Abstract
The ubiquitin-conjugating enzyme (UbcH10) plays important roles in the regulation of cell cycle progression. Recently, UbcH10 expression has been demonstrated in several human and experimental tumors, and proteasome inhibitors have been tested in trials for pulmonary neoplasms; however, the underlying mechanisms as well as the clinicopathological relevance of UbcH10 in the genesis and progression of lung cancer remain largely unknown. Therefore, the authors evaluated the expression of UbcH10 in human lung cancer and evaluated its possible diagnostic and prognostic use. They found that most cases of lung adenocarcinoma, squamous cell carcinoma, and large cell and small cell carcinoma were positive for UbcH10. The expression levels of UbcH10 progressively increased with decreasing degree of tumor differentiation. There was a statistically significant difference of UbcH10 positivity between grade I/III of lung adenocarcinoma (p=0.013) and squamous cell carcinoma (p=0.002). No significant differences were found between histological types (p=0.072). In the case of cell blocks prepared from pleural effusions, inflammatory and reactive mesothelial elements did not show appreciable UbcH10 expression, whereas neoplastic cells exhibited clear UbcH10 positivity. The results suggest that UbcH10 might represent a new and promising diagnostic and prognostic marker in both histologic and cytologic specimens of lung cancer.
Keywords: UbcH10, lung, carcinomas, immunochemistry, cancer markers
Lung cancer is the leading cause of cancer-related mortality around the world (Molina et al. 2008), but many problems concerning its therapy and diagnosis are at present unsolved. A series of reports have focused attention on the potential role of the ubiquitin-mediated proteolysis and ubiquitin- conjugating enzyme (UbcH10) in cell cycle regulation and malignant transformation (Wagner et al. 2004; Pallante et al. 2005; Berlingieri, Pallante, Sboner, et al. 2007; Berlingieri, Pallante, Guida, et al. 2007; Donato et al. 2008). Pulmonary tumors are among neoplasms believed to be triggered and regulated in their growth from high levels of UbcH10 (van Ree et al. 2010). Recently, proteasome inhibitors have been developed as therapeutic agents in cancer therapy (Rossi et al. 2005; Orlowski and Kuhn 2008; Testa 2009; Crawford et al. 2011), and several phase I and phase II clinical trials in non–small cell and small cell lung cancer are in progress (Bunn 2004; Li et al. 2010; Escobar et al. 2011). Paradoxically, the role of UbcH10 in human lung cancer is not yet well explored, and very little is known regarding the histopathological and immunohistochemical pattern of UbcH10 in the most important subtypes of lung cancer. To extend the knowledge about the relationship between lung cancer and UbcH10 and to better define the diagnostic and prognostic potentials of UbcH10 on histologic and/or cytologic specimens, we have evaluated the expression profile of the enzyme in normal lung tissue, low-grade and high-grade lung carcinomas, and pleural effusions. We suggest that a tailored lung cancer treatment might require, in the future, a preliminary study of the expression of UbcH10 by a simple immunohistochemical or immunocytochemical analysis.
Materials and Methods
Study Materials
In this article, a retrospective evaluation of histopathological (bronchoscopic biopsy and surgical resections) and cytopathological (cell blocks) specimens diagnosed at the Department of Pathology of the University of Catanzaro during 2006 to 2011 was done. Archival formalin-fixed and paraffin-embedded tissues were used. Cancer tissues obtained from 140 patients (94 men and 46 women; mean age 62 ± 8) with lung cancer encompassed 57 lung adenocarcinomas, 54 squamous cell carcinomas (SCCs), 8 large cell carcinomas, and 21 small cell neuroendocrine lung tumors. The diagnostic accuracy of classification of lung carcinomas has been assessed based on the 2004 World Health Organization classification (Beasley et al. 2005). Grading of adenocarcinoma and squamous cells carcinoma was obtained according current criteria (Suster et al. 2007). Five normal lung specimens obtained at postmortem examination were also used as controls. The cytopathological study was carried out on formalin-fixed, paraffin-embedded archival cell block preparations from pleural effusions submitted for routine diagnosis at the Department of Pathology, University of Catanzaro. The cell blocks, each with histologic confirmation (n=47), included 15 reactive effusions, 14 pleural effusions of patients with malignant mesotheliomas, and 18 pleural effusions of patients with primary lung adenocarcinomas. Appropriate informed consent has been obtained from the patients involved in the study.
