Abstract
Sensitive non-heme iron histochemistry—namely, the perfusion-Perls method and perfusion-Turnbull method—was applied to study the distribution and age-related accumulation of non-heme ferric iron and ferrous iron in mouse ovary. Light and electron microscopic studies revealed that non-heme ferric iron is distributed predominantly in stromal tissue, especially in macrophages. By contrast, the distribution of non-heme ferrous iron was restricted to a few ovoid macrophages. Aged ovaries exhibited remarkable non-heme iron accumulation in all stromal cells. In particular, non-heme ferrous iron level was increased in stromal tissue, suggestive of increased levels of redox-active iron, which can promote oxidative stress. Moreover, intense localization of both non-heme ferric and ferrous iron was observed in aggregated large stromal cells that were then characterized as ceroid-laden enlarged macrophages with frothy cytoplasm. Intraperitoneal iron overload in adult mice resulted in non-heme iron deposition in the entire stroma and generation of enlarged macrophages, suggesting that excessive iron accumulation induced macrophage morphological changes. The data indicated that non-heme iron accumulation in ovarian stromal tissue may be related to aging of the ovary due to increasing oxidative stress.
Keywords: non-heme iron, ovary, aging, macrophage, sensitive non-heme iron histochemistry
In the ovary, continuous growth and differentiation of cells occur during reproductive age of mammals because of folliculogenesis and corpus luteum formation. The requirement of iron is expected to give the enormous increase in mass of oocyte and its associated granulosa cells (Briggs et al. 1999). The presence of transferrin receptors on granulosa cells (Aleshire et al. 1989) and transferrin production by granulosa cells (Briggs et al. 1999) are evidence of the demand for iron for folliculogenesis. Simultaneously, cellular degeneration occurs in follicular atresia and luteal regression due to apoptosis (Shikone et al. 1996; Kaipia and Hsueh 1997; McCormack et al. 1998; Harada et al. 2004; Luz et al. 2006). Along these processes, the expression of heme oxygenase–1 (HO-1), an enzyme-catalyzing heme degradation, was observed in apoptotic granulosa cells in atretic follicles (Harada et al. 2004); thus, the release of iron ions from heme structures can occur. Free iron ions, particularly ferrous iron ions, can catalyze the generation of toxic free radicals such as hydroxyl radicals through the Haber-Weiss reaction (Halliwell and Gutteridge 1990, 2007; Crichton et al. 2002). To protect the tissue from oxidative damage, free iron ions would be sequestrated as stable forms (i.e., associated with ferritin or transferrin) and then processed to be stored in ovarian tissue or excreted from the ovary. These processes continuously occur during the reproductive period of mammals and, therefore, non-heme iron accumulation in ovarian tissue as aging can be considered.
Abnormal iron deposition in several organs, including the heart, liver, and pancreas, results in oxidative tissue injury and fibrosis (Ramm and Ruddell 2010). Iron accumulation during aging is due to neuropathogenesis of the brain in association with reactive oxygen species (ROS) and oxidative stress (Moos 2002; Schipper 2004). The accumulation of damage exerted by increased levels of ROS is believed to be involved in ovarian aging (Tarin 1995, 1996). Furthermore, it has been proposed that ROS play a role in the decline of ovarian function due to the aging of oocytes or reduced ability of granulosa cells (Agarwal et al. 2005; Yin and Chen 2005; Tatone et al. 2008). Although the mechanisms of ovarian functional decline due to ROS have been suggested by a decrease in enzymatic or nonenzymatic antioxidant defense, the details of the mechanisms are unclear (Tatone et al. 2008). In this study, we focused on non-heme iron accumulation in ovarian tissue as a factor potentially associated with several aspects of ovarian aging and functional decline.
Currently, knowledge of iron distribution and accumulation in the ovary is restricted. In addition, there are no reports regarding changes in non-heme iron distribution during aging and its relevance to oxidative damage and the decline of ovarian function in aged ovaries. The aim of this study was to provide the basic knowledge of non-heme iron distribution in ovarian tissue and its transition during aging. We visualized non-heme ferric iron (NHF[III]) and non-heme ferrous iron (NHF[II]) in ovarian tissue from reproductive adult mice using sensitive non-heme iron histochemistry for light microscopic and electron microscopic analyses (Asano et al. 2006; Meguro et al. 2007). We also reported here the transition of NHF[III] and NHF[II] distribution in mouse ovarian tissue during aging in association with changes in ovarian stromal structure. Moreover, intraperitoneal iron overload was performed to evaluate acute iron accumulation in mouse ovarian tissue.
Materials and Methods
Animals
Female C57B/6J mice were used for all experiments. All animal experiments in this study were approved by the Animal Research Committee at Hirosaki University and conducted according to the Guidelines for Animal Experimentation, Hirosaki University. The mice were maintained under controlled light (12-h light:dark cycle) and temperature (21C). In this study, the ages of each animal group were considered according to murine average reproductive life of 2 to 12 months of age (Rugh 1968): Mice at 4 to 7 weeks of age represented prereproductive age (termed immature), mice at 10 to 14 weeks of age represented the adult reproductive age (termed adult), mice at 34 weeks of age were considered middle-aged between adult and old ages (termed middle-aged), and mice at 46 to 52 weeks of age represented the late-reproductive age (termed aged). For quantitative analysis of non-heme iron in ovarian tissue, mice at 7 weeks of age (immature), at 13 weeks of age (adult), and at 46 and 63 weeks of age (aged, including late- and postreproductive age) were used for the experiment.
Sensitive Non-heme Iron Histochemistry for Light Microscopy and Electron Microscopy
Sensitive non-heme iron histochemistry—namely, the perfusion-Perls method (PPIrH) and perfusion Turnbull method (PTIrH), both of which were supplemented by 3,3′-diaminobenzidine·4HCl (DAB) intensification—was performed according to previously published methods (Asano et al. 2006; Meguro et al. 2007) with some modifications for optimization in mouse ovarian tissue. NHF[III] and NHF[II] were stained by PPIrH and PTIrH, respectively. The animals were anesthetized with intraperitoneal pentobarbital sodium (50 mg/kg) and transcardially perfused with 0.9% sodium chloride containing 5 U/ml heparin to flush out the blood and 25 ml of 4% paraformaldehyde (PFA) and 1% glutaraldehyde (GA) in phosphate-buffered saline (PBS); (pH 7.4) as a fixative. Then, non-heme iron staining solution was perfused as follows. For PPIrH, 200 ml of staining solution containing 1% potassium ferrocyanide, 4% PFA, 0.25% GA, and 0.9% sodium chloride in distilled water (pH 1.0) was perfused. For PTIrH, the staining solution had the same components as that for PPIrH excluding the substitution of 1% potassium ferricyanide for potassium ferrocyanide. Next, ovaries were extracted and processed for paraffin embedding. The sections were cut at a thickness of 5 µm. To visualize the staining, DAB intensification was performed. After deparaffinization, the sections were treated with PBS containing 3% hydrogen peroxide for 30 min to inhibit peroxidase and catalase activities and eliminate pseudoperoxidase activity in the erythrocytes. After washing with PBS (pH 7.4), the sections were incubated in 0.025% DAB (Sigma Aldrich, St. Louis, MO) and 0.005% hydrogen peroxide in PBS for 30 min at room temperature. Thereafter, the sections were counterstained with hematoxylin, dehydrated, and cover-slipped. Customary non-heme iron staining for NHF[III] was performed according to the Perls method supplemented by DAB intensification for normally fixed tissue sections (Nguyen-Legros 1980). For iron histochemistries in light microscopy, at least four female mice in each age group were used, with the representative data shown.
