Abstract
As yet no unifying grading system for meningiomas has been adopted. We evaluate epidemiologic factors of meningioma in Iran & degree of agreement between the two commonly used grading systems namely WHO (2000) and Mahmood systems. During a 6-year period 238 meningiomas were selected and reviewed by two independent pathologists using both grading systems. 205(86.1%) cases were benign, 19(8%) atypical and 14(5.9%) malignant. 181(18%) cases were primary and 51(27%) secondary; 35(68%) of the latter benign, 7(14%) atypical and 9(18%) malignant. All intraspinal meningiomas were benign. In benign cranial and spinal types female to male ratios were 1.9: 1 and 1.3: 1 ; while in atypical and malignant types were 1 :1.4 and 1:3.1 respectively. Mean ages were 49.9 for benign. 41.1 for atypical and 50 for malignant types. The most frequent site of involvement in all grades of intracranial tumors was cerebral convexity (31.1 %). The most common subtype was menigothelial (65.1%). Female preponderance seen in benign nonrecurrent meningioma became increasingly less prominent and even reversed in recurrent, atypical and malignant forms. Benign recurrent tumors were similar to non-recurrent tumors microscopically. Kappa value comparing two grading systems was 0.947, so good agreements were found between Mahmood and WHO grading systems.
Keywords: meningioma, brain tumor, intracranial, intraspinal, Mahmood grading system, WHO grading system
Introduction
Meningiomas are the most frequently encountered primary non-glial tumors of the central nervous system and constitute about 20% of all primary brain tumors. (1) They are benign in most instances and may be cured with gross total resection; however, approximately 9–22% of patients experience recurrence and rarely are they frankly malignant leading to metastasis. (2) Incidence of typical (benign) intracranial meningiomas in women exceeds that in men by a ratio of 3:2, in contrast atypical and malignant meningiomas are somewhat more frequent in males. (2) Intraspinal meningiomas has female to male ratio of more than cranial type; about 4:1 in some series. (3) The distribution of intracranial meningiomas is as following in most instances: cerebral convexity (35%), parasagital (20%), sphenoid wing (20%), infra-tentorial (13%), interventricular (5%), tuberculum sella (3%) and other sites (4%). (4) It has several subtypes including meningotheliomatous, fibrous, transitional, secretory, Chordoid, clear cell, papillary, rhabdoid, psammomatous, microcystic, lymphoplasma cell rich and metaplastic types. (5) The correlation between clinical behavior and histologic grading of meningiomas has been of much interest in recent years. Several grading systems have been used for menjngioma. One of the most objective systems was introduced by Mahmood who modified the initial WHO grading system accomplished by numeric scoring. (6) In the recently revised WHO grading system (2000) comparing to its initial version some criteria has been changed.(7) Many factors considered to have prognostic significance such as sheeting, hypercellularity, cytologic atypia, increased mitotic index, necrosis, small cell change, brain invasion and elevated proliferative index of MIB-l.(4) Extent of resection is also a powerful prognostic indicator. In some areas such as skull base difficulties in achieving gross total resection exists, therefore the tumor site is also another important prognostic factor. (8) Meningioma may be the presenting feature of neurofibromatosis type 2 (NF2) particularly in childhood. (9) Finding of meningioma in an individual younger than 25 years of age should prompt an evaluation for an underlying genetic condition. (10) The aims of this study were: 9) - to take an estimate of meningioma in this region according to age, gender, location, histologic subtypes, and other clinical data b) - to evaluate the less well defined category of recurrent meningiomas with respect to demographic and histopathologic data i a c) -To compare the recent WHO grading system with Mahmood’s modified system.
Materials and methods
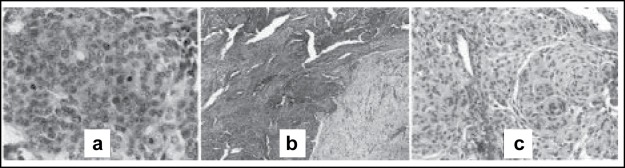
In this retrospective study, 238 cases of meningiomas operated from 1997 to 2002 at Sina hospital Tehran were reviewed. Histological assessment and grading of tumors performed blindly according to both WHO 2000 schema and Mahmood’s modified WHO grading system by two independent pathologists. In Mahnood’s system, grading performed according to numeric scores based on 6 predetermined criteria (table 1). According to WHO 2000 schema the most important criteria for malignancy (anaplasia) are the number of mitotic figures per 10 high power microscopic fields (figure 1a) and loss of differentiation (table 2). Presence of brain invasion (figure 1b) or coexistence of 3 out of 5 certain histologic features also define a tumor as atypical (table 2). The usual variants of meningioma (i.e. fibrous, meningothelial and transitional) lacking the above criteria are grouped as benign (figure 1c). Chordoid and clear cell meningiomas graded as atypical and papillary and rhabdoid variants if presented focally graded as atypical and diffuse forms graded as malignant. Clinical data (age, sex, site of tumor, history of neurofibromatosis and presenting sign & symptoms) and history of recurrence till the end of 2003 were recorded and compared between groups. Two grading systems were compared and degree of agreement (kappa value) was determined. None of the patients had preoperative tumor embolization or chemotherapy.