Immunochemical Staining on Tissue Sections and Cell Blocks
Xylene dewaxed and alcohol-rehydrated paraffin sections (4 µm thick) were irradiated in a microwave oven in 10 mM citrate buffer (pH 6.0) for 10 min according to the antigen retrieval method. After a washing step in PBS, rabbit polyclonal anti-α-UbcH10 (A-650) (Boston Biochem, Cambridge, MA) was applied at the appropriate concentration (1:100) and then incubated overnight at 4C. This polyclonal antibody has been used in previous studies to detect UbcH10 on Western blots (Pallante et al. 2005; Berlingieri, Pallante, Sboner, et al. 2007). Secondary antibody (biotinylated anti–primary antibody species) was applied at a 1:100 dilution. Peroxidase-conjugated avidin-biotin complex was allowed to react with secondary antibody for 30 min, and antibody complexes were visualized after addition of 0.01% DAB (3,3′-diaminobenzidine tetrahydrochloride; Sigma-Aldrich, St. Louis, MO) and 0.01% H2O2 in Tris (pH 7.2) for 4 to 6 min. Finally, sections were rinsed in tap water, dehydrated in graded alcohol, cleared with xylene, and mounted on glass slides with coverslips using Eukitt mounting medium (Calibrated Instruments, Inc., Ardsley, NY). Negative control sections for immunohistochemistry were processed without the primary antibody.
Statistical Analysis
A semiquantitative analysis was performed by an evaluation of the percentage of UbcH10-positive stained cells and staining intensity using the score system of Allred (Allred et al. 1998; Mohsin et al. 2004; Henderson-Jackson et al. 2011). A proportion score was assigned representing the estimated proportion of positively stained tumor cells (0 = none; 1 = <1/100; 2 = 1/100 to <1/10; 3 = 1/10 to 1/3; 4 = 1/3 to 2/3; 5 = >2/3). Average estimated intensity of staining in positive cells was assigned as an intensity score (0 = none; 1 = weak; 2 = intermediate; 3 = strong). Proportion score and intensity score were added to obtain a total score ranging from 0 to 8. Statistical analysis was performed using analysis of variance (ANOVA) to compare adenocarcinoma and squamous cells carcinoma of different grades and the three groups of non–small cell lung cancer (large cell carcinoma, adenocarcinoma, and squamous cell carcinoma) with small cell lung carcinoma. The tests were significant when the p-value was <0.05.
Results
Immunohistochemistry
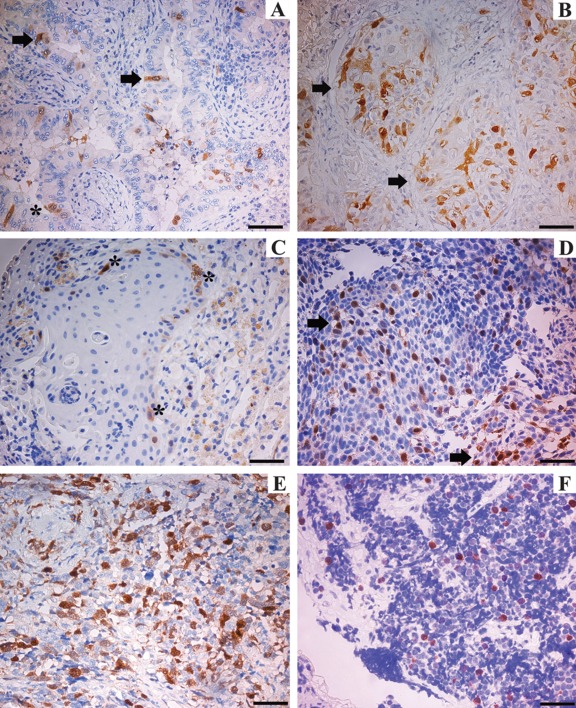
The results of immunohistochemical staining on biopsy specimens demonstrated positive UbcH10 expression in the four types of pulmonary cancer analyzed in our series and represented by lung adenocarcinoma (Fig. 1A,B), squamous cell carcinoma (Fig. 1C,D), and large cell (Fig. 1E) and small cell carcinoma (Fig. 1F). The UbcH10-positive immunoreactivity appeared as a brownish color and was predominantly located in the nucleus of neoplastic cells with only a faint cytoplasmic signal.
