Abstract
Since 1892, anatomical studies have demonstrated that the retinas of mammals, including humans, receive input from the brain via axons emerging from the optic nerve. There are only a small number of these retinopetal axons, but their branches in the inner retina are very extensive. More recently, the neurons in the brain stem that give rise to these axons have been localized, and their neurotransmitters have been identified. One set of retinopetal axons arises from perikarya in the posterior hypothalamus and uses histamine, and the other arises from perikarya in the dorsal raphe and uses serotonin. These serotonergic and histaminergic neurons are not specialized to supply the retina; rather, they are a subset of the neurons that project via collaterals to many other targets in the central nervous system, as well. They are components of the ascending arousal system, firing most rapidly when the animal is awake and active. The contributions of these retinopetal axons to vision may be predicted from the known effects of serotonin and histamine on retinal neurons. There is also evidence suggesting that retinopetal axons play a role in the etiology of retinal diseases.
Keywords: centrifugal, efferent, histamine, primate, serotonin
MORPHOLOGY AND FUNCTIONS OF RETINOPETAL AXONS IN MAMMALS
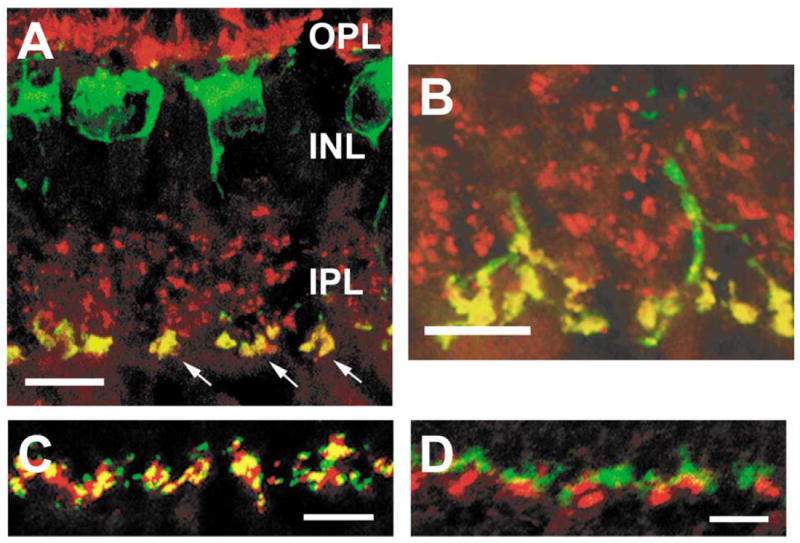
In mammalian retinas, three types of retinopetal axons have been distinguished by morphological criteria. The first type gives rise to terminals in the outer strata of the inner plexiform layer (IPL). Typically, the axons terminate along the border with the inner nuclear layer (INL) (Figs. 1A and 1C). Using the Golgi method, axons with this pattern of branching have been identified in dog1 and chimpanzee2 retinas. The second type of retinopetal axon is larger in diameter, varicose, and terminates in the outer plexiform layer (OPL). In rats, these axons are labeled with antibodies to serotonin after loading the retina with a related indoleamine, 5,7-dihydroxytryptamine (5,7-DHT). They originate from perikarya in the optic chiasm or the preoptic area of the hypothalamus.3 Retinopetal axons similar to these are labeled with neurofilament antibodies in the mouse retina.4 Neurons that project from the anterior hypothalamus to the retina have been identified in a prosimian,5 but their terminals in the retina have not been described.
FIGURE 1.
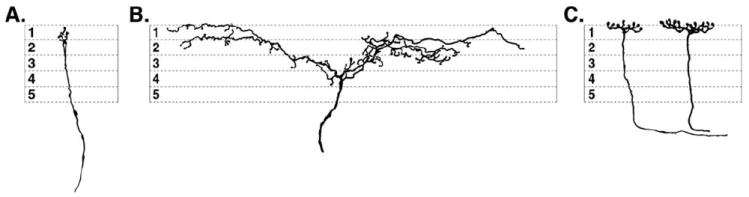
Two of the three types of retinopetal axons in mammalian retinas. (A, B) Two types of retinopetal axon terminals were redrawn from plates 74 and 93 of Polyak, respectively.2 One type from a chimpanzee (A), terminates near the border of the inner plexiform (IPL) and inner nuclear layer (INL). A second type from a macaque has more broadly stratified branches in the center of the IPL (B). (C) In the dog retina, two retinopetal axons were redrawn from plate AI-36 from Ramon y Cajal.1 They have large diameters, terminate in the outer strata of the IPL, and end in large swellings. The orientation of the drawing is photoreceptors are up; subdivisions 1–5 represent the sublamina of the IPL.
The third type of retinopetal axon branches extensively in IPL (Fig. 1B). Using the Golgi method, Polyak described axons like these in macaque retinas.2 They run in the optic fiber layer (OFL), and they give off a nearly vertical branch to the IPL, where they branch extensively in a broad band in the center of the layer. In the mouse retina, axons with a similar branching pattern are labeled with neurofilament antibodies.4 These axons are also labeled using reduced silver stains in monkey6,7 and human8,9 retinas. These studies confirmed and extended Polyak’s descriptions, and they also demonstrated clear morphological differences between retinopetal axons and those of ganglion cells with intraretinal collaterals. These ganglion cell axons have several long, thin branches that extend into the outer half of the IPL.7,10 Retinopetal axons have a larger diameter, branch more extensively, and do not originate from perikarya in the retina.
Retinopetal axons have also been studied using anterograde labeling techniques. After lesions of cat optic nerves, degenerating terminals are found in the IPL near the perikarya of amacrine cells.11,12 After lesions of macaque optic nerves, degenerating terminals are found in the outer third of the IPL.11 Retinopetal axons are labeled anterogradely with horseradish peroxidase (HRP) applied to the cut optic nerve in macaques13 and guinea pigs,14 or else with phaseolus vulgaris leucoagglutinin injected in the oculomotor nucleus in rats.15 Retinopetal axons have also been observed in transgenic mice that express yellow fluorescent protein (YFP) in a subset of projection neurons under control of the Thy-1 promoter.16 An axon labeled by this technique is illustrated in Fig. 2. Thus, the retinopetal axons are seen with a variety of anatomical techniques, ranging from one of the oldest, the Golgi method, to one of the newest.
FIGURE 2.
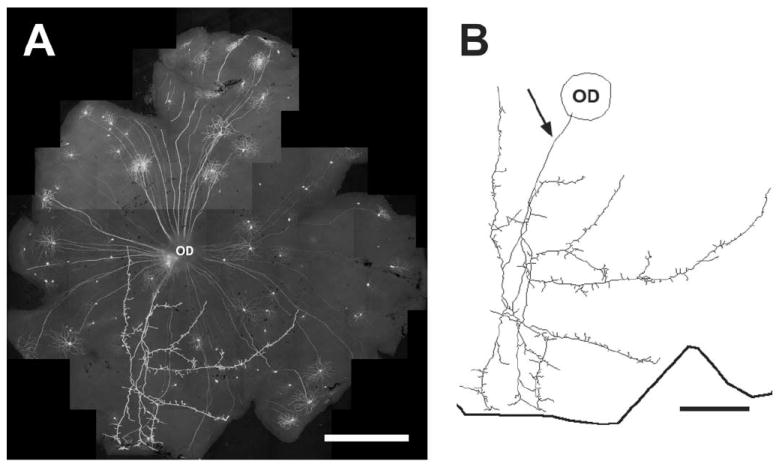
A THY1-YFP positive retinopetal axon in the mouse retina labeled from a transgenic mouse (line H, Jackson Laboratories) that expresses THY1-YFP in a subset of neurons.16 The labeling in the retinas was enhanced with a rabbit polyclonal antibody to GFP conjugated to Alexa Fluor 488 (Molecular Probes). (A) A low-power photomontage of a whole-mounted retina. (B) A Neurolucida (Microbrightfield, Williston, VT, USA) drawing of a retinopetal axon that emerges from the optic disk (arrow) and branches in the retina. Axon branches reach to the ora serrata, indicated by the thick line. Scale bar = 0.5 mm.
Mammalian retinas receive input from a relatively small number of neurons in the brain. In dogs, for example, 176–260 neurons in the hypothalamus are retrogradely labeled after HRP is applied to the optic nerve.17 In macaques and guinea pigs, only 25–35 neurons are labeled using a similar technique.18 The branches of retinopetal axons are very extensive, however.6-9,19,20 The axon in Fig. 2 occupies approximately one-fourth of the retinal area, for example.
