Abstract
Several heterocycles such as furanones, pyrrolones, and indolizines, which are of pharmacological importance, are easily accessed via the Pt(II)-catalyzed heterocyclization/1,2-migration of propargylic ketols or hydroxy imine derivatives. This method sidesteps the challenges of traditional heteroaromatic oxygenation strategies such as regioselectivity and functional group tolerance in the syntheses of these heterocycles.
1. Introduction
The development of efficient and versatile strategies for the synthesis of heterocycles continues to be of significance in synthetic organic chemistry. The 3(2H)-furanones are a class of important heterocycles that are found in a variety of biologically active natural products such as the eremantholides1 and jatrophone2 as well as several medicinally active agents.3 Various methods have been developed for the synthesis of these heterocycles, including the conversion of 3-alkoxy-furans to 2-alkoxy furanones4 and the reaction of 3-silyloxyfurans with electrophiles5 such as aldehydes. Most of the current approaches to furanones remain rather specialized or demand considerable investment in the preparation of the requisite precursor substrates.
For example, we have considered a route to 3(2H)-furanones via the oxygenation/rearrangement of furans (e.g., 1, Scheme 1), which may proceed via the intermediacy of epoxide 2. However, this approach possesses several distinct challenges such as (A) the difficulty of synthesis of the requisite furan (i.e., 1), (B) the inherent instability of the furan and other functional groups (e.g., sulfides, aldehydes, etc.) to the oxygenation (epoxidation) conditions, and (C) the regioselectivity of the initial epoxidation.
Scheme 1.
Proposed access to 3(2H)-furanones.
As a result of these challenges, we were encouraged to pursue alternate strategies that would provide ready access to 2 (or an equivalent) in one or two steps en route to an expedient synthesis of 3(2H)-furanones (i.e., 4). In this article, we present our efforts that have led to the realization of 3(2H)-furanone compounds in short order.6 An extension of this transformation to the expedient synthesis of other heterocycles such as pyrrolones, indolizines, and indolizidinones using nitrogen nucleophiles was subsequently developed and communicated by us.7 A comprehensive account of these studies is presented herein.
2. Results and discussion
As illustrated in Scheme 1, it was envisaged that intermediates such as 2 could be readily accessed from propargylic alcohols (e.g., 3) via a carbophilic Lewis acid-mediated cyclization followed by proton transfer. The viability of this transformation is supported by several related literature reports using Ag(I) by Marshall,8 and Au(III) by Liu,9 Larock,10 and Hashmi11 among others. In turn, rearrangement of 2 could yield 3(2H)-furanone 4. Investigations of this transformation began with alkynyl ketone 5 (Scheme 2), which was available in one-step from 3,4-hexanedione using the method of Chisholm.12 Our past success using PtCl2 as a carbophilic Lewis acid to mediate transformations involving alkynes13 made it a starting point of our investigations.
Scheme 2.
A proposed mechanism for the synthesis of 3(2H)-furanones.
Although various Lewis acids (e.g., InCl3, BF3 · OEt2) and Brønsted acids such as HCl were also investigated for the conversion of 5 to 3(2H)-furanone 9, in the end, PtCl2 proved to be the ideal catalyst. This was due in part to the operational simplicity of using the air- and moisture-tolerant Pt(II) salts, as well as the consistency in yield and amenability to scale up when this catalyst was used. The reaction can be run at low catalyst loadings (2 mol % PtCl2), although a longer period of time is required for the reaction to reach completion. However, the use of a 2 mol % PtCl2 loading under an atmosphere of CO, following the precedent of Fürstner,14 leads to comparable reaction rates as to when a 10 mol % loading is used. Furthermore, the reaction gave comparable yields in the presence of 1 equiv of H2O.
As illustrated in Table 1, the reaction is general for a range of substrates. Significant variability can be achieved at the alkyne terminus as alkyl (3a and 3b) and aryl substituents (3c–f) are well tolerated (entry 1). In addition, the formation of spiro-furanone products appears to be general if cyclic substrates are employed (3g–j, entry 2). The migrating group is not restricted to alkyl fragments as phenyl groups migrate just as readily (entry 3). Furthermore, a high level of versatility may be achieved in the furanone product by beginning with an appropriately functionalized propargylic alcohol (3l and 3m, entry 4). Importantly, sensitive functional groups such as sulfides, which will not survive the oxidative transformation of furans to 3(2H)-furanones (i.e., 1 → 3, Scheme 1), remain intact under the PtCl2-catalyzed conditions (see 3j, entry 2).
