Abstract
To determine the practicality of controlled ovulation of the dominant follicle as a technique to study meiotic maturation of oocytes during contraceptive research, we developed a technique for aspiration of the single dominant follicle using a dual-needle continuous irrigation technique 27 hours after an ovulatory stimulus. All of the oocytes (3/3) recovered from control animals, but only 1/6 (17%) of oocytes from animals treated with the meiotic inhibitor ORG 9935 exhibited germinal vesicle breakdown, indicating resumption of meiosis.
Our understanding of the events surrounding maturation of the primate oocyte within the dominant follicle stems primarily from investigations of oocytes obtained from gonadotropin-stimulated cycles designed to produce multiple follicles (1, 2). The limitations of controlled ovarian hyperstimulation (COH) protocols include the heterogeneity of follicles (and presumably oocytes) produced using supraphysiologic levels of gonadotropins and GnRH antagonists (GnRH-a) designed to support development of a pool of follicles that would normally undergo apoptosis as the naturally selected dominant follicle develops (3). Therefore, the use of pharmacologic interventions designed to manipulate oocyte maturation for either fertility enhancing or contraceptive purposes in natural cycles rest on knowledge gained from an inherently artificial model.
Ideally, investigations on the cellular and molecular events surrounding oocyte maturation would use the naturally selected dominant follicle of the spontaneous menstrual cycle. Unfortunately, normal variation in the interval for follicle maturation (e.g., the length of the follicular phase) and the timing of the preovulatory LH surge among non-human primates and women makes follicle sampling during the spontaneous cycle logistically difficult. Mistimed aspirations due to variability in follicular phase development can result in tissue/oocyte collection from an immature or postovulatory follicle (4).
To help overcome the difficulties inherent in studying development of the naturally selected dominant follicle, Young et al. (4) developed a successful technique to investigate periovulatory events and their regulation by gonadotropins and local factors during the natural menstrual cycle in primates. Under this protocol of controlled ovulation, menstrual cycles of rhesus monkeys are monitored, and a 2-day treatment consisting of a GnRH-a plus gonadotropins is initiated at the late follicular phase (after dominant follicle selection, but before ovulation). Administration of an ovulatory stimulus (hCG) allows for precise timing of surgery for retrieval of tissues to study molecular events in the periovulatory follicle. This technique has been used to precisely time tissue recovery during experiments investigating the role of gonadotropins and local factors in the ovulatory process and luteal development (4). To date, no studies have been performed applying this technique to assess oocyte maturation or quality.
Retrieval of a single oocyte from the dominant follicle presents another technical challenge to the investigator. In contrast to the multiple somatic cells of the periovulatory follicle, the single oocyte must be recovered efficiently for the experiment to yield data in a cost-effective manner. Due to the high cost and scarcity of the primate research model, oophorectomy to recovery the oocyte from the intact follicle represents an unacceptable means that precludes the use of the animal for future experiments. Alternatively, monkeys tolerate repeated laparoscopic surgery for aspiration of multiple antral follicles during gonadotropin-stimulated cycles in IVF-type protocols, but the effectiveness of aspirating the dominant follicle to collect the single oocyte during natural cycles has not been reported.
Therefore, we conducted a pilot study to determine whether controlled ovulation, followed by laparoscopic aspiration of a single dominant follicle, could be used for reliable recovery of the oocyte during studies of meiotic inhibitors. We report on a novel technique of simultaneous follicle aspiration/irrigation to assist in the retrieval of the oocyte from the dominant follicle during natural menstrual cycles.
MATERIALS AND METHODS
The general care and housing of rhesus monkeys at the Oregon National Primate Research Center (ONPRC) was described previously (5). The ONPRC Institutional Animal Care and Use Committee approved all study protocols and experiments before initiation.
The controlled ovulation protocols (4) were initiated in conjunction with studies of agents affecting meiosis. In this protocol, after a period of observation to confirm occur-rence of normal menstrual cycles, adult female rhesus macaques (n = 8) underwent daily venipuncture during the follicular phase (4, 6) until serum E2 levels reached levels ≥80 but <120 pg/mL. At this point, animals were started on a 2-day treatment regimen of human recombinant gonadotropins to control the timing of ovulation. On day 1 of treatment, a GnRH-a (Antide; 0.5 mg/kg; Ares Serono Group, Ltd., Aubonne, Switzerland) and recombinant hFSH and recombinant hLH (30 IU each; Repronex, Fer-ring Pharmaceuticals, Suffern, NY) were administered at noon, and gonadotropins alone at 10 pm. On day 2, GnRH-a and an ovulatory stimulus (1,000 IU hCG, Ovidrel, Ares Serono Group, Ltd.) were administered at 6 AM with laparoscopy performed 27 hours later. Control animals received no additional treatment. Another group of animals (ORG 9935) received the phosphodiesterase 3 inhibitor ORG 9935, an agent that has been shown to inhibit both spontaneous (7) and gonadotropin-induced (8) resumption of meiosis in macaque oocytes during the follicular phase (9).
