Abstract
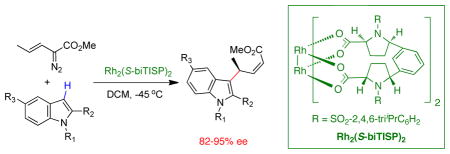
Asymmetric functionalization of N-heterocycles by vinylcarbenoids in the presence of catalytic amounts of Rh2 (S-biTISP)2 has been successfully developed. This bridged dirhodium catalyst not only selectively enforces the reaction to occur at the vinylogous position of the carbenoid, but also, affords high levels of asymmetric induction.
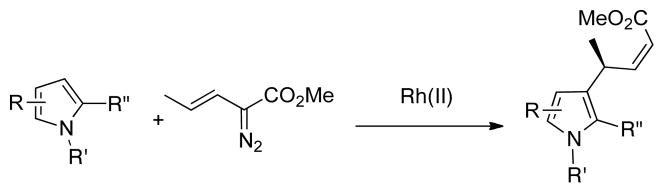
Asymmetric methods for the selective functionalization of indoles or pyrroles are in great demand1 because these electron-rich heterocycles are constituents of many natural products and pharmaceutical agents.2 The most widely used approach has been the conjugate addition of heterocycles to α,β-unsaturated carbonyl compounds using chiral transition-metal catalysts3 or organocatalysts.4 An alternative approach has been to use carbenoid intermediates.5 We have demonstrated that chiral 4-substituted indoles can be generated in a cascade sequence involving a combined C—H functionalization/Cope rearrangement.5a Fox5b and Hashimoto5g have shown that the electrophilic substitution reactions of methyl 2-diazoalkanoate generate chiral 3-substituted indoles with high levels of asymmetric induction. In this paper we describe an alternative carbenoid approach for the asymmetric synthesis of 3-substituted indoles by exploiting the vinylogous electrophilic character of vinylcarbenoids (Scheme 1). This transformation occurs with substrates that are too sterically crowded to react with the carbenoid site of the vinylcarbenoid
Scheme 1.
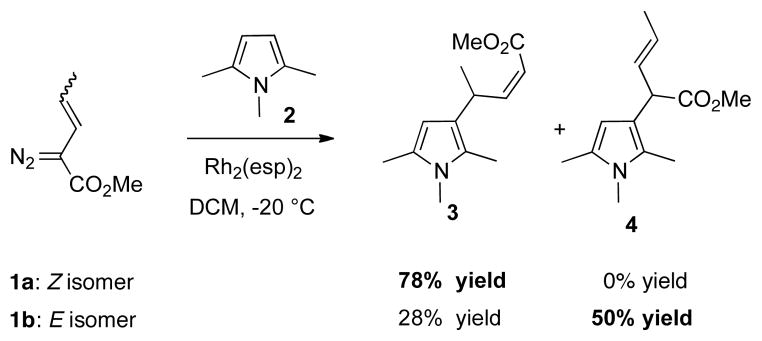
We have recently described the functionalization of electron rich heterocycles using 2-diazo-3-pentenoates as the carbenoid source.6 These reactions proceed by attack of the heterocycles at the vinylogous position of the vinylcarbenoid.6,7 Vinylogous reactivity of the carbenoid is more pronounced in Z-vinylcarbenoids than E-vinylcarbenoids.6 For example, the reaction of Z-2-diazopentenoate 1a with 1,2,5-trimethylpyrrole (2) gave the vinylogous alkylation product 3 in 78% yield exclusively (Scheme 2), whereas the major product from the reaction with the E-2-diazopentenoate 1b with 2 was 4, derived from electrophilic attack at the carbenoid center.6 The objective of the current study was to identify suitable chiral catalysts that enable the application of this unusual vinylogous reactivity to the asymmetric functionalization of electron rich heterocycles.
Scheme 2.