Table 1.
PtCl2-catalyzed formation of furanone productsa
| Entry | Substrate | Product | Yield (%) |
|---|---|---|---|
| 1 |  |
 |
|
| 65 | |||
| 48 | |||
| 38 | |||
| 56 | |||
| 65 | |||
| 48 | |||
| 2 |  |
 |
|
| 76 | |||
| 66 | |||
| 89 | |||
| 76 | |||
| 3 |  |
 |
77 |
| 4b |  |
 |
60 |
Reaction conditions: substrate (0.1 M of substrate), 5 mol % PtCl2 in PhMe at 100 °C for 8 h.
An inseparable 1:2 mixture (as determined by 1H NMR) of propargylic alcohols 3l and 3m was used for the formation of 4l.
In a subset of the transformations of propargylic alcohols (see 10a and 10b, Scheme 3) studied to date, significant amounts of enedione products (12a and 12b) were obtained in addition to the desired 3(2H)-furanone products.15 This appears to correlate with an increase in the rate of other competing fragmentation processes relative to the migratory aptitude of the alkyl substituent alpha to the tertiary alcohol (e.g., the ethyl group, see 10).
Scheme 3.
Divergent reactivity in the PtCl2-catalyzed transformation.
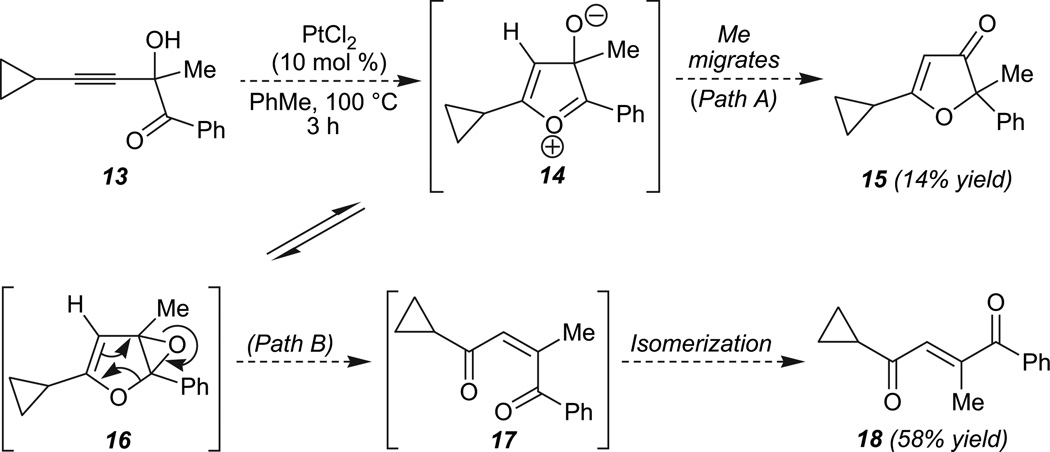
Insight into the mechanism for the formation of the enedione products was gained upon reaction of 13 (Scheme 4). Consistent with our initial proposal, whereas 3(2H)-furanone products such as 15 (14% yield) may arise via a concerted migration16 involving the Me substituent (see 14, Path A), competing epoxidation by collapse of the zwitterionic intermediate 14 may lead to epoxydihydrofuran 16. In turn, 16 may be converted to the observed enedione products via epoxide opening and ring fragmentation (path B).17,18 The major E-alkene diastereomer observed for the enedione product (18, 58% yield)19 presumably arises via Pt(II)-catalyzed or thermal isomerization of 17 following ring opening under the reaction conditions.20
Scheme 4.
A possible rationale for the formation of enediones.
Subsequent to the studies on the formation of 3(2H)-furanones, we have discovered that these novel transformations of tertiary propargylic alcohols are not restricted to cyclizations involving oxygen nucleophiles (i.e., carbonyl groups), as nitrogen nucleophiles readily participate to provide important azaheterocycles. For example, indolizines (e.g., 21, Scheme 5) and related derivatives, which have significant pharmacological potential,21 may be realized if one begins with the pyridinyl propargylic alcohol 19. Because of the importance of indolizines from a pharmacological standpoint, a variety of methods for their syntheses have emerged.22 On the basis of our precedent for the formation of furanones, which was achieved prior to the initiation of our work with nitrogen nucleophiles, we reasoned that substrates such as 19 (Scheme 5), which possess a pyridine fragment, could participate in metal-catalyzed cycloisomerizations to access a range of nitrogen-containing heterocycles (e.g., indolizines related to 21).