The early experiments on the functions of retinopetal axons are controversial,21 but a number of recent studies suggest that inputs from the brain to the retina make important contributions to vision. In humans, retinal neurons respond more rapidly during the day than during the night, regardless of the short-term adaptation state. Exposing one retina to a long, bright stimulus at night returns that retina to the daytime state, and the effect transfers, in part, to the unstimulated eye.22,23 The latter finding suggests that retinopetal axons play a role. There is both electrophysiological24 and psychophysical25,26 evidence for a decrease in the sensitivity of humans to light stimuli during the day, also independent of adaptation state, and retinopetal axons may play a role in these sensitivity changes as well. In rats, retinopetal axons might influence the timing of rod outer segment disk shedding. The optic nerves must be intact in order to reset the shedding rhythm in response to changes in illumination.27 Changes in retinal ganglion cell activity and the amplitude of the electroretinogram (ERG) during sleep have also been attributed to retinopetal axons.28-30
Several neurotransmitters of retinopetal axons in other vertebrates have not been found there in mammals. Nitric oxide is unlikely to be used as a transmitter because no retinopetal axons are labeled with NADPH diaphorase histochemistry or with antibodies to nitric oxide synthase.31 The neuropeptide FMRFamide is not present in macaque retinas.32 Another neuropeptide, gonadotropin releasing hormone (GnRH), is found in the optic nerves of rats,33,34 voles,35 and fetal macaques,36 but not in their retinas. One population of retinopetal axons is labeled with antibodies to tyrosine hydroxylase, but the catecholamine they presumably contain has not been identified.37,38 Two other candidates for retinopetal neurotransmitters, histamine and serotonin, are discussed below.
RETINOPETAL AXONS CONTAINING HISTAMINE
Retinopetal axons containing histamine were first described in guinea pigs.39 Retinopetal axons of the third morphological type are also labeled with antiserum to histamine in macaque retinas.19 Some make collateral branches that return to the optic disk; these may be the source of the histamine-immunoreactive (IR) axons seen in the superior colliculus and lateral geniculate nucleus of macaques.40 Other histamine-IR axons descend orthogonally through the ganglion cell layer (GCL) into the IPL and branch extensively in a broad band in the center of the layer (Fig. 3). Histamine-IR axons run alongside some of the larger blood vessels in the OFL and IPL. Varicose histamine-IR axons are also found among the ganglion cell axons in the optic nerve. No cell bodies are labeled in the retina, and two lines of evidence suggest that they are located in the tuberomamillary nucleus of the posterior hypothalamus (Fig. 4). First, neurons there are retrogradely labeled after HRP application to the proximal stump of the cut optic nerve.18 Second, this is the only area of the macaque brain where cell bodies containing histamine are found.40
FIGURE 3.
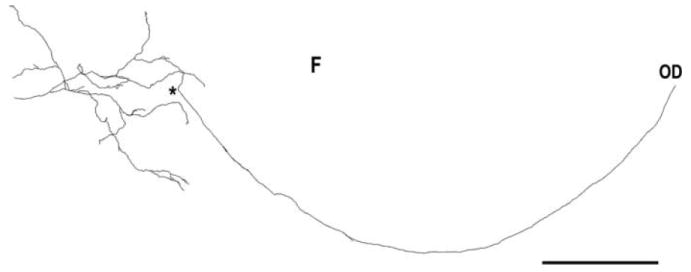
A histamine-immunoreactive retinopetal axon in the macaque retina. This axon was labeled with a histamine antiserum in whole-mounted retina, as previously described in Gastinger et al.19 It emerged from the optic disk (OD) and ran to the temporal retina where it made an orthogonally projecting branch (*) to the inner plexiform layer. The axon branches were studded with varicosities and stratified in the center of the inner plexiform layer. Scale bar = 1.0 mm.
FIGURE 4.
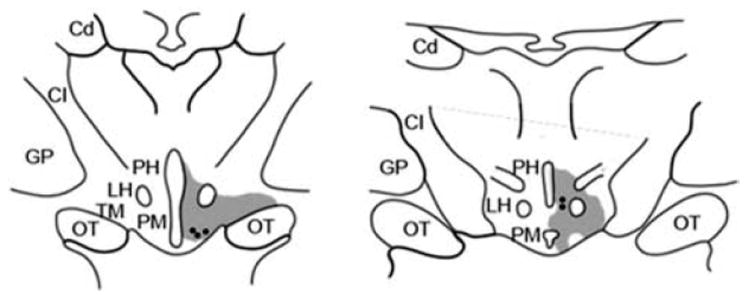
Localization of histamine-immunoreactive neurons and retinopetal neurons in the macaque hypothalamus. Two coronal sections redrawn from Labandiera-Garcia et al.18 show the regions where retrogradely labeled neurons from the cut optic nerve (black circles) are found. The sites of histamine-immunoreactive neurons were superimposed on these sections (shaded areas), based on descriptions by Manning et al.40 TM, tuberomamillary nucleus; PH, posterior hypothalamus; LH, lateral hypothalamus; OT, optic tract; PM, premamillary; CI, capsula interna; Cd, caudate nucleus; GP, globus pallidus.
The histamine-IR retinopetal axons in rats are similar to those in monkeys in most respects. A few histamine-IR axons terminate in the GCL and OFL, but the majority of axons do not have branches there. Most axons branch in the center of the IPL, and a few terminal branches extend into the inner nuclear layer (INL). Histamine-IR terminals in the IPL typically have large varicosities; varicosities on branches in the OFL and GCL are smaller and less common. A few histamine-IR branches are closely associated with large retinal blood vessels in the IPL. Histamine-IR axons are also found in the optic nerve.20
Histamine levels have been measured in the retinas of several mammals, and the values are similar to those of brain regions, such as the cerebral cortex, that receive modest levels of histaminergic input.41 Mast cells are another source of histamine in the brain, but they cannot account for the histamine detected in the retina because none are present there.42 Histamine is synthesized in one step from L-histidine by the enzyme histidine decarboxylase (HDC). HDC activity has been detected in the retinas of rabbits,43 rats,44,45 and monkeys.45
The histaminergic neurons that project to the retina are a subset of the “waking-on” neurons recorded in the tuberomamillary nucleus of the posterior hypothalamus of rats46 and cats.47,48 They have a slow, tonic firing rate during waking, followed by a gradual decrease during slow-wave sleep, and a complete a cessation of firing during paradoxical sleep (Fig. 5). Thus, the release of histamine in the retina should vary according to the sleep/wake cycle of the animal. Histamine is expected to be released from retinopetal axons during the day in diurnal animals and during the night in nocturnal animals.
FIGURE 5.
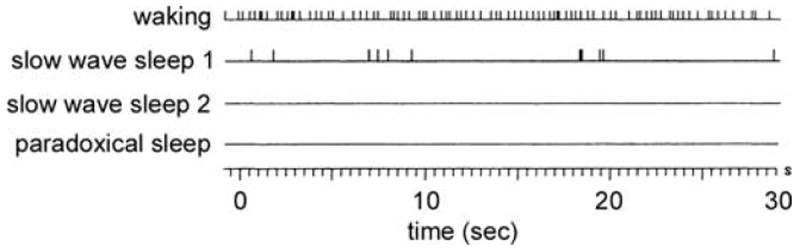
Histaminergic neurons in the posterior hypothalamus are most active during waking in the cat. A single histaminergic neuron, recorded in vivo, fires action potentials at a steady rate of 4–6 Hz during waking, more slowly early in slow wave sleep, and is silent during paradoxical sleep. Modified and reprinted from Vanni-Mercier et al.48
In the central nervous system, histamine acts on three different G-protein–coupled receptors: histamine receptor 1 (HR1), histamine receptor 2 (HR2), and histamine receptor 3 (HR3). HR1 are present on bovine retinal blood vessels,49 and histamine increases the permeability of the blood-retinal barrier in rats.50 Histamine also produces a relaxation of bovine retinal blood vessels mediated primarily by HR1, but also by HR2,51 and histamine increases the diameter of rat retinal capillaries.52 Some of the histamine receptors mediating these effects may be localized on pericytes. Histamine depolarizes isolated pericytes from rat retinas53 and increases the ratio of free to bound calcium in isolated pericytes from bovine retinas.54 Histamine receptors are also present on vascular endothelial cells from rat51 and bovine55 retinas. Because they are expected to be active during the day in humans, histaminergic retinopetal axons may contribute to the increase in optic nerve blood flow observed during the day.56
The binding of ligands to HR1 has been characterized in membrane preparations from mammalian retinas,41,57 and HR1 have been localized to the IPL in macaque retinas.58 There is indirect evidence suggesting that histamine acting at HR1 enables humans to respond to light stimuli at higher temporal frequencies. HR1 antagonists decrease the critical flicker fusion frequency.59-62 The responses of macaque parasol ganglion cells to luminance flicker are identical to those of human subjects under the same conditions,63 and it is possible that the antihistamines act on the neural circuit providing input to parasol cells. In rats, dopaminergic neurons express HR1 (Fig. 6),64 and these receptors would be activated by tonic release of histamine at night. Histamine strongly inhibits the release of dopamine from guinea pig retinas.65
FIGURE 6.
