incubated with biotin-SP-conjugated F(ab′)2 fragment donkey anti-rabbit antibodies (711-066-152; Jackson ImmunoResearch, West Grove, PA) followed by streptavidin-conjugated Alexa Fluor 594 (S11227; Molecular Probes, Eugene, OR) and then coverslipped.
Double staining for YKL-40 and OCT4 or HNF-3β during later investigations
The same staining protocol for YKL-40 was used in both staining reactions. Prior to staining, nonspecific binding was inhibited by incubation with 0.2% casein (C-7078; Sigma) for 30 min at room temperature. The sections were then incubated with YKL-40 diluted in 2% casein for 1½ hr at room temperature and then for 15 min in 0.3% hydrogen peroxide, incubated for 30 min with labeled polymer–HRP anti-mouse (EnVision+ System/HRP K4007; DakoCytomation), and followed by TSA Kit 2 with Alexa Fluor 488 Tyramide (T20912; Invitrogen, Molecular Probes).
OCT4
After staining for YKL-40, the sections were subjected to antigen retrieval in TEG buffer and incubated with OCT4 overnight. Finally, they were incubated with biotin-SP-conjugated F(ab′)2 fragment donkey anti-rabbit antibodies (711-066-152; Jackson ImmunoResearch) followed by streptavidin-conjugated Dylight 594 (SA5594; Vector Laboratories, Burlingame, CA) and then coverslipped.
HNF-3β
After staining for YKL-40, the sections were subjected to antigen retrieval in TEG buffer. Nonspecific binding was inhibited by incubation for 30 min with 10% donkey serum (017-000-1210; Jackson ImmunoResearch). Sections were then incubated with HNF-3β for 1½ hr at room temperature and then in biotin-SP-conjugated F(ab′)2 fragment donkey anti-goat (705-066-147; Jackson ImmunoResearch) followed by streptavidin-conjugated Dylight 594 (SA5594; Vector Laboratories) before being coverslipped.
Double staining for YKL-40 and PAX6
Prior to staining, nonspecific binding was inhibited by incubation for 30 min with 0.2% casein (C-7078; Sigma) at room temperature. The sections were incubated with a mixture of YKL-40 and PAX6 diluted in 0.2% casein for 1½ hr at room temperature and then for 15 min in 0.3% hydrogen peroxide subsequently for 30 min with labeled polymer–HRP anti-mouse (EnVision+ System/HRP K4007; DakoCytomation), followed by TSA Kit 2 with Alexa Fluor 488 Tyramide (T20912; Invitrogen, Molecular Probes). Finally, they were incubated with biotin-SP-conjugated F(ab′)2 fragment donkey anti-rabbit antibodies (711-066-152; Jackson ImmunoResearch), followed by streptavidin-conjugated Dylight 594 (SA5594; Vector Laboratories), and then coverslipped.
A Zeiss Axioplan 2 microscope was used for fluorescence microscopy, and the confocal images were obtained with a Carl Zeiss LSM 510 and LSM 780. In the case of LSM 510, a C-Apochromat 40×/1.2-W objective was used, a 488-nm argon laser with an emission filter of 505 to 550 was used for excitation of Alexa Fluor 488, and a 543-nm HeNe laser with an emission filter of 560 to 615 was used for excitation of Alexa Fluor 594. In the case of LSM 780, a Plan-Apochromat 63/1.40 Oil DIC M27 objective was used together with the following lasers and emission filters: For excitation of Alexa Fluor 488, a 488-nm argon laser with an emission filter of 493 to 590 was used, and for excitation of Dylight 594, an In Tune laser with an emission filter of 596 to 692 using the GaAsP spectral detector was used.
Results
Colonies grown on MEF or hFF feeders in medium with or without bFGF and at low (7%) or high (18%) oxygen tension developed well, with cells showing undifferentiated stem cell morphology—that is, single large nucleated cells with prominent nucleoli and scanty cytoplasm interspersed with cells of more differentiated morphology depending on culture age. Following mRNA analysis by QPCR, there was no significant change of expression of the short or long isoform of YKL-40 in relation to culture age. Analysis of the culture medium using ELISA revealed an inverse expression of YKL-40 protein and OCT4, and the embedding techniques applied made it possible to perform IHC on serial sections of the entire cultures for YKL-40, pluripotent stem cell markers, and germ layer markers (Brøchner et al. 2010). The IHC findings correlated well with the QPCR and ELISA results of YKL-40 expression. Parallel studies were carried out on all LRB cell lines used in the present study, and similar data were obtained with the different cell lines. For simplicity, the data presented in Figure 1, obtained by parallel investigations of QPCR, ELISA, and IHC over culture time, are derived from cell line LRB03.
YKL-40 and OCT4 mRNA Expression and Protein Secretion in hESC Cultures
In a series of experiments, hESC cultures were grown for 28 days in either proliferation medium (with bFGF) or differentiation medium (without bFGF) in either 7% O2 or 18% O2. The expressions of two isoforms of YKL-40 mRNA were analyzed by QPCR, and YKL-40 protein secretion was determined by ELISA. All colonies from the six different hESC lines showed expression of YKL-40 mRNA, and all secreted YKL-40 to the medium. The YKL-40 concentration in the medium from hESC line LRB03 was analyzed at different time points from culture days 0 to 28. When cells were cultured without bFGF, YKL-40 was detectable already at day 9, and the production rate increased up to day 28. In the more undifferentiated cell colonies cultured with 4 ng/ml bFGF, the secretion of YKL-40 to the medium was first detectable after 27 days. Irrespective of oxygen tension, there was no difference of YKL-40 protein concentration in the medium or of YKL-40 mRNA expression (Fig. 1A). At culture day 28, a significant upregulation of YKL-40 mRNA expression was observed in the hESC cultures grown without bFGF, compared to the cultures grown in the presence of bFGF (172-fold and 62-fold for the long and short YKL-40 mRNA isoform, respectively), whereas OCT4 mRNA expression remained high only in the presence of bFGF (Fig. 1B, same experiment as in Fig. 1A).