Scheme 5.
Proposed hetero-cycloisomerization.
Optimization of the general transformation 19 → 21 was conducted with propargylic ester 22a (Table 2) prepared from pyridine-2-carboxaldehyde by the addition of ethynyl Grignard reagent and subsequent acylation.23 Initially, we found that both PtCl4 (entry 1) and PtCl2 (entry 2) at 5 mol % loading effected the transformation of 22a (0.20 M in PhH, 70 °C) to the desired C-1 substituted indolizine 23a under similar conditions.24 Because PtCl2 is less sensitive to moisture as compared to PtCl4, we continued our studies with this salt, which greatly simplified the experimental setup.
Table 2.
Cycloisomerization of terminal alkyne propargylic ester substrates
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Catalyst | Additive | Temp (°C) |
Time (h) |
Yield of 23 (%) |
| 1 | 22a | PtCl4 | — | 70 | 18 | 34 |
| 2 | 22a | PtCl2 | — | 70 | 20 | 31 |
| 3 | 22a | PtCl2 |  |
70 | 20 | 43 |
| 4 | 22a | PtCl2 | 70 | 8 | 79 | |
| 5 | 22b | PtCl2 | — | 70 | 10 | 81 |
| 6 | 22b | PtCl2 | 24 | 70 | 3 | 95 |
| 7 | 22b | PtCl2 | 25 | 70 | 9 | 95 |
| 8 | 22b | PtCl2 | — | 40 | 36 | No reaction |
| 9 | 22b | PtCl2 | 24 | 40 | 12 | 95 |
| 10 | 22b | PtCl2 | 25 | 40 | 36 | 33 |
| 11 | 22b | InCl3 | — | 40 | 4 | 85 |
Further optimization revealed that the introduction of bulky, electron-rich phosphine ligands such as 2-(di-tert-butyl-phosphino) biphenyl25 (24, entry 3) or 2-(di-cyclohexylphosphino) biphenyl (25, entry 4) led to a pronounced increase in the yield of the indolizine product 23a, with 25 proving to be the best additive of those surveyed (79% yield). Phosphine ligands have been shown to facilitate Pt(II)-catalyzed reactions involving nitrogen nucleophiles in studies by Widenhoefer on the hydroamination of olefins.26 In these studies, Widenhoefer reported that a 1:1 ratio of Pt(II) salt to exogenous phosphine (Pt/PR3) was critical to success.27 We reasoned that the use of bulky phosphines would favor the formation of a 1:1 Pt/PR3 complex. It should be noted that in our studies, these ligands were consistently superior to PPh3 for the formation of indolizines from the corresponding propargylic alcohols.
The reaction efficiency was substantially different when internal alkyne substrates (e.g., 22b) were used (entries 5–11). Similarly in these cases, bulky phosphine additives led to higher yields of the indolizine products (i.e., 23b, entries 6 and 7) as compared to the cases when phosphines were not added (entry 5) or when triphenylphosphine was used as the additive (not shown). For example at 40 °C, there was no reaction with PtCl2 alone as catalyst (entry 8) whereas with 24 and 25 as additives (entries 9 and 10, respectively), product formation was observed, with 24 proving to be superior. Indium trichloride also catalyzes the indolizine-forming reaction (entry 11) but the scope was limited to internal alkyne substrates. We believe that the lack of success of InCl3 in catalyzing the cycloisomerization of terminal alkyne substrates may be the result of the competing formation of indium acetylides under the reaction conditions, consistent with the observations of Shibasaki.28
As shown in Figure 1, a range of indolizines are obtained utilizing either Pt(II) (5 mol % of PtCl2, 10 mol % of 2-(di-tert-butylphosphino)biphenyl (24), 0.2 M in PhH, 70 °C) or In(III) (5 mol % of InCl3, 0.2 M in PhH, 70 °C). The pivalate protective group was found to give the highest yields (see 26–29) compared to other acyl protecting groups (e.g., acetate and benzoate) for a range of alkyl-, cycloalkyl-, aryl-, and alkenyl-substituted indolizines. Silyl protective groups (e.g, TBS) may also be employed, but the products are generally isolated in lower yield (57% yield of 30).29 This is likely due to the relative instability of the corresponding silylated indolizines toward purification.