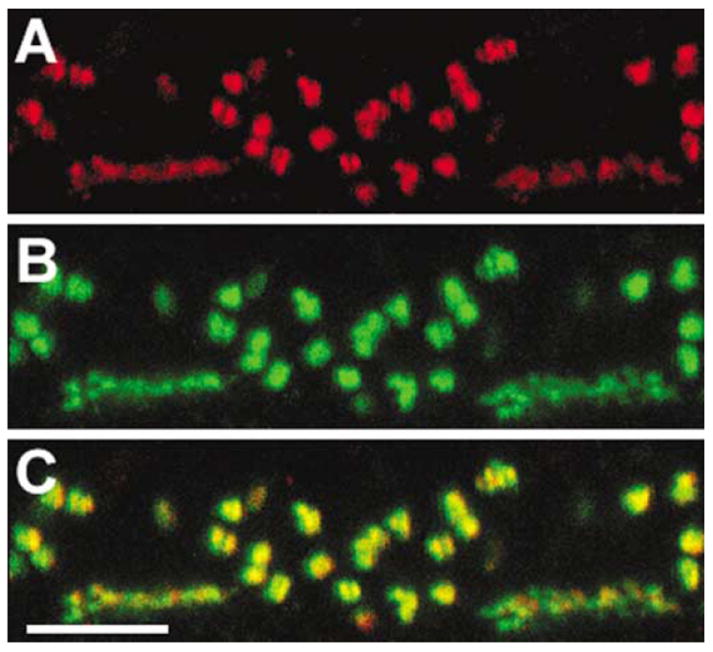
HR3 localization in the outer plexiform layer of a macaque retina. This vertical section was double-labeled with a rabbit anti-HR3 (red, Chemicon) and mouse anti-mGluR6 (a gift from Noga Vardi73) antibodies. Both HR3 and mGluR6 were found on the tips of ON-bipolar cell dendrites. Reprinted from Gastinger et al.64 Scale bar = 5 μm.
HR2 have not been localized in mammalian retinas, but there is indirect evidence that they are present. Histamine inhibits a forskolin-induced increase in cAMP in rabbit retina via HR2.66,67 Histamine also increases a GABAA-mediated chloride current in amacrine cells in rat retinas via protein kinase A, a signaling pathway frequently used by HR2.68,69 If amacrine cells are inhibited more effectively, there would be less tonic inhibition of the ganglion cells and bipolar cells. Accordingly, both histamine and the HR2 agonist dimaprit increase the maintained firing rates of rat retinal ganglion cells in vitro. Because histamine has no effect when synaptic transmission is blocked, this effect on ganglion cells must be indirect.70
In monkeys, HR3 are found on the tips of the dendrites of ON-bipolar cells64 (Fig. 7), but the effects of histamine on primate bipolar cells have not yet been studied. Histamine and the HR3 agonist methylhistamine modulate the maintained activity of most monkey ganglion cells, and they also decrease the responses of some cells to full-field light stimuli.70 Histamine might act via the same Go as the metabotropic glutamate receptor, closing nonselective cation channels,71 or it might open K+ channels.72 Both would hyperpolarize ON-bipolar cells and decrease the maintained firing rates and light responses of ON-ganglion cells. HR3 might produce opposite effects on maintained activity and light responses if they increase the efficacy of GABAA-mediated input from horizontal cells.73,74 In the dark, this would depolarize ON-bipolar cells and increase the maintained activity of ON-ganglion cells. However, with a full-field light stimulus, the responses of ON-ganglion cells would be decreased because lateral inhibition is more effective.
FIGURE 7.
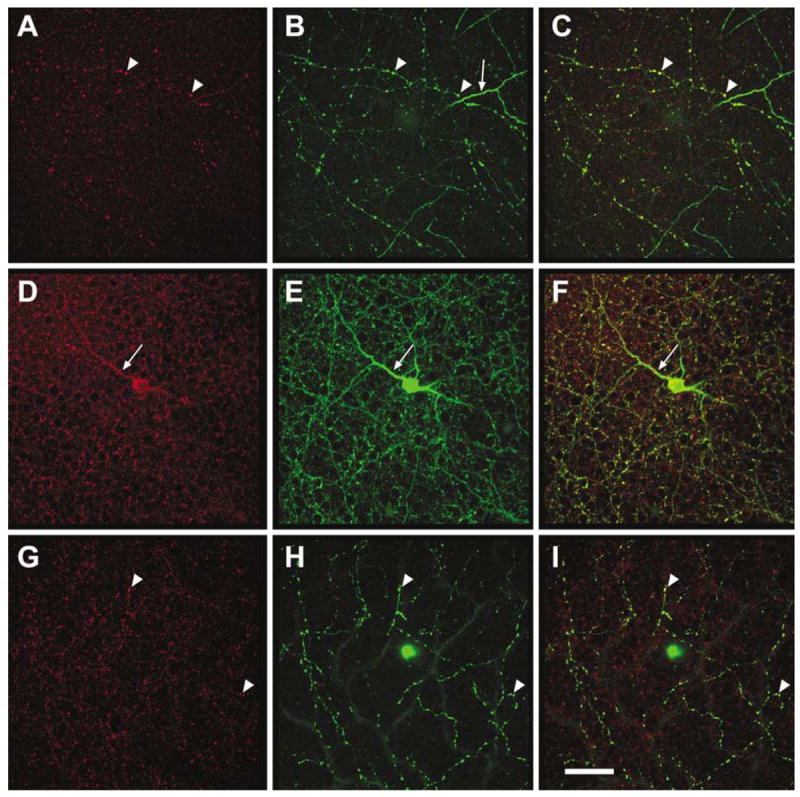
HR1 localization in rat retina. This rat retina was double-labeled with a rabbit anti-HR1 (red, Chemicon) and mouse anti-tyrosine hydroxylase (green, Sigma). All retinal layers were scanned using a confocal microscope, and HR1 receptors were found in all parts of the TH-IR amacrine cells in stratum 4 of the IPL (A), in S1 of the IPL (B), and in the INL (C). Reprinted from Gastinger et al.64 Scale bar = 50 μm.
The retina inactivates histamine by three mechanisms: reuptake, methylation, and oxidation. Retinal slices take up [3H]histamine, and uptake is inhibited by ouabain, an inhibitor of Na+/K+-dependent ATPase.75 The primary inactivation pathway for histamine is methylation by N-methyltransferase, an enzyme present in the retina.76 The product in this pathway is tele-methylhistamine, which is biologically inactive. Endogenous histamine is also oxidized via diamine oxidase to form imidazoleacetic acid (IAA),77 a known GABAC receptor antagonist.78,79 Diamine oxidase mRNA is expressed in human retinas,80,81 and this pathway may be particularly important in the retina because of the prevalence of GABAC receptors on bipolar cell axon terminals.82 Because the ratios of GABAC to GABAA receptors vary depending on the bipolar cell type,83 the efficacy of disinhibition of bipolar cells by IAA would be expected to vary as well.
In summary, histamine released from retinopetal axons in mammalian retinas would be expected to modulate the maintained activity of most retinal ganglion cells and to influence the light responses of some ganglion cells as well. In some instances, the effects of histamine appear to optimize retinal function at the ambient light intensities prevailing when the animal is awake. In monkeys, which are diurnal, histamine decreases the responses of some ganglion cells to light stimulation, a finding suggesting that retinopetal axons contribute to light adaptation. In guinea pigs, a nocturnal species, histamine inhibits dopamine release from amacrine cells, a finding consistent with a role for retinopetal axons in dark adaptation.
RETINOPETAL AXONS CONTAINING SEROTONIN
Retinas of macaques and baboons contain serotonin-immunoreactive (5-HT-IR) retinopetal axons.84 They emerge from the optic nerve head, run in the OFL and terminate, mainly in the GCL. There is also a plexus of 5-HT axons in the OFL near the optic disk, and some axons that supply other regions of the retina originate from there. Some of the 5-HT-IR axons terminate in the distal IPL and in the INL; these may be the first type described by Polyak. 5-HT-IR axons are also associated with a subset of retinal blood vessels, particularly in the central retina. The area covered by each axon is quite large; for example, the branches of one 5-HT-IR axon in a macaque retina occupy an area of 23 mm2.
The 5-HT-IR axons are similar in some respects to the histamine-IR axons, but there are clear morphological differences: (1) the 5-HT axons mainly branch in the GCL, whereas histamine-IR axons terminate in the IPL; (2) the 5-HT-IR axons are narrower in diameter than the histamine-IR axons; (3) unlike the histamine-IR axons, the 5-HT-IR axons do not surround the fovea or return to the optic disk; (4) the serotonin-IR axons form a plexus near the optic disk, but the histamine-IR axons do not; and (5) although both types of retinopetal axons run alongside retinal blood vessels, the 5-HT-IR axons make more branches associated with blood vessels.
There are indoleamine-accumulating neurons in retinas of New World monkeys.85,86 In rabbits, there are amacrine cells that take up 5-HT so avidly that they are labeled with antibodies to 5-HT after contamination with blood.87,88 However, because these cells do not contain detectable amounts of endogenous serotonin, they are thought to use other molecules as their neuro-transmitters. It is unlikely that platelets are the source of the immunoreactive serotonin in retinopetal axons of macaque and baboon retinas, however, because the animals were exsanguinated or perfused before the eyes were removed, and there was no detectable blood in the retinal vessels.