Table 1 :
Mahmood’s modified grading system for meningioma (6)
| Score | Brain invasion | Mitosis | Nuclear pleomorphism | Necrosis* | Hypercellularity | Loss of architecture |
|---|---|---|---|---|---|---|
| 0 | Absent | none | Uniform, bland nuclei, no nucleoli | none | 10 whorls fascicles/ HPF | none |
| 1 | Tumor pushing the brain without intervening meaning | 1-1/10HPH | Occasional larger nuclei, 2–3 times larger with irregular contours | Rare, each involving less than 1/2 of HPF | 10 whorls-fascicles/ HPF or increased cellularity in perivascular area | Incipient loss |
| 2 | Cords infiltrating the brain | 3–4/10HPF | Many cells with large pale nuclei, small non prominent nucleoli | Frequent foci involving more than 1/2 but less than 1 HPF | Less defined, small, more closely packed whorls (up to 30/ HPF) | Involving 1–2 HPF |
| 3 | >5/10HPF | Most cells with nuclei, variable size, prominent nucleoli | Large confluent areas of necrosis >1 HPF | Densely crowded overlapping nuclei with loss of whorls | Involving more than 2 adjacent HPF |
Score 0–4 =benign, Score 5–11 =atypical, Score>11 =malignant
In the absence of preoperative tumor embolization and radiation therapy
Figure 1 :
Some histologic features of different grades of meningioma: 1a numerous mitotic figures seen in malignant meningioma. 1b invasion of atypical meningioma (darker area) into the brain parenchyma (lighter area). 1c characteristic whorls of benign meningothelial meningioma.
Table 2 :
WHO histologic grading scheme for meningioma (2000) (7)
Atypical meningloma: any of the following three criteria:
|
Anaplastic meningloma: Either of the following criteria
|
Results
Overall comparison of two grading system considering all 3 groups; revealed good agreement (kappa = 0.947).The kappa values were 0.945 for benign, 0.908 for atypical and 1 for malignant meningiomas. Only 3 atypical meningiomas according to Mahmood’s system did not meet the WHO criteria for atypical grade. Due to good agreement of the two systems and more objectivity of the Mahmood’s system, we preferred to use it in this study. Of total 238 cases, 205(86.1%) were classified as benign, 19(8%) as atypical and 14(5.9%) as malignant. One hundred cud eight seven (78.6%) cases were primary and 51(21.4%) cases were secondary. Distribution of patients’ age, sex, tumor grade, location and other clinical data are presented in Table 3. Mean age for benign, atypical & malignant forms were 49.4, 41.1 and 50 respectively. There was no significant difference in the mean age between all grades although the mean age of malignancy was slightly higher. Female to male ratio in benign form was 1.81:1 but atypical and malignant variants were more common in men (p = 0.048 and 0.001) with female to male ratios of 1:1.3 in atypical and 1:3.7 in malignant forms. Considering all grades in all age groups there was female predominance (female to male ratio 1.5:1); however under the age 40 this ratio was reverse (1:1.14). The most common site of involvement in all grades was cerebral convexity (Table 4).
Table 3-.
