Abstract
To determine the prevalence of goblet cell metaplasia in endocervical and endometrial adenocarcinomas by histochemial staining and to investigate the most sensitive histochemical staining method to detect this metaplasia, a total of 90 tissue blocks representing 30 non-neoplastic cervix, 30 non-neoplastic endometrium, 30 endocervical and endometrial adenocarcinoma cases were obtained for histochemical staining with Toluidine Blue (TB), Methylene Blue (MB), Mucicarmine (MUC), Periodic Acid Schiff before and after Diastase digestion (PAS, PAS-D), Alcian Blue pH 2.5 (AB), and Periodic Acid Schiff after Alcian Blue pH 2.5 (PAB). The cases were blinded and evaluated by a pathologist [NHO] for the presence of goblet cell metaplasia, the amount of goblet cells present and the histochemical differentiation of the goblet cells compared with its surrounding glandular epithelium. Goblet cell metaplasia was present in 2 out of 30 cases in non-neoplastic cervix, 0 out of 30 cases in non-neoplastic endometrium, 7 out of 15 cases in endocervical adenocarcinoma and in 2 out of 15 cases in endometrial adenocarcinoma. Relatively few goblet cells were seen in endometrial adenocarcinoma, few to moderate amounts were seen in endocervical adenocarcinoma and relatively more goblet cells were seen in non-neoplastic cervix. The differentiation of the goblet cells with its surrounding glandular epithelium was moderate to strong in non-neoplastic cervix and endocervical adenocarcinoma, while the differentiation in endometrial adenocarcinoma was weak to moderate. The various staining methods showed differences in presence, amount and differentiation of the goblet cells. Goblet cell metaplasia of the reproductive organs is not as rare as previously reported. There was no statistical difference in presence, amount and differentiation of goblet cells according to the various cases. The must optimum staining methods for staining goblet cells in non-neoplastic cervix, endocervical adenocarcinoma and endometrial adenocarcinoma were PAS, PASD and AB.
Keywords: endocervical adenocarinoma, endometrial adenocarcinoma, histochemical staining, intestinal metaplasia, goblet cell metaplasia, prevalence
Introduction
Goblet cell metaplasia is often synonymously referred as intestinal metaplasia. It has been described in endocervical (1–4) and endometrial adenocarcinomas (5–7). Savargaonkar et al (1) found intestinal metaplasia in 32% of endocervical adenocarcinoma cases, while McCluggage et al (6) found intestinal metaplasia in 17% of endometrial adenocarcinoma cases.
Intestinal metaplasia has also been reported in non-neoplastic cervix (8,9) and endometrium (10), mucinous tumors of the ovary (11, 12), adenocarcinomas of the vulva (13) and in the vagina (14), in adenoma malignum of the endocervix (15, 16).
The aim of our study was to determine the prevalence of goblet cell metaplasia in nonneoplastic endocervix and endometrium and their malignant counterparts. We also wanted to investigate the most sensitive histochemical staining method to detect this metaplasia. To the best of our knowledge similar studies has not been carried out before.
Materials and methods
Endocervix and endometrium tissues from the year 1998 to 2004 were obtained from the archived tissue block registry at the Department of Pathology, Universiti Sains Malaysia (USM). We randomly chose 30 non-neoplastic cervix, 15 non-neoplastic endometrium, 15 endocervical adenocarcinoma and 15 endometrial adenocarcinoma cases. The nonneoplastic cervix used in this study was acute/ chronic endocervicitis and the non-neoplastic endometrium was proliferative/secretory endometrium.
Seven 4-μm sections were obtained for each case. The sections were then stained with Toluidine Blue (TB), Methylene Blue (MB), Mucicarmine (MUC), Periodic Acid Schiff before and after Diastase digestion (PAS, PAS-D), Alcian Blue pH 2.5 (AB), and Periodic acid schiff after Alcian Blue pH 2.5 (PAB) based on the standard techniques for each stain (17).
The sections were examined under 100X microscopic magnification by a pathologist (NHO). Goblet cell metaplasia was defined as the presence of goblet cells in the glandular epithelium. The results were graded as negative (−) or positive (+). In cases of positive results the degree of severity of the metaplasia and the staining intensity of the goblet cells were evaluated. The degree of severity of metaplasia was defined as the amount of goblet cells present compared to the amount of normal glandular cells. This was arbitrarily expressed as <10% or >10%. The differentiation of the goblet cells compared with its surrounding glandular epithelium was evaluated as weak (+), moderate (++), or strong (+++). In endocervical and endometrial adenocarcinoma the histological grade of the carcinoma was also evaluated. This was graded as well, moderate or poor. Statistical analysis was done by Chi-Square test and Mann-Whitney test.