The levels of endogenous serotonin in mammalian retinas are relatively low, approximately 10% of the levels of an amacrine cell neurotransmitter, dopamine.89-93 These low levels of endogenous serotonin suggest that serotonin is used as a neurotransmitter by a sparse population of retinopetal axons rather than amacrine cells. Serotonin is synthesized from tryptophan in two steps by tryptophan hydroxylase and amino acid decarboxylase. Tryptophan hydroxylase and its mRNA have been detected in mammalian retinas, but most retinal serotonin is synthesized by photoreceptors as a precursor for melatonin.94,95
The serotonergic neurons that project to the retina were identified in vervets, another species of Old World monkey, using methods described recently.96 After intravitreal injection of cholera toxin subunit B, retrogradely labeled perikarya are found in the dorsal raphe, and all of these neurons contain immunoreactive serotonin (Fig. 8). These findings are not attributable to transneuronal transport because no retinal ganglion cell axons are labeled in the dorsal raphe. Rodent retinas are also innervated by axons from neurons in the dorsal raphe. Five studies have shown that neurons in the dorsal raphe of various rodents are labeled after intraocular injections of retrograde tracers.97-101 In rats, retinal serotonin levels are reduced after electrolytic lesions97 or injections of 5,7-DHT, which is toxic to serotonergic neurons,102 in the dorsal raphe. Based on recordings from serotonergic neurons in awake, behaving mammals, retinopetal axons are expected to fire tonically. Their firing rate would be highest when the animal is awake and active, lower when the animal is quiet or during slow wave sleep, and lowest during paradoxical sleep.103 Thus, retinopetal axons in diurnal animals would be expected to release more serotonin during the day, and the opposite would be true in nocturnal animals.
FIGURE 8.
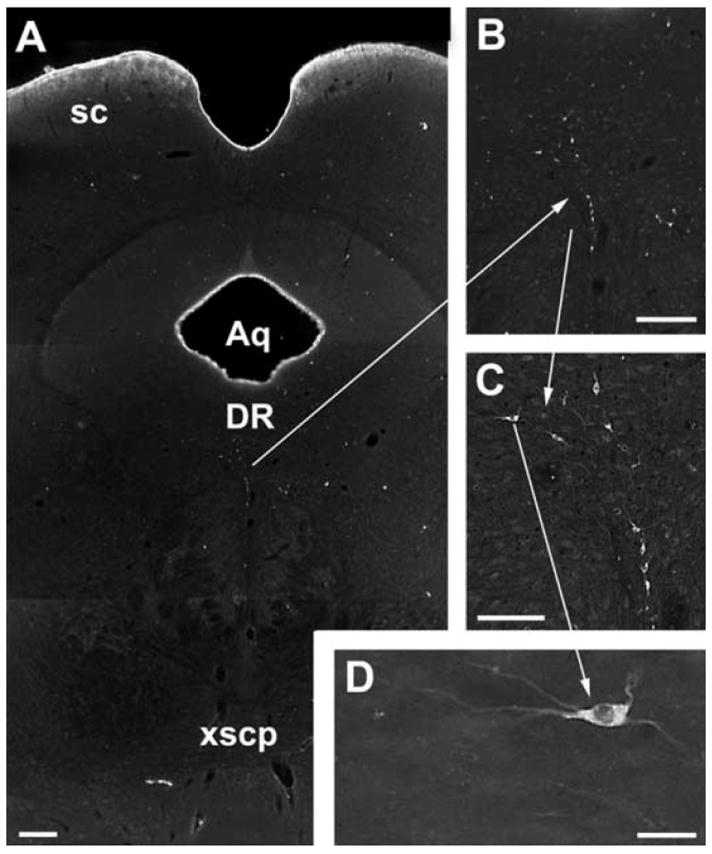
Darkfield photomicrographs of retrogradely labeled neurons in the dorsal raphe of a green monkey. St. Kitts vervet monkeys (n =2) were given a 60-μl intravitreal injection of cholera toxin subunit B (CTB; 1 mg/ml) with 10% dimethyl sulfoxide. After a 2-week survival, the animals were perfusion fixed with 4% paraformaldehyde. Coronal sections of the brain were cut on a Vibratome, incubated in a goat anti-CTB antibody (1:10000, List Biological Laboratories), and visualized using an immunoperoxidase technique.96 (A–D) CTB-labeled neurons were found in the dorsal raphe. Each panel represents an increasing level of magnification. Scale bar = 200 μm (A, B), scale bar = 100 μm (C). (D) Retrogradely labeled neurons like this one (arrow) also contained immunoreactive serotonin (not illustrated). Scale bar = 20 μm. DR, dorsal raphe; Aq, cerebral aqueduct; SC, superior colliculus.
There are 14 types of receptors for serotonin, and they are divided into 7 families of related molecules, 5-HT1 to 5-HT7. 5-HT3 is an ionotropic receptor, but the rest are G-protein–coupled receptors.104 One possible function of the 5-HT-IR axons associated with retinal blood vessels is vasoconstriction. Serotonin constricts blood vessels in bovine105 and atherosclerotic monkey106 retinas, and serotonin produces retinal ischemia in rats.107 In humans, topical application of a 5-HT2 antagonist increases blood flow in the posterior ciliary arteries supplying the optic disk.108 In monkeys, the distribution of 5-HT-IR axon terminals on retinal blood vessels suggests that they may also have a chemosensory function. Serotonergic neurons in the dorsal raphe are stimulated by increases in arterial CO2 concentration,109 and according to this hypothesis, the 5-HT-IR axons convey a signal to the brain reflecting the activity of neurons in the retina.
The physiological effects of serotonin and the localization of serotonin receptors suggest that retinal neurons are the major targets of serotonergic retinopetal axons. In cat retinas, serotonin decreases the maintained firing rates of ON center ganglion cells and increases the firing rates of OFF center cells; it also has similar, but smaller, effects on their light responses. The effects on maintained activity may contribute to light adaptation by counteracting the effect of background light.110 Serotonin also increases the maintained firing rates of rabbit retinal ganglion cells111,112 and increases the amplitude of the b-wave of the ERG in cats.113 In rabbit retinas, serotonin stimulates the release of purines in normal saline but inhibits their release in saline with elevated K+.114 Serotonin also inhibits the release of thyrotropin releasing hormone from rat retinas.115
5-HT1 receptors are present on rabbit retinal membranes,92 and mRNA for 5-HT1A is present in rabbit and rat retinas.116,117 The effects of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino) tetralin(8-OH-DPAT) are particularly well-characterized in rabbit retinas.88 8-OH-DPAT reduces the ON responses of retinal ganglion cells; it either has no effect or increases the responses of OFF cells. Although 8-OH-DPAT has no effect on horizontal cells or the b-wave of the ERG, it blocks the inhibitory effects of horizontal cell stimulation on retinal ganglion cells.118 Thus, serotonin acting at 5-HT1A would be expected to reduce the responsiveness of receptive field surrounds in rabbit retinas. Some of the effects of serotonin and 8-OH-DPAT might be mediated by 5-HT7,104 which are also expressed in rat and rabbit retinas.116,117 For example, the increases in rabbit retinal cAMP in response to serotonin119 and 8-OH-DPAT120 originally attributed to 5-HT1 are now thought to be mediated by 5-HT7.116 Serotonin acting at 5-HT7 also inhibits dopamine release from rabbit retinas.93
In rabbits, 5-HT2 receptors are found on retinal membranes,92 and serotonin acting through 5-HT2 stimulates inositol phosphate formation in retinal slices.121 5-HT2A are localized to the axon terminals of rod bipolar cells, rod spherules, cone pedicles, and unidentified puncta in the IPL122 (Fig. 9 A,B), and serotonin acting at 5-HT2 inhibits dopamine release.93 The effects mediated by 5-HT2 on rabbit retinal ganglion cells are essentially the opposite of those mediated by 5-HT1A.88 In rat retinas, the mRNA for 5-HT2C has been localized to a subset of perikarya in the proximal INL and in the GCL by in situ hybridization, a finding suggesting that these receptors are expressed by amacrine cells, ganglion cells, or both types.123 Acting through 5-HT2, serotonin decreases the Cl− current through GABAC receptors of rat bipolar cells.124 Serotonin released from retinopetal axons would, therefore, be expected to reduce the efficacy of inhibitory inputs from amacrine cells to bipolar cells, increase the amplitude of bipolar cell light responses, and increase the rate of spontaneous glutamate release. If the effects of serotonin are similar in other mammalian retinas, this may account for the increase in the amplitude of the b-wave of the cat ERG113 and the increases in the maintained firing rates of rabbit retinal ganglion cells.111,112 5-HT3A receptors are localized to human, rat, and rabbit rod spherules.117 (Figs. 10 C,D). Accordingly, the effects of serotonin via 5-HT3 receptors are observed only in dark-adapted retinas; it enhances the responses of ON ganglion cells and decreases the responses of OFF ganglion cells.125
FIGURE 9.
