Abstract
We hypothesized that under high glucose conditions, activation of the hexosamine pathway leads to impaired nitric oxide (NO)-dependent arteriolar dilation. Skeletal muscle arterioles (diameter: ~160 μm) isolated from male Wistar rats were exposed to normal glucose (NG, 5.5 mmol/L) or high glucose concentrations (HG, 30 mmol/L, for 2 h) and agonist-induced diameter changes were measured with videomicroscopy. Western blots were performed to identify the vascular levels of protein O-linked-N-acetyl-glucosamine (O-GlcNAc) and phosphorylated endothelial NO synthase (eNOS). In arterioles exposed to HG, dilations to histamine were abolished compared to those exposed to NG (max: −6±6% and 69±9%, respectively), while acetylcholine-induced responses were not affected. Inhibition of NO synthesis with NG-nitro-L-arginine methyl ester (L-NAME) reduced histamine-induced dilations in NG arterioles, but it had no effect on microvessels exposed to HG. Dilations to the NO donor, sodium nitroprusside and constrictions to norepinephrine and serotonin were similar in the two groups. In the presence of the inhibitor of hexosamine pathway, azaserine, histamine-induced dilations were significantly augmented in arterioles exposed to HG (max: 67±2%). Moreover, exposure of vessels to glucosamine (5 mmol/L, for 2 h) resulted in reduced histamine-induced arteriolar dilations (max: 26±3%). The level of protein O-GlcNAcylation was increased, whereas the P-eNOS (Ser-1177) was decreased in HG exposed vessels. These findings indicate that a high concentration of glucose may lead to glucosamine formation, which impairs histamine-induced, NO-mediated arteriolar dilations. We propose that interfering with the hexosamine pathway may prevent microvascular complications in diabetes.
Keywords: Hyperglycemia, Glucosamine, Hexosamine pathway, Diabetes mellitus, O-GlcNAcylation, Microvessel
1. Introduction
Hyperglycemia is recognized as the primary cause in the pathogenesis of diabetic complications, such as abnormal microvascular reactivity, including impaired endothelium-dependent relaxation (Fulop et al., 2007a; Wells et al., 2003). Elevated blood glucose concentration results in increased intracellular glucose levels in various cell types, such as endothelial cells, because they are unable to limit glucose intake (Brownlee, 2005; Dominiczak, 2003; Gugliucci, 2000). Increased glucose concentration may lead to augmented glycosylation of various proteins that are important in the regulation of normal cellular homeostasis (Fulop et al., 2007b). Glycosylation is considered to be one of the key mechanisms responsible for the long-term consequences of diabetes. The O-linked enzymatic attachment of N-acetyl-glucosamine (O-GlcNAc) on serine and threonine residues of nuclear and cytoplasmic proteins is a specific form of glycosylation, which is a highly dynamic post-translational modification process (Hart et al., 2007). An increased O-GlcNAcylation of proteins is considered to be a major contributor to the etiology of various human diseases, such as hypertension (Lima et al., 2009b) and diabetes mellitus (Fulop et al., 2007a).
The level of O-GlcNAc formation is regulated, in part, by the metabolism of glucose via the hexosamine biosynthetic pathway (HBP) (Buse et al., 2002; McClain, 2002; Ngoh et al., 2010; Patti et al., 1999; Ross et al., 2000). HBP is highly sensitive to changes in intracellular glucose concentration. Earlier studies in rodents have demonstrated that chronically elevated flux through the HBP leads to insulin resistance and glucose toxicity by hyperglycemia (Dias and Hart, 2007; Wang et al., 2007). There are two major nutrient inputs of glucose and glucosamine, and one rate-limiting enzyme glutamine fructose-6-phosphate amidotransferase (GFAT) in the HBP (Hawkins et al., 1997; Traxinger and Marshall, 1991). It has been shown that adipocytes and muscle cells exposed to chronic high glucose levels in the presence of insulin develop insulin resistance, which can be prevented when adipocytes are incubated with a the inhibitor of GFAT (Marshall et al., 1991; McClain et al., 2002). An excessive flux through the HBP, results in many of the phenotypic characteristics of diabetes (McClain, 2002); however, the functional consequence of O-GlcNAcylation in microvessels is not clear.
It has been shown that hyperglycemia impairs endothelium-dependent vasodilation in diabetic patients (Beleznai et al., 2011) and healthy human subjects (Picchi et al., 2010). Previously, we demonstrated that in the presence of chronic hyperglycemia (experimental type 1 diabetes) (Bagi and Koller, 2003) or acute elevation of glucose concentration elicits reduction of NO-mediated dilations in skeletal muscle arterioles of the rat, due to the impaired synthesis of NO (Bagi et al., 2004). The endothelial NO synthase (eNOS) is classified as a constitutive and Ca2+/calmodulin-dependent enzyme. In addition to changes in intra-cellular levels of Ca2+ a number of post-translational mechanisms have been proposed to regulate eNOS activity, including phosphorylation of eNOS (Fleming and Busse, 1999). Interestingly, it has been found that high glucose and glucosamine caused a reduction in insulin-induced eNOS activity as a result of increased O-GlcNAcylation in human coronary endothelial cells in culture (Federici et al., 2002). Whether increased activation of HBP and augmented O-GlcNAcylation by high glucose concentrations affects NO-mediated vasomotor responses in resistance arteries is not known.