Figure 1.
Pt(II)- and In(III)-catalyzed cycloisomerizations. Yields are indicated for reactions using PtCl2 and InCl3 (in parentheses). For a full description of reaction details, including the identity of propargylic ester substrates, see Supplementary data.
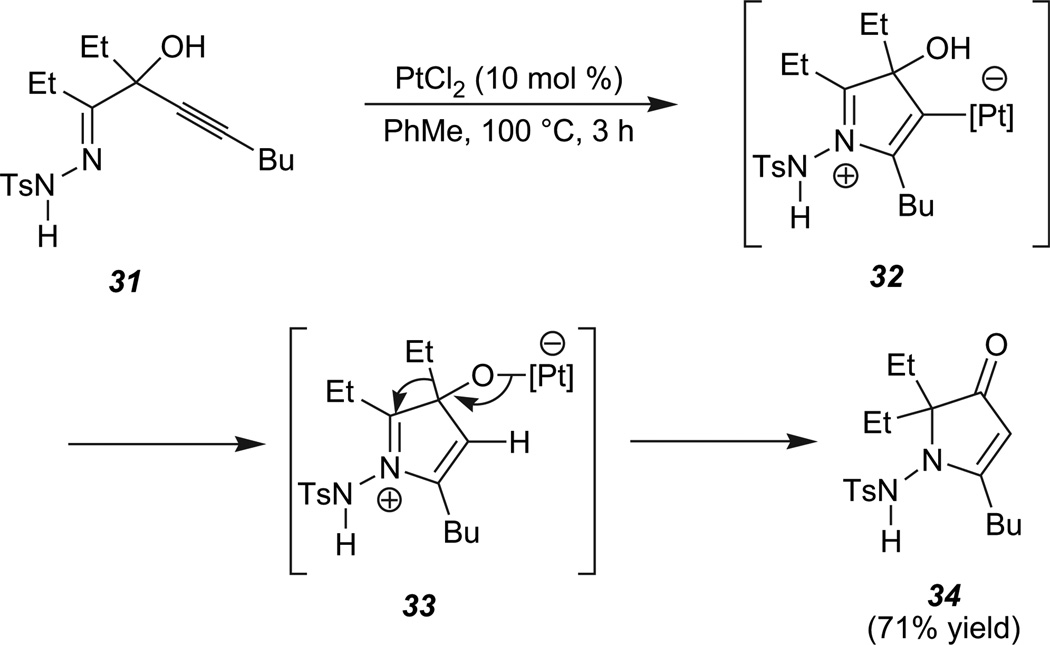
By direct analogy to our observations involving the furanone-forming reactions, we hypothesized that tertiary propargylic alcohol substrates such as 31 (Scheme 6) could provide a platform for heterocyclizations that involve a 1,2-shift.30
Scheme 6.
Tandem cyclization/1,2-migration of 31.
In a preliminary study, upon treatment of hydrazone 31 with PtCl2 (10 mol %) for 3 h at 100 °C, pyrrolone 34 was formed in 71% yield. This transformation likely proceeds via zwitterion 32 and platina-alkoxide 33, in an analogous fashion to the furanones.31
Despite our initial success in transforming 31 to pyrrolone 34, the reaction conditions were ineffective at low catalyst loadings especially for substrates containing a pyridine fragment. Following a comprehensive study of various additives, solvents, and temperatures, optimized conditions (5 mol % PtCl2, 10 mol % 2-(di-tert-butylphosphino)biphenyl, 0.1 equiv Cs2CO3, 100 °C), which were readily applicable to several tertiary propargylic alcohol substrates (35a–e, Table 3) were identified. This provided the corresponding indolizinones (36a–e) in modest to good yields. The addition of substoichiometric quantities of a base (Cs2CO3), which may facilitate proton transfer events prior to the 1,2-migration event, was found to be critical.32 To probe the nature of the 1,2-migrations, enantioenriched 35a (99.9% ee, Eq. 1) was subjected to the optimized reaction conditions and provided 36a in 97% ee, which corroborated a highly stereoselective 1,2-migration.33–35
 |
(1) |
Table 3.