For electron microscopy, PPIrH and PTIrH were performed according to the methods for light microscopy, and then ovaries were extracted and incubated in 10% gelatin in 0.1 M phosphate buffer (pH 7.4) at 60C for 4 hr with gentle mixing and embedded in gelatin at 4C for 1 hr. The gelatin blocks containing ovaries were fixed with 4% PFA in 0.1 M phosphate buffer (pH 7.4) at 4C overnight and sliced at 50-µm thickness on a microslicer; then, the intensification of non-heme iron staining was performed in free-floating slices. Sections were treated with PBS containing 3% hydrogen peroxide and 0.01 M sodium azide for 30 min at room temperature. After washing with PBS, sections were incubated in 0.025% DAB, 0.04% ammonium nickel sulfate hexahydrate, and 0.005% hydrogen peroxide in PBS for 30 min at 25C to develop a black color with high electron density and washed with distilled water. Then, the sections were embedded in Epon 812. The ultra-thin sections (70 nm thick) were prepared and observed under an electron microscope (JEM1250; JEOL, Tokyo, Japan). Electron staining was omitted to observe the staining of NHF[III] or NHF[II].
Quantitative Analysis of Non-heme Iron in Ovarian Tissue
The mice were killed by cervical dislocation, and the tissues, including the ovaries, were immediately extracted. The ovaries were isolated from the adipose tissue, uterus, and oviduct under stereoscopic microscopy and stocked at −80C. The analysis of total non-heme iron in ovarian tissue was performed according to the method of Rebouche et al. (2004). Data were expressed as means ± standard deviations, and Student’s t-test was used to determine the significance of the differences between ages.
Immunohistochemistry
Mice were fixed by transcardiac perfusion of 4% PFA in 0.1 M phosphate buffer (pH 7.4), followed by paraffin embedding of ovaries and preparation of 5-µm-thick sections. After deparaffinization, antigen retrieval was performed by twice boiling the sections on glass slides in 10 mM citric acid (pH 6.0) using a microwave oven for 5 min each. Then the sections were treated with 3% goat normal serum (Wako Pure Chemical Industries, Ltd.; Osaka, Japan), 0.05% Tween 20 in 0.1 M phosphate buffer (pH 7.4) for 1 hr at room temperature, and rat anti-F4/80 monoclonal antibody (BMA Biomedicals AG; Rheinstrasse, Switzerland) to denote mouse macrophages (Hume et al. 1984), rabbit anticonjugated malondialdehyde (MDA) polyclonal antibody (Advanced Targeting Systems; San Diego, CA) to monitor lipid peroxidation (Dei et al. 2002), or rabbit antiferritin light chain polyclonal antibody (Wako Pure Chemical Industries, Ltd.) overnight at 4C. The sections were washed with 0.05% Tween 20 in PBS (PBST) and treated with PBS containing 3% hydrogen peroxide for 30 min to inhibit endogenous peroxidase activity. After washing, the sections were incubated with biotin-conjugated goat anti-rat IgG or biotin-conjugated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) as the secondary antibody for 1 hr at room temperature. Detection of bound antibodies was performed using an ABC staining kit (Vector Laboratories) according to the manufacturer’s protocol. Then the sections were counterstained with hematoxylin, dehydrated, and cover-slipped.
Ceroid Deposition
Ceroid deposits on ovarian tissue were examined by either lipid staining with Sudan III or direct visualization of autofluorescence (Lee et al. 1998). The paraffin sections of ovaries were prepared according to the method described previously. For Sudan III staining, deparaffinized sections were soaked in 50% ethanol for 1 min and then stained with 1% Sudan III in 70% ethanol for 1 hr at 37C. The sections were washed in 50% ethanol and distilled water, counterstained with hematoxylin, and cover-slipped with 50% glycerol in 0.01 M phosphate buffer (pH 7.4). The autofluorescence of ceroid on deparaffinized ovarian tissue was observed under a fluorescent microscope BX50 (Olympus, Tokyo, Japan) by using a 480-nm filter.
Iron Overload
Adult mice (10–14 weeks of age) were used for iron overload experiments. Iron-dextran (Sigma Aldrich) in PBS was intraperitoneally injected (100 mg/kg/d) for 16 days. Then, a customary Perls method, PPIrH or PTIrH, was performed for ovarian tissue.
Results
Non-heme Iron Distribution in Ovarian Tissue
The staining of adult mouse ovarian tissue by the customary Perls method, PPIrH and PTIrH, was observed under light microscope. The results are shown in Table 1. PPIrH clearly visualized NHF[III] distribution in ovarian tissue (Table 1, Fig. 1A–C). Intense staining was observed in stroma, especially in macrophages with an ovoid shape and vacuoles in the cytoplasm, which were characterized as “ovoid macrophages” (Fig. 1C, arrows), and thin and branch-shaped macrophages, which were characterized as “thin-branched macrophages” (Fig. 1C, arrowheads). These macrophages were further identified by immunohistochemistry against F4/80 as a mouse macrophage marker (data not shown). The staining of macrophages was observed also in theca externa (Table 1) and atretic follicles (Fig. 1B, inset). Intense staining was also observed in lutein cells in degenerating corpora lutea (Table 1). In contrast, customary Perls method visualized NHF[III] only in a few structures, including stromal ovoid macrophages (Table 1).
Table 1.
Distribution of Non-heme Iron Staining in Ovarian Structures
| Immature |
Adult |
Aged |
Iron Overload |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPIrH |
PTIrH |
Perls |
PPIrH |
PTIrH |
Perls |
PPIrH |
PTIrH |
Perls |
PPIrH |
PTIrH |
|
| Anatomic Region | NHF[III] | NHF[II] | NHF[III] | NHF[III] | NHF[II] | NHF[III] | NHF[III] | NHF[II] | NHF[III] | NHF[III] | NHF[II] |
| Tunica albuginea | − | − | − | − | − | − | − | − | + | + | − |
| Primordial and secondary follicle | − | − | − | − | − | − | − | − | − | − | − |
| Graafian follicle | |||||||||||
| Oocyte | − | − | − | ± | − | − | ± | − | − | ± | − |
| Granulosa cell | ± | − | − | ± | − | − | ± | − | − | ± | − |
| Folliclar fluid | ± | − | − | ± | − | − | ± | − | − | ± | − |
| Theca interna | − | − | − | ± | − | − | ± | − | − | ± | − |
| Theca externa | − | − | − | ± | − | − | ± | − | + | ++ | − |
| Macrophage in theca | − | − | + | + | + | + | ++ | + | + | ++ | + |
| Atretic follicle | N | N | ± | ++ | + | ± | ++ | ++ | ++ | ++ | ++ |
| Corpus luteum | |||||||||||
| Lutein cell (active) | N | N | − | ± | − | − | ± | − | − | − | − |
| Lutein cell (degenerating) | N | N | ± | ++ | − | − | ++ | − | ± | ± | − |
| Stroma | |||||||||||
| Macrophage (thin-branched) | ± | − | ± | ++ | − | ± | ++ | + | +++ | +++ | ± |
| Macrophage (ovoid) | − | − | + | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ |
| Macrophage (enlarged) | N | N | N | N | N | + | +++ | +++ | +++ | +++ | ++ |
| Fibroblast | − | − | − | ± | − | − | + | + | + | + | − |
| Interstitial gland cell | N | N | − | ± | − | − | + | + | + | + | − |
| Blood vessels | − | − | − | − | ± | − | − | ± | + | + | ± |
The results of non-heme iron staining for immature, adult, aged, and iron-overloaded mice are summarized. Perls, customary Perls method for staining of NHF[III]; PPIrH, perfusion-Perls method for staining of NHF[III]; PTIrH, perfusion-Turnbull method for staining of NHF[II]. N = absence of the structure or cell in the indicated mouse; – = absence of staining; ± = faint positive staining; + = positive staining; ++ = strong positive staining; +++ = extremely strong positive staining.