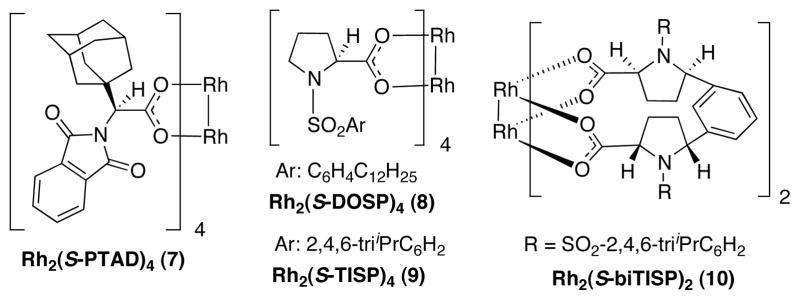
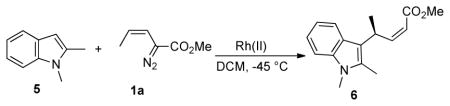
We initiated this study by exploring the enantioinduction by some of the standard chiral dirhodium catalysts that have been developed for the reactions of vinyldiazoacetates (Figure 1).8 The alkylation of indole 5 with Z-vinyldiazoacetate 1a was used as the standard screening reaction and the results are summarized in Table 1. All the catalysts in our study produced the alkylation product 6 in excellent yields (up to 93%). The two most versatile chiral catalysts for the asymmetric transformations of donor/acceptor carbenoids, Rh2(S-PTAD)4 (7) and Rh2(S-DOSP)4 (8), failed to give high levels of asymmetric induction in the test reaction. The more bulky catalysts Rh2(S-TISP)4 (9) gave slightly higher enantioselectvity than Rh2(S-DOSP)4 (26% ee vs 10% ee) but the bulky and conformationally constrained catalyst Rh2(S-biTISP)2 (10)9 gave 0% ee.
Figure 1.
Chiral dirhodium catalysts
Table 1.
Asymmetric alkylation of indole 5 by Z-vinyldiazoacetate 1a
 | |||
|---|---|---|---|
| entry | catalyst | yield (%) | ee (%)a |
| 1 | Rh2(S-PTAD)4 | 88 | 48 |
| 2 | Rh2(S-DOSP)4 | 93 | −10 |
| 3 | Rh2(S-TISP)4 | 84 | −26 |
| 4 | Rh2(S-biTISP)2 | 90 | 0 |
Negative value indicates opposite asymmetric induction.
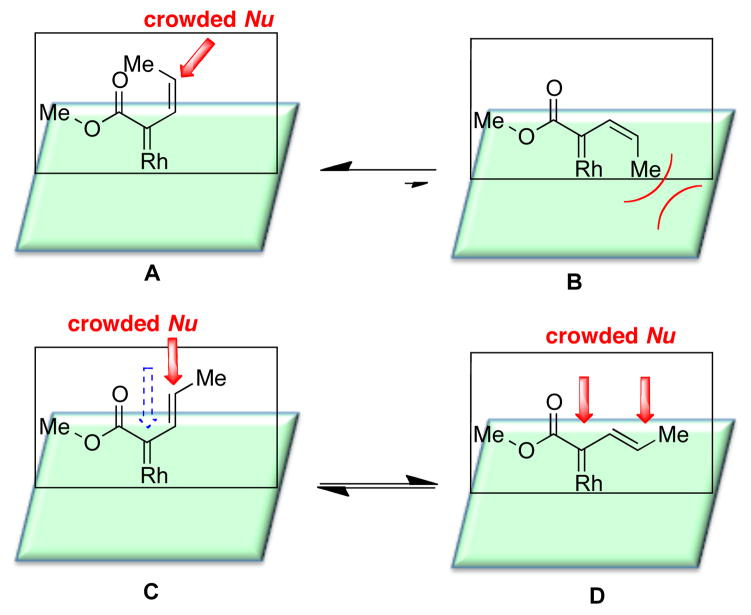
Having failed to achieve high levels of asymmetric induction with the Z-vinyldiazoacetate 1a, we decided to re-explore the possibility of using E-vinyldiazoacetate 1b as an effective reagent for vinylogous reactivity. Recently, we calculated that the s-cis configuration (conformer B) of Z-vinyldiazoacetates is sterically unfavorable.10 In contrast E-vinyldiazoacetates exist as equilibrating mixture of s-trans and s-cis conformers (conformers C and D) (Figure 2). On the basis of the reactivity patterns we have observed to date,6,10 we propose that carbenoids in s-trans configurations (conformers A and C) are more likely to display vinylogous reactivity than carbenoids in s-cis configurations (conformers B and D). The double bond geometry of the products derived from vinylogous reactivity is indicative of the reacting conformation of the carbenoid.6 Thus, the reaction of Z-vinyldiazoacetate with indole 5 proceeds through the s-trans conformer, leading to the formation of Z-6. Donor/acceptor carbenoids are known to be sensitive to steric effects, and if the nucleophile is sterically demanding, reactions at the vinylogous position of the carbenoid are enhanced.7c We, therefore, hypothesized that it would be possible to enhance the vinylogous reactivity of E-vinyldiazoacetates by re-enforcing the s-trans conformation of the carbenoid through the use of highly bulky catalysts.