Pt-catalyzed formation of indolizinones
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Time (h) | Product | Yield (%) |
| 1 |  |
 |
||
| 48 | 66 | |||
| 168 | 40 | |||
| 120 | 66 | |||
| 48 | 70a | |||
| 144 | 44 | |||
| 2 |  |
48 |  |
36 |
Cs2CO3 was not used as an additive.
3. Conclusion
In conclusion, we report an expedient synthesis of 3(2H)-furanones from propargylic alcohols that may be accessed in one step from 1,2-diketones using an efficient Rh(I)-catalyzed alkyne addition developed in the laboratories of Chisholm. The reaction tolerates sensitive functional groups such as nitro groups and sulfides, which may be unstable or easily oxidized (sulfides) under traditional protocols for the formation of these heterocycles from the oxygenation of furans or other related heteroaromatic compounds. The overall transformation is not restricted to the synthesis of 3(2H)-furanones as indolizines and indolizinones may also be formed using substrates bearing a nitrogen nucleophile. While PtCl2 alone, as well as in the presence of phosphine additives, has been found to be generally effective, InCl3 has also proven to be a highly competent catalyst for a subset of these transformations involving internal alkyne substrates. The work reported herein presents an observed significant effect of bulky electron-rich phosphines on the hetero-cycloisomerization transformations involving Pt(II) catalysis, which we anticipate will find wide applicability in other cycloisomerization reactions. This work has also described the competing formation of enedione byproducts in the Pt-catalyzed cycloisomerization of keto propargyl alcohols, and a tentative mechanism for the formation of these compounds is proposed. Further studies to probe the mechanisms of these transformations, broaden the scope to include other examples of chirality transfer, and identify conditions to shorten the reaction times are underway. Additionally, applications of these heterocycles in natural product synthesis are currently under investigation and will be reported in due course.
4. Experimental section
4.1. Materials and methods
All air or moisture sensitive reactions were conducted in flame-dried glassware under an atmosphere of nitrogen using dry, deoxygenated solvents. Toluene and benzene were distilled over calcium hydride. Platinum catalysts and phosphine ligands were purchased from Strem or Johnson Matthey. All other reagents were purchased from Aldrich, Acros, or Lancaster and used without further purification. Reaction temperatures were controlled by an IKAmag® or OptiChem® temperature modulator. Thin layer chromatography (TLC) was performed using E. Merck silica gel 60 F254 precoated plates (0.25 mm) and visualized by UV and anisaldehyde stain. Fisher silica gel 240–400 mesh (particle size 0.032–0.063) was used for flash chromatography. Pt-catalyzed reactions were performed in Schlenk flasks unless otherwise noted. 1H and 13C NMR spectra were recorded on a Bruker AV-500 (at 500 MHz and 125 MHz, respectively), Bruker DRX-500 (at 500 MHz and 125 MHz, respectively) or on a Bruker AVB-400 (at 400 MHz and 100 MHz, respectively) in chloroform-d or benzene-d6 at 23 °C, unless otherwise stated. Chemical shifts were referenced to the residual solvent peak, which was set at δ=7.26 for 1H NMR and δ=77.0 for 13C NMR, for CDCl3; and δ=7.15 for 1H NMR and δ=128.6 for 13C NMR, for C6D6. Data for 1H NMR are reported as follows: chemical shifts (δ ppm); multiplicity, (s=singlet, d=doublet, t=triplet, q=quartet, dd=doublet of doublet, dt=doublet of triplet, dq=doublet of quartet, qd=quartet of doublet, m=multiplet, br=broad resonance), coupling constants (Hz), and integration in parentheses. Data for 13C NMR are reported in terms of chemical shift. IR spectra were recorded on a Nicolet MAGNA-IR 850 spectrometer and are reported in frequency of absorption (cm−1). Low and high resolution mass spectral data were obtained from the University of California, Berkeley Mass Spectral Facility, on a VG 70-Se Micromass spectrometer for FAB, and a VG Prospec Micromass spectrometer for EI. Enantiomeric excess was determined by HPLC using a Shimatzu 10A VP series chiral HPLC. Optical rotation data was obtained using a Perkin–Elmer 241 Polarimeter with a 589 nm sodium lamp.