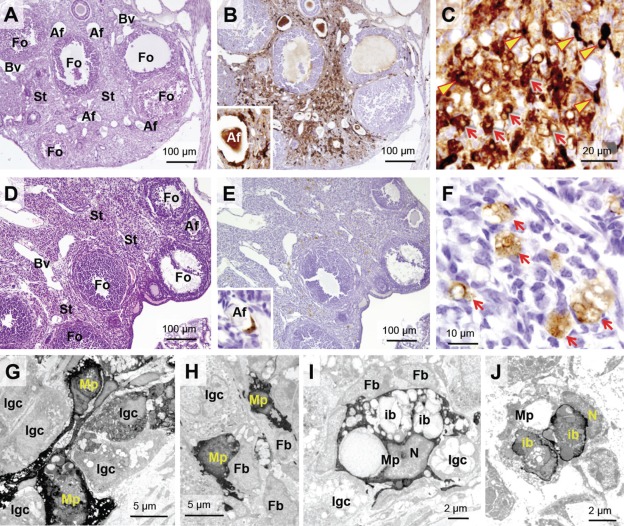
Figure 1.
Distribution of NHF[III] and NHF[II] in ovarian tissue of adult mice visualized by sensitive non-heme iron histochemistries. (A–C) Visualization of NHF[III] in adult mice (14 weeks old) by perfusion-Perls method (PPIrH) under light microscope. (A) PPIrH and hematoxylin and eosin (HE) staining. (B, C) PPIrH and 3,3′-diaminobenzidine·4HCl (DAB) intensification. (A, B) NHF[III]-positive staining (brown color) was mainly observed in stroma (St). Around atretic follicles (Af), intense staining was observed by localization of macrophages with NHF[III] positive (B, inset). Granulosa cells of growing follicles (Fo) were negative or palely positive. Follicular fluid was weakly stained. (C) Macrophages with thin-branched shape (thin-branched macrophages) were densely stained (arrowheads). The other stained cells were macrophages with an ovoid shape or interstitial gland cells (arrows). (D–F) Visualization of NHF[II] in adult mice (14 weeks old) by perfusion Turnbull method (PTIrH). (D) PTIrH and HE staining. (E, F) PTIrH and DAB intensification. NHF[II]-positive staining (brown color) was observed in only a few cells in the stroma and atretic follicles (inset). No staining was observed in follicles. (F) Macrophages with ovoid shape and vacuoles (ovoid macrophages) were stained (arrows). (G–I) Electron microscopy of ovarian stroma stained using PPIrH. The staining of NHF[III] (high electron density) was strongly observed in thin-branched macrophages (G and H, Mp). Some of interstitial gland cells were slightly stained (G and H, Igc). Fibroblasts were negative (H and I, Fb). (I) Ovoid macrophages (Mp) were densely stained by PPIrH. The staining was observed in the cytosol, rather than inclusion bodies (ib) or lipid droplets. (J) Electron microscopy of stromal ovoid macrophages (Mp) stained by PTIrH. Intense staining of NHF[II] with high electron density was observed at peripheral parts of inclusion bodies (ib). N, nucleus; Bv, blood vessels. For sensitive non-heme iron histochemistries in light microscopy, at least four female mice in each age group were used, with the representative data shown.
Electron microscopy of ovarian stroma stained by PPIrH revealed intense staining in macrophages. The thin-blanched macrophages had projections and a few inclusion bodies in cytoplasm (Fig. 1G,H). The ovoid macrophages had abundant lipid droplets and polymorphic inclusion bodies in the cytoplasm (Fig. 1I). Dense staining of NHF[III] was mainly observed in the cytosol of both macrophages (Fig. 1G–I, Mp). Regarding other types of stromal cells, little or no staining was observed. Some interstitial gland cells were weakly stained in the cytoplasm (Fig. 1G–I, Igc), whereas fibroblasts did not exhibit NHF[III] staining (Fig. 1H,I, Fb).
In growing follicles, granulosa cells, oocytes, and follicular fluid were NHF[III] negative or only palely stained (Table 1, Fig. 1B), and theca externa and theca interna did not exhibit staining excluding intermediating macrophages between the theca cells (Table 1). The NHF[III]-positive macrophages surrounded atretic follicles (Fig. 1B, inset). In corpora lutea, active lutein cells were NHF[III] negative. In contrast, lutein cells in degenerating corpora lutea exhibited strongly positive staining, suggesting the release of non-heme iron due to apoptosis (Table 1).
NHF[II], as visualized by PTIrH, was distributed only in ovoid macrophages in the stroma (Table 1, Fig. 1D–F) and atretic follicles (Fig. 1E, inset). In electron microscopy, intense staining was observed in peripheral parts of polymorphic inclusions in ovoid macrophages (Fig. 1J, ib).
Changes in Non-heme Iron Distribution in Ovarian Tissue during Aging
The distribution of non-heme iron in the ovarian tissue of immature (prereproductive age) mice, adult (reproductive age) mice, and aged (late-reproductive age) mice was compared. The results are shown in Table 1 and Figure 2. Ovaries from immature mice had only a few stromal masses (Fig. 2A,F), and no intense staining by PPIrH was observed in any structures (Table 1, Fig. 2A), although granulosa cells in grown follicles were faintly stained (Fig. 2A, Fo). By contrast, extremely intense staining of NHF[III] was observed in the expanded stroma of ovarian tissue from aged mice (Fig. 2C,E) compared to the staining in ovaries from adult mice (Fig. 2B,D). In observations of the stroma, macrophages, interstitial gland cells, and fibroblasts, but not blood vessels, were NHF[III] positive (Fig. 2E). Moreover, stromal cells with large round cell bodies with dense staining were observed in aged ovaries (Fig. 2E, asterisks). No increase of NHF[III] was observed in growing follicles (excluding macrophages in the theca) and active lutein cells (Table 1, Fig. 2C, Fo) compared to the findings in ovaries from adult mice (Fig. 2B).