In this study we have tested the hypothesis that under high glucose conditions activation of the hexosamine pathway leads to O-GlcNAcylation of eNOS. This mechanism may prevent phosphorylation-dependent activation of eNOS and leads to a diminished NO-dependent arteriolar dilation. Isolated skeletal muscle arterioles were exposed to high glucose concentrations and agonist-induced changes in diameter were measured with videomicroscopy before and after interfering with NO synthesis and HBP pathway. Phosphorylation of eNOS was also detected to provide evidence for changes in phosphorylation of eNOS at Ser-1177, known to be associated with activation of enzyme activity.
2. Material and methods
Experiments were carried out on male Wistar rats (n=22, weighing ~300 g). The animals were housed in the animal care facility and were fed standard rat chow and drank tap water ad libitum with a 12-h light–dark cycle. All experimental procedures were in compliance with the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. After overnight fasting, the rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg kg−1) and the gracilis muscle was removed. The animals were then euthanized by an additional injection of sodium pentobarbital (150 mg kg−1).
2.1. Measurment of diameter of isolated, cannulated and pressurized arterioles
With the use of microsurgical instruments and an operating microscope the gracilis muscle arteriole (~1.5 mm in length) was isolated and transferred into organ chambers containing two glass micropipettes filled with physiological salt solution (PSS), composed of (in mm) 110.0 NaCl, 5.0 KCl, 2.5 CaCl2, 1.0 MgSO4, 1.0 KH2PO4, 5.5 glucose and 24.0 NaHCO3, equilibrated with a gas mixture of 10% O2 and 5% CO2, balanced with nitrogen, at pH 7.4. The vessels were cannulated at both ends and the micropipettes were connected with silicone tubing to a pressure servo control system (Living Systems Instrumentation, Burlington, VT, U.S.A.) to set the intraluminal pressure to 80 mm Hg. The temperature was set at 37 °C by a circulating bath temperature controller (Cole Parmer, Vernon Hills, IL, U.S.A.). Images were collected with a digital camera (CFW1310, Scion Corp., Frederick, MO, U.S.A.) connected to a microscope (Nikon, Eclipse 80i). The internal diameter at the midpoint of the isolated arteriole was measured offline by Image J software (NIH Image, Bethesda)(Bagi et al., 2002; Koller and Bagi, 2004).
2.2. Dilation to agonist
In the first series of experiments the gracilis muscle arterioles were exposed to PSS that contained 5.5 mmol/L glucose. During an incubation period of 1 h at 37 °C, spontaneous myogenic tone developed in the isolated arterioles in response to the intraluminal pressure of 80 mm Hg. Cumulative concentrations of the endothelium-dependent vasodilator histamine (1 nmol/L–10 μmol/L), acetylcholine (Ach, 1 nmol/L–1 μm/L) or endothelium-independent vasodilator sodium nitroprusside (SNP, 1 nmol/L–1 μmol/L) were administrated to the vessels and changes in diameter were measured. Furthermore, vasoconstrictors that primarily act on smooth muscle cells, such as norepinephrine (NE, 0.3 nmol/L–1 μm/L) and serotonin (5-HT, 0.1 nmol/L–1 μmol/L) were also applied. In a separate set of experiments the vessels were incubated with 30 mmol/L glucose or 5 mmol/L glucosamine (incubation time: 2 h) to investigate changes of agonist-induced arteriolar responses. As an osmotic control, mannitol (25 mmol/L) was administered and histamine-induced vasodilator response was reassessed. Histamine-induced dilations were also observed in the presence of inhibitor of NO-synthase, L-NAME (200 μmol/L, incubation for 20 min) with or without azaserine (20 μmol, incubation for 20 min), which is a glutamine analog and known to irreversibly inhibit fructose-6-phosphate amidotransferase (GFAT)(Liu et al., 2007). Azaserine was also employed in glucosamine exposed arterioles and histamine-induced responses were reassessed.
2.3. Immunoblots
Branches of femoral arteries were dissected from Wistar rats, cleared of connective tissue. Vessels were exposed to normal and high glucose concentrations as well as glucosamine and incubated for 2 h after which they were snap frozen in liquid nitrogen. After the addition of 20 μl RIPA buffer (containing protease and phosphatase inhibitors) arteries were homogenized and 20 μl of Laemmli sample buffer was added (from Sigma Inc.). Immunoblot analysis was carried out as described before (Jebelovszki et al., 2008).