4.2. Representative procedure for the formation of furanones
4.2.1. 5-Butyl-2,2-diethylfuran-3(2H)-one (4a)
A 20 mL Schlenk tube equipped with a stir bar was charged with platinum(II) chloride (5 mg, 0.02 mmol, 0.1 equiv). To the tube was added a solution of 3a (39 mg, 0.2 mmol) in toluene (2.0 mL). The Schlenk tube was sealed, and the reaction mixture was heated to 100 °C for 3 h, then cooled to 23 °C. Silica gel (250 mg) was added to the reaction mixture and the solvent was removed by rotary evaporation. The adsorbed product was purified by flash chromatography (20 mL silica gel, 8:1 hexanes/ethyl acetate), to yield 25 mg (65%) of a light yellow oil. 1H NMR (CDCl3, 500 MHz) δ 5.40 (s, 1H), 2.50 (t, J=7.0 Hz, 2H), 1.75 (qd, J=7.3, 1.2 Hz, 4H), 1.65 (dt, J=15.3, 7.6 Hz, 2H), 1.41 (sext., J=7.4 Hz, 2H), 0.94 (t, J=7.4 Hz, 3H), 0.78 (t, J=7.4 Hz, 6H); 13C NMR (CDCl3, 125 MHz) δ 207.1, 193.5, 104.1, 94.4, 30.5, 28.8, 28.4, 22.3, 13.7, 7.2; IR (film) νmax 2970, 2937, 1702, 1596, 1460 cm−1; HRMS (EI+) calcd for [C12H20O2]+: m/z 196.1463, found 196.1469.
4.3. Representative procedure for the formation of indolizines
4.3.1. 3-Butylindolizin-1-yl pivalate (23b)
Platinum(II) chloride (5 mg, 18 µmol) and 2-(di-tert-butylphosphino) biphenyl (11 mg, 36 µmol) were added to a solution of pivalate 22b (100 mg, 0.36 mmol) in benzene (2 mL) in a 1-dram vial. The vial was sealed and heated at 70 °C for 3 h. The mixture was then filtered, concentrated by rotary evaporation, and purified by flash chromatography (4:1 hexanes/EtOAc) to yield 95 mg (95%) of 23b as a yellow oil. 1H NMR (500 MHz, C6D6) δ 7.30 (d, J=9.01 Hz, 1H), 7.04 (d, J=7.10 Hz, 1H), 6.76 (s, 1H), 6.32 (dd, J=8.99, 6.42 Hz, 1H), 6.06 (t, J=6.76 Hz, 1H), 2.26 (t, J=7.65 Hz, 2H), 1.40–1.32 (m, 2H), 1.27 (s, 9H), 1.18–1.10 (m, 2H), 0.74 (t, J=7.34 Hz, 3H); 13C NMR (125 MHz, C6D6) δ 175.7, 127.1, 121.3, 120.9, 120.7, 116.1, 114.0, 109.6, 105.0, 38.9, 29.0, 27.0, 25.2, 22.4, 13.6; IR (film) νmax 2958, 2931, 2871, 1749, 1278, 1120, 728 cm−1; HRMS (EI) calcd for [C17H23NO2]+: m/z 273.1729, found 273.1732.
4.4. Representative procedure for the formation of indolizinones
4.4.1. (±)-3-Butyl-8a-ethylindolizin-1(8aH)-one (36a)
Platinum(II) chloride (3 mg, 12 µmol), 2-(di-tert-butylphosphino) biphenyl (7 mg, 23 µmol), and cesium carbonate (7 mg, 23 µmol) were added to a solution of (±)-35a (50 mg, 0.23 mmol) in benzene (1 mL) and the solution was heated at 100 °C in a sealed vial with stirring. Once the reaction was judged complete by TLC (48 h) the mixture was concentrated by rotary evaporation and the residue purified by flash chromatography (4:1 hexanes/EtOAc) to yield 33 mg (0.15 mmol, 66%) of 36a as a yellow oil. 1H NMR (500 MHz, C6D6) δ 5.95 (d, J=9.28 Hz, 1H), 5.84 (d, J=7.07 Hz, 1H), 5.61 (dd, J=9.26, 5.37 Hz, 1H), 4.99 (t, J=6.21 Hz, 1H), 4.82 (s, 1H), 1.92–1.82 (m, 1H), 1.80–1.64 (m, 3H), 1.16–1.04 (m, 2H), 1.03–0.93 (m, 2H), 0.80 (t, J=7.41 Hz, 3H), 0.65 (t, J=7.29 Hz, 2H); 13C NMR (125 MHz, C6D6) δ 201.0, 175.0, 123.7, 122.2, 121.8, 108.1, 98.3, 70.3, 31.4, 28.4, 26.2, 22.1, 13.3, 6.5; IR (film) νmax 2960, 2932, 2873, 1675, 1534, 1433, 725, 689 cm−1; HRMS (EI) calcd for [C14H19NO]+: m/z 217.1467, found 217.1467.