Figure 2.
Steric influence on the conformation and reactivity of vinylcarbenoids
In order to explore this hypothesis, three standard achiral catalysts and four chiral catalysts were screened in the rhodium(II)-catalyzed decomposition of the (E)-vinyldiazoacetate 1b with 1,2-dimethylindole 5 (Table 2). These reactions afforded variable mixtures of three products (6, 11 and 12). Products 6 and 11 arise from the vinylogous reactivity of the s-trans and s-cis conformers of the vinylcarbenoid, respectively. Product 12 is derived from attack of the heterocycle at the carbenoid center and could, in principle, be derived from reaction with either conformer of the vinylcarbenoid. The product ratio is dependent on the catalyst structure. While the sterically less crowded catalysts Rh2(OAc)4 and Rh2(OAc)4 give considerable amounts of 11 and 12 (entries 1,2), the bulkier catalysts Rh2(esp)4, Rh2(S-PTAD)4, Rh2(S-TISP)4 and Rh2(S-biTISP)2 show a strong preference for the formation of 6 (entries 3,4,6,7). Most notable is the comparison between Rh2(S-DOSP)4, which gives near equimolar amounts of the three compounds (entry 5), and Rh2(S-TISP)4, which gives about a 8:1 preference for 6 over the two other products (entry 6). Furthermore, in the Rh2(S-biTISP)2 catalyzed reaction, 6 is isolated in 66% yield (after purification) and in 89% ee (entry 7).
Table 2.
Asymmetric alkylation of indole 5 with E-vinyldiazoacetate 1b
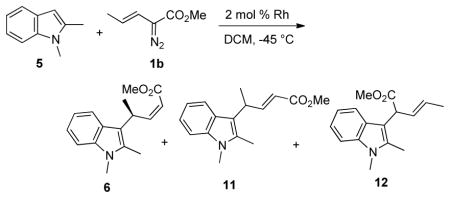 | ||||
|---|---|---|---|---|
| entry | catalyst | ratioa of 6/11/12 | yieldb of 6 (%) | ee of 6 (%) |
| 1 | Rh2(OAc)4 | 62/33/5 | 57 | --- |
| 2 | Rh2(TFA)4 | 49/44/7 | 43 | --- |
| 3 | Rh2(esp)2 | 74/26/0 | 52 | --- |
| 4 | Rh2(S-PTAD)4 | 76/16/8 | 55 | −8 |
| 5 | Rh2(S-DOSP)4 | 26/38/36 | 22 | 17 |
| 6 | Rh2(S-TISP)4 | 89/6/5 | 66 | 39 |
| 7 | Rh2(S-biTISP)2 | 88/9/3 | 66 | 89 |
ratio was the average of two runs and determined from the 1H NMR of the crude reaction mixture.