The upregulation of YKL-40 mRNA expression in differentiating hESCs was studied further by analyzing the expression of YKL-40 and OCT4 at different time points during differentiation of hESC cultures, from culture days 0 to 15. The relative expression of mRNA of the short and long isoforms of YKL-40 showed no significant change in expression. QPCR analysis of the expression of OCT4 was clearly negatively associated with the expression of both YKL-40 isoforms, with upregulation of YKL-40 from around day 7 (Fig. 1C). Exemplified by cell line LRB03, different methods of analysis related to cell culture age and culture conditions are depicted in Figure 1D.
YKL-40 and OCT4 Protein Expression in hESC Cultures Evaluated by Immunohistochemistry
The hESC cultures were well preserved and exhibited either good or excellent morphology. Immunostaining for YKL-40 resulted in a well-defined staining pattern, which could be a combination of uniformly or granular cytoplasmic staining and/or membrane-associated staining. The pattern of reactivity was similar following the two entirely different staining protocols for bright-field microscopy and confocal microscopy. In late cultures (28–32 days and 42 days) grown in the presence of bFGF, IHC analysis demonstrated that less than 2% of the cells expressed OCT4 and showed morphology in toluidine blue–stained sections compatible with undifferentiated hESCs (i.e., large nuclei with 1–3 reticulated dense nucleoli in a scanty cytoplasm).
Control sections incubated with mouse IgG1, IgG2a, IgG2b, and irrelevant goat or rabbit antibodies, as well as subjected to omission of primary or secondary antibodies, showed no reactivity. Preabsorption with human YKL-40 antigen showed a lack of or a diminished reactivity in accordance with previously described results (see Fig. 1 in Johansen et al. 2007). The control sections for the OCT4 antibody, which included preabsorption with OCT4 peptide, showed a complete lack of reactivity.
YKL-40 and OCT4 Protein Expression in hESCs from the Onset of Cellular Differentiation
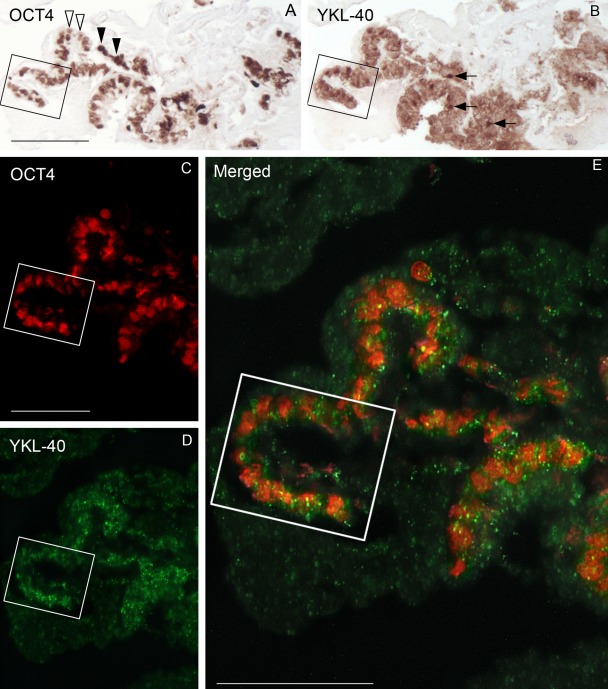
Colonies grown in the presence of bFGF showed OCT4-positive cell clusters even after 28 days. However, colonies grown without bFGF had fewer visible OCT4-positive cells from day 15. Virtually all cells in colonies that served as the “starting point” (culture day 0) for the individual cultures were positive for OCT4 and also for YKL-40 (Fig. 2A,B). Double labeling of sections from culture day 0 followed by confocal microscopy showed the presence of cells simultaneously positive for both YKL-40 and OCT4 protein (Fig. 2C–E).
Figure 2.
Staining patterns for OCT4 (A), YKL-40 (B), and OCT4/YKL-40 (C–E) in three consecutive sections of a human embryonic stem cell colony (LRB03) from culture day 0 visualized by immunohistochemistry and confocal fluorescence microscopy. The “U-turn” in the boxed area is easy to identify in the three sections. (A) All cell nuclei exhibit OCT4 positivity, although a varying intensity of the staining is evident. Open arrowheads point to weakly stained nuclei, and solid arrowheads show strongly labeled nuclei. (B) The adjacent section stained for YKL-40 shows a marked reactivity in the cytoplasm with occasionally denser areas (arrows). (C–E) Confocal microscopy of a neighboring section stained simultaneously with antibodies against OCT4 and YKL-40. Cells with nuclear reactivity (red) for OCT4 (C) also exhibit cytoplasmic reactivity (green) for YKL-40 (D), verified at higher magnification by the merged image (E). Yellow dots correspond to YKL-40 located directly below or above the OCT4-positive red nucleus. Scale bars: 100 µm.