Serotonin receptors in the rabbit retina. (A-B) Serotonin receptors of the 5-HT2A subtype are localized to rod bipolar cell terminals in the rabbit retina. These vertical sections were labeled with antibodies to 5-HT2A (red) and protein kinase C (PKC, green). 5-HT2A-IR puncta were found in the inner plexiform layer (IPL, arrows) (A) and outer plexiform layer (OPL) (B). All of the PKC-IR rod bipolar cells expressed 5-HT2A in both the IPL and OPL. (C-D) 5-HT3A receptors in the rabbit retina are localized to rod spherules in the outer plexiform layer (OPL). Using an antibody to 5-HT3A (red), large puncta were labeled in the OPL. The yellow regions in panel (C) demonstrate that the 5-HT3A-IR were colocalized with a marker for rod spherules, B16 (green). Alternatively in panel (D), a marker of cone pedicles, peanut agglutin (green), was not associated with 5-HT3A-IR puncta. (A-B) Modified and reprinted from Pootanakit and Brunken.122 (C-D) Modified and reprinted from Pootanakit et al.117
In summary, serotonin released from retinopetal axons is expected to act on at least four types of receptors, and the three types that have been localized each have a different distribution within the retina. To date, the effects of serotonin, its agonists, and its antagonists on retinal physiology have been studied only in nocturnal or crepuscular animals. The results suggest that serotonin released from retinopetal axons improves the performance of neural circuits in the retina at scotopic or mesopic levels of illumination, the conditions prevailing when these animals are awake. Acting via 5-HT1, serotonin would be expected to increase absolute sensitivity of rabbit retinal ganglion cells by decreasing surround inhibition. Serotonin also increases the responses to increments in light intensity in cat and rabbit retinal ganglion cells, probably through its effects on bipolar cells via 5-HT2. Acting through 5-HT3, serotonin increases the sensitivity of ON retinal ganglion cells to light stimuli in dark-adapted rabbit retinas. Serotonin also inhibits dopamine release from rabbit amacrine cells via 5-HT2 and 5-HT7, and this may contribute to dark adaptation. To understand how serotonergic retinopetal axons contribute to retinal information processing in diurnal animals, it will be necessary to extend the physiological studies to other species.
CONCLUSIONS
Mammalian retinas receive input from neurons in the brain, and in this respect, they are like other vertebrate retinas and sensory systems in general. In mammals, the two populations of retinopetal axons that have been studied most thoroughly originate from cell bodies in the hypothalamus and midbrain that contain histamine or serotonin, respectively. These neurons also project to many other targets in the central nervous system. They fire tonically according to the sleep/wake cycle, with the highest rates when the animal is awake.126 With one exception, the receptors for these neurotransmitters are G-protein–coupled receptors. The axons containing histamine and serotonin are morphologically similar, and there are also some similarities in the localization of their receptors. Although these retinopetal axons terminate in the inner retina, their targets are often in the outer retina. Serotonin receptors are localized to photoreceptor terminals, and histamine receptors are localized to ON bipolar cell dendrites. These findings suggest that at least some effects of retinopetal axons are mediated by volume transmission. As a result, retinopetal axons are able to influence many types of retinal neurons. Dopaminergic amacrine cells are also targets in some species, and because dopamine influences so many types of retinal neurons, this greatly amplifies the effects of the retinopetal axons.
Many of the effects of histamine and serotonin on light responses suggest that retinopetal axons optimize retinal function at the ambient light intensity during the animal’s waking period. Histamine reduces the amplitude of light responses in a subset of monkey retinal ganglion cells, a finding consistent with a role for retinopetal axons in light adaptation in these diurnal animals. Several of the effects of serotonin in retinas of nocturnal and crepuscular animals suggest that retinopetal axons play a role in dark adaptation in these species. Serotonin reduces the strength of surround inhibition in rabbit retinal ganglion cells, disinhibits rat bipolar cells, and increases the amplitude of the b-wave of the cat ERG. Both neurotransmitters of retinopetal axons also inhibit dopaminergic neurons in nocturnal or crepuscular animals. Dopaminergic neurons express HR1 in rats, and histamine inhibits dopamine release from guinea-pig retinas. Serotonin inhibits dopamine release from rabbit retinas through both 5-HT2 and 5-HT7. Because dopamine plays a major role in light adaptation,127 a reduction in dopamine release at night may facilitate dark adaptation.
Histamine and serotonin released from retinopetal axons may contribute to vision in other ways as well. Both neurotransmitters modulate the maintained rate of firing in retinal ganglion cells, and this should increase their operating ranges. Cells that have no maintained activity can signal only by increasing their firing rates, and cells with high rates can signal only by decreasing their rates. On the other hand, cells with intermediate maintained firing rates can signal by either increasing or decreasing their firing rates.128,129
Acting through 5-HT1B, serotonin increases the sensitivity of mice to the effects of constant light on their circadian rhythm of activity.130 Increasing the sensitivity of the retina to light might be detrimental under pathological conditions, however. The photoreceptors of rats are more sensitive to light damage with the optic nerves intact than after the optic nerves have been sectioned.131 There might be neuroprotective effects mediated by the dying ganglion cells or by the reduction of visual input to the brain, but another possibility is that sectioning the optic nerve removes retinopetal axons that normally increase the sensitivity of the rat retina to light during the night. The neuroprotective effects of 5-HT2 antagonists in rats suggest that serotonin increases the sensitivity of ionotropic glutamate receptors in the retina but also makes the retina more vulnerable to ischemia.132
Histaminergic retinopetal axons might contribute to the etiology of diabetic retinopathy through their interactions with retinal blood vessels. The axons undergo degenerative changes in the retinas of streptozotocin-diabetic rats,20 and in a preliminary clinical trial, a combination of HR1 and HR2 antagonists decreased the permeability of the blood-retinal barrier in diabetic patients with nonproliferative diabetic retinopathy.133 Although a larger trial with HR1 antagonist alone did not conclusively demonstrate a beneficial effect in similar patients, there were robust trends toward improvement in the areas of the retina where the density of histaminergic retinopetal axons is expected to be highest based on results in non-human primates.19,134
Further investigations of the cellular localization of receptors for retinopetal neurotransmitters and their effects at each site are likely to yield other examples of contributions of retinopetal axons to vision or to the etiology of retinal diseases. It is also essential to identify more neurotransmitters and co-transmitters of retinopetal axons. Anatomical studies indicate that there are additional types of retinopetal axons whose origins and functions are still unknown.
Acknowledgments
Support for this study was provided by National Eye Institute grants EY06472 and EY12610, Core Grant EY10608, and grants from the Juvenile Diabetes Research Foundation and the Plum Foundation.
Contributor Information
Matthew J. Gastinger, Graduate School of Biomedical Sciences, The University of Texas Health Science Center Houston, Houston, Texas, USA; and Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, Houston, Texas, USA
Ning Tian, Department of Ophthalmology and Visual Sciences, Yale University School of Medicine, New Haven, Connecticut, USA.
Tamas Horvath, Department of Obstetrics and Gynecology, Yale University School of Medicine, New Haven, Connecticut, USA.
David W. Marshak, Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, Houston, Texas, USA
References
- 1.Ramon y, Cajal S. The Structure of the Retina. Springfield: Charles C Thomas; 1892. p. 196. [Google Scholar]
- 2.Polyak SL. The Retina. Chicago: The University of Chicago Press; 1941. [Google Scholar]
- 3.Schütte M. Centrifugal innervation of the rat retina. Vis Neurosci. 1995;12:1083–1092. doi: 10.1017/s0952523800006738. [DOI] [PubMed] [Google Scholar]