Primary antibodies were used for detection of O-GlcNAc (dilution 1:1000, CTD110.6, Convance, USA) as well as for detection of protein expression of eNOS (anti-eNOS, Transduction, dilution 1:1000) and P-eNOS levels (anti-P-eNOS-Ser-1177, dilution 1:500, BD Bioscieses). Anti-β-actin IgG obtained from Abcam Inc was used as loading control. Corresponding horseradish peroxidase laballed secondary antibodies were used and signals were revealed with chemiluminescence and visualized autoradiographically. Optical density of bands was quantified by using NIH Image software.
2.4. Drugs
All salts and chemicals were purchased from Sigma-Aldrich Co. (St Louis, MO, U.S.A). All solutions were prepared in destilled water and on the day of the experiment and final concentrations are reported.
2.5. Data analysis
Statistical analyses were performed using GraphPad Prism Software (San Diego California USA) by two-way ANOVA repeated measures followed by Tukey’s post-hoc test or Student’s t-test as appropriate. Data are expressed as means + SEM. Agonist-induced arteriolar dilations were expressed as changes in arteriolar diameter as a percentage of the maximal dilation defined as the passive diameter of the vessel at 80-mm Hg intraluminal pressure in a Ca2+-free medium. P<0.05 was considered statistically significant.
3. Results
3.1. Effect of high glucose concentration on arteriolar dilations
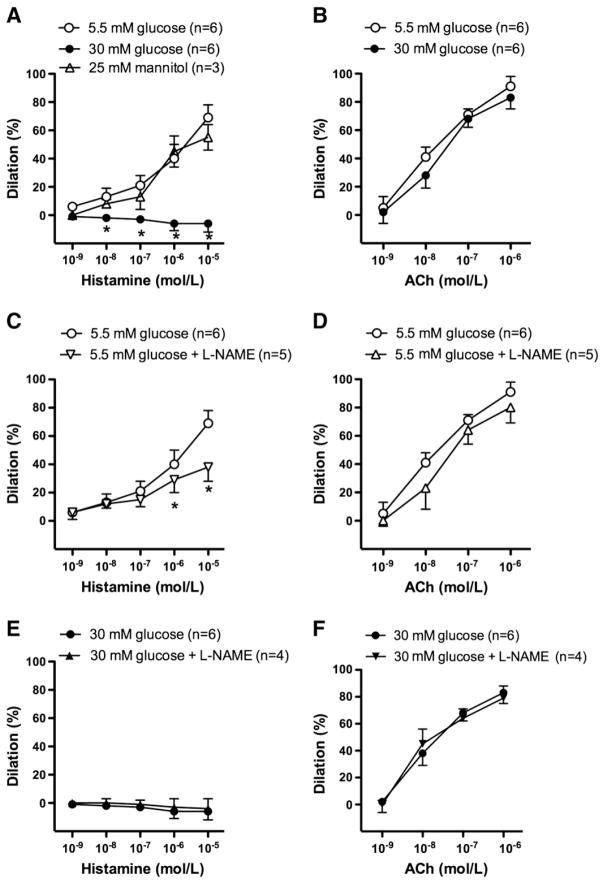
In isolated, pressurized (80 mm Hg) gracilis muscle arterioles of the rat, active arteriolar tone developed (~30%) in response to intraluminal pressure without the use of any vasoactive agent. In comparison with the control responses, histamine-induced dilations were abolished in the presence of 30 mmol/L glucose (Fig. 1A), whilst response to another endothelium-dependent agonist, acetylcholine (ACh) was not affected (Fig. 1B). As an osmotic control, mannitol (25 mmol/L) was used and found without any effects on histamine-induced vasodilation (Fig. 1). Histamine-induced dilations were significantly reduced in the presence of the inhibitor of NO synthase (L-NAME, 200 μmol/L) (Fig. 1C), whereas L-NAME had no effect on high glucose-exposed vessels (Fig. 1E). L-NAME had no effect on ACh-induced arteriolar responses either in normal (Fig. 1D) or high glucose conditions (Fig. 1F).
Fig. 1.
Percent changes in diameter of gracilis muscle arterioles in response to cumulative concentration of histamine (1 nmol/L–10 μmol/L, Panels A, C and E) and acetylcholine (ACh, 1 nmol/L–1 μmol/L, Panels B, D and F) in the presence of normal (5.5 mmol/L) or high glucose concentrations (30 mmol/L) and in the absence or in the presence of NO synthase inhibitor L-NAME (100 μM). Data are in means ± S.E.M, significant differences: * P≤0.05.
There were no significant differences observed in response to the endothelium-independent vasodilator, sodium nitroprusside (Fig. 2A); furthermore, no differences were observed in vasoconstriction to norepinephrine (Fig. 2B) and serotonin (Fig. 2C) in normal and high glucose condition.
Fig. 2.