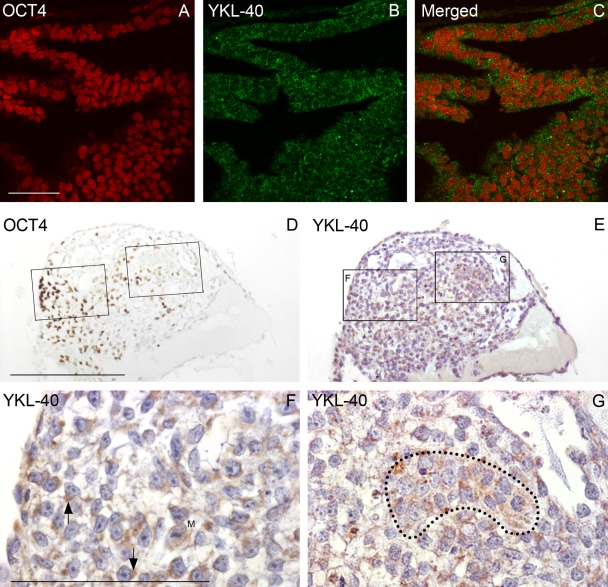
After 4 to 7 days of culture without bFGF, IHC revealed a transition of expression patterns of OCT4 and YKL-40 protein. Initially, the vast majority of cells showed both OCT4 and YKL-40 positivity, exemplified in Figure 3A–C with cell line LRB03 grown for 7 days. Additional time in culture resulted in the appearance of more cells being OCT4 negative and YKL-40 positive, which correlates well with the results shown in Figure 1C. Similar results were found in all six cell lines examined. In the cell line LRB010, this pattern of expression was maintained in colonies grown for 10 to 17 days. A small group of OCT4-positive cells gathered toward one edge of a colony (17 days) with few weakly OCT4-positive cells scattered toward the middle (Fig. 3D). A neighboring section stained for YKL-40 (Fig. 3E) showed YKL-40 in the cytoplasm, often in close proximity to the nucleus (higher magnification in Fig. 3F and Fig. 3G). The appearance of the cells corresponded well with typical hESCs. However, the cell pattern was less epithelial and more mesenchymal, suggesting an initial cryptic differentiation prior to overt differentiation. In the rather OCT4-negative field, the epithelial-like cell group showed a dense granular cytoplasmic YKL-40 reactivity and nuclei without dense nucleoli (Fig. 3G).
Figure 3.
Early and later differentiating colonies. (A–C) Confocal microscopy of a 7-day-old colony from LRB03 grown without basic fibroblast growth factor shows early differentiating human embryonic stem cells (hESCs) stained for OCT4 (A) and YKL-40 (B). The merged picture (C) depicts strongly OCT4-reacting stem cell nuclei surrounded by a fine granular cytoplasmic YKL-40 staining. A later differentiating colony (shown in D–G) in bright-field microscopy is from a 17-day-old culture from LRB010 also stained for OCT4 (D) and YKL-40 (E). In (D), a small group of strongly OCT4-positive cells is present toward one edge of the colony with few weakly OCT4-positive cells scattered toward the middle. A neighboring section stained for YKL-40 (E) with boxed areas shown at higher magnification in (F) and (G) depicts YKL-40 in the cytoplasm of loosely packed cells, often in close proximity to the nucleus (arrows in F). Morphology of the individual cells is corresponding well to hESC morphology with large nuclei with one to three dense nucleoli in a scanty cytoplasm. However, the cell pattern is less epithelial and more mesenchymal. In the rather OCT4-negative field in (G), the epithelial-like cell group marked by a dotted line shows a dense granular cytoplasmic YKL-40 reactivity and nuclei without dense nucleoli. M shows a mitotic figure. A–C: same magnification, scale bar: 50 µm. D, E: same magnification, scale bar: 250 µm. F, G: same magnification, scale bar: 100 µm.
Differentiated Cells and Tissues
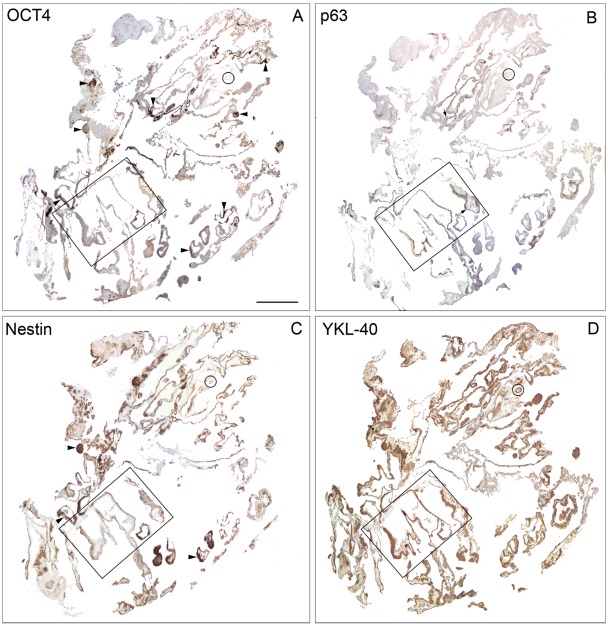
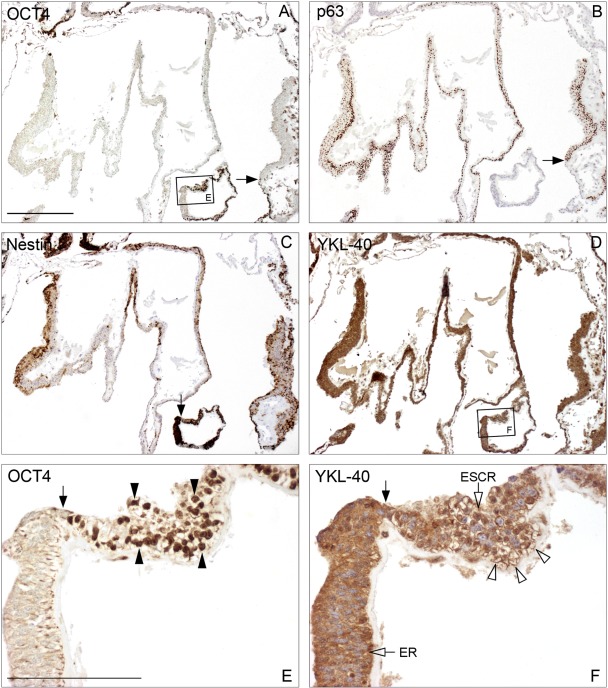
During the subsequent formation of complex tissue morphologies comprising cell populations representative of all three germ layers, the differentiating progeny from colonies grown for 28 to 32 days all showed a strong YKL-40 protein expression. All cells from one culture dish (cell line LRB03), grown in the presence of bFGF, were embedded using the technique for bulging colonies at culture day 32. Adjacent sections were stained for OCT4, p63, nestin, and YKL-40 (Fig. 4A–D, higher magnification in Fig. 5A–D). Small OCT4-positive areas were distributed throughout the continuum of cells from the dish in contrast to the vast OCT4-negative areas (Fig. 4A). The ectodermal marker p63 was positive in well-defined regions of the colony, with a distinct borderline to the OCT4-positive areas (Fig. 4B and