- 4.Drager UC, Edwards DL, Barnstable CJ. Antibodies against filamentous components in discrete cell types of the mouse retina. J Neurosci. 1984;4:2025–2042. doi: 10.1523/JNEUROSCI.04-08-02025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bons N, Petter A. Retinal afferents of hypothalamic origin in a prosimian primate: Microcebus murinus. Study using retrograde fluorescent tracers. Comptes rendus de l’Academia des sciences Serie III. 1986;303:719–722. [PubMed] [Google Scholar]
- 6.Honrubia FM, Elliott JH. Efferent innervation of the retina. II. Morphologic study of the monkey retina. Invest Ophthal. 1970;9:971–976. [PubMed] [Google Scholar]
- 7.Usai C, Ratto GM, Bisti S. Two systems of branching axons in monkey’s retina. J Comp Neurol. 1991;308:149–161. doi: 10.1002/cne.903080202. [DOI] [PubMed] [Google Scholar]
- 8.Honrubia FM, Elliott JH. Efferent innervation of the retina. I. Morphologic study of the human retina. Arch Ophthalmol. 1968;80:98–103. doi: 10.1001/archopht.1968.00980050100017. [DOI] [PubMed] [Google Scholar]
- 9.Repérant J, Miceli D, Vesselkin NP, Molotchnikoff S. The centrifugal visual system of vertebrates: a century-old search reviewed. Int Rev Cytol. 1989;118:115–171. doi: 10.1016/s0074-7696(08)60874-8. [DOI] [PubMed] [Google Scholar]
- 10.Peterson BB, Dacey DM. Morphology of human retinal ganglion cells with intraretinal axon collaterals. Vis Neurosci. 1998;15:377–387. doi: 10.1017/s0952523898152161. [DOI] [PubMed] [Google Scholar]
- 11.Brooke RN, Downer JdC, Powell TP. Centrifugal fibres to the retina in the monkey and cat. Nature. 1965;207:1365–1367. doi: 10.1038/2071365a0. [DOI] [PubMed] [Google Scholar]
- 12.Wakakura M, Ishikawa S. Ultrastructural study on centrifugal fibers in the feline retina. Jap J Ophthal. 1982;26:282–291. [PubMed] [Google Scholar]
- 13.Perry VH, Oehler V, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- 14.Larsen JN, MØller M. The presence of retinopetal fibres in the optic nerve of the Mongolian gerbil (Meriones unguiculatus): a horseradish peroxidase in vitro study. Exp Eye Res. 1987;45:763–768. doi: 10.1016/s0014-4835(87)80093-3. [DOI] [PubMed] [Google Scholar]
- 15.Hoogland PV, Vanderkrans A, Koole FD, Groenewegen HJ. A direct projection from the nucleus oculomotorius to the retina in rats. Neurosci Lett. 1985;56:323–328. doi: 10.1016/0304-3940(85)90263-0. [DOI] [PubMed] [Google Scholar]
- 16.Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 17.Terubayashi H, Fujisawa H, Itoi M, Ibata Y. Hypothalamo-retinal centrifugal projection in the dog. Neurosci Lett. 1983;40:1–6. doi: 10.1016/0304-3940(83)90082-4. [DOI] [PubMed] [Google Scholar]
- 18.Labandeira-Garcia JL, Guerra-Seijas MJ, Gonzalez F, et al. Location of neurons projecting to the retina in mammals. Neurosci Res. 1990;8:291–302. doi: 10.1016/0168-0102(90)90035-d. [DOI] [PubMed] [Google Scholar]
- 19.Gastinger MJ, O’Brien JJ, Larsen NB, Marshak DW. Histamine immunoreactive axons in the macaque retina. Invest Ophthal Vis Sci. 1999;40:487–495. [PMC free article] [PubMed] [Google Scholar]
- 20.Gastinger MJ, Barber AJ, Khin SA, et al. Abnormal centrifugal axons in streptozotocin-diabetic rat retinas. Invest Ophthal Vis Sci. 2001;42:2679–2685. [PMC free article] [PubMed] [Google Scholar]
- 21.van Hasselt P. The centrifugal control of retinal function. Ophthalmic Res. 1972;4:298–320. [Google Scholar]
- 22.Hankins MW, Jones RJM, Ruddock KH. Diurnal variation in the B-wave implicit time of the human electroretinogram. Vis Neurosci. 1998;15:55–67. doi: 10.1017/s0952523898151118. [DOI] [PubMed] [Google Scholar]
- 23.Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- 24.Birch DG, Berson EL, Sandberg MA. Diurnal rhythm in the human rod ERG. Invest Ophthal Vis Sci. 1984;25:236–238. [PubMed] [Google Scholar]
- 25.Bassi CJ, Powers MK. Daily fluctuations in the detectability of dim lights by humans. Physiol Behav. 1986;38:871–877. doi: 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- 26.Roenneberg T, Lotze M, von Steinbuchel N. Diurnal variation in human visual sensitivity determined by incremental thresholds. Clin Vis Sci. 1992;7:83–91. [Google Scholar]
- 27.Goldman AI, Teirstein PS, O’Brien PJ. The role of ambient lighting in circadian disc shedding in the rod outer segment of the rat retina. Invest Ophthal Vis Sci. 1980;19:1257–1267. [PubMed] [Google Scholar]
- 28.Galambos R, Juhász G, Kékesi AK, et al. Natural sleep modifies the rat electroretinogram. Proc Nat Acadl Sci USA. 1994;91:5153–5157. doi: 10.1073/pnas.91.11.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pena M, Birch D, Uauy R, Peirano P. The effect of sleep state on electroretinographic (ERG) activity during early human development. Early Hum Dev. 1999;55:51–62. doi: 10.1016/s0378-3782(99)00006-7. [DOI] [PubMed] [Google Scholar]
- 30.Galambos R, Szabó-Salfay O, Szatmári E, et al. Sleep modifies retinal ganglion cell responses in the normal rat. Proc Natl Acad Sci USA. 2001;98:2083–2088. doi: 10.1073/pnas.98.4.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim IB, Oh SJ, Chun MH. Neuronal nitric oxide synthase immunoreactive neurons in the mammalian retina. Microsc Res Tech. 2000;50:112–1123. doi: 10.1002/1097-0029(20000715)50:2<112::AID-JEMT3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Rusoff AC, Hendrickson AE. Some mammalian retinae lack FMRF-amide-like immunoreactive efferents. Invest Ophthal Vis Sci. 1989;30:791–794. [PubMed] [Google Scholar]
- 33.Witkin JW. Immunocytochemical demonstration of luteinizing hormone-releasing hormone in optic nerve and nasal region of fetal rhesus macaque. Neurosci Lett. 1987;79:73–77. doi: 10.1016/0304-3940(87)90674-4. [DOI] [PubMed] [Google Scholar]
- 34.Santacana M, de la Vega AG, Heredia M, Valverde F. Presence of LHRH (luteinizing hormone-releasing hormone) fibers in the optic nerve, optic chiasm and optic tract of the adult rat. Brain Res Dev Brain Res. 1996;91:292–299. doi: 10.1016/0165-3806(95)00199-9. [DOI] [PubMed] [Google Scholar]
- 35.Wirsig-Wiechmann CR, Wiechmann AF. Vole retina is a target for gonadotropin-releasing hormone. Brain Res. 2002;950:210–217. doi: 10.1016/s0006-8993(02)03039-1. [DOI] [PubMed] [Google Scholar]
- 36.Witkin JW. Nervus terminalis, olfactory nerve, and optic nerve representation of luteinizing hormone-releasing hormone in primates. Ann NY Acad Sci. 1987;519:174–183. doi: 10.1111/j.1749-6632.1987.tb36296.x. [DOI] [PubMed] [Google Scholar]
- 37.Simon A, Martin-Martinelli E, Savy C, et al. Confirmation of the retinopetal/centrifugal nature of the tyrosine hydroxylase-immunoreactive fibers of the retina and optic nerve in the weaver mouse. Brain Res Dev Brain Res. 2001;127:87–93. doi: 10.1016/s0165-3806(01)00103-1. [DOI] [PubMed] [Google Scholar]
- 38.Simon A, Savy C, Martin-Martinelli E, et al. Paradoxical increase of tyrosine hydroxylase-immunoreactive retinopetal fibers in the weaver mouse. Brain Res Dev Brain Res. 2000;121:113–117. doi: 10.1016/s0165-3806(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 39.Airaksinen MS, Panula P. The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol. 1988;273:163–186. doi: 10.1002/cne.902730204. [DOI] [PubMed] [Google Scholar]
- 40.Manning KA, Wilson JR, Uhlrich DJ. Histamine-immunoreactive neurons and their innervation of visual regions in the cortex, tectum, and thalamus in the primate Macaca mulatta. J Comp Neurol. 1996;373:271–282. doi: 10.1002/(SICI)1096-9861(19960916)373:2<271::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Nowak JZ. Histamine in the retina and some other components of the visual system. Prog Ret Res. 1993;12:41–74. [Google Scholar]
- 42.Smelser GK, Silver S. The distribution of mast cells in the normal eye. Exp Eye Res. 1963;2:134–141. doi: 10.1016/s0014-4835(63)80005-6. [DOI] [PubMed] [Google Scholar]
- 43.Nowak JZ, Nawrocki J, Malinski C. Histamine in the rabbit eye: distribution, synthesis, catabolism, and changes by light stimulation. Agents Actions. 1985;16:80–83. doi: 10.1007/BF01983106. [DOI] [PubMed] [Google Scholar]
- 44.Arbones L, Garcia-Verdugo J, Picatoste F, Garcia A. Presence and distribution of histaminergic components in rat and bovine retina. Neurochem J. 1988;13:97–104. doi: 10.1016/0197-0186(88)90108-8. [DOI] [PubMed] [Google Scholar]
- 45.Sawai S, Fukui H, Imamura I, et al. Histamine and its synthesis in mammalian retinas. J Ocular Pharmacol. 1991a;7:213–219. [PubMed] [Google Scholar]
- 46.Steininger TL, Alam MN, Gong H, et al. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- 47.Lin JS, Sakai K, Vanni-Mercier G, et al. Involvement of histaminergic neurons in arousal mechanisms demonstrated with H3-receptor ligands in the cat. Brain Res. 1990;523:325–330. doi: 10.1016/0006-8993(90)91508-e. [DOI] [PubMed] [Google Scholar]