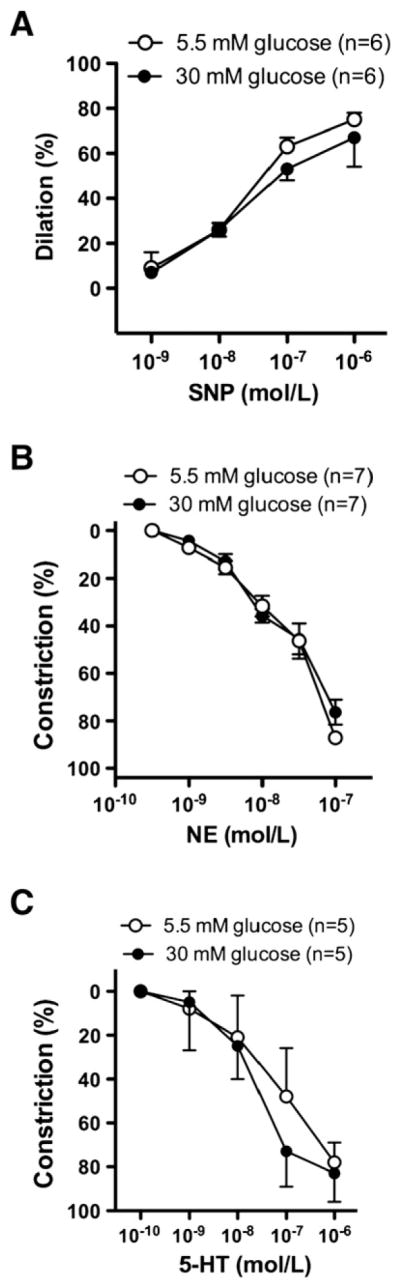
Percent changes in diameter of gracilis muscle arterioles in response to cumulative concentration of sodium nitroprusside (SNP, 1 nmol/L–1 μmol/L) (Panel A), norepinephrine (NE, 0.3 nmol/L–0.1 μmol/L) (Panel B) and serotonine (0.1 nmol/L–1 μmol/L) (Panel C) in the presence of normal (5.5 mmol/L) or high glucose concentration (30 mmol/L). Data are in means ± S.E.M, significant differences: * P<0.05.
3.2. Activation of hexosamine-pathway contributes to abolished histamine-induced vasodilation in high glucose condition
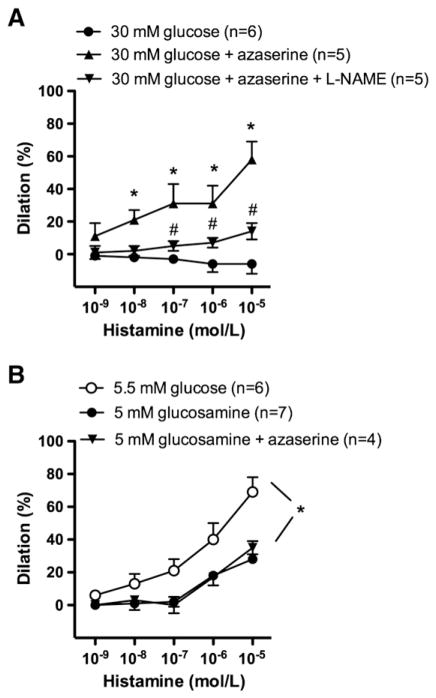
The glutamine fructose-6-phosphate amidotransferase (GFAT) is the rate-limiting enzyme in the conversion of fructose-6-phosphate to glucosamine-6-phosphate and can be irreversible blocked by aza-serine (Liu et al., 2007). We found that in the presence of azaserine (20 μmol/L), histamine-induced dilations were restored to the control level, even in the presence of high glucose (Fig. 3A). In the presence of azaserine, additional administarion of NO synthase inhibitor, L-NAME significanly reduced the magnitude of histamine-induced dilations (Fig. 3A).
Fig. 3.
Percent changes in diameter of gracilis muscle arterioles in response to cumulative concentration of histamine (1 nM–10 μM) in the presence of high glucose concentration before and after incubations with GFAT inhibitor, azaserine (20 μmol/L) or in combination with NO synthase inhibitor, L-NAME (Panel A). Percent changes in diameter of gracilis muscle arterioles in the presence of glucosamine (5 mmol/L) and in the simultaneous presence of glucosamine and the GFAT inhibitor, azaserine (Panel B). Data are in means±S.E.M, significant differences: * P<0.05.
In separate experiments arterioles were incubated with glucosamine (5 mmol/L), a direct substrate of hexosamine-pathway. Glucosamine significantly reduced histamine-induced arteriolar dilations, to similar extent, which was elicited by high glucose exposure (Fig. 3B). To test the specificity of azaserine we have examined histamine-induced dilations in the simultaneous presence of glucosamine and the GFAT-inhibitor azaserine. We have found that in the presence of glucosamine the abolished histamine-induced dilations were not significantly affected by azaserine treatment (Fig. 3B).