Fig. 5B). Nestin-positive staining was partly overlapping with OCT4-positive areas (Fig. 4C). YKL-40 showed a varying expression in the colony both in the remaining pluripotent cells and in the more differentiated ectodermal lineages (Fig. 4D and Fig. 5D). No OCT4-positive cells showed simultaneously expression of ecto-, meso-, or endoderm layer markers, whereas some coexpression of OCT4 and the neuroectodermal marker nestin was observed. A strong uniform cytoplasmic YKL-40 reaction was present in areas without OCT4-positive cells, whereas YKL-40 showed both a membrane association and a coarse granular perinuclear reactivity (suggesting a close relation to the epithelial region [ER] and Golgi complex) in the cells of OCT4-positive areas, with pluripotent stem cell morphology (see, e.g., Fig. 5E,F).
Figure 4.
Survey of entire human embryonic stem cell (hESC) culture from LRB03 embedded at culture day 32 with neighboring sections stained for OCT4 (A), p63 (B), nestin (C), and YKL-40 (D). Boxed areas are shown at higher magnification in Figure 5. (A) Small OCT4-positive areas are distributed widely across the entire culture (arrowheads) in contrast to the vast OCT4-negative areas. (B) Ectodermal marker p63 is positive in well-defined regions of the culture, with a distinct borderline to OCT4 areas (arrows), but this is better appreciated at higher magnification (Fig. 5B). (C) A moderate nestin-positive staining is seen in areas overlapping the OCT4 stained areas (arrowheads). The stronger nestin-reacting subregions of the culture are not OCT4 positive (see also Fig. 5C). (D) YKL-40 is present in all regions and subregions throughout the culture, both in the pluripotent OCT4-positive cells and in the various progenitor cells. YKL-40 reactivity is also seen in structures that show no OCT4, p63, or nestin reactivity (see encircled area). A–D: same magnification, scale bar: 1 mm.
Figure 5.
(A–D) Higher magnification of boxed areas in Figure 4. Transition points from OCT4-positive cells to p63-positive ectodermal and nestin-positive neuroectodermal cells in the same layer are indicated by single arrows (A, B, C). YKL-40 is positive in areas with pluripotent undifferentiated cells and the more differentiated ectodermal lineages (D). Cells with nuclear OCT4 (dense arrowheads) and cytoplasmic and membranous YKL-40 staining at higher magnification of boxed areas from A and D (E, F). YKL-40 is seen along the membranes (open arrowheads) of the OCT4-positive cells, with pluripotent stem cell morphology in contrast to the strong cytoplasmic reactivity of the adjacent epithelial region (ER). The borderline between the embryonic stem cell region (ESCR) and the epithelial region is indicated by the dense arrow. A–D: same magnification, scale bar: 500 µm. E, F: same magnification, scale bar: 100 µm.
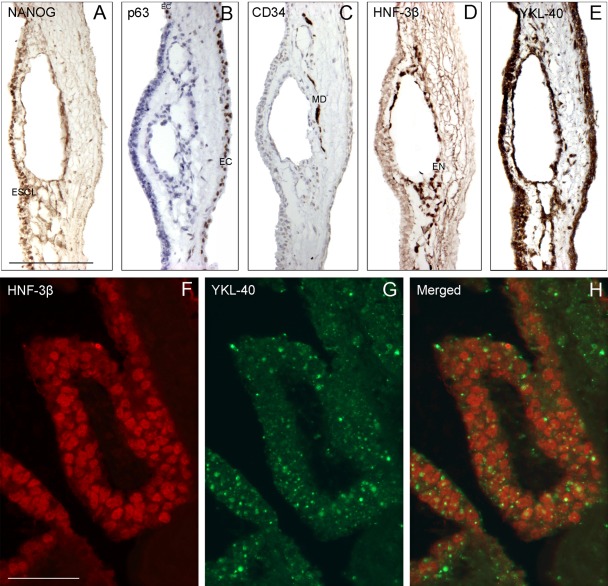
Adjacent sections from a morphologically more differentiated hESC colony (LRB01) grown in the presence of bFGF for 42 days showed positive IHC staining for hESC marker NANOG, as well as for ectoderm (p63), mesoderm (CD34), and endoderm (HNF-3β) markers and YKL-40 protein. NANOG-positive cells and p63-positive cells were located in the outermost epithelial-like stratum of the colony, whereas the CD34-positive cells were present in an area deeper within the colony. The HNF-3β-positive cells encircled a tube-like structure in the innermost part (Fig. 6A–D). YKL-40-positive cells were found both in the outermost stratum and in the mesendodermal cells (Fig. 6E). An earlier stage (28 days) of the same LRB01 colony double stained for HNF-3β and YKL-40 showed HNF-3β-positive endodermal cell nuclei surrounded by cytoplasmic coarse YKL-40-positive granules (Fig. 6F–H).