Supplementary Material
Acknowledgements
The authors are grateful to UC Berkeley, Abbott Laboratories, and GlaxoSmithKline for generous financial support, Johnson Matthey for a gift of PtCl2, Dr. Herman van Halbeek for help with NOE studies, and to Dr. John Greaves (Mass Spectral Facility, University of California, Irvine) for HRMS data for compounds 3b, 10a, and 11.
Footnotes
Supplementary data
Supplementary data associated with this article can be found in the online version, at doi:10.1016/j.tet.2008.02.103.
References and notes
- 1.Le Quesne PW, Levery SB, Menachery MD, Brennan TF, Raffauf RF. J. Chem. Soc. Perkin Trans. 1. 1978:1572–1580. [Google Scholar]
- 2.(a) Kupchan SM, Sigel CW, Matz MJ, Gilmore CJ, Bryan RF. J. Am. Chem. Soc. 1976;98:2295–2300. doi: 10.1021/ja00424a050. [DOI] [PubMed] [Google Scholar]; (b) Smith AB, III, Guaciaro MA, Schow SR, Wovkulich PM, Toder BH, Hall TW. J. Am. Chem. Soc. 1981;103:219–222. [Google Scholar]
- 3.Shin SS, Byun Y, Lim KM, Choi JK, Lee K-W, Moh JH, Kim JK, Jeong YS, Kim JY, Choi YH, Koh H-J, Park Y-H, Oh YI, Noh M-S, Chung S. J. Med. Chem. 2004;47:792–804. doi: 10.1021/jm020545z. [DOI] [PubMed] [Google Scholar]
- 4.Sayama S. Heterocycles. 2005;65:1347–1358. [Google Scholar]
- 5.Winkler JD, Oh K, Asselin SM. Org. Lett. 2005;7:387–389. doi: 10.1021/ol047810q. [DOI] [PubMed] [Google Scholar]
- 6.A synthesis of 3(2H)-furanones closely related to our work appeared while this work was in progress, see: Kirsch SF, Binder JT, Liébert C, Menz H. Angew. Chem. Int. Ed. 2006;45:5878–5880. doi: 10.1002/anie.200601836..
- 7.For our initial report on these transformations, see: Smith CR, Bunnelle EM, Rhodes AJ, Sarpong R. Org. Lett. 2007;9:1169–1171. doi: 10.1021/ol0701971..
- 8.Precedent for a closely related strategy in the synthesis of furans and butenolides can be found in the work of Marshall, see: Marshall JA, Wang X-J. J. Org. Chem. 1991;56:960–969..
- 9.Liu Y, Liu M, Guo S, Tu H, Zhou Y, Gao H. Org. Lett. 2006;8:3445–3448. doi: 10.1021/ol061059z. [DOI] [PubMed] [Google Scholar]
- 10.Yao T, Zhang X, Larock RC. J. Am. Chem. Soc. 2004;126:11164–11165. doi: 10.1021/ja0466964. [DOI] [PubMed] [Google Scholar]
- 11.Hashmi ASK, Schwarz L, Choi J-H, Frost TM. Angew. Chem. Int. Ed. 2000;39:2285–2288. doi: 10.1002/1521-3773(20000703)39:13<2285::aid-anie2285>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12. Dhondi PK, Chisholm JD. Org. Lett. 2006;8:67–69. doi: 10.1021/ol0525260.. For more details, see Supplementary data.
- 13.(a) Bhanu Prasad BA, Yoshimoto FK, Sarpong R. J. Am. Chem. Soc. 2005;127:12468–12469. doi: 10.1021/ja053192c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pujanauski BG, Bhanu Prasad BA, Sarpong R. J. Am. Chem. Soc. 2006;128:6786–6787. doi: 10.1021/ja061549m. [DOI] [PubMed] [Google Scholar]
- 14.Fürstner A, Davies PW, Gress T. J. Am. Chem. Soc. 2005;127:8244–8245. doi: 10.1021/ja050845g. [DOI] [PubMed] [Google Scholar]
- 15.Minor amounts of enedione products were observed during the reactions of substrates 3a–f.