3.3. O-GlcNAc modi3cation of proteins in arteries
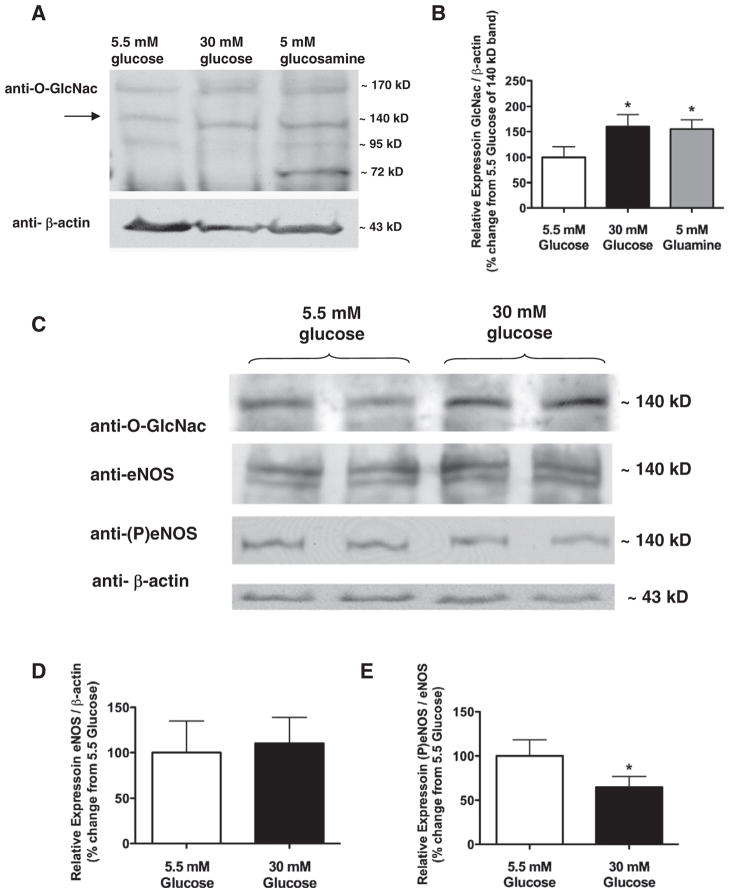
In order to quantify the level of the protein O-GlcNAcylation, western blot analysis was performed in normal and high glucose conditions as well as in arteries exposed to glucosamine. For this assay we used larger femoral arteries for a more efficient and reliable detection of O-GlcNAcylation and P-eNOS levels in the vascular wall; therefore these data could not directly be extrapolated to microvessels and as such should be interpreted cautiosly. We found increases in protein O-GlcNAcylation in high glucose and also glucosamine exposed femoral arteries, when compared with the normal glucose condition (Fig. 4A and B). We observed increased protein O-GlcNAcylation of various proteins, including those ~140 KDa molecular weight proteins that could represent eNOS.
Fig. 4.
Representative Western blots (Panel A) and summary data (Panel B) show O-GlcNac formation in skeletal muscle arteries exposed to normal (5.5 mmol/L), high glucose concentration (30 mmol/L) and also to glucosamine (5 mmol/L). Western blots (Panel C) and summary data (Panel D and E) of normalized densitometry ratios from four independent experiments show O-GlcNac, eNOS and P-eNOS-Ser1177 levels. β-actin was used for normalizing of loading variations.
3.4. Detection of P-eNOS (Ser-1177) in rat arteries in the presence of high glucose
We sought to determine the effect of high glucose on the phosphorylation state of eNOS. To this end, arteries were exposed to 5.5 mmol/L and 30 mmol/L glucose concentration and then P-eNOS (Ser-1177) levels were detected by Western immunoblots. Whilst the level of eNOS protein remained unchanged (Fig. 4D), the level of P-eNOS (Ser-1177) was significantly decreased in high glucose exposed arteries compared to that of normal glucose exposed vessels (Fig. 4C and E).
4. Discussion
This study demonstrates that increased flux through the hexosamine biosynthetic pathway (HBP) leads to enhanced O-GlcNAcylation of eNOS under condition of high glucose, which interferes with NO-dependent arteriolar dilation (Beleznai et al., 2008). This conclusion is supported by the findings that 1) in gracilis muscle arterioles of the rat histamine caused an abolished vasodilation in the presence of high glucose, 2) which was restored by incubation with azaserine, an inhibitor of GFAT. 3) A significant increase in protein O-GlcNAcylation was found in high glucose- and glucosamine-exposed vessels, when compared with the normal glucose. 4) Arteries exposed to high glucose exhibited a decreased phosphorylation of eNOS on the site of Ser-1177, whilst the level of eNOS remained unchanged.