Figure 6.
(A–E) Markers for human embryonic stem cells (NANOG), ectoderm (p63), mesoderm (CD34), endoderm (HNF-3b), and YKL-40 applied to neighboring sections from an LRB01 colony cultured for 42 days. The p63-positive cells line the outermost area of the culture, whereas the CD34-positive cells are in the middle of the culture. The HNF-3b-positive cells are encircling a tube-like structure in the innermost part of the culture. YKL-40-positive cells are in all three germ layer–like areas as well as in the NANOG-positive cells lining the edge. (F–H) Confocal microscopy of a section from an earlier stage (28 days) of the same LRB01 colony stained for HNF-3b (F) and YKL-40 (G). The merged picture (H) shows the red HNF-3b endodermal cell nuclei surrounded by coarse green granules in the YKL-40-stained cytoplasm. (A–E) Same magnification, scale bar: 200 µm. (F–H) Same magnification, scale bar: 50 µm. ESCL, embryonic stem cell layer; EC, ectodermal layer; MD, mesodermal layer; EN, endodermal layer.
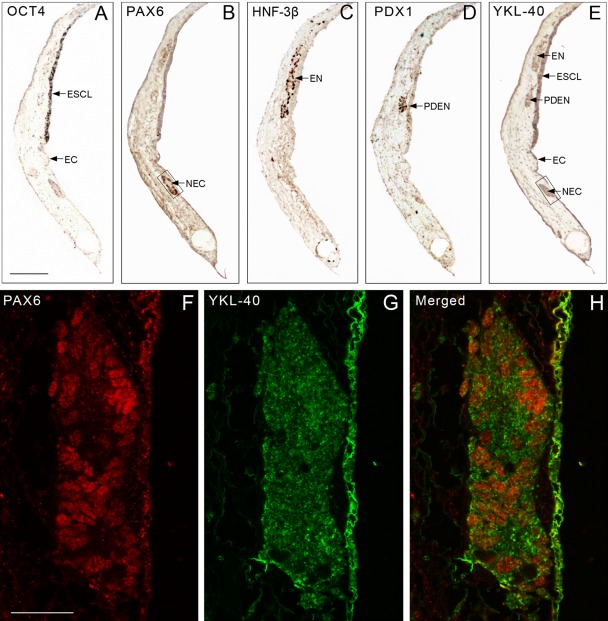
In another series of adjacent sections from LRB01 grown for 42 days, IHC analysis was performed using antibodies against OCT4, the neuroectodermal marker PAX6, the endoderm markers HNF-3β and PDX1, and YKL-40. Well-defined areas consisting of pluripotent OCT4-positive cells at the outermost epithelial-like stratum—an embryonic stem cell layer—were in direct continuation with a layer of p63-positive ectodermal (not shown) and PAX6-positive neuroectodermal cells, whereas cells positive for endoderm markers were situated below this area (see, e.g., Fig. 7A–D). YKL-40-positive staining co-localized in corresponding areas (Fig. 7E). From the same series, sections double labeled for PAX6 and YKL-40 showed a similar pattern of reactivity as that seen in Figure 7B,E (bright-field microscopy), with PAX6-positive neuroectodermal cell nuclei surrounded by a fine granular YKL-40-positive cytoplasm (Fig. 7F–H). Note that two entirely different staining protocols lead to very similar results. A strongly varying intensity of YKL-40 staining throughout the germ layers was always found. Thus, the data presented in Figures 5, 6, and 7 demonstrate a direct and distinct epithelial transformation from undifferentiated hESCs in the outermost epithelial-like layer of the colony into ectodermal p63-positive cells and to neuroectodermal nestin-positive cells within the same cell layer (Fig. 5), whereas a similar direct transformation into either meso- or endodermal marker–positive cells was not observed (Figs. 6 and 7).
Figure 7.
(A–E) A series of sections adjacent to those shown in Figure 6A–E (i.e., also 42 days old), stained for markers for undifferentiated stem cells (OCT4); neuroectodermal cells (PAX6); endodermal germ layer (HNF-3β), including the pancreas-duodenum part (PDX1); and YKL-40. A well-defined area consisting of pluripotent OCT4-positive cells is confined to the outermost lining—an embryonic stem cell layer (ESCL), which continues into an ectodermal layer (EC), which extends to a PAX6-positive neuroectoderm (NEC). Cells positive for endodermal germ layer markers (EN) are localized in a deeper layer. The transcription factor PDX1 is seen in the endoderm, differentiating into the pancreas-duodenum anlage (PDEN). YKL-40-positive staining is found in the ESCL, the germ layers, and the PDX1-positive cell aggregate (arrows in E). (F–H) Confocal microscopy of a section from the same series as that shown in A–E, stained for PAX6 (F) and YKL-40 (G). The boxed areas in B and E correspond to F and G (i.e., two entirely different staining protocols lead to very similar results). The merged picture (H) shows the red PAX6-positive neuroectodermal cell nuclei surrounded by a fine granular green YKL-40-positive cytoplasm. Note the stronger YKL-40 staining of the ectoderm. A–E: same magnification, scale bar: 200 µm. F–H: same magnification, scale bar: 20 µm.