Clinical data of study group
| Benign | Atypical | malignant | Total | ||
|---|---|---|---|---|---|
| Number (%) | 205 (86.1) | 19 (8) | 14 (5.9) | 238 | |
| Mean age | 49.4 | 41.1 | 50 | 48.8 | |
| Sex | Female (%) | 132 (92.3) | 8 (5.6) | 3 (2.1) | 143 |
| Male (%) | 73 (76.8) | 11 (11.6) | 11 (11.6) | 95 | |
| Location | Intracranial (%) | 189 (85.1) | 19 (8.6) | 14 (6.3) | 222 |
| Intraspinal (%) | 16 (100) | 0 | 0 | 16 | |
| Recurrence | Primary (%) | 170 (90.9) | 12 (6.4) | 5 (2.7) | 187 |
| Secondary (%) | 35 (68.6) | 7 (13.7) | 9 (17.7) | 51 | |
| NF2* (%) | 8 (88.9) | 1 (11.1) | 0 | 9 | |
NF 2: neurofibromatosis type 2
Table 4 :
Frequency of tumor location in study groups
| Sites | Benign (%) | Atypical (%) | Malignant (%) |
|---|---|---|---|
| Cerbral convexity | 68(31.1) | 6(31.6) | 7(50) |
| Sphenoid ridge | 26(12) | 4(21) | 0 |
| CP angle | 25(11.5) | 3(15.7) | 2(14.3) |
| Parasagital | 19(8.7) | 2(10.5) | 2(14.3) |
| Olfactory groove | 14(6.4) | 1(5.3) | 0 |
| Parafalx | 13(6) | 1(5.3) | 1(7.1) |
| Petroclivus | 12(5.5) | 1(5.3) | 0 |
| Orbital | 7(3.2) | 0 | 0 |
| Cerebellum | 3(1.4) | 0 | 2(14.3) |
| Tentorial | 3(1.4) | 1(5.3) | 0 |
| Tuberculum sella | 4(1.8) | 0 | 0 |
| Foramen magnum | 3(1.4) | 0 | 0 |
| Anterior clenoidal | 3(1.4) | 0 | 0 |
| Cavernous sinus | 2(0.9) | 0 | 0 |
| Spinal | 16(7.3) | 0 | 0 |
| Total | 218 | 19 | 14 |
Note: In multiple meningiomas each tumor counted separately
The most common histologic subtypes were meningothelial (65.5%), transitional (17.2%)and fibrous (9.2%). The most common variants were metaplastic, chordoid, angiomatous, and lymphocyte-rich, each one constituting about 3% (Table 5).
Table 5 :
Frequency of histopathologic subtypes of evaluated meningiomas
| Subtypes | Number | Percent |
|---|---|---|
| Menigotheliomatous | 155 | 65.1 |
| Transitional | 41 | 17.2 |
| Fibrous | 22 | 9.2 |
| Metaplastic | 3 | 1.3 |
| Chord roid | 3 | 1.3 |
| Angiomatous | 3 | 1.3 |
| Lymphoplasma cell rich | 3 | 1.3 |
| Clear cell | 2 | 0.8 |
| Psammomatous | 2 | 0.8 |
| Rhabdoid | 2 | 0.8 |
| Papillary | 1 | 0.4 |
| Microcystic | 1 | 0.4 |
| Secretory | 0 | 0 |
| Total | 238 | 100 |
Local bone invasion - although not a malignant feature per se - was seen in 26 patients (10.9%), 24 of them were in benign, one in atypical and one in malignant groups. 15 patients with benign primary meningioma 8.4% showed recurrence within the study period; 6 of them (40 %) were male and 9(60%) were female. Mean age was 41.4. None of recurrent tumors revealed necrosis, mitosis or brain invasion. Thirteen of them were of benign histology in recurrences as their primaries; one of them was benign at first biopsy in 1998, atypical in first recurrence in 2001 and malignant in second recurrence in 2002. The other one was benign in 1998 and subsequently recurred as malignant in 2001. In recurrent tumors the most frequent sites were as the primaries. For 49.2% of the patients the tumor could not be resected totally due to location and local adhesions.
Sixteen patients (6.7%) had intraspinal meningiomas with mean age of 48.6 and female to male ratio of 1.3:1 .All of them were benign with small size and no atypical features.
Of total 238 cases, 8 patients (3.4%) had multiple (2 to 3) benign meningiomas at presentation, with female to male ratio of 3:4 and mean age of 37 years. All of them were primary nonrecurrent with no history of radiation and located in cerebral convexity, parasagittal, CP angle and petroclival region. Four out of them were known cases of neurofibromatosis type 2 (NF2). All of 9 patients (3.8 %) with NF2 included in this study had intracranial meningiomas. They were between 16 and 22 years old (mean = 18.67) and their tumors were located at cerebral convexity (6), CP angle (4), parasagital (1), parafalx (1), sphenoid ridge (1) and orbital region (1). Eight of them were male with benign meningioma and the only 1 female had atypical brain-invasive meningioma. Four NF2 patients (44%) had multiple meningiomas. Two NF2 patients (22%) showed histologically benign recurrences in the study period at the same place and one of them had schwannoma of CP angle at the same time.
One patient (male 33 years-old) had tuberous sclerosis syndrome with 2 tumor types (meningioma and low grade astrocytoma) at the same time.