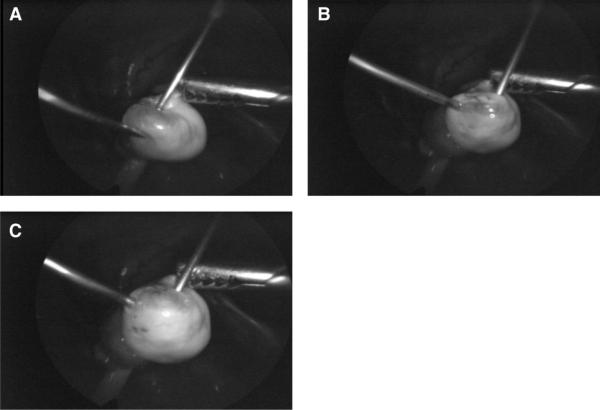
Follicle aspiration was performed by laparoscopy on anesthetized animals (10) using a double-needle, continuous irrigation technique to recover the oocyte from the single dominant follicle. The follicular contents were collected in a physiologic buffered solution (TALP-HEPES with 0.3% bovine serum albumin [BSA] and 0.5% heparin), which was also used as the irrigant. Using a 5-mm, 0-degree laparoscope with continuous video monitoring (Richard Wolf Medical Instruments Corporation, Vernon Hills, IL), two small graspers are inserted through accessory ports and the ovary containing the dominant follicle is located and stabilized. Two 22-gauge spinal needles are then inserted percutaneously (Fig. 1A). One of these, the aspiration needle is connected through tubing to a 15-mL sterile collection tube fitted with a double-hole stopper that allows for a further connection to an adjustable suction of 80–140 psi. The second, or flush needle, is attached to a 10-cc syringe containing media for the experiment. The surgeon and assistant work to position the two needles, and enter the follicle simultaneously (Fig. 1B). The follicle is initially aspirated using suction, and then continuously flushed with heparinized media (~10 mL) under constant visualization (Fig. 1C). To prevent loss of the oocyte, care must be taken throughout the procedure to prevent either needle from moving once the fragile follicle is entered.
FIGURE 1.
(A) The 22-gage aspiration and irrigation needles are inserted percutaneously, and positioned for simultaneous entry into the follicle. The follicle is then aspirated (B) and irrigated (C) continuously to ensure recovery of the oocyte.
RESULTS
In 10 successful cycles, monkeys had initiation of the standard controlled ovulation protocol beginning on or after cycle day 5. A single dominant follicle was noted at laparoscopy, with an oocyte recovered by follicle aspiration in all but one (90%) of these cases. In the one experiment where the oocyte was not recovered, the suction equipment malfunctioned during the follicle aspiration. Six monkeys received ORG 9935 and four received no additional treatments (controls). As expected, all of the oocytes recovered from control controlled ovulation cycles were noted to have resumed meiosis. Significantly, 5/6 (83%) of the ORG 9935-treated oocytes were arrested at the germinal vesicle-intact stage, consistent with results seen during COH cycles (8).
DISCUSSION
Our results demonstrate that the controlled ovulation protocol represents a practical technique in studies that require evaluation of oocyte maturation during the periovulatory period in natural menstrual cycles. The dual-needle, continuous irrigation follicle aspiration technique successfully recovered an oocyte suitable for evaluation of meiotic stage in all but one case. The lone failure occurred due to equipment malfunction. Furthermore, the controlled ovulation technique does not appear to adversely affect events occurring within the periovulatory interval. As expected, oocytes recovered at the end of the periovulatory interval from control animals resumed meiosis, whereas most oocytes from animals treated with the meiotic inhibitor ORG 9935 did not exhibit germinal vesicle breakdown.
These findings support the use of single follicle aspiration using a dual-needle, continuous irrigation technique following a protocol of controlled ovulation as a research tool for the study of novel contraceptive agents that affect meiosis.
Acknowledgments
ORG 9935 was provided as a gift of N.V. Organon through the kind assistance of Pieter M. Verbost, Ph.D. The authors wish to thank the staff of the Division of Animal Resources at ONPRC for excellent surgical and animal care, and David Hess, Ph.D., and the Endocrine Services Core Laboratory for steroid assays.
Supported by the National Institute of Child Health and Human Development through grants HD042710 (R01-042710); U54 HD18185; U54 HD 031398; and NCRR RR00163.
REFERENCES
- 1.Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol (Orlando) 1996;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekher YA, Hutchison JS, Zelinski-Wooten MB, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in primate follicles using recombinant and native human luteinizing hormone to mimic the midcycle gonadotropin surge. J Clin Endocrinol Metab. 1994;79:298–306. doi: 10.1210/jcem.79.1.8027245. [DOI] [PubMed] [Google Scholar]
- 3.Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32. doi: 10.1186/1477-7827-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod. 2003;18:2257–63. doi: 10.1093/humrep/deg467. [DOI] [PubMed] [Google Scholar]
- 5.Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev. 1990;27:261–80. doi: 10.1002/mrd.1080270313. [DOI] [PubMed] [Google Scholar]
- 6.Zelinski-Wooten MB, Hutchison JS, Trinchard-Lugan I, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in gonadotrophin-stimulated macaques with varying doses of recombinant human chorionic gonadotrophin. Hum Reprod. 1997;12:1877–85. doi: 10.1093/humrep/12.9.1877. [DOI] [PubMed] [Google Scholar]
- 7.Jensen JT, Schwinof KM, Zelinski-Wooten MB, Conti M, DePaolo LV, Stouffer RL. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod. 2002;17:2079–84. doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- 8.Jensen JT, Zelinski-Wooten MB, Schwinof KM, Vance JE, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation during gonadotropin-stimulated ovarian cycles in rhesus macaques. Contraception. 2005;71:68–73. doi: 10.1016/j.contraception.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Young KA, Hennebold JD, Stouffer RL. Dynamic expression of mRNAs and proteins for matrix metalloproteinases and their tissue inhibitors in the primate corpus luteum during the menstrual cycle. Mol Hum Reprod. 2002;8:833–40. doi: 10.1093/molehr/8.9.833. [DOI] [PubMed] [Google Scholar]
- 10.Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA. 1996;93:1897–901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]