Discussion
High YKL-40 protein expression is reported in cell populations relevant for morphogenesis during early human development (Johansen et al. 2007). In the present study, we examined the expression of YKL-40 in six different hESC lines from the day of transfer (day 0) with entirely undifferentiated hESCs to days 28 to 32 and, in the case of LRB01, to day 42 with more or less totally differentiated cells. The expression of YKL-40 was related to the cellular differentiation and the initial morphogenesis in the individual colonies. The used monoclonal YKL-40 antibody has been demonstrated by epitope mapping to bind the epitope GAWRGTTGHHS corresponding to amino acids 210 to 220 of the human YKL-40 protein (Johansen et al. 2007). This antibody has previously shown YKL-40 immunoreactivity in different types of cancer cells, embryonic carcinoma cells, and fetal cells (Schultz and Johansen 2010).
Our studies demonstrate that YKL-40 is consistently expressed in both undifferentiated and early differentiating hESCs in all cell lines investigated. YKL-40 is abundant in both OCT4-positive pluripotent cells and in the more differentiated progenitor cells positive for all germ layer markers used. Interestingly, OCT4/YKL-40-positive cells change expression pattern as differentiation takes place. Within the same hESC colony, different areas show OCT4/YKL-40-positive cells separated from areas containing only YKL-40-positive cells. However, as differentiation progresses, OCT4 expression diminishes, whereas YKL-40 expression increases in cells lacking basic morphological criteria for hESCs and confirmed with IHC. At later stages of differentiation, the subcellular distribution of YKL-40 expression is associated with the differentiation stage of the cell, with a more membrane-associated immunopositive staining in the undifferentiated cells and a more cytoplasmic YKL-40 protein expression in the differentiated cells. A marked coarse granular reactivity characterizes the endodermal HNF-3β-positive cells in contrast to a fine cytoplasmic YKL-40 reactivity in the neuroectodermal PAX6-positive cells. A varying intensity of the YKL-40 staining is noticeable in particular when comparing the very strong reactivity in the ectoderm with the weaker reactivity of the neuroectoderm and the mesoderm.
Furthermore, YKL-40 protein is secreted extracellularly into the culture medium with increasing concentrations as the colony grows older and becomes more differentiated. Specific culture conditions such as alteration of oxygen tension and source of feeder cells have no impact on YKL-40 synthesis and secretion. Neither are significant changes of expression of the short or long isoform of YKL-40 in relation to days of culture observed. The use of differentiation medium compared to proliferation medium leads to early differentiation of the cells with subsequent rapid increases of YKL-40 secreted to the cell culture medium.
YKL-40 was first described in 1992 as being secreted in large amounts in serum-free conditioned media of the human MG-63 osteosarcoma cell line (Johansen et al. 1992). Recently, it has been reported that stem-like cells can be isolated from the MG-63 osteosarcoma cell line (Lou et al. 2010). High YKL-40 expression is associated with organ fibrosis and cancer (Johansen et al. 2009; Schultz and Johansen 2010; Lee et al. 2011). Tissue biopsies from patients with cancer have demonstrated that YKL-40 is produced by different types of cancer cells, including embryonic carcinoma, different types of adeno- and squamous cell carcinoma, renal cell cancer, small cell lung cancer, melanoma, and glioblastoma (Schultz and Johansen 2010). Highest plasma concentrations of YKL-40 are found in patients with metastatic diseases and with the poorest prognosis (Johansen 2006; Johansen et al. 2009; Schultz and Johansen 2010). Cancer stem cells have several similarities with hESCs, and cancer stem cells play an important role in cancer development, different cancer phenotypes, and cancer cell metastasis potential (Chaffer and Weinberg 2011; Visvader 2011). Little is known whether cancer stem cells produce YKL-40. Mesenchymal stem cells derived from human glioblastoma express YKL-40 (Lang et al. 2008), and CD133+ glioblastoma stem cells express YKL-40 (Liu et al. 2009).
Although the present study does not identify a specific function of YKL-40, its wide distribution and expression in hESCs and their progeny suggest that YKL-40 is involved in a change of commitment and thereby in directing further differentiation of pluripotent hESCs toward more differentiated germ layer lineages. In a previous study of regional differences in the expression of markers during in vitro differentiation of hESC lines (LRB01-04), a direct change of expression from undifferentiated OCT4-positive hESCs to SSEA1-positive fully differentiated epithelial cells was noticed (Laursen et al. 2007). Very recently, early colonies from one of the LRB cell lines included in the present study (LRB010) were investigated using markers such as E-cadherin, β-catenin, vimentin, and N-cadherin, known to be involved in epithelial-mesenchymal transition and shown to contain a central compartment characterized by hESC-like cells of typical undifferentiated morphology (Jacobsen et al. 2011). In older colonies, a gradual change of differentiation was noted from the central compartment toward both apical and basal compartments, indicating a direct shift from hESCs to columnar epithelial cells and to nestin-positive neuroectodermal cells in the basal compartment (Jacobsen et al. 2011).