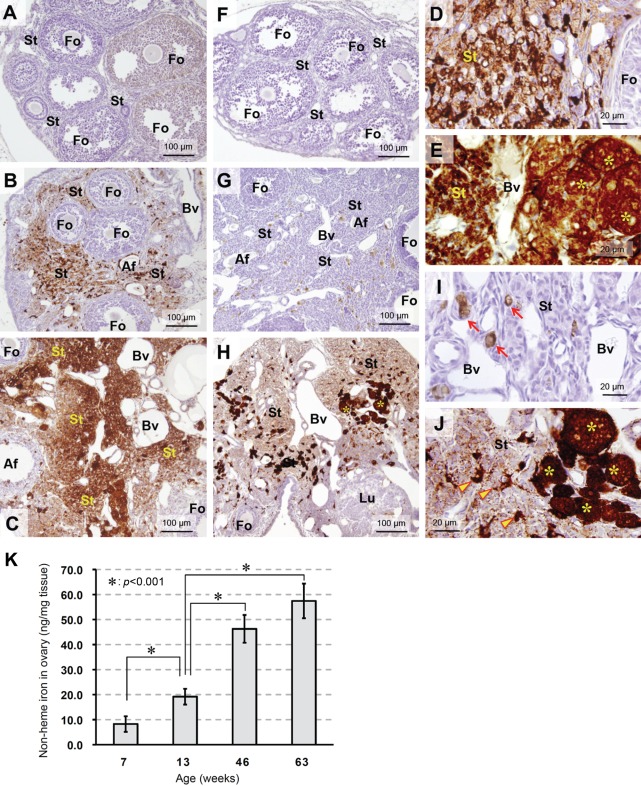
Figure 2.
Change of NHF[III] and NHF[II] distribution in ovarian tissue as aging. (A–C) Change of NHF[III] distribution as aging visualized by perfusion-Perls method (PPIrH) and 3,3′-diaminobenzidine·4HCl (DAB) intensification. (F–H) Change of NHF[II] distribution as aging visualized by perfusion Turnbull method (PTIrH) and DAB intensification. (A, F) Ovary from immature mouse (4 weeks old). (A) No staining of NHF[III] was found in the stroma (St). In some follicles (Fo), weak staining was observed in granulosa cells. (F) No staining of NHF[II] was found. (B, G) Ovary from adult mouse (14 weeks old). In the expanded stroma, distribution of NHF[III] and NHF[II] was observed as in Figure 1B,E. (C, H) Ovary from aged mouse (52 weeks old). (C) Stroma (St) further expanded with aging. The density of NHF[III] staining was higher than that of adult ovary, suggesting accumulation of non-heme iron. Growing follicles did not show increase of staining compared to adult ovary. Af, atretic follicle; Bv, blood vessels. (H) Increase of NHF[II] staining was observed in entire of stroma (St), including enlarged macrophages with extremely dense staining (asterisks). Growing follicles (Fo) and corpora lutea (Lu) were not stained. (D, E) Ovarian stroma of adult mouse (D) and aged mouse (E) stained by PPIrH. In aged mouse (E), the staining was intense and homologous among stromal cells except for blood vessels. Clusters of large round cells, characterized as macrophages by F4/80 immunostaining (Fig. 3), were intensely stained (asterisks). (I, J) Ovarian stroma of adult mouse (I) and aged mouse (J) stained by PTIrH. In the aged ovary (J), NHF[II] staining was increased over the stroma, compared to adult ovary (I). Clusters of large and round macrophages were intensely stained (asterisks). Also, positively stained thin-branched macrophages increased (arrowheads). (K) Quantitative analysis of non-heme iron in ovarian tissue from immature mice (7 weeks old), adult mice (13 weeks old), and aged mice (46 and 63 weeks old). Results are mean ± SD (n=4). The amount of non-heme iron in ovarian tissue clearly increased with age (p<0.001 in 13 weeks old vs 7, 46, or 63 weeks old).
Regarding the distribution of NHF[II] as visualized by PTIrH, immature ovaries were not stained (Fig. 2F). In aged ovaries, increased staining was observed in stroma (Fig. 2H,J) compared to that in adult mice (Fig. 2G,I). Interestingly, intense NHF[II] staining was detected in stromal large round cells (Fig. 2J, asterisks). In other parts of the stroma, the numbers of positively stained thin-branched macrophages increased (Fig. 2J, arrowheads). Moreover, sparse staining was observed in the entire stroma (Fig. 2H,J), suggestive of increased NHF[II] levels in the stroma of aged ovaries. No increase in NHF[II] levels was observed in growing follicles and active lutein cells (Table 1, Fig. 2H, Fo) compared to the findings in ovaries from adult mice (Fig. 2G). In electron microscopic observation of aged ovaries, stained using PPIrH or PTIrH, an increase of thin-branched macrophages and enlarged macrophages with intense staining was observed. Furthermore, positively stained granular structures in the cytoplasm of fibroblasts and interstitial gland cells or intercellular spaces were also detected (data not shown).
The increase in non-heme iron levels in ovarian tissue during aging was also confirmed by quantitative analysis using a biochemical technique (Fig. 2K). In prereproductive immature age (7 weeks old) and reproductive adult age (13 weeks old), the amount of non-heme iron in ovarian tissue was approximately 10 ng/mg and 20 ng/mg, respectively. At 46 weeks of age in the late-reproductive age, the amount of non-heme iron was approximately 46 ng/mg, which is almost 2.5-fold higher than the levels at 13 weeks of age. Further high level was also detected at postreproductive age (63 weeks old).
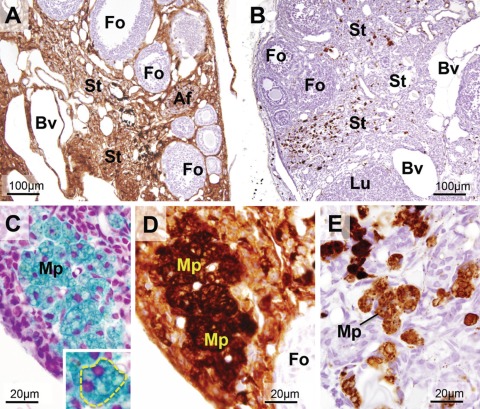
Characterization of Stromal Large Round Cells in Aged Ovaries
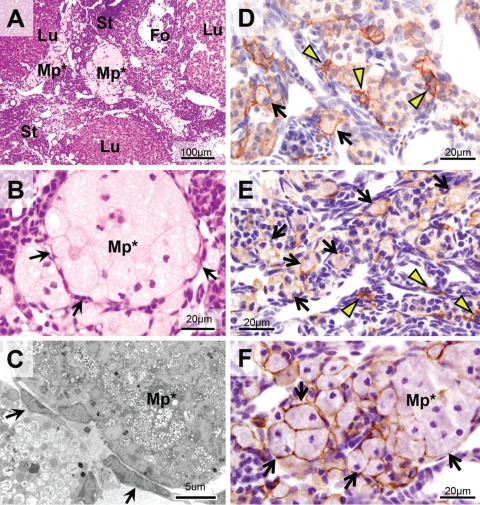
The large stromal cells with dense non-heme iron staining, observed in aged mouse ovaries, were further characterized. Upon hematoxylin and eosin staining, these large cells with multiple nuclei were palely stained by eosin. Their size was maximally 60 µm in diameter. The cells were surrounded by thin stromal cells that are considered mechanically compressed fibroblasts (Fig. 3B,C, arrows). The cytoplasm of these cells was filled with frothy vacuoles (Fig. 3B, Mp*). As shown in Figure 3F (Mp* and arrows), the surface of these cells was positively stained by the anti-F4/80 antibody, which denotes mouse macrophages (Hume et al. 1984). Therefore, these cells were characterized as enlarged macrophages. In customary electron microscopy, the cytoplasm of these cells was filled with lipid droplets, polymorphic inclusion bodies, and electron-dense lysosomes (Fig. 3C). The characteristics of these cells were similar to those of macrophage foam cells (Lee et al. 1998). The ovoid macrophages in ovarian stroma from adult and middle-aged mice were similarly stained by the anti-F4/80 antibody as enlarged macrophages (Fig. 3D,E, arrows). In middle-aged ovaries, increases in the number of ovoid macrophages were observed (Fig. 3E) compared the findings in adult ovaries (Fig. 3D). These results strongly suggest that the enlarged macrophages in aged ovaries exhibiting frothy cytoplasm and multiple nuclei are derived from ovoid macrophages in younger ovaries.