The hexosamine biosynthetic pathway is a unique form of glycosylation and exemplifies one such accessory pathway for glucose metabolism (Marshall et al., 1991). The relative flux through HBP and consecutive O-GlcNAcylation of proteins is still not entirely understood in the vasculature. It has been shown that increased O-GlcNAc level is associated with increased vasoconstrictor reactivity and impaired endothelium-dependent relaxation in aorta of deoxycorticosterone acetate (DOCA)-induced hypertensive rats (Lima et al., 2009a). Interestingly, aorta from these hypertensive rats also exhibited decreased levels of P-eNOS(Ser-1177) and P-Akt(Ser-473) (Lima et al., 2009a). There is experimental evidence of competitive occupancy at the same site for eNOS, which can be either O-GlcNAc modified or phosphorylated (Du et al., 2001). In this context, it was shown that elevated O-GlcNAcylation of eNOS inhibited its phosphorylation-dependent activation by Akt, suggesting that the site of O-GlcNAcylation is at or near the Akt phosphorylation site (Du et al., 2001). In diabetes, O-GlcNAcylation of eNOS has been implicated in erectile dysfunction; as it was demonstrated that erectile dysfunction is associated with hyperglycemia-induced increase in O-GlcNAc modification and decreased phosphorylation of eNOS at Ser-1177 in the penis of diabetic rats (Musicki et al., 2005). Furthermore, in the aorta of rats with streptozotocin (STZ)-induced diabetes, increased O-GlcNAc modification of eNOS and inhibition of its activity was observed (Du et al., 2001). In the study by Federici et al. impaired activation of eNOS by Akt was found in cultured human coronary artery endothelial cells exposed to hyperglycemia (Federici et al., 2002). Taken together, evidence indicates that eNOS activation could be limited by O-GlcNAcylation and consequent phosphorylation deficit. Yet, no study has demonstrated whether these mechanisms contribute to impaired endothelium-dependent dilation of intact microvessels and that this is responsible for high glucose-induced impairment of NO production.
Previously, we demonstrated that hyperglycemia impairs endothelium-dependent vasodilation in coronary arterioles of diabetic patients (Beleznai et al., 2011). We also showed that the presence of chronic hyperglycemia (Bagi and Koller, 2003) or acute, 1–2 hour elevation of glucose concentration elicits reduction of NO-mediated dilations in skeletal muscle arterioles of the rat, which is due to the impaired synthesis of NO (Bagi et al., 2004). Histamine has been shown to cause the release of NO in several vascular beds (Weksler et al., 1978). The histamine-induced relaxation is endothelium-dependent and it is mediated by endothelial H1 receptors (Jansen-Olesen et al., 1997; Toda, 1990). Histamine was chosen to examine the effect of high glucose on eNOS activity because we have shown previously that NO synthase inhibitor, L-NAME had a more pronounced effect on histamine-induced dilation in skeletal muscle arterioles, which suggests NO as an important mediator of the histamine response (Erdei et al., 2006).
In the present study we found that exposure of skeletal muscle arterioles to high glucose or to glucosamine leads to abolished, histamine-induced, NO-mediated dilations. In contrast, arteriolar dilation to ACh, which is less dependent on NO, but mediated primarily by endothelium-derived hyperpolarizing factor (EDHF) in skeletal muscle microvessel (Erdei et al., 2006), was not affected by high glucose concentrations. The smooth muscle-dependent vasoregulatory pathways, such as those induced by NO donor or elicited by vasoconstrictors, such as norepinephrine or serotonin also were not affected by high glucose. Thus, it plausible that high glucose-induced changes in vasomotor responsiveness of skeletal muscle arterioles are specific for altered NO synthesis and do not impair EDHF type of dilation or augment vasoconstrictor signaling pathways.
In addition to these functional changes we demonstrated an increased O-GlcNAcylation of proteins in the vascular wall upon exposure the vessels to high glucose. In parallel, a reduced phosphorylation of Ser-1177 of eNOS has been detected. In this context, Zhang et al. has recently demonstrated that exposure of porcine aortic endothelial cells to high glucose concentrations inhibited eNOS phosphorylation at Ser-1177 and also dephosphorylation at Thr-495 induced by bradykinin (Zhang et al., 2010). Thus, it is possible that O-GlcNacylation interferes with both activation and inhibitory sites of eNOS. Based on our functional experiments it seems that the net results of high glucose-induced O-GlcNacylation are the inhibitions of NO synthesis. Whether the Thr-495 inhibitory site is O-GlcNacylated and whether this affects NO synthesis in microvessels has yet to be elucidated. Because inhibition of GFAT by azaserine restored the impaired NO-mediated dilation in high glucose condition, but did not prevent detrimental effects of glucosamine, we concluded that eNOS activation could be limited by the activation hexosamine pathway and subsequent O-GlcNAcylation of eNOS in skeletal muscle arterioles (Fig. 5). Our results are in accordance with previous observations obtained in large vessels (Du et al., 2001) or in cultured endothelial cells (Federici et al., 2002; Zhang et al., 2010) and extend these findings towards those intact resistance arteries. Further studies are needed to provide experimental evidence whether O-GlcNAcylation of eNOS and consequently impaired NO synthesis alters vasoreactivity of resistance arteries in vivo and whether this mechanism could contribute to increased vascular resistance and the development of hypertension or other microvascular complications that are associated with diabetes mellitus.