The most frequent presenting symptoms and signs were headache and vomiting (46.4%), visual problems (27.9%), paresis (24%), seizure (13.3%) and proptosis (6%).(Table 6) In two patients CT scan performed for evaluation of head trauma incidentally found the tumor.
Table 6 :
The most frequent presenting signs and symptoms
| Signs and symptoms | Percent (%) | Signs and symptoms | Percent (%) |
|---|---|---|---|
| Headache and vomiting | 46.4 | Hearing loss | 4.3 |
| Visual problem | 27.9 | Sensory loss | 3.4 |
| Paresis | 24 | Personality change | 3 |
| Seizure | 13.3 | plegia | 2.1 |
| Proptosis | 6 | Speech disorder | 1.3 |
| Ataxia | 5.2 | Urinary incontinence | 0.9 |
| Cranial nerve palsy | 4.7 | dysphagia | 0.9 |
Discussion
Meningiomas as brain tumors have been recognized for nearly 200 years. (11) Initially all of them were considered benign. Recognition of their recurrent and malignant potential has encouraged some authors to classify them according to their histology. Despite introduction of new subtypes in WHO grading system such as clear cell and Chordoid (assumed as atypical) and rhabdoid (assumed as malignant); disagreement with Mahmood’s system was observed only in 3 cases that had mild nuclear pleomorphism taking them to “atypical” group of Mahmood’s system while according to WHO system which considers only prominent nucleoli as important, these tumors were classified as “benign”.
The relative ratios of benign, atypical and malignant tumors in this study were about 14.3:1.3:1 respectively (Table 3). This is similar to another study2 indicating the ratio of 16:2:1.
In our study female preponderance (1.3:1) was less obvious in spinal meningiomas compared to others (4:1). (3) This may be due to different genetic, environmental or other factors .In our study female predominance could be seen after 4th decade while in another report it was seen after 5th decade. (4) In atypical and anaplastic meningiomas we found obvious male predominance. This correlates with the study of Perry et al (12) and may indicate male sex as a negative prognostic factor. Perry also indicated that patients younger than 40 years had higher likelihood of recurrence independent of sex, grade or extent or resection; however; we found no significant age difference between recurrent and non-recurrent tumors.
Reportedly 4–15% of patients experience recurrence due to unclear mechanisms that may include continued proliferation of residual tumor cells left behind at surgery, other factors such as tumor proliferative activity and vascular endothelial growth factor (VEGF) (8, 13) or multicentric tumors (tumor diathesis). In our study period recurrence rate was about 8.4% occurring 1 to 6 years following surgery; more than half of them recurred after apparently gross total resection, although in less than half of them it may also have been occurred due to incomplete resection. In recurrent group female to male ratio was 1.5:1 that was slightly lower than non-recurrent group; However; no significant difference was identified in routine histopathologic indices, age and involved sites compared to nonrecurrent group.
The most common sites of involvement in our intracranial meningiomas were compatible with most previous studies i.e., cerebral convexity followed by sphenoid wing, CP angle, parasagittal region, olfactory groove, parafalcine, petroclivus and other sites. (4, 8)
About 3.3% of our cases were multiple, all of them with benign histology and half of them occurring in NF2 patients. In another reference its incidence was about 1–6% (14). It may be related to neurofibromatosis 2 or radiation (5). Rare instances of multiple meningiomas without vestibular schwannoma segregating as an autosomal dominant disorder have also been reported(15). In many instances however; no obvious etiology can be identified. (2)
Approximately half of individuals with NF2 develop meningiomas(16). As in our study most of NF2 meningiomas are intracranial; however, spinal meningiomas may also occur(17). In our NF2 patients the most common sites and type were cerebral convexity followed by skull base and meningothelial type but in other reports skull base is less frequent and they are usually of fibroblastic type. (18)
It should be mentioned that our limited study interval and limited numbers of intraspinal and rare subtypes of intracranial cases may necessitate more extended population studies and longer follow up periods to validate these results.
Conclusion
In this study the prevalence of tumor location, histologic subtypes and grades as well as age and sex distribution were similar to other studies. When recurrent tumors compared to non-recurrents we found no difference in age, site predilection and routine histologic difference. Compared to cranial tumors, spinal tumors showed less obvious female preponderance, lower recurrence rates and no atypicality or malignancy. In NF2 patients we found strict male preponderance (F:M ratio of 1:7.7), more common recurrence rate and tumor multiplicity. Finally in 238 cases of meningiomas studied, WHO and Mahmood grading systems had agreement in 235 cases which indicates excellent concordance rate (k = 0.947).