Figure 3.
Enlarged macrophages in ovarian stroma of aged mice. (A, B) Hematoxylin and eosin (HE) staining. Enlarged macrophages (Mp*) located in stroma as clusters. Their cytoplasm filled with vacuoles was palely stained with eosin. Multiple nuclei and incomplete cellular borders were observed, suggesting fusion of the cells. The clusters were surrounded by thin and flat cells (arrows). (C) Electron microscopy of enlarged macrophages (Mp*) with customary electron staining. Cytoplasm was filled with polymorphic inclusion bodies and lipid droplets. Granules with high electron density, probably lysosomes, were also observed. The flat cells surrounding enlarged macrophages were considered mechanically pressured fibroblasts according to their morphological characteristics. (D–F) Ovarian stroma stained with F4/80 immunostaining as a marker for mouse macrophages. (D) Ovary of an adult mouse (14 weeks old). The staining was observed at the surface of thin-branched macrophages (arrowheads) and ovoid macrophages (arrows). (E) Ovary of a middle-aged mouse (34 weeks old). The number of ovoid macrophages with F4/80 immunostaining was increased in stroma (arrows). (F) Ovary of an aged mouse (52 weeks old). The immunostaining was observed at the periphery of enlarged macrophages (arrows). These observations suggest that the enlarged macrophages are derived from ovoid macrophages in the stroma of adult and middle-aged ovaries. St, stroma; Fo, follicles; Lu, corpus luteum.
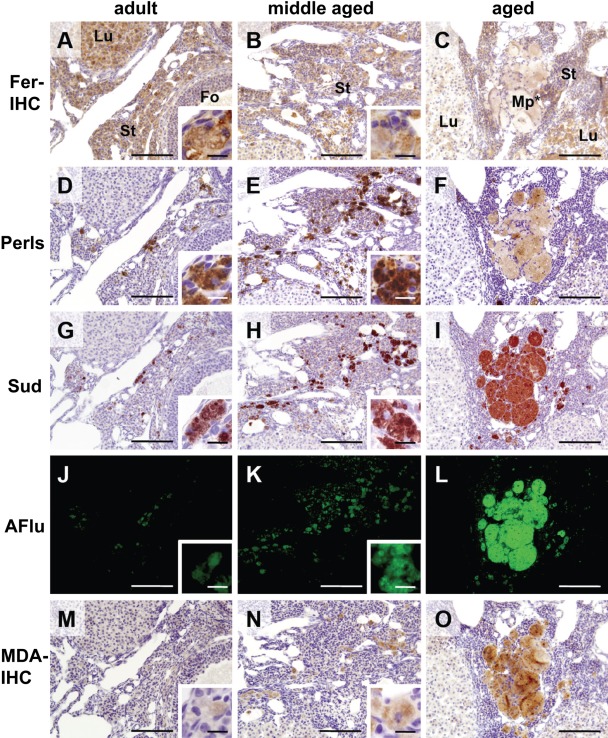
The characteristics of macrophage foam cells were further elucidated by Sudan III staining for lipids (Fig. 4G–H) and autofluorescence observation (Fig. 4J–L). Both methods demonstrate localization of the ceroid, a yellowish and fluorescent substance of aggregated polymers that is derived from the oxidation products of proteins and lipids (Lee et al. 1998; Sitte et al. 2000). The ovoid macrophages in stroma of adult and middle-aged ovaries, but not in thin-branched macrophages, were clearly stained by Sudan III (Fig. 4G,H, insets) and were autofluorescent (Fig. 4J,K, insets). In the enlarged macrophages of aged ovaries, strong and specific staining by Sudan III and strong autofluorescence were also observed (Fig. 4I).
Figure 4.
Co-localization of iron and ceroid in ovoid macrophages and enlarged macrophages. Serial sections of ovaries from adult mouse (14 weeks old, left column), middle-aged mouse (34 weeks old, center column), and aged mouse (52 weeks old, right column) were stained by ferritin immunohistochemistry (Fer-IHC, A–C), customary Perls method (Perls, D–F), Sudan III staining (Sud, G–I), and malondialdehyde immunohistochemistry (MDA-IHC, M–O). Autofluorescence was also observed (AFlu, J–L). Ferritin immunohistochemistry demonstrated the staining in almost every part of ovarian tissue of adult (A) and middle-aged (B) ovary. Ovoid macrophages were also positively stained (insets of A and B), although only pale staining was observed in enlarged macrophages in aged ovary (C, Mp*). Customary Perls method to adjoining sections showed intense staining of NHF[III] in ovoid macrophages in adult and middle-aged ovary (D and E, insets). In aged ovary, clusters of enlarged macrophages were weakly stained (F). Note that these cells were densely stained by perfusion-Perls method (PPIrH) and perfusion Turnbull method (PTIrH) (Fig. 2E,J). On the other hand, ovoid macrophages in adult (G and J) and middle-aged (H and K) ovary and enlarged macrophages in aged ovary (I and L) were intensely stained by Sudan III and emitted strong autofluorescence by 480 nm excitation light, demonstrating presence of ceroid. MDA immunohistochemistry also showed the staining of ovoid macrophages in adult ovary (M, inset), middle-aged ovary (N, inset), and enlarged macrophages in aged ovary (O), with gradual increase of staining intensity with age, suggesting accumulation of oxidative stress. Fo, follicle; Lu, corpus luteum; St, stroma. Scale bar: 100 µm (inset: 10 µm).
Co-localization of non-heme iron and ferritin was also examined by the customary Perls method and ferritin immunohistochemistry. In cells with positive Sudan III staining and autofluorescence, the presence of non-heme iron was found (Fig. 4D–F). Although ovoid macrophages in adult and middle-aged ovary were densely stained (Fig. 4D,E, insets), enlarged macrophages in aged ovaries were moderately and heterogeneously stained (Fig. 4F). Corresponding to this finding, the immunoreaction of ferritin light chain was also intense in ovoid macrophages in adult and middle-aged ovaries (Fig. 4A,B, insets) and weak in enlarged macrophages in aged ovaries (Fig. 4C, Mp*). The immunohistochemistry for ferritin heavy chain also showed a similar pattern of staining (data not shown). Furthermore, staining with MDA, a marker of lipid peroxidation and oxidative stress, was performed. The immunoreaction was observed in ovoid macrophages in adult and middle-aged ovaries (Fig. 4M,N, insets) and in enlarged macrophages in aged ovaries (Fig. 4O). The intensity of MDA localization was gradually increasing with aging, suggesting accumulation of oxidative stress in these cells.
Non-heme Iron Accumulation in Ovarian Tissue after Iron Overload
To evaluate non-heme iron accumulation in ovarian tissue after iron overload, ovaries from adult mice that were intraperitoneally injected with iron-dextran were stained for non-heme iron. The results of staining are shown in Table 1 and Figure 5.
Figure 5.