During in vivo embryogenesis and organ development, the process initially termed epithelial-mesenchymal transformation (Hay 2005), now referred to as epithelial-mesenchymal transition (EMT) (Kalluri and Weinberg 2009; Thiery et al. 2009), is a key event. EMT is a biological process that allows an epithelial cell to undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype, which includes enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and increased production of extracellular matrix components (Kalluri and Weinberg 2009). There seems to be a direct transformation, with the term reflecting the presumed irreversibility of this process, of pluripotent hESCs into ecto- and neuroectodermal germ layer cells (Thiery et al. 2009). In contrast, the transition, with the term reflecting a presumed reversibility of the process, of pluripotent cells into meso- and endoderm requires an EMT, known from the gastrulation during early human embryonic development (Eastham et al. 2007; Thiery et al. 2009). Early in the third week of human embryonic development, epiblast cells lateral to the primitive streak begin to move into the streak, where they undergo an EMT. The epithelial epiblast cells elongate and detach from their neighbors and then migrate away below the primitive streak as individual, loosely organized mesendodermal progenitors. This process of gastrulation represents the first EMT after implantation (Thiery et al. 2009). However, what has not really been emphasized so far is the lack of EMT in the initial differentiation of epiblast cells to the remaining ecto- and neuroectodermal germ layer—a process of epiblast-ectodermal transformation (EET).
During the in vitro differentiation of hESCs, we observed an ESC-ectodermal transformation from undifferentiated hESCs toward ectodermal p63-positive cells and to neuroectodermal nestin-positive cells. Meso- and endodermal marker–positive cells were consistently found in deeper layers within the colony. These findings suggest that a direct epithelial transformation from OCT4-positive hESCs into ecto- and neuroectodermal lineages and an epithelial-mesenchymal transition-like process from hESCs to meso- and endoderm progenitors take place in cell culture. A direct role of YKL-40 in ESC-ectodermal transformation and in the transition from hESCs to meso- and endodermal cells is yet to be fully elucidated, but YKL-40 seems to be important in both of these processes. Further studies involving both an EMT setup and in vitro and in vivo functional experiments with, for example, siRNA or blocking antibodies are necessary to obtain further insights into the function of YKL-40.
In conclusion, our studies suggest that YKL-40 plays an important role during early differentiation, both in the observed direct epithelial transformation of hESCs to ecto- and neuroectoderm and in the transition from undifferentiated pluripotent hESCs toward multipotent progenitor cells in endo- and mesoderm. The already known roles of YKL-40 in the adult organism and its distribution during in vivo differentiation in human embryonic development, in concert with the present results, relating YKL-40 to early differentiation and initial morphogenesis in individual hESC colonies, indicate that YKL-40 has pleiotropic effects in different cell systems.
Acknowledgments
The expert technical assistance of Sussi Forchhammer, Hanne Hadberg, Pernille S. Froh, Ha Nguyen, and Lillian Rasmussen (Department of Cellular and Molecular Medicine, Faculty of Health Sciences, University of Copenhagen, Copenhagen) and Marjo Westerdahl (Laboratory of Reproductive Biology, Rigshospitalet, University Hospital of Copenhagen, Copenhagen) through all stages of the project is gratefully acknowledged. The Core Facility for Integrated Microscopy (Faculty of Health Sciences, University of Copenhagen, Copenhagen) is acknowledged for use of LSM 780 confocal microscopy. Keld B. Ottosen (Department of Cellular and Molecular Medicine, Faculty of Health Sciences, University of Copenhagen, Copenhagen) is thanked for the final layout of the figures.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by grants from The Vera and Carl Johan Michaelsens Foundation.
References
- Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, et al. 2007. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 25:803–816 [DOI] [PubMed] [Google Scholar]
- Awan A, Oliveri RS, Jensen PL, Christensen ST, Andersen CY. 2010. Immunoflourescence and mRNA analysis of human embryonic stem cells (hESCs) grown under feeder-free conditions. Methods Mol Biol. 584:195–210 [DOI] [PubMed] [Google Scholar]
- Baeten D, Boots AM, Steenbakkers PG, Elewaut D, Bos E, Verheijden GF, Berheijden G, Miltenburg AM, Rijnders AW, Veys EM, et al. 2000. Human cartilage gp-39+,CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 43:1233–1243 [DOI] [PubMed] [Google Scholar]
- Bigg HF, Wait R, Rowan AD, Cawston TE. 2006. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem. 281:21082–21095 [DOI] [PubMed] [Google Scholar]
- Brøchner CB, Vestentoft PS, Lynnerup N, Andersen CY, Møllgård K. 2010. A two- and three-dimensional approach for visualizing human embryonic stem cell differentiation. Methods Mol Biol. 584:179–193 [DOI] [PubMed] [Google Scholar]
- Bussink AP, Speijer D, Aerts JM, Boot RG. 2007. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics 177:959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. 2011. A perspective on cancer cell metastasis. Science 331:1559–1564 [DOI] [PubMed] [Google Scholar]
- Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, et al. 2007. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 357:2016–2027 [DOI] [PubMed] [Google Scholar]
- Dickey BF. 2007. Exoskeletons and exhalation. N Engl J Med. 357:2082–2084 [DOI] [PubMed] [Google Scholar]
- Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stern PL, Ward CM. 2007. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 67:11254–11262 [DOI] [PubMed] [Google Scholar]