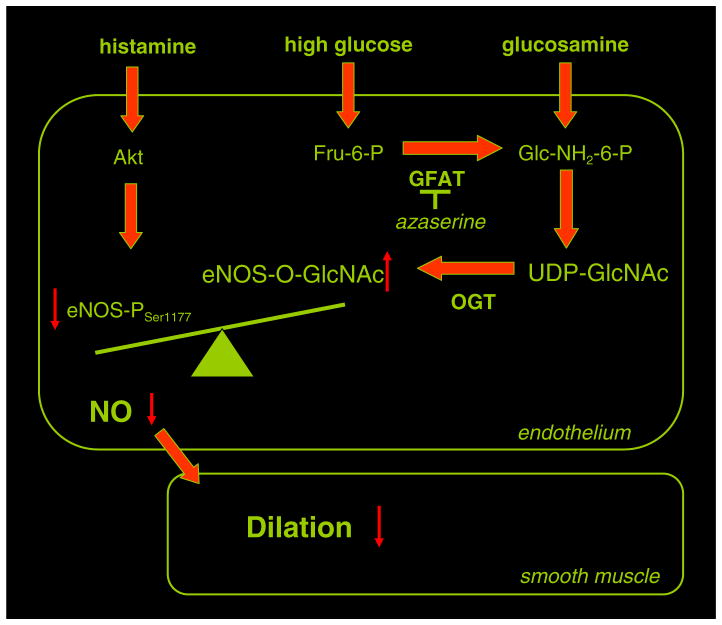
Fig. 5.
Schematic draw demonstrates that increased flux through the hexosamine biosynthetic pathway leads to enhanced O-GlcNAcylation of eNOS in condition of high glucose, which interferes with eNOS-Ser1177 phosphorylation by Akt to reduce histamine-induced, NO-dependent dilation of skeletal muscle arterioles. GFAT: glutamine fructose-6-phosphate amidotransferase, O-GlcNAc: O-linked attachment of N-acetyl-glucosamine, OGT: O-linked N-acetylglucosaminyl-transferase.
5. Conclusions
Collectively, our present findings suggest an important functional role for O-GlcNAcylation in interfering with the phosphorylation of Ser-1177 site on eNOS in microvessels. Thus, in diabetes, interfering with hexosamine biosynthetic pathway may prevent or delay the development of dysfunction in resistance arteries.
Acknowledgments
The technical assistance of Marta Balogh and Ibolya Rutkai is gratefully acknowledged. ZB acknowledges the support from the British Heart Foundation, Centre of Research Excellence, Oxford (RE/08/004).
Abbreviations
- HBP
hexosamine biosynthetic pathway
- GFAT
glutamine fructose-6-phosphate amidotransferase
- eNOS
endothelial NO synthase
- O-GlcNAc
O-linked attachment of N-acetyl-glucosamine
- OGT
O-linked N-acetylglucosaminyl-transferase
Footnotes
Conflict of interest statement
For both authors, no conflict of interest is declared.
References
- Bagi Z, Koller A. Lack of nitric oxide mediation of flow-dependent arteriolar dilation in type I diabetes is restored by sepiapterin. J Vasc Res. 2003;40 (1):47–57. doi: 10.1159/000068938. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Ungvari Z, Koller A. Xanthine oxidase-derived reactive oxygen species convert flow-induced arteriolar dilation to constriction in hyperhomocysteinemia: possible role of peroxynitrite. Arterioscler Thromb Vasc Biol. 2002;22 (1):28–33. doi: 10.1161/hq0102.101127. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Toth E, Koller A, Kaley G. Microvascular dysfunction after transient high glucose is caused by superoxide-dependent reduction in the bioavailability of NO and BH(4) Am J Physiol Heart Circ Physiol. 2004;287 (2):H626–H633. doi: 10.1152/ajpheart.00074.2004. [DOI] [PubMed] [Google Scholar]
- Beleznai T, Feher A, Rutkai I, Edes I, Bagi Z. O-linked-n-acetylglucosamine formation reduces nitric oxide-dependent dilation in arterioles exposed to high glucose concentrations. Circulation. 2008;118:S_552. [Google Scholar]
- Beleznai T, Feher A, Spielvogel D, Lansman SL, Bagi Z. Arginase 1 contributes to diminished coronary arteriolar dilation in patients with diabetes. Am J Physiol Heart Circ Physiol. 2011;300 (3):H777–H783. doi: 10.1152/ajpheart.00831.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54 (6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab. 2002;283 (2):E241–E250. doi: 10.1152/ajpendo.00060.2002. [DOI] [PubMed] [Google Scholar]
- Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol Biosyst. 2007;3 (11):766–772. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- Dominiczak MH. Obesity, glucose intolerance and diabetes and their links to cardiovascular disease. Implications for laboratory medicine. Clin Chem Lab Med. 2003;41 (9):1266–1278. doi: 10.1515/CCLM.2003.194. [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108 (9):1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdei N, Toth A, Pasztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol. 2006;291 (5):H2107–H2115. doi: 10.1152/ajpheart.00389.2006. [DOI] [PubMed] [Google Scholar]
- Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106 (4):466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res. 1999;43 (3):532–541. doi: 10.1016/s0008-6363(99)00094-2. [DOI] [PubMed] [Google Scholar]
- Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007a;73 (2):288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol. 2007b;292 (4):C1370–C1378. doi: 10.1152/ajpcell.00422.2006. [DOI] [PubMed] [Google Scholar]