Results
Prevalence
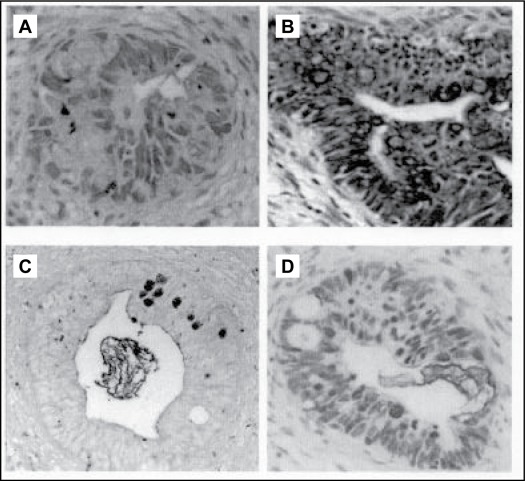
A total of 630 sections representing 90 cases were evaluated. Goblet cell metaplasia was present in 56 (0.09%) sections, representing 11 cases. Goblet cell metaplasia was present in 2 out of 30 (6.67%) cases in non-neoplastic cervix, 0 out of 30 cases in non-neoplastic endometrium, 7 out of 15 (46.7%) cases in endocervical adenocarcinoma and in 2 out of 15 (13.3%) cases in endometrial adenocarcinoma. Correlation for histological grade and presence of goblet cell metaplasia was not statistically significant. The endometrial adenocarcinomas, which displayed goblet cell metaplasia were graded as moderately differentiated adenocarcinomas. Some examples of the positive cases are depicted in Figure 1. There was no statistical difference in the amount of goblet cells of various histological diagnosis (Table 1).
Figure 1 :
Goblet Cell Metaplasia according to various staining methods under 100X (A) Endocervical adenocarcinoma with Alcian Blue pH 2.5 (AB), (B) Endocervical adenocarcinoma with Toluidine Blue (TB), (C) Endocervical adenocarcinoma with Periodic Acid Schiff after Alcian Blue pH 2.5 (PAB), (D) Endocervical adenocarcinoma with Periodic Acid Schiff (PAS).
Table 1 :
Shows the list of histological diagnosis, the amount of goblet cells and degree of epithelial - stromal differentiation
| amount of goblet cells | epithelial-stromal cell differentiation | |||||
|---|---|---|---|---|---|---|
| Tissue and histological diagnosis | Case No | <10% | >10% | + | ++ | +++ |
| Non-neoplastic cervix | ||||||
| 1 | 1 | 6 | 1 | 6 | ||
| 2 | 3 | 3 | ||||
| Endocervical adenoccarcinoma | ||||||
| - well differentiated | 3 | 6 | 2 | 4 | ||
| - well differentiated | 4 | 5 | 1 | 2 | 2 | |
| - moderately differentiated | 5 | 2 | 1 | 1 | ||
| - moderately differentiated | 6 | 4 | 1 | 3 | ||
| - moderately differentiated | 7 | 4 | 1 | 2 | 1 | |
| - moderately differentiated | 8 | 6 | 1 | 5 | 2 | |
| - moderately differentiated | 9 | 7 | 1 | 1 | 5 | |
| Endometrial edenocarcinoma | ||||||
| - moderately differentiated | 10 | 5 | 1 | 4 | ||
| - moderately differentiated | 11 | 6 | 1 | 4 | 1 | |
(Every case consists of one section per staining method, 7 sections per case)
The p values for all groups were >0.5
Histochemical staining
The staining differentiation of the goblet cells with its surrounding glandular epithelium was moderate to strong in non-neoplastic cervix and endocervical adenocarcinoma, while the staining differentiation in endometrial adenocarcinoma was weak to moderate. The various staining methods showed differences in presence, amount and differentiation of the goblet cells (Table 2).