- 16.The α-ketol migration has been shown to be stereospecific. See Ref. 6.
- 17.This may occur in a stepwise as opposed to a concerted manner. The role of the cyclopropyl group at the terminus of the alkyne in promoting the formation of significant amounts of enedione products is under active investigation.
- 18.A Meyere–Schuster mechanism for the formation of 18 from 13 cannot be ruled out at this time.
- 19.The E-diastereomer is supported by NOE analysis of the product 18. For more information, see Supplementary data.
- 20.For a recent report of protic acid-catalyzed isomerization of Z-enediones, see: Crone B, Kirsch SF. Chem. Commun. 2006;764 doi: 10.1039/b515838a..
- 21.Michael JP. Nat. Prod. Rep. 1999;16:675–696. doi: 10.1039/a809408j. [DOI] [PubMed] [Google Scholar]
- 22.For recent examples, see: Kaloko J, Jr, Hayford A. Org. Lett. 2005;7:4305–4308. doi: 10.1021/ol051860t. Kel’in AV, Sromek AW, Gevorgyan VJ. Am. Chem. Soc. 2001;123:2074–2075. doi: 10.1021/ja0058684. Seregin IV, Gevorgyan V. J. Am. Chem. Soc. 2006;128:12050–12051. doi: 10.1021/ja063278l. Marchalin S, Baumlová B, Baran P, Oulyadi H, Daïch A. J. Org. Chem. 2006;71:9114–9127. doi: 10.1021/jo0615044. Yan B, Liu Y. Org. Lett. 2007;9:4323–4326. doi: 10.1021/ol701886e..
- 23.For details, see Supplementary data.
- 24.Heating 22a without added catalyst at 120 °C over 48 h yielded minor amounts of 23a (ca. 15% yield) along with significant byproducts. This points to a slow and inefficient background reaction.
- 25.(a) Tomori H, Fox JM, Buchwald SL. J. Org. Chem. 2000;65:5334–5341. doi: 10.1021/jo000691h. [DOI] [PubMed] [Google Scholar]; (b) Fox JM, Huang X, Chieffi A, Buchwald SL. J. Am. Chem. Soc. 2000;122:1360–1370. [Google Scholar]
- 26.(a) Bender CF, Widenhoefer RA. J. Am. Chem. Soc. 2005;127:1070–1071. doi: 10.1021/ja043278q. [DOI] [PubMed] [Google Scholar]; (b) Qian H, Widenhoefer RA. J. Org. Lett. 2005;7:2635–2638. doi: 10.1021/ol050745f. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Widenhoefer RA. Organometallics. 2004;23:1649–1651. [Google Scholar]
- 28.Takita R, Yakura K, Ohshima T, Shibasaki M. J. Am. Chem. Soc. 2005;127:13760–13761. doi: 10.1021/ja053946n. [DOI] [PubMed] [Google Scholar]
- 29.Previously, PtCl2 was reported to be an ineffective catalyst for the cycloisomerization that results in 30. See Ref. 22c.
- 30.For an example of α-hydroxyiminium 1,2-migration, see: Fenster MDB, Patrick BO, Dake GR. Org. Lett. 2001;3:2109–2112. doi: 10.1021/ol0160708..
- 31.More recently, following our initial report (Ref. 7), Kirsch and co-workers have reported that imines may participate in similar transformations, see: Binder JT, Crone B, Kirsch SF, Liébert C, Menz H. Eur. J. Org. Chem. 2007:1636–1647. and Table 4 therein.
- 32.The formation of a cesium alkoxide prior to 1,2-migration may also be important.
- 33.Enantioenriched 35a was obtained via preparative chiral column chromatography of the racemate.23
- 34.Efforts to delineate whether this transformation is stereospecific and the loss of ee in forming enantioenriched 36a occurs via a competing process are currently ongoing.
- 35.Liu has recently reported that these reactions may be effected with Cu(I) salts, see: Yan B, Zhou Y, Chen J, Liu Y. J. Org. Chem. 2007;72:7783–7786. doi: 10.1021/jo070983j..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.