- Eiselleova L, Peterkova I, Neradil J, Slaninova I, Hampl A, Dvorak P. 2008. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol. 52:353–363 [DOI] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM. 2005. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 102:4783–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faibish M, Francescone RA, Bentley B, Yan W, Shao R. 2011. A YKL-40 neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 10:742–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. 2010. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 139:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescone RA, Scully S, Faibish M, Taylor SL, Oh D, Moral L, Yan W, Bentley B, Shao R. 2011. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 286:15332–15343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. 2003. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 278:37753–37760 [DOI] [PubMed] [Google Scholar]
- Hakala BE, White C, Recklies AD. 1993. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 268:25803–25810 [PubMed] [Google Scholar]
- Harvey S, Weisman M, O’Dell J, Scott T, Krusemeier M, Visor J, Swindlehurst C. 1998. Chondrex: new marker of joint disease. Clin Chem. 44:509–516 [PubMed] [Google Scholar]
- Hay ED. 2005. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 233:706–720 [DOI] [PubMed] [Google Scholar]
- Hough SR, Laslett AL, Grimmond SB, Kolle G, Pera MF. 2009. A continuum of cell states spans pluripotency and lineage commitment in human embryonic stem cells. PLoS One. 4:e7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DR, Recklies AD, Krupa JC, van Aalten DM. 2003. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes. J Biol Chem. 278:30206–30212 [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. 2000. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 6:88–95 [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JV, Andersen CY, Hyttel P, Møllgård K. 2011. From pluripotency to early differentiation of human embryonic stem cell cultures evaluated by electron microscopy and immunohistochemistry. In: Kallos MS. editor. Embryonic stem cells: basic biology to bioengineering. Rijeka, Croatia: InTech; p. 171–190 [Google Scholar]
- Johansen JS. 2006. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 53:172–209 [PubMed] [Google Scholar]
- Johansen JS, Høyer PE, Larsen LA, Price PA, Møllgård K. 2007. YKL-40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem. 55:1213–1228 [DOI] [PubMed] [Google Scholar]
- Johansen JS, Schultz NA, Jensen BV. 2009. Plasma YKL-40: a potential new cancer biomarker? Future Oncol. 5:1065–1082 [DOI] [PubMed] [Google Scholar]
- Johansen JS, Williamson MK, Rice JS, Price PA. 1992. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res. 7:501–512 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. 2009. The basics of epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang FF, Gumin J, Amano T, Hata N, Heimberger AB, Marini F, Andreeff M, Aldape KD. 2008. Tumor-derived mesenchymal stem cells in human gliomas: isolation and biological properties. J Clin Oncol. 26(Suppl):abstract 2001 [Google Scholar]
- Laursen SB, Møllgård K, Olesen C, Oliveri RS, Brøchner CB, Byskov AG, Andersen AN, Høyer PE, Tommerup N, Yding Andersen C. 2007. Regional differences in expression of specific markers for human embryonic stem cells. Reprod Biomed Online. 15:89–98 [DOI] [PubMed] [Google Scholar]
- Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. 2011. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 73:479–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Recklies AD. 2004. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor–alpha. Biochem J. 380:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Nguyen DH, Dong Q, Shitaku P, Chung K, Liu OY, Tso JL, Liu JY, Konkankit V, Cloughesy TF, et al. 2009. Molecular properties of CD133+ glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. J Neurooncol. 94:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou N, Wang Y, Sun D, Zhao J, Wang Y, Gao Z. 2010. Isolation of stem-like cells from human MG-63 osteosarcoma cells using limiting dilution in combination with vincristine selection. Indian J Biochem Biophys. 47:340–347 [PubMed] [Google Scholar]
- Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. 1999. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 250:168–173 [DOI] [PubMed] [Google Scholar]
- Millman JR, Tan JH, Colton CK. 2009. The effects of low oxygen on self-renewal and differentiation of embryonic stem cells. Curr Opin Organ Transplant. 14:694–700 [DOI] [PubMed] [Google Scholar]
- Nishikawa KC, Millis AJ. 2003. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res. 287:79–87 [DOI] [PubMed] [Google Scholar]
- Recklies AD, Ling H, White C, Bernier SM. 2005. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem. 280:41213–41221 [DOI] [PubMed] [Google Scholar]
- Recklies AD, White C, Ling H. 2002. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B–mediated signalling pathways. Biochem J. 365:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehli M, Krause SW, Andreesen R. 1997. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 43:221–225 [DOI] [PubMed] [Google Scholar]
- Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, Krause SW. 2003. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem. 278:44058–44067 [DOI] [PubMed] [Google Scholar]
- Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, Hrebicek M, Aerts JM. 1998. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 251:504–509 [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. 2000. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 18:399–404 [DOI] [PubMed] [Google Scholar]
- Saidi A, Javerzat S, Bellahcene A, De VJ, Bello L, Castronovo V, Deprez M, Loiseau H, Bikfalvi A, Hagedorn M. 2008. Experimental anti-angiogenesis causes upregulation of genes associated with poor survival in glioblastoma. Int J Cancer. 122:2187–2198 [DOI] [PubMed] [Google Scholar]
- Schultz NA, Johansen JS. 2010. YKL-40—a protein in the field of translational medicine: a role as a biomarker in cancer patients? Cancers. 2:1453–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelton LM, Mann DM, Millis AJ. 1995. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem. 270:13076–13083 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial-mesenchymal transitions in development and disease. Cell. 139:871–890 [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998. Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- Visvader JE. 2011. Cells of origin in cancer. Nature. 469:314–322 [DOI] [PubMed] [Google Scholar]