Figure 1.
Immunohistochemical analysis of UbcH10 protein in biopsy specimens of lung cancer. UbcH10-positive staining was brown, mostly located in the cell nucleus. (A) Well-differentiated adenocarcinoma (asterisk indicates transformed epithelium; arrows indicate UbcH10 stained cells). (B) Poorly differentiated adenocarcinoma (arrows indicate strongly labeled neoplastic elements). (C) Well-differentiated squamous cell carcinoma (asterisks indicate positively stained cells). (D) Poorly differentiated squamous cell carcinoma (arrows point to some of the positively stained areas). (E) Large cell lung carcinoma. (F) Small cell carcinoma. In the well-differentiated tumors (A–C), UbcH10 immune reaction was weaker than in poorly differentiated ones (B–D). Scale bars = 80 µm.
Protein expression exhibited a close inverse correlation with the degree of tumor differentiation in both adenocarcinoma (Fig. 1A,B) and squamous cell carcinoma (Fig. 1C,D).
Moderate expression of UbcH10 was seen in the well-differentiated lung adenocarcinoma (Fig. 1A) and squamous cell carcinoma (Fig. 1C) with no, or only scarce, immunolabeling in the transformed epithelium (asterisk in Fig. 1A). UbcH10-positive cells in the poorly differentiated adenocarcinoma (Fig. 1B) and squamous cell carcinoma (Fig. 1D) were significantly higher than those in the well-differentiated variants.
Moderate UbcH10 expression was detected in 14 of 18 cases of grade I adenocarcinoma (77.8%) and in 10 of 19 cases of grade III adenocarcinoma (52.6%). Strong UbcH10 immunolabeling was observed in 4 of 18 cases of grade I adenocarcinoma (22.2%) and in 9 of 19 cases of grade III adenocarcinoma (47.4%). With regard to lung squamous cell carcinoma, moderate UbcH10 expression was detected in 11 of 15 cases of grade I tumor (73.3%) and in 6 of 17 cases of grade III tumor (35.3%); strong UbcH10 reactivity was observed in 4 of 15 cases of grade I squamous cell carcinoma (26.7%) and in 11 of 17 cases of grade III squamous cell carcinoma (64.7%). Therefore, the expression levels of UbcH10 protein progressively increased with decreasing degree of tumor differentiation (Table 1).
Table 1.
UbcH10 Protein Expression in Different Histologic Grades of Lung Carcinomas
| Number (%) of Cases | Moderate UbcH10 Immunoreactivity, No. (%) | Strong UbcH10 Immunoreactivity, No. (%) | |
|---|---|---|---|
| Adenocarcinoma | 57 | ||
| Grade I | 18 (31.6) | 14 (77.8) | 4 (22.2) |
| Grade II | 20 (35.1) | 15 (75.0) | 5 (25.0) |
| Grade III | 19 (33.3) | 10 (52.6) | 9 (47.4) |
| Squamous cell carcinoma | 54 | ||
| Grade I | 15 (27.8) | 11 (73.3) | 4 (26.7) |
| Grade II | 22 (40.7) | 14 (63.6) | 8 (36.4) |
| Grade III | 17 (31.5) | 6 (35.3) | 11 (64.7) |
| Large cell carcinoma | 8 | 5 (62.5) | 3 (37.5) |
| Small cell neuroendocrine carcinoma | 21 | 12 (57.1) | 9 (42.9) |
There was no difference in the subcellular localization of UbcH10 between the histological subtypes and the grade of differentiation of neoplasms. Also, a large proportion of large cell