isolated yield
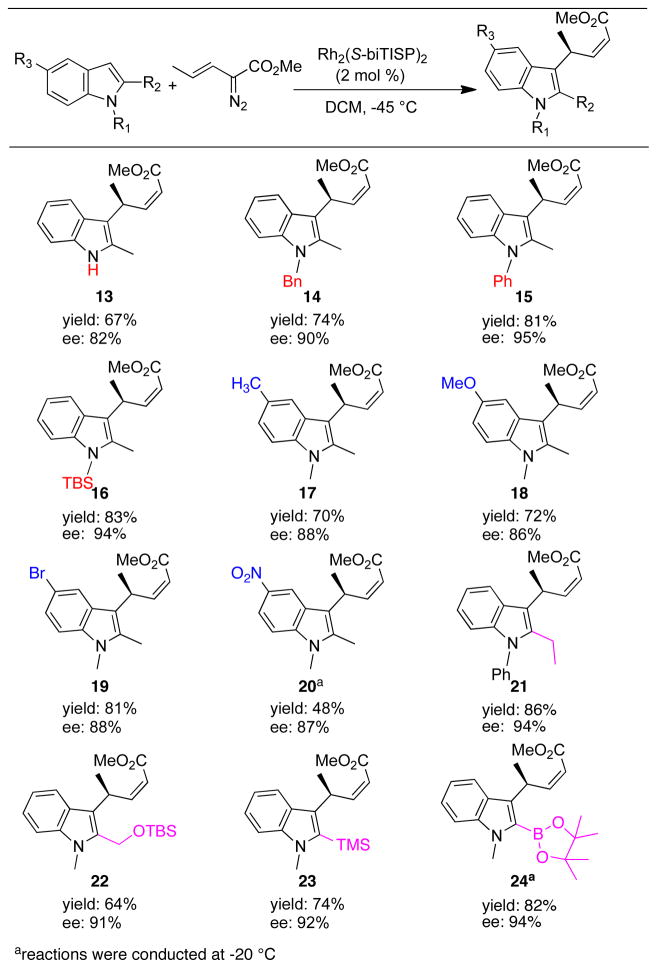
The Rh2(S-biTISP)2-catalyzed asymmetric vinylogous alkylation is applicable to a range of substituted indoles as illustrated in Figure 3. The desired transformation was observed for all substrates tested and the (Z)-pent-2-enoates 13–24 were produced in 48–86% yields with good levels of enantioselectivity (82–95% ee). Protected and unprotected 2-methylindoles are both effective in producing the vinylogous alkylation products, although increasing the size of the protecting group enhanced the asymmetric induction slightly (compare 13, 6, 14–16). This transformation was also successful with 2-methylindoles bearing different functionalities on the 5-position. However, 3-substituted indoles did not results in an efficient transformation. Good yields and excellent enantioselectivities were achieved with indoles containing bulky groups at the 2-position, such as TMS and pinacol boronate ester (23 and 24). The generation of 23 and 24 may offer the opportunity for further functionalization. The absolute configuration of 17 was unambiguously assigned by X-ray crystallography11 and the other products were assigned by analogy.
Figure 3.
Substrate scope of asymmetric vinylogous reactivity
areactions were conducted at −20 °C
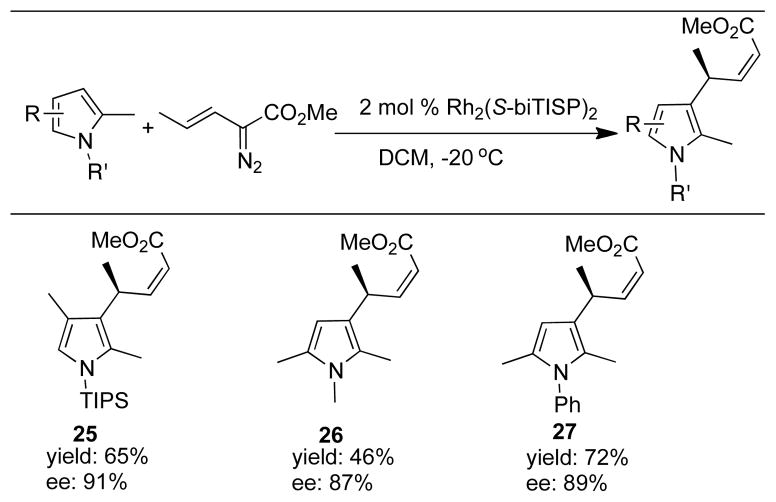
This reaction can be extended to pyrrole derivatives as shown in Figure 4. Due to the decreased reactivity of pyrroles, the reaction was conducted at −20 °C instead of −45 °C.12 Even so, the alkylation products 25–27 were obtained in good yield with high levels of enantioselectivity (87–91% ee). The absolute configuration of these products was assigned by analogy to the absolute configuration of 17.