Distribution of NHF[III] and NHF[II] in ovarian tissue of iron-overloaded adult mouse. (A) Distribution of NHF[III] visualized by perfusion-Perls method (PPIrH) and 3,3′-diaminobenzidine·4HCl (DAB) intensification. Intense staining was observed in the entire stroma and theca externa except for growing follicles. (B) Distribution of NHF[II] visualized by perfusion Turnbull method (PTIrH) and DAB intensification. The increased staining was restricted in the stromal ovoid macrophages (brown spots). (C, D) The cluster of enlarged macrophages (Mp) in stroma of iron-overloaded ovary. (C) PPIrH and hematoxylin and eosin (HE) staining. (D) PPIrH and DAB intensification. These enlarged macrophages showed dense staining of NHF[III] (blue color in C and brown color in D), frothy cytoplasm, and multiple nuclei (inset of C; nuclei were stained by hematoxylin). (E) Stroma in iron-overloaded ovary stained by PTIrH with DAB intensification. Enlarged macrophages (Mp) were NHF[II] positive. Fo, follicle; Af, atretic follicles; Lu, corpus luteum; St, stroma; Bv, blood vessels.
By comparing with non-iron-overloaded control mice (Fig. 2B), PPIrH for ovaries from iron overloaded mice showed that growing follicles and active lutein cells were not different from control mice, whereas the entire stroma showed extremely intense staining (Fig. 5A, St). In particular, ovoid and thin-branched macrophages were densely stained, suggesting acute NHF[III] accumulation. In contrast, PTIrH staining in ovarian tissue was similar between iron-overloaded and non-iron-overloaded adult mice, excluding the intensification of staining in ovoid macrophages and a few thin-branched macrophages in stroma, suggesting that the increase in NHF[II] after iron overloading was moderate (Fig. 5B).
Interestingly, a few clusters of enlarged ovoid macrophages with intense NHF[III] staining were found in the stroma of iron-overloaded ovaries (Fig. 5C,D). These macrophages also exhibited frothy cytoplasm and multiple nuclei (Fig. 5C, inset) and positive staining for NHF[II] (Fig. 5E). These characteristics corresponded to those of enlarged macrophages in aged ovaries (Fig. 2H).
Discussion
The present study demonstrated the distribution and age-associated accumulation of NHF[III] and NHF[II] in mouse ovarian tissue. This is the first report describing the histological visualization of non-heme iron in ovarian tissue, which had not been fully observed by customary non-heme iron-staining methods.
Using the customary Perls or Turnbull method supplemented with DAB intensification for tissue section staining, the detection of weakly bound iron is difficult because of a considerable loss of such iron in the process of tissue treatments. Furthermore, the Turnbull method cannot visualize the precise distribution of NHF[II] because of the autoxidation of NHF[II] in the process of tissue treatments. In PPIrH and PTIrH, the NHF[III] and NHF[II], which are ionically bound to and chelated by cytoplasmic organic substances, are released as free iron in the acidic perfusate, after which they immediately react with ferrocyanide or ferricyanide ions in the perfusate to form insoluble precipitates (Asano et al. 2006; Meguro et al. 2007). These methods can prevent the oxidation or reduction of tissue non-heme iron during tissue treatments. However, it should be stressed that there are still some possible redox changes of iron due to the denaturation of iron-reductive or iron-oxidative enzymes and iron-binding or chelating proteins caused by aldehydes before and during the specific staining of non-heme iron (Asano et al. 2006).
In the ovaries of adult mice (10–14 weeks old), NHF[III] was distributed in stromal tissue and localized in macrophages. NHF[III] was particularly localized in macrophages around the rudiments of atretic follicles, suggesting that non-heme iron from apoptotic cells is accumulated. Non-heme iron can be released from heme by heme oxygenases. HO-1 is an inducible isoform that is upregulated by heme, oxidative stress, metal ions, and hormones (Poss and Tonegawa 1997; Chen 2003). In the porcine ovary, strong HO-1 expression has been detected in the granulosa cells of atretic follicles (Harada et al. 2004). We also found intense HO-1 in macrophages surrounding atretic follicles by immunostaining (data not shown), demonstrating the degeneration of incorporated heme and storage in these cells.
In ovarian stroma, thin-branched and ovoid macrophages, two morphologically classified subpopulations, were positive for non-heme iron staining and anti-F4/80 immunohistochemistry. Thin-branched macrophages were densely NHF[III] positive in the cytosol, whereas most of them were NHF[II] negative. These macrophages extended NHF[III]-positive branches between fibroblasts, interstitial gland cells, and blood vessels. On the other hand, ovoid macrophages had frothy cytoplasm in which abundant inclusion bodies and lipid droplets were observed by electron microscopy. This type of macrophage was both NHF[III] and NHF[II] positive. The staining of NHF[II] in ovoid macrophages was observed at inclusion bodies and their peripheral parts. The co-localization of NHF[III] and NHF[II] could indicate the presence of redox-active non-heme iron, which weakly binds to carrier molecules and is easy to be non-enzymatically liberated (Das et al. 1997; Asano et al. 2006). The redox-active iron ion acts as a catalyst in the Haber-Weiss reaction in the presence of superoxide and hydrogen peroxide to generate hydroxyl radicals, which are known to attack a wide range of cellular constituents, including protein, DNA, and membrane lipids to initiate a free radical chain reaction (Crichton et al. 2002; Asano et al. 2006). Thus, the presence of NHF[II] can be considered a risk factor for oxidative damage in the tissue. Our data suggested that the stromal ovoid macrophages have redox-active iron and harbor oxidative stress, which was also confirmed by the presence of ceroid and MDA in Figure 4. The role of thin-branched and ovoid macrophages may be different in modes of iron metabolism, and further investigations are required.
Some interstitial gland cells also exhibited NHF[III] staining as detected by PPIrH. This result suggested that these cells have a moderate capability to incorporate non-heme iron.
In this study, increased levels of NHF[III] and NHF[II] were clearly demonstrated in the ovarian stroma from aged mice around 50 weeks old. Moreover, the increase of non-heme iron levels during aging was confirmed by a quantitative method. This finding correlated with the expansion of stromal tissue during aging, which suggested that the accumulation of non-heme iron occurred along with the repeated processing of apoptotic cells under follicular atresia and luteolysis. In ovaries from aged mice or iron-overloaded mice, NHF[III] staining was detected in all types of stromal cells, including fibroblasts and interstitial gland cells. In contrast, in both aged and iron-overloaded ovaries, the theca interna, granulosa cells, oocytes, and follicular fluid in growing follicles did not exhibit increased NHF[III] staining. Inversion of the accumulated non-heme iron to follicles or active corpora lutea may be strictly regulated; in addition, theca cells may act as a barrier to regulate the influx of stromal substances, including iron, and maintain the homeostasis of follicles.
The increase of NHF[II] in stroma was clearly observed along with aging not only in increasing ovoid and enlarged macrophages but also in fibroblast, interstitial gland cells, and intracellular spaces. These findings strongly suggested that, in a stromal environment surrounding growing follicles, the elevation of oxidative stress occurs in the aged ovary. During aging, non-heme iron may increase excessively beyond the capacity of macrophages, and then it may be incorporated in fibroblasts and interstitial gland cells. This incorporation was confirmed by iron overloading in adult mice as intense accumulations of NHF[III] in the entire stroma. A portion of accumulated NHF[III] may not only stably bind a carrier such as ferritin but also unstably bind other low-molecular-weight carriers such as redox-active iron, which was observed as NHF[II]-positive staining. Age-related alteration of iron metabolism may result in NHF[II] accumulation due to insufficient sequestration of free iron by ferritin. Further molecular studies of iron metabolism in ovarian aging are needed to understand the mechanism of non-heme iron accumulation.