- 48.Vanni-Mercier G, Gigout S, Debilly G, Lin JS. Waking selective neurons in the posterior hypothalamus and their response to histamine H3-receptor ligands: an electrophysiological study in freely moving cats. Behav Brain Res. 2003;144:227–241. doi: 10.1016/s0166-4328(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 49.Sawai S, Fukui H, Fukuda M, et al. [3H]mepyramine binding sites, histamine H1-receptors, in bovine retinal blood vessels. Curr Eye Res. 1991b;10:713–718. doi: 10.3109/02713689109013865. [DOI] [PubMed] [Google Scholar]
- 50.Bamforth SD, Lightman SL, Greenwood J. Interleukin-1 beta-induced disruption of the retinal vascular barrier of the central nervous system is mediated through leukocyte recruitment and histamine. Am J Pathol. 1997;150:329–340. [PMC free article] [PubMed] [Google Scholar]
- 51.Benedito S, Prieto D, Nielsen PJ, Nyborg NC. Histamine induces endothelium-dependent relaxation of bovine retinal arteries. Invest Ophthal Vis Sci. 1991;32:32–38. [PubMed] [Google Scholar]
- 52.Schönfelder U, Hofer A, Paul M, Funk RH. In situ observation of living pericytes in rat retinal capillaries. Microvas Res. 1998;56:22–29. doi: 10.1006/mvre.1998.2086. [DOI] [PubMed] [Google Scholar]
- 53.Wagner U, Wiederholt M. Membrane voltage and whole-cell currents in cultured pericytes of control rats and rats with retinal dystrophy. Curr Eye Res. 1996;15:1045–1053. doi: 10.3109/02713689609017654. [DOI] [PubMed] [Google Scholar]
- 54.Ramachandran E, Frank RN, Kennedy A. Effects of endothelin on cultured bovine retinal microvascular pericytes. Invest Ophthal Vis Sci. 1993;34:586–595. [PubMed] [Google Scholar]
- 55.Gardner TW, Lesher T, Khin S, et al. Histamine reduces ZO-1 tight-junction protein expression in cultured retinal microvascular endothelial cells. Biochem J. 1996;320:717–721. doi: 10.1042/bj3200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osusky R, Rohr P, Schotzau A, Flammer J. Nocturnal dip in the optic nerve head perfusion. Jpn J Ophthalmol. 2000;44:128–131. doi: 10.1016/s0021-5155(99)00188-4. [DOI] [PubMed] [Google Scholar]
- 57.Sawai S, Wang NP, Fukui H, et al. Histamine H1-receptor in the retina: species differences. Biochem Biophys Res Comm. 1988;150:316–322. doi: 10.1016/0006-291x(88)90522-0. [DOI] [PubMed] [Google Scholar]
- 58.Gastinger MJ, Vardi N, Marshak DW. Localization of histamine receptors in mammalian retinas. Washington, DC: Society for Neuroscience; 2004. Program No. 934.3. 2004 Abstract Viewer/Itinerary Planner http://web.sfn.org. [Google Scholar]
- 59.Turner P. Critical flicker frequency and centrally-acting drugs. Br J Ophthalmol. 1968;52:245–250. doi: 10.1136/bjo.52.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Large AT, Wayte G, Turner P. Promethazine on hand-eye co-ordination and visual function. J Pharmacy Pharmacol. 1971;23:134–135. doi: 10.1111/j.2042-7158.1971.tb08627.x. [DOI] [PubMed] [Google Scholar]
- 61.Nicholson AN, Smith PA, Spencer MB. Antihistamines and visual function: studies on dynamic acuity and the pupillary response to light. Br J Clin Pharmacol. 1982;14:683–690. doi: 10.1111/j.1365-2125.1982.tb04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicholson AN, Stone BM. The H1-antagonist mequitazine: studies on performance and visual function. Eur J Clin Pharmacol. 1983;25:563–566. doi: 10.1007/BF00542129. [DOI] [PubMed] [Google Scholar]
- 63.Lee BB, Martin PR, Valberg AV. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gastinger MJ, Barber AJ, Vardi N, Marshak DW. Histamine receptors in mammalian retinas. J Comp Neurol. 2006;496:658–667. doi: 10.1002/cne.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber B, Schlicker E. Modulation of dopamine release in the guinea-pig retina by G(i)- but not by G(s)- or G(q)-protein-coupled receptors. Fund Clin Pharmacol. 2001;15:393–400. doi: 10.1046/j.1472-8206.2001.00056.x. [DOI] [PubMed] [Google Scholar]
- 66.Nowak JZ, Sek B, Szkiel B. Histamine-mediated regulation of cAMP levels and inositol phosphate metabolism in isolated rabbit retina. Agents Actions. 1989;27:131–134. doi: 10.1007/BF02222219. [DOI] [PubMed] [Google Scholar]
- 67.Nowak JZ. Histamine in the retina. In: Watanabe T, Wada H, editors. Histaminergic Neurons: Morphology and Function. Boca Raton: CRC Press; 1991. pp. 365–382. [Google Scholar]
- 68.Gantz I, Munzert G, Tashiro T, Schaffer M, Wang L, DelValle J, Yamada T. Molecular cloning of the human histamine H2 receptor. Biochem Biophys Res Commun. 1991;178:1386–1392. doi: 10.1016/0006-291x(91)91047-g. [DOI] [PubMed] [Google Scholar]
- 69.Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Nat Acadl Sci USA. 1994;91:10893–10897. doi: 10.1073/pnas.91.23.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gastinger MJ, Yusupov RG, Glickman RD, Marshak DW. The effects of histamine on rat and monkey retinal ganglion cells. Vis Neurosci. 2004;21:935–943. doi: 10.1017/S0952523804216133. [DOI] [PubMed] [Google Scholar]
- 71.Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+ J Neurosci. 2000;20:4471–4479. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinto LH, Klumpp DJ. Localization of potassium channels in the retina. Prog Ret Eye Res. 1998;17:207–230. doi: 10.1016/s1350-9462(97)00011-6. [DOI] [PubMed] [Google Scholar]
- 73.Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 74.Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 75.Nowak JZ, Kulinski JZ. The origin and fate of histamine in the rabbit retina. Neurochem Int. 1986;8:53–58. doi: 10.1016/0197-0186(86)90100-2. [DOI] [PubMed] [Google Scholar]
- 76.Nowak JZ. Histamine in retina, optic nerve, choroid and brain of albino and pigmented rabbits. Pol J Pharmacol Pharm. 1985b;37:663–671. [PubMed] [Google Scholar]
- 77.Prell GD, Morrishow AM, Duoyon E, Lee WS. Inhibitors of histamine methylation in brain promote fromation of imidazoleacetic acid, which interacts with GABA receptors. J Neurochem. 1997;68:142–151. doi: 10.1046/j.1471-4159.1997.68010142.x. [DOI] [PubMed] [Google Scholar]
- 78.Qian H, Dowling JE. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994;14:4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian H, Dowling JE. GABAa and GABAc receptors on hybrid bass retinal bipolar cells. J Neurophysiol. 1995;74:1920–1928. doi: 10.1152/jn.1995.74.5.1920. [DOI] [PubMed] [Google Scholar]
- 80.Imamura Y, Kubota R, Wang Y, et al. Human retina-specific amine oxidase (RAO): cDNA cloning, tissue expression, and chromosomal mapping. Genomics. 1997;40:277–283. doi: 10.1006/geno.1996.4570. [DOI] [PubMed] [Google Scholar]
- 81.Imamura Y, Noda S, Mashima Y, Kudoh J, Oguchi Y, Shimizu N. Human retina-specific amine oxidase: genomic structure of the gene (AOC2), alternatively spliced variant, and mRNA expression in retina. Genomics. 1998;51:293–298. doi: 10.1006/geno.1998.5357. [DOI] [PubMed] [Google Scholar]
- 82.Enz R, Brandstatter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAc receptor p subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gastinger MJ, Marshak DW. A second retinopetal pathway in monkeys containing endogenous serotonin. Curr Eye Res. 2005;30:1089–1095. doi: 10.1080/02713680500371532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Florén I, Hendrickson A. Indoleamine-accumulating horizontal cells in the squirrel monkey retina. Invest Ophthal Vis Sci. 1984;25:997–1006. [PubMed] [Google Scholar]
- 86.Dowling JE, Ehinger B, Florén I. Fluorescence and electron microscopical observations on the amine-accumulating neurons of the cebus monkey retina. J Comp Neurol. 1980;192:665–685. doi: 10.1002/cne.901920404. [DOI] [PubMed] [Google Scholar]
- 87.Sandell JH, Masland RH. Indoleamine accumulation by retinal neurons exposed to blood. Histochem. 1989;92:57–60. doi: 10.1007/BF00495016. [DOI] [PubMed] [Google Scholar]
- 88.Brunken WJ, Jin XT, Pis-Lopez AM. The properties of the serotoninergic system in the retina. Prog Ret Res. 1993;12:75–99. [Google Scholar]
- 89.Ehinger B, Hansson C, Tornqvist K. 5-Hydroxytryptamine in the retina of some mammals. Exp Eye Res. 1981;33:663–672. doi: 10.1016/s0014-4835(81)80106-6. [DOI] [PubMed] [Google Scholar]
- 90.Florén I, Hansson HC. Investigations into whether 5-hydroxytryptamine is a neurotransmitter in the retina of rabbit and chicken. Invest Ophthal Vis Sci. 1980;19:117–125. [PubMed] [Google Scholar]
- 91.Osborne NN, Nesselhut T, Nicholas DA, et al. Serotonin-containing neurones in vertebrate retinas. J Neurochem. 1982;39:1519–1528. doi: 10.1111/j.1471-4159.1982.tb07984.x. [DOI] [PubMed] [Google Scholar]