Figure 4.
Scope of asymmetric vinylogous reactivity with pyrroles
In conclusion, the Rh2(S-biTISP)2-catalyzed asymmetric vinylogous alkylation between N-heterocycles and methyl E-2-diazo-3pentenoate is an effective method for C-3 functionalization of indoles and pyrroles. This work illustrates the subtle controlling elements of dirhodium catalysts on the chemistry of donor/acceptor carbenoids.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (GM099142). We thank Dr. Kenneth I. Hardcastle (Emory University) for the X-ray crystallographic analysis.
Footnotes
Supporting Information Available. Full experimental data, X-ray crystallographic data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For some reviews, see: Cacchi S, Fabrizi G. Chem Rev. 2005;105:2873. doi: 10.1021/cr040639b.Maryanoff BE, Zhang H, Cohen JH, Turchi IJ, Maryanoff CA. Chem Rev. 2004;104:1431. doi: 10.1021/cr0306182.Saracoglu N. Top Heterocycl Chem. 2007;11:1.Humphrey GR, Kuethe JT. Chem Rev. 2006;106:2875. doi: 10.1021/cr0505270.Poulsen TB, Jørgensen KA. Chem Rev. 2008;108:2903. doi: 10.1021/cr078372e.Thirumalairajan S, Pearce BM, Thompson A. Chem Commum. 2010;46:1797. doi: 10.1039/b926045e.
- 2.(a) Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. John Wiley& Sons Inc; Chichester: 2009. [Google Scholar]; (b) Barton DHR, Nakanishi K, MethCohn O, Kelly JW. Comprehensive Natural Products Chemistry. Pergamon Press; Oxford: 1999. [Google Scholar]; (c) Kochanowska-Karamyan AJ, Hamann MT. Chem Rev. 2010;110:4489. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Batt DG, Qiao JX, Modi DP, Houghton GC, Pierson DA, Rossi KA, Luettgen JM, Knabb RM, Jadhav PK, Wexler RR. Biorg Med Chem Lett. 2004;14:5269. doi: 10.1016/j.bmcl.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 3.(a) Evans DA, Fandrick KR, Song H, Scheidt KA, Xu R. J Am Chem Soc. 2007;129:10029. doi: 10.1021/ja072976i. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Müller C. J Am Chem Soc. 2008;130:2438. doi: 10.1021/ja711080y. [DOI] [PubMed] [Google Scholar]; (c) Arai T, Yokoyama N. Angew Chem Int Ed. 2008;47:4989. doi: 10.1002/anie.200801373. [DOI] [PubMed] [Google Scholar]; (d) Rasappan R, Hager M, Gissibl A, Reiser O. Org Lett. 2006;8:6099. doi: 10.1021/ol062697k. [DOI] [PubMed] [Google Scholar]; (f) Zhou J, Tang Y. J Am Chem Soc. 2002;124:9030. doi: 10.1021/ja026936k. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bartoli G, Bencivenni G, Dalpozzo R. Chem Soc Rev. 2010;39:4449. doi: 10.1039/b923063g. [DOI] [PubMed] [Google Scholar]; (b) Doyle AG, Jacobsen EN. Chem Rev. 2007;107:5713. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; (c) Austin JF, MacMillan DWC. J Am Chem Soc. 2002;124:1172. doi: 10.1021/ja017255c. [DOI] [PubMed] [Google Scholar]; (d) Galzerano P, Pesciaioli F, Mazzanti A, Bartoli G, Melchiorre P. Angew Chem Int Ed. 2009;48:7892. doi: 10.1002/anie.200903803. [DOI] [PubMed] [Google Scholar]; (e) Herrera RP, Sgarzani V, Bernardi L, Ricci A. Angew Chem Int Ed. 2005;44:6576. doi: 10.1002/anie.200500227. [DOI] [PubMed] [Google Scholar]; (f) Austin JF, Kim SG, Sinz CJ, Xiao WJ, MacMillan DWC. Proc Natl Acad Sci U S A. 2004;101:5482. doi: 10.1073/pnas.0308177101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ganesh M, Seidel D. J Am Chem Soc. 2008;130:16464. doi: 10.1021/ja8063292. [DOI] [PubMed] [Google Scholar]