Although the accumulation of ovarian damage induced by increased levels of ROS is believed to be related to aging (Tarin 1995, 1996; Tatone et al. 2008), the mechanism has not been elucidated. In humans, oxidative damage of oocytes and granulosa cells was described in the cohort of primordial follicles in women of advanced age (de Bruin et al. 2004). ROS perturb the microenvironment in and around ova and granulosa cells and decrease the viability of oocytes and embryos (Miyamoto et al. 2010; Van Blerkom et al. 1997; Yang et al. 1998; Schallreuter et al. 1999). By contrast, it has been also demonstrated that ROS, including superoxide and hydrogen peroxide, play critical roles in the regulation of ovarian functions, such as oocyte maturation, folliculogenesis, ovarian steroidogenesis, and luteolysis. Thus, there is a delicate balance between ROS and antioxidant enzymes in the ovarian tissue (Shiotani et al. 1991; Behrman et al. 2001; Sugino et al. 2004; Agarwal et al. 2005; Agarwal et al. 2006; Tatone et al. 2008). The imbalance of ROS and antioxidants has been suggested as a mechanism of ovarian aging, as shown by decreased levels of glutathione and glutathione S-transferase in ovulated oocytes from aged mouse or downregulation of superoxide dismutase (Cu/ZnSOD and MnSOD) and catalase genes in human granulosa cells (Tarin et al. 2004; Tatone et al. 2006; Tatone et al. 2008). Under these conditions, increased redox-active iron in aged ovaries would be a pro-oxidant for the generation of toxic ROS such as hydroxyl radicals, resulting in a loss of the balance between ROS and antioxidants. To prove this hypothesis, oxidative stress level in aged or iron-overloaded ovarian tissue can be further investigated using reliable markers such as protein carbonyls and 8-hydroxydeoxyguanosine in mitochondrial DNA (Chao et al. 2005; Miyamoto et al. 2010; Tarin et al. 2004). Moreover, the levels of antioxidants, including superoxide dismutases, catalase, and glutathione, also can be investigated to assess the pro-oxidative condition.
We found aggregations of enlarged cells with remarkable accumulations of NHF[III] and NHF[II] in the stromal tissue of ovaries from aged mice. These cells featured multiple nuclei and frothy cytoplasm with Sudan III staining and autofluorescence. These stromal enlarged cells obviously immunoreacted with the anti-F4/80 antibody, a common marker of mouse macrophages (Hume et al. 1984). The characteristics of these cells in aged mouse ovaries were shared with foam cells, which are located in atherosclerotic foci. In foam cells, lipid peroxidation, ceroid accumulation, and autofluorescence have been observed (Lee et al. 1998; Shashkin et al. 2005; Levy et al. 2007). Therefore, we identified the enlarged stromal cells in aged ovaries as macrophages with characteristics of foam cells. The co-localization of ceroid and iron also has been detected in atherosclerotic foam cells (Lee et al. 1998; Kockx et al. 2003; Levy et al. 2007). Erythrophagocytosis and iron uptake by macrophages in hemorrhagic microvessels in atherosclerosis result in low-density lipoprotein peroxidation and ceroid formation (Yuan et al. 1996; Lee et al. 1998; Kockx et al. 2003). We hypothesized that ovarian stromal ovoid macrophages might accumulate non-heme iron released from engulfed apoptotic bodies, and then redox-active iron would promote lipid peroxidation and ceroid formation. Under these conditions, the fusion of macrophages as demonstrated by multiple nuclei might occur in a mechanism similar to that of foam cell formation (Shashkin et al. 2005). This hypothesis is supported by the results of the iron overload experiment for adult mouse ovaries that showed generation of enlarged macrophages with frothy cytoplasm and multiple nuclei.
The nature of NHF[III] and NHF[II] in enlarged macrophages is still unclear. The intensity of non-heme iron staining by the customary Perls method and immunostaining of ferritin was moderate in these macrophages compared to the intense staining in ovoid macrophages of adult or middle-aged mice. These findings suggested that, in a low-ferritin background, abundant redox-active non-heme iron localizes in enlarged macrophages. This intracellular environment can promote oxidative stress in these cells, which was demonstrated by high MDA immunoreactivity. The contribution of the highly pro-oxidative environment in foam cells to the development of atherosclerotic lesions, with the co-localization of iron and ceroid, has been discussed (Lee et al. 1998; Kockx et al. 2003; Levy et al. 2007). However, we did not observe significant oxidative effects in the tissue near the aggregations of enlarged macrophages, as there were no increased MDA immunostaining or degenerative changes. These macrophages were surrounded by flattened fibroblasts. This structure may segregate enlarged macrophages exhibiting oxidative characteristics from other ovarian structures.
In summary, we clearly demonstrated the accumulation of non-heme iron in mouse ovarian stromal tissue during aging. In addition, the co-localization of NHF[III] and NHF[II] strongly suggested the elevation of redox-active iron levels in aged ovaries. Moreover, we found stromal foam cell-like enlarged macrophages concomitantly with the co-localization of redox-active iron and ceroid. From the results of the present study, we propose that redox-active iron accumulation may promote oxidative stress in ovarian structures and contribute to the process of ovarian aging and decline of ovarian function. Regarding human ovarian aging, iron accumulation in healthy females has not been investigated. The profile of non-heme iron accumulation as aging and its correlation to decline of human ovarian functions should be further examined. The elucidation of the molecular mechanisms of non-heme iron accumulation in the aging ovary may contribute to the improvement of declined ovarian function in aged females.
Acknowledgments
The author thanks Dr. Kazuhiko Shoumura for establishment of sensitive non-heme iron histochemistries; Dr. Hiroshi Kijima and Dr. Noritaka Ichinohe for useful comments and discussions; and Mr. Takahiro Sakano, Miss Tamaki Fujita, and Mr. Masamichi Nakata for technical supports and discussions.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by a grant from the Karoji Memorial Fund for Medical Research (Grant B to Y. Asano, 2007).