References
- 1.Beatriz M, Lopes S, Horten BC. central nervous system tumors: meningiomas. In: Weidner N, Cote RJ, Suster S, Weiss LM, editors. “modern surgical pathology”. 1st ed. Vol. 2. philadelphia: Saunders; 2003. pp. 2091–2094. [Google Scholar]
- 2.Burger P, Scheihauer BW, Vogel FC. Surgical pathology of nervous system and its coverings. 4th ed. Philadelphia: Churchill Livingston; 2002. Intracranial menings; pp. 49–71. [Google Scholar]
- 3.Burger P, Scheihauer BW, Vogel FC. Surgical pathology of nervous system and its coverings. 4th ed. Philadelphia: Churchill Livingston; 2002. Spinal menings, spinal nerve roots and spinal cord; pp. 527–531. [Google Scholar]
- 4.Haddad GF, Al-Mefty O, Aboulrauf SI. meningiomas. In: Winn HR, editor. “Youmans neurological surgery”. 5th ed. Philadelphia: Saunders; 2004. pp. 1099–1131. [Google Scholar]
- 5.Rosenblum MK, Bilbao JM, Ang LC. neuromuscular System: meningiomas. In: Rosai J, editor. Rosai & Ackerman’s surgical pathology. 9th edition. Vol. 1. Philadelphia: Mosby; 2004. pp. 2564–2572. [Google Scholar]
- 6.Mahmood A, Caccamo DV, Tomecec FJ, Malik GM. Atypical and malignant meningiomas: A clinicopathologic review. Neurosurg. 1993;33:955–63. doi: 10.1227/00006123-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Perry A, Scheithauer B, Stafford S, Lohse CM, Wollan PC. Malignancy in meningiomas a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046–56. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Ming-Tak Ho D, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas, a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94:1537–47. doi: 10.1002/cncr.10351. [DOI] [PubMed] [Google Scholar]
- 9.Perry A, Giannini C, Raghavan R, Scheithauer BW, Banerjee R, Margraf L, Bowers DC, Lytle RA. Newsham IF, Gutmann DH Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60:994–1003. doi: 10.1093/jnen/60.10.994. [DOI] [PubMed] [Google Scholar]
- 10.Evans DG, Huson SM, Donnai D, Neary W, Blair V, Teare D, Newton V, Strachan T, Ramsden R, Harris R. A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 1992;29:841–6. doi: 10.1136/jmg.29.12.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph E, Sandhyamani S, Rao MB, Nair S, Radhakrishnan VV. Atypical meningioma: A clinicopathologic analysis. Neurol India. 2000;48:338–42. [PubMed] [Google Scholar]
- 12.Perry A, Stafford S, Scheithauer B, Bernard W, Suman VJ. Meningioma grading: an analysis of histopathologic parameters. Am J Surg Pathol. 1997;21(12):1445–65. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki F, Yoshioka H, Hama S, Sugiyama K, Arita K, Kurisu K. recurrence of meningiomas: influence of vascular endothelial growth factor expression. Cancer. 2000;89:1102–10. doi: 10.1002/1097-0142(20000901)89:5<1102::aid-cncr20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Mckeever PE. The brain, Spinal cord and menings. In: Sternberg SS, Antonioli DA, Carter SE, Oberman HA, editors. Diagnostic surgical pathology. 3rd ed. USA; 1999. pp. 438–445. [Google Scholar]
- 15.Maxwell M, Shih SD, Galanopoulos T, Hedley-Whyte ET, Cosgrove GR. Familial meningioma: analysis of expression of neurofibromatosis 2 protein Merlin. Report of two cases. J Neurosurg. 1998;2–9 doi: 10.3171/jns.1998.88.3.0562. [DOI] [PubMed] [Google Scholar]
- 16.Perry DM, Eldridge R, Kaiser-Kupfer MI, Bouzas EA, Pikus A, Patronas N. Neurofibromatosis 2 (NF2): clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet. 1994;52:450–61. doi: 10.1002/ajmg.1320520411. [DOI] [PubMed] [Google Scholar]
- 17.Argenyi ZB, Thieberg MD, Hayes CM, Whitaker DC. Primary cutaneous meningioma associated with von Recklinghausen’s disease. J Cutan Pathol. 1994;21:549–56. doi: 10.1111/j.1600-0560.1994.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 18.Kros J, de Greve K, van Tilborg A, Hop W, Pieterman H, Avezaat C, Lekanne Dit Deprez R, Zwarthoff E. NF2 status of meningiomas is associated with tumor localization and histology. J Pathol. 2001;194:367–72. doi: 10.1002/path.909. [DOI] [PubMed] [Google Scholar]