- 5.(a) Davies HML, Manning JR. J Am Chem Soc. 2006;128:1060. doi: 10.1021/ja057768+. [DOI] [PubMed] [Google Scholar]; (b) DeAngelis A, Shurtleff VW, Dmitrenko O, Fox JM. J Am Chem Soc. 2011;133:1650. doi: 10.1021/ja1093309. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Davies HML, Hedley SJ. Chem Soc Rev. 2007;36:1109. doi: 10.1039/b607983k. [DOI] [PubMed] [Google Scholar]; (d) Johansen MB, Kerr MA. Org Lett. 2010;12:4956. doi: 10.1021/ol1020948. [DOI] [PubMed] [Google Scholar]; (e) Chan WW, Yeung SH, Zhou Z, Chan ASC, Yu WY. Org Lett. 2010;12:604. doi: 10.1021/ol9028226. [DOI] [PubMed] [Google Scholar]; (f) Cai Y, Zhu SF, Wang GP, Zhou QL. Adv Synth Catal. 2011;353:2939. [Google Scholar]; (g) Goto T, Natori Y, Takeda K, Nambu H, Hashimoto S. Tetrahedron-Asymm. 2011;22:907. [Google Scholar]; (h) Wood JL, Stoltz BM, Dietrich H, Pflum DA, Petsch DT. J Am Chem Soc. 1997;119:9641. [Google Scholar]
- 6.Lian Y, Davies HML. Org Lett. 2010;12:924. doi: 10.1021/ol9028385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For other examples of vinylcarbenoids displaying electrophilic character at the vinylogous position, see: Davies HML, Saikali E, Clark TJ, Chee EH. Tetrahedron Lett. 1990;31:6299.Davies HML, Saikali E, Young WB. J Org Chem. 1991;56:5696.Davies HML, Hu B, Saikali E, Bruzinski PR. J Org Chem. 1994;59:4535.Davies HML, Yokota Y. Tetrahedron Lett. 2000;41:4851.Yue Y, Wang Y, Hu W. Tetrahedron Lett. 2007;48:3975.Sevryugina Y, Weaver B, Hansen J, Thompson J, Davies HML, Petrukhina MA. Organometallics. 2008;27:1750.Hansen JH, Davies HML. Chem Sci. 2011;2:457.Wang XC, Xu XF, Zavalij PY, Doyle MP. J Am Chem Soc. 2011;133:16402. doi: 10.1021/ja207664r.Xu XF, Ratnikov MO, Zavalij PY, Doyle MP. Org Lett. 2011;13:6122. doi: 10.1021/ol2026125.
- 8.Hansen JH, Davies HML. Coord Chem Rev. 2008;252:545. doi: 10.1016/j.ccr.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies HML, Panaro SA. Tetrahedron Lett. 1999;40:5287. [Google Scholar]
- 10.(a) Lian Y, Davies HML. J Am Chem Soc. 2010;132:440. doi: 10.1021/ja9078094. [DOI] [PubMed] [Google Scholar]; (b) Hansen JH, Gregg TM, Ovalles SR, Lian Y, Autschnach J, Davies HML. J Am Chem Soc. 2011;133:5076. doi: 10.1021/ja111408v. [DOI] [PubMed] [Google Scholar]
- 11.The crystal structure of 17 has been deposited at the Cambridge Crystallographic Data Centre and the deposition number CCDC #830585 has been allocated.
- 12.Mayer H, Kempf B, Ofial AR. Acc Chem Res. 2003;36:66. doi: 10.1021/ar020094c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.