References
- Agarwal A, Gupta S, Sharma RK. 2005. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sikka S. 2006. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 18:325–332 [DOI] [PubMed] [Google Scholar]
- Aleshire SL, Osteen KG, Maxson WS, Entman SS, Bradley CA, Parl FF. 1989. Localization of transferrin and its receptor in ovarian follicular cells: morphologic studies in relation to follicular development. Fertil Steril. 51:444–449 [DOI] [PubMed] [Google Scholar]
- Asano Y, Meguro R, Odagiri S, Li C, Iwatsuki H, Shoumura K. 2006. Visualization of non-heme ferric and ferrous iron by highly sensitive non-heme iron histochemistry in the stress-induced acute gastric lesions in the rat. Histochem Cell Biol. 125:515–525 [DOI] [PubMed] [Google Scholar]
- Behrman HR, Kodaman PH, Preston SL, Gao S. 2001. Oxidative stress and the ovary. J Soc Gynecol Investig. 8(1 Suppl Proceedings):40–42 [DOI] [PubMed] [Google Scholar]
- Briggs DA, Sharp DJ, Miller D, Gosden RG. 1999. Transferrin in the developing ovarian follicle: evidence for de-novo expression by granulosa cells. Mol Hum Reprod. 5:1107–1114 [DOI] [PubMed] [Google Scholar]
- Chao HT, Lee SY, Lee HM, Liao TL, Wei YH, Kao SH. 2005. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Ann N Y Acad Sci. 1042:148–156 [DOI] [PubMed] [Google Scholar]
- Chen YH, Yet SF, Perrella MA. 2003. Role of heme oxygenase-1 in the regulation of blood pressure and cardiac function. Exp Biol Med. 228:447–453 [DOI] [PubMed] [Google Scholar]
- Crichton RR, Wilmet S, Regssyer R, Ward RJ. 2002. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorg Biochem. 91:9–18 [DOI] [PubMed] [Google Scholar]
- Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. 1997. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med. 23:8–18 [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, te Velde ER. 2004. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod. 70:419–424 [DOI] [PubMed] [Google Scholar]
- Dei R, Takeda A, Niwa H, Li M, Nakagomi Y, Watanabe M, Inagaki T, Washimi Y, Yasuda Y, Horie K, et al. 2002. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer’s disease. Acta Neuropathol. 104:113–122 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. 1990. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186:1–85 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. 2007. Free radicals in biology and medicine. New York: Oxford University Press [Google Scholar]
- Harada T, Koi H, Kubota T, Aso T. 2004. Haem oxygenase augments porcine granulosa cell apoptosis in vitro. J Endocrinol. 181:191–205 [DOI] [PubMed] [Google Scholar]
- Hume DA, Halpin D, Charlton H, Gordon S. 1984. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of endocrine organs. Proc Natl Acad Sci U S A. 81:4174–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipia A, Hsueh AJ. 1997. Regulation of ovarian follicle atresia. Annu Rev Physiol. 59:349–363 [DOI] [PubMed] [Google Scholar]
- Kockx MM, Cromheeke KM, Knaapen MW, Bosmans JM, De Meyer GR, Herman AG, Bult H. 2003. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol. 23:440–446 [DOI] [PubMed] [Google Scholar]
- Lee FY, Lee TS, Pan CC, Huang AL, Chau LY. 1998. Colocalization of iron and ceroid in human atherosclerotic lesions. Atherosclerosis. 138:281–288 [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy JE, Kalet-Litman S, Miller-Lotan R, Levy NS, Asaf R, Guetta J, Yang C, Purushothaman KR, Fuster V, et al. 2007. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 27:134–140 [DOI] [PubMed] [Google Scholar]
- Luz MR, Cesário MD, Binelli M, Lopes MD. 2006. Canine corpus luteum regression: apoptosis and caspase-3 activity. Theriogenology. 66:1448–1453 [DOI] [PubMed] [Google Scholar]
- McCormack JT, Friederichs MG, Limback SD, Greenwald GS. 1998. Apoptosis during spontaneous luteolysis in the cyclic golden hamster: biochemical and morphological evidence. Biol Reprod. 58:255–260 [DOI] [PubMed] [Google Scholar]
- Meguro R, Asano Y, Odagiri S, Li C, Iwatsuki H, Shoumura K. 2007. Nonheme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Arch Histol Cytol. 70:1–19 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Sato EF, Kasahara E, Jikumaru M, Hiramoto K, Tabata H, Katsuragi M, Odo S, Utsumi K, Inoue M. 2010. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic Biol Med. 49:674–681 [DOI] [PubMed] [Google Scholar]
- Moos T. 2002. Brain iron homeostasis. Dan Med Bull. 49:279–301 [PubMed] [Google Scholar]
- Nguyen-Legros J, Bizot J, Bolesse M, Pulicani JP. 1980. [‘Diaminobenzidine black’ as a new histochemical demonstration of exogenous iron]. Histochemistry. 66:239–244 French [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. 1997. Reduced stress defence in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 94:10925–10930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm GA, Ruddell RG. 2010. Iron homeostasis, hepatocellular injury, and fibrogenesis in hemochromatosis: the role of inflammation in a noninflammatory liver disease. Semin Liver Dis. 30:271–287 [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Wilcox CL, Widness JA. 2004. Microanalysis of non-heme iron in animal tissues. J Biochem Biophys Methods. 58:239–251 [DOI] [PubMed] [Google Scholar]
- Rugh R. 1968. Reproductive systems of adult mice. In: Rugh R, editor. The mouse: its reproduction and development. Minneapolis: Burgess Publishing; p. 7–43 [Google Scholar]
- Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, Marshall HS, Panske A, Panzig E, Hibberts NA. 1999. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 4:91–96 [DOI] [PubMed] [Google Scholar]
- Schipper HM. 2004. Brain iron deposition and the free radical-mitochondrial theory of aging. Aging Res Rev. 3:265–301 [DOI] [PubMed] [Google Scholar]
- Shashkin P, Dragulev B, Ley K. 2005. Macrophage differentiation to foam cells. Curr Pharm Des. 11:3061–3072 [DOI] [PubMed] [Google Scholar]
- Shikone T, Yamoto M, Kokawa K, Yamashita K, Nishimori K, Nakano R. 1996. Apoptosis of human corpora lutea during cyclic luteal regression and early pregnancy. J Clin Endocrinol Metab. 81:2376–2380 [DOI] [PubMed] [Google Scholar]
- Shiotani M, Noda Y, Narimoto K, Imai K, Mori T, Fujimoto K, Ogawa K. 1991. Immunohistochemical localization of superoxide dismutase in the human ovary. Hum Reprod. 6: 1349–1353 [DOI] [PubMed] [Google Scholar]
- Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von Zglinicki T, Davies KJ. 2000. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 14: 1490–1498 [DOI] [PubMed] [Google Scholar]
- Sugino N, Karube-Harada A, Taketani T, Sakata A, Nakamura Y. 2004. Withdrawal of ovarian steroids stimulates prostaglandin F2alpha production through nuclear factor-kappaB activation via oxygen radicals in human endometrial stromal cells: potential relevance to menstruation. J Reprod Dev. 50:215–225 [DOI] [PubMed] [Google Scholar]
- Tarin JJ. 1995. Aetiology of age-associated aneuploidy: a mechanism based on the ‘free radical theory of aging.’ Hum Reprod. 10:1563–1565 [DOI] [PubMed] [Google Scholar]
- Tarin JJ. 1996. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod. 2:717–724 [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V, Pertusa JF, Hermenegildo C, Cano A. 2004. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Mol Reprod Dev. 69:402–410 [DOI] [PubMed] [Google Scholar]
- Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. 2008. Cellular and molecular aspects of ovarian follicle aging. Hum Reprod Update. 14:131–142 [DOI] [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. 2006. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 12:655–660 [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Antczak M, Schrader R. 1997. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 12:1047–1055 [DOI] [PubMed] [Google Scholar]
- Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. 1998. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 13:998–1002 [DOI] [PubMed] [Google Scholar]
- Yin D, Chen K. 2005. The essential mechanisms of aging: irreparable damage accumulation of biochemical side-reactions. Exp Gerontol. 40:455–465 [DOI] [PubMed] [Google Scholar]
- Yuan XM, Anders WL, Olsson AG, Brunk UT. 1996. Iron in human atheroma and LDL oxidation by macrophages following erythrophagocytosis. Atherosclerosis. 124:61–73 [DOI] [PubMed] [Google Scholar]