Table 2 :
The presence, amount and differentiation of the goblet cells for the various staining methods
| Staining method | Tissue | Staining method | Tissue | ||||
|---|---|---|---|---|---|---|---|
| cervix | cervixca | endometriumca | cervix | cervixca | endometriumca | ||
| TB | PAS-D | ||||||
| presence | 0/2 | 4/7 | 1/2 | presence | 2/2 | 7/7 | 1/2 |
| amount | >10% | 0–10% | <10% | amount | 0–>10% | 0–>10% | <10% |
| differentiation | +++ | +/++/+++ | ++ | differentiation | ++/+++ | +/++/+++ | ++ |
| MB | AB | ||||||
| presence | 1/2 | 2/7 | 1/2 | presence | 2/2 | 6/7 | 2/2 |
| amount | >10% | <10% | <10% | amount | 0–>10% | 0–>10% | <10% |
| differentiation | +++ | ++ | + | differentiation | +/+++ | +/++/+++ | +/++ |
| MUC | PAB | ||||||
| presence | 1/2 | 6/7 | 2/2 | presence | 1/2 | 4/7 | 2/2 |
| amount | >10% | 0–>10% | <10% | amount | 0–>10% | 0–>10% | <10% |
| differentiation | +++ | ++/+++ | +/++ | differentiation | ++/+++ | ++/+++ | +/++ |
| PAS | |||||||
| presence | 1/2 | 6/7 | 2/2 | ||||
| amount | >10% | 0–>10% | <10% | ||||
| differentiation | +++ | ++/+++ | +/+++ | ||||
‘Cervix’ is non-neoplastic cervix; ‘cervixca’ is endocervical adenocarcinoma; ‘endometriumca’ is endometrial adenocarcinoma. ‘Presence’ indicate the number of positive cases/total cases. ‘Amount’ indicate the number of goblet cells in percentage in comparison with the amount of glandular epithelium. ‘Differentiation’ refers to the degree of differentiation of the goblet cells compared to the rest of glandular celss. The (+) is weak differentiation, (++) is moderate differentiation, and (+++) is strong differentiation.
Discussion
The presence of goblet cell metaplasia in endometrium and endocervix is often overlooked by pathologists unlike in the stomach. Our study showed a prevalence of intestinal metaplasia to be 6.67% (2/30 cases) in non-neoplastic cervix, 46.7% in endocervical adenocarcinoma (7/15cases), 13.3% in endometrial adenocarcinoma (2/15 cases) and none in non-neoplastic endometrium (0/30 cases). This confirms that intestinal metaplasia is not as rare as previously reported. This is supported by the study of Savargaonkar et al (1) and McCluggage et al (6). Our study showed no correlation between the prevalence of goblet cell metaplasia and the histological grade in endocervical adenocarcinoma. McCluggage et al (7) noted similar findings but for endometrial adenocarcinoma.
The amount of goblet cells seemed to differ among the various histological groups. In this study relatively few goblet cells were seen in endometrial adenocarcinoma, few to moderate amounts were seen in endocervical adenocarcinoma and relatively more goblet cells were seen in non-neoplastic cervix. The differentiation of the goblet cells with its surrounding glandular epithelium was moderate to strong in non-neoplastic cervix and in endocervical adenocarcinoma, while the differentiation in endometrial adenocarcinoma was weak to moderate. There was no statistical difference in differentiation between the various histological groups. Such findings were also shown by Savargaonkar et al (1) using PB/KOH/PAS-staining.
In this study we demonstrated that the most optimal overall staining methods for staining goblet cells in non-neoplastic cervix, endocervical adenocarcinoma and endometrial adenocarcinoma were PAS, PAS-D and AB. These stains highlighted the cells exhibiting goblet cell metaplasia from the adjacent glandular epithelium. Mucincarmine has a relatively strong detection rate, but the differentiation is of a lower degree compared to the other staining methods. Our study is in line with the findings of Mikami et al (15), who described a strong staining of the intestinal metaplasia in endocervical glandular hyperplasia for AB.
In conclusion, the presence of goblet cell metaplasia in reproductive organs is not as rare as previously reported. Routine histochemical stains could be used to highlight its presence. Although the presence of goblet cell metaplasia in neoplastic cervix and endometrium gives no added prognostic implication, when in abundance could lead to diagnostic difficulty.