- 92.Mitchell CK, Redburn DA. Analysis of pre- and postsynaptic factors of the serotonin system in rabbit retina. J Cell Biol. 1985;100:64–73. doi: 10.1083/jcb.100.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neal MJ, Cunningham JR, Matthews KL. Activation of nicotinic receptors on GABAergic amacrine cells in the rabbit retina indirectly stimulates dopamine release. Vis Neurosci. 2001;18:55–64. doi: 10.1017/s0952523801181058. [DOI] [PubMed] [Google Scholar]
- 94.Chanut E, Nguyen-Legros J, Labarthe B, et al. Serotonin synthesis and its light-dark variation in the rat retina. J Neurochem. 2002;83:863–869. doi: 10.1046/j.1471-4159.2002.01194.x. [DOI] [PubMed] [Google Scholar]
- 95.Liang J, Wessel JH, 3rd, Iuvone PM, et al. Diurnal rhythms of tryptophan hydroxylase 1 and 2 mRNA expression in the rat retina. Neuroreport. 2004;15:1497–1500. doi: 10.1097/01.wnr.0000131007.59315.66. [DOI] [PubMed] [Google Scholar]
- 96.Abizaid A, Horvath B, Keefe DL, et al. Direct visual and circadian pathways target neuroendocrine cells in primates. Eur J J Neurosci. 2004;20:2767–2776. doi: 10.1111/j.1460-9568.2004.03737.x. [DOI] [PubMed] [Google Scholar]
- 97.Villar MJ, Vitale ML, Parisi MN. Dorsal raphe serotonergic projection to the retina. A combined peroxidase tracing-neurochemical/high-performance liquid chromatography study in the rat. Neuroscience. 1987;22:681–686. doi: 10.1016/0306-4522(87)90364-2. [DOI] [PubMed] [Google Scholar]
- 98.Labandeira-Garcia JL. The retinopetal system in the rat. Neurosci Res. 1988;6:88–95. doi: 10.1016/0168-0102(88)90010-7. [DOI] [PubMed] [Google Scholar]
- 99.Fite KV, Janusonis S, Foote W, Bengston L. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. J Comp Neurol. 1999;414:469–484. doi: 10.1002/(sici)1096-9861(19991129)414:4<469::aid-cne4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 100.Repérant J, Araneda S, Miceli D, et al. Serotonergic retinopetal projections from the dorsal raphe nucleus in the mouse demonstrated by combined [(3)H] 5-HT retrograde tracing and immunolabeling of endogenous 5-HT. Brain Res. 2000;878:213–217. doi: 10.1016/s0006-8993(00)02706-2. [DOI] [PubMed] [Google Scholar]
- 101.Fite KV, Janusonis S. Retinal projection to the dorsal raphe nucleus in the Chilean degus (Octodon degus) Brain Res. 2001;895:139–145. doi: 10.1016/s0006-8993(01)02061-3. [DOI] [PubMed] [Google Scholar]
- 102.Lima L, Urbina M. Serotonergic projections to the retina of rat and goldfish. Neurochem Int. 1998;32:133–141. doi: 10.1016/s0197-0186(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 103.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 104.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 105.Delaey C, Van de Voorde J. Retinal arterial tone is controlled by a retinal-derived relaxing factor. Circ Res. 1998;83:714–720. doi: 10.1161/01.res.83.7.714. [DOI] [PubMed] [Google Scholar]
- 106.Hayreh SS. Retinal and optic nerve head ischemic disorders and atherosclerosis: role of serotonin. Prog Ret Eye Res. 1999;18:191–221. doi: 10.1016/s1350-9462(98)00016-0. [DOI] [PubMed] [Google Scholar]
- 107.Boerrigter RM, Siertsema JV, Kema IP. Serotonin (5-HT) and the rat’s eye. Some pilot studies. Doc Ophthalmol. 1992;82:141–150. doi: 10.1007/BF00157004. [DOI] [PubMed] [Google Scholar]
- 108.Cellini M, Caramazza R. Color doppler imaging of ocular blood flow after topical ketanserin. Ophthalmologica. 1999;213:286–289. doi: 10.1159/000027440. [DOI] [PubMed] [Google Scholar]
- 109.Severson CA, Wang W, Pieribone VA, et al. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 110.Thier P, Wässle H. Indoleamine-mediated reciprocal modulation of on-centre and off-centre ganglion cell activity in the retina of the cat. J Physiol. 1984;351:613–630. doi: 10.1113/jphysiol.1984.sp015266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ames A, III, Pollen DA. Neurotransmission in central nervous tissue: a study of isolated rabbit retina. J Neurophysiol. 1969;32:424–442. doi: 10.1152/jn.1969.32.3.424. [DOI] [PubMed] [Google Scholar]
- 112.Brunken WJ, Daw NW. The effects of serotonin agonists and antagonists on the response properties of complex ganglion cells in the rabbit’s retina. Vis Neurosci. 1988;1:181–188. doi: 10.1017/s0952523800001450. [DOI] [PubMed] [Google Scholar]
- 113.Skrandies W, Wässle H. Dopamine and serotonin in cat retina: electroretinography and histology. Exp Brain Res. 1988;71:231–240. doi: 10.1007/BF00247483. [DOI] [PubMed] [Google Scholar]
- 114.Perez MT, Ehinger B. Multiple neurotransmitter systems influence the release of adenosine derivatives from the rabbit retina. Neurochem Int. 1989;15:411–420. doi: 10.1016/0197-0186(89)90158-7. [DOI] [PubMed] [Google Scholar]
- 115.Mitsuma T, Kayama M, Yokoi Y, et al. Effects of serotonin on the release of thyrotropin-releasing hormone from the rat retina in vitro. Hormone Metab Res. 1996;28:220–222. doi: 10.1055/s-2007-979168. [DOI] [PubMed] [Google Scholar]
- 116.Chidlow G, Le Corre S, Osborne NN. Localization of 5-hydroxytryptamine1A and 5-hydroxytryptamine7 receptors in rabbit ocular and brain tissues. Neuroscience. 1998;87:675–689. doi: 10.1016/s0306-4522(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 117.Pootanakit K, Brunken WJ. Identification of 5-HT(3A) and 5-HT(3B) receptor subunits in mammalian retinae: potential presynaptic modulators of photoreceptors. Brain Res. 2001;896:77–85. doi: 10.1016/s0006-8993(01)01998-9. [DOI] [PubMed] [Google Scholar]
- 118.Mangel SC, Brunken WJ. The effects of serotonin drugs on horizontal and ganglion cells in the rabbit retina. Vis Neurosci. 1992;8:213–218. doi: 10.1017/s0952523800002868. [DOI] [PubMed] [Google Scholar]
- 119.Blazynski C, Ferrendelli JA, Cohen AI. Indoleamine-sensitive adenylate cyclase in rabbit retina: characterization and distribution. J Neurochem. 1985;45:440–447. doi: 10.1111/j.1471-4159.1985.tb04007.x. [DOI] [PubMed] [Google Scholar]
- 120.Ghazi H, Osborne NN. Agonist-induced stimulation of inositol phosphates in primary rabbit retinal cultures. J Neurochem. 1988;50:1851–1858. doi: 10.1111/j.1471-4159.1988.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 121.Cutcliffe N, Osborne NN. Serotonergic and cholinergic stimulation of inositol phosphate formation in the rabbit retina. Evidence for the presence of serotonin and muscarinic receptors. Brain Res. 1987;421:95–104. doi: 10.1016/0006-8993(87)91279-0. [DOI] [PubMed] [Google Scholar]
- 122.Pootanakit K, Prior KJ, Hunter DD, Brunken WJ. 5-HT2a receptors in the rabbit retina: potential presynaptic modulators. Vis Neurosci. 1999;16:221–230. doi: 10.1017/s0952523899162035. [DOI] [PubMed] [Google Scholar]
- 123.Perez-Leon JA, Sarabia G, Miledi R, Garcia-Alcocer G. Distribution of 5-hydroxytriptamine2C receptor mRNA in rat retina. Brain Res Mol Brain Res. 2004;125:140–142. doi: 10.1016/j.molbrainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 124.Feigenspan A, Bormann J. Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J Physiol. 1994;481:325–330. doi: 10.1113/jphysiol.1994.sp020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin XT, Brunken WJ. Serotonin receptors modulate rod signals: a neuropharmacological comparison of light- and dark-adapted retinas. Vis Neurosci. 1998;15:891–902. doi: 10.1017/s0952523898155116. [DOI] [PubMed] [Google Scholar]
- 126.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 127.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 128.Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002;22:2737–2747. doi: 10.1523/JNEUROSCI.22-07-02737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sollars PJ, Ogilvie MD, Rea MA, Pickard GE. 5-HT1B receptor knock-out mice exhibit an enhanced response to constant light. J Biol Rhyth. 2002;17:428–437. doi: 10.1177/074873002237137. [DOI] [PubMed] [Google Scholar]
- 131.Bush RA, Williams TP. The effect of unilateral optic nerve section on retinal light damage in rats. Exp Eye Res. 1991;52:139–153. doi: 10.1016/0014-4835(91)90254-c. [DOI] [PubMed] [Google Scholar]
- 132.Inoue-Matsuhisa E, Sogo S, Mizota A, et al. Effect of MCI-9042, a 5-HT2 receptor antagonist, on retinal ganglion cell death and retinal ischemia. Exp Eye Res. 2003;76:445–452. doi: 10.1016/s0014-4835(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 133.Gardner TW, Eller AW, Friberg TR, DA JA, Hollis TM. Antihistamines reduce blood-retinal barrier permeability in type I (insulin-dependent) diabetic patients with nonproliferative retinopathy. A pilot study. Retina. 1995;15:134–140. [PubMed] [Google Scholar]
- 134.Gardener TW, Sander B, Larsen ML, Kunselman A, Tenhave T, Lund-Anderson H, Reimers J, Hubbard L, Blankenship GW, Quillen DA, Brod RD, Wilmarth MH, Post-Hansen H, Parving HH, Davis MD. An extension of the early treatment diabetic retinopathy study (ETDRS) system for grading of diabetic macular edema in the astemizole retinopathy trial. Curr Eye Res. 2006;31:535–547. doi: 10.1080/02713680600746112. [DOI] [PubMed] [Google Scholar]