- Gugliucci A. Glycation as the glucose link to diabetic complications. J Am Osteopath Assoc. 2000;100 (10):621–634. [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446 (7139):1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest. 1997;99 (9):2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen-Olesen I, Ottosson A, Cantera L, Strunk S, Lassen LH, Olesen J, Mortensen A, Engel U, Edvinsson L. Role of endothelium and nitric oxide in histamine-induced responses in human cranial arteries and detection of mRNA encoding H1- and H2-receptors by RT-PCR. Br J Pharmacol. 1997;121 (1):41–48. doi: 10.1038/sj.bjp.0701097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebelovszki E, Kiraly C, Erdei N, Feher A, Pasztor ET, Rutkai I, Forster T, Edes I, Koller A, Bagi Z. High-fat diet-induced obesity leads to increased NO sensitivity of rat coronary arterioles: role of soluble guanylate cyclase activation. Am J Physiol Heart Circ Physiol. 2008;294 (6):H2558–H2564. doi: 10.1152/ajpheart.01198.2007. [DOI] [PubMed] [Google Scholar]
- Koller A, Bagi Z. Nitric oxide and H2O2 contribute to reactive dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 2004;287 (6):H2461–H2467. doi: 10.1152/ajpheart.00295.2004. [DOI] [PubMed] [Google Scholar]
- Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension. 2009a;53 (2):166–174. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VV, Rigsby CS, Hardy DM, Webb RC, Tostes RC. O-GlcNAcylation: a novel post-translational mechanism to alter vascular cellular signaling in health and disease: focus on hypertension. J Am Soc Hypertens. 2009b;3 (6):374–387. doi: 10.1016/j.jash.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42 (1):177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266 (8):4706–4712. [PubMed] [Google Scholar]
- McClain DA. Hexosamines as mediators of nutrient sensing and regulation in diabetes. J Diabetes Complications. 2002;16 (1):72–80. doi: 10.1016/s1056-8727(01)00188-x. [DOI] [PubMed] [Google Scholar]
- McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci U S A. 2002;99 (16):10695–10699. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005;102 (33):11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107 (2):171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Virkamaki A, Landaker EJ, Kahn CR, Yki-Jarvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes. 1999;48 (8):1562–1571. doi: 10.2337/diabetes.48.8.1562. [DOI] [PubMed] [Google Scholar]
- Picchi A, Capobianco S, Qiu T, Focardi M, Zou X, Cao JM, Zhang C. Coronary microvascular dysfunction in diabetes mellitus: a review. World J Cardiol. 2010;2 (11):377–390. doi: 10.4330/wjc.v2.i11.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Chen X, Hope HR, Sun S, McMahon EG, Broschat K, Gulve EA. Development and comparison of two 3T3-L1 adipocyte models of insulin resistance: increased glucose flux vs glucosamine treatment. Biochem Biophys Res Commun. 2000;273 (3):1033–1041. doi: 10.1006/bbrc.2000.3082. [DOI] [PubMed] [Google Scholar]
- Toda N. Mechanism underlying responses to histamine of isolated monkey and human cerebral arteries. Am J Physiol. 1990;258 (2 Pt 2):H311–H317. doi: 10.1152/ajpheart.1990.258.2.H311. [DOI] [PubMed] [Google Scholar]
- Traxinger RR, Marshall S. Coordinated regulation of glutamine:fructose-6-phosphate amidotransferase activity by insulin, glucose, and glutamine. 1991. [PubMed] [Google Scholar]
- Role of hexosamine biosynthesis in enzyme regulation. J Biol Chem. 266(16):10148–10154. [PubMed] [Google Scholar]
- Wang Z, Pandey A, Hart GW. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol Cell Proteomics. 2007;6 (8):1365–1379. doi: 10.1074/mcp.M600453-MCP200. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Ley CW, Jaffe EA. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978;62 (5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci. 2003;60 (2):222–228. doi: 10.1007/s000180300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Yokoo H, Nishioka H, Fujii H, Matsuda N, Hayashi T, Hattori Y. Beneficial effect of the oligomerized polyphenol oligonol on high glucose-induced changes in eNOS phosphorylation and dephosphorylation in endothelial cells. Br J Pharmacol. 2010;159 (4):928–938. doi: 10.1111/j.1476-5381.2009.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]