Footnotes
Drs. Laurens Nieuwenhuizen was a postdoctoral (hon) researcher at the department of pathology, USM, Kubang Kerian, Malaysia. His home institution is Faculty of Medicine, University Maastricht, Netherlands. This research was conducted under sponsorship of grant number 305/ PPSP/6112246 of the Ministry of Science, Technology and Innovation, Malaysia
References
- 1.Savargaonkar PR, Hale RT, Pope R, Fox H, Buckley H. Enteric differentiation in cervical adenocarcinoma and its prognostic significance. Histopathology. 1993;23:275–277. doi: 10.1111/j.1365-2559.1993.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 2.Fox H, Wells M, Harris M, McWilliam LJ, Anderson GS. Enteric tumours of the lower female genital tract: a report of three cases. Histopathology. 1988;12:167–176. doi: 10.1111/j.1365-2559.1988.tb01927.x. [DOI] [PubMed] [Google Scholar]
- 3.Lapertosa G, Baracchini P, Fulcher E, Tanzi R. Patterns of mucous secretion in normal and pathological conditions of the endocervix. Eur J Gynecol Oncol. 1986;7:113–119. [PubMed] [Google Scholar]
- 4.Azzopardi JG, Hou LT. Intestinal metaplasia with argentaffin cells in cervical adenocarcinoma. J Pathol Bacteriol. 1965;90:686–690. doi: 10.1002/path.1700900245. [DOI] [PubMed] [Google Scholar]
- 5.Wells M, Tiltman A. Intestinal metaplasia of the endometrium. Histopathology. 1989;15:431–3. doi: 10.1111/j.1365-2559.1989.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 6.McCluggage WG, Roberts N, Bharucha H. Enteric differentiation in endometrial adenocarcinomas: a mucin histochemical study. Int J of Gynecol Pathol. 1995;14:250–254. doi: 10.1097/00004347-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W, Yang GC, Godwin TA, Caputo TA, Zuna RE. Mucinous adenocarcinoma of the endometrium with intestinal differentiation: a case report. Hum Pathol. 1995;26:1358–1368. doi: 10.1016/0046-8177(95)90307-0. [DOI] [PubMed] [Google Scholar]
- 8.Trowell JE. Intestinal metaplasia with argentaffin cells in the uterine cervix. Histopathology. 1985;9:551–559. doi: 10.1111/j.1365-2559.1985.tb02836.x. [DOI] [PubMed] [Google Scholar]
- 9.Michael H, Sutton G, Hull MT, Roth LM. Villous adenoma of the uterine cervix associated with invasive adenocarcinoma: a histochemical, ultrastructural and immunohistochemical study. Int J Gyaecol Pathol. 1986;5:163–169. [PubMed] [Google Scholar]
- 10.Moore WF, Bentley RC, Kim KR, Olatidoye B, Gray SR, Robboy SJ. Goblet-cell mucinous epithelium lining the endometrium and endocervix: evidence of metastasis from an appendiceal primary tumour through the use of cytokeratin-7 and -20 immunostains. Int J Gynecol Pathol. 1998;17:363–367. doi: 10.1097/00004347-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Szymanska K, Szamborski J, Miechowiecka N, Czerwinski W. Malignant transformation of mucinous ovarian cystadenomas of intestinal epithelial type. Histopathology. 1986;7:497–509. doi: 10.1111/j.1365-2559.1983.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 12.Lapertosa G, Baracchini P, Fulcher E, Tanzi R. Oacetylated sialic acid variants in mucinous tumours of the ovary. Histopathol. 1986;10:707–712. doi: 10.1007/BF00707697. [DOI] [PubMed] [Google Scholar]
- 13.Tiltman AJ, Knutzen VK. Primary adenocarcinoma of the vulva originating in misplaced cloacal tissue. Obstet Gynecol. 1978;51:30–3. [PubMed] [Google Scholar]
- 14.Fox H, Wells M, Harris M, McWilliam LJ, Andersson GS. Enteric tumours of the lower female genital tract: a report of three cases. Histopathology. 1988;12:167–176. doi: 10.1111/j.1365-2559.1988.tb01927.x. [DOI] [PubMed] [Google Scholar]
- 15.Mikami Y, et al. Florid endocervical glandular hyperplasia with intestinal and pyloric gland metaplasia: worrisome benign mimic of “adenoma malginum”. Gynecol Oncol. 1999;74:504–511. doi: 10.1006/gyno.1999.5462. [DOI] [PubMed] [Google Scholar]
- 16.Ishii K, et al. A new view of the so-called adenoma malignum of the uterine cervix. Virchows Arch. 1998;432:315–322. doi: 10.1007/s004280050172. [DOI] [PubMed] [Google Scholar]
- 17.Bancroft J, De Steven A. Theory and Practice of Histological Techniques. New York: Churchill Livingstone; 1996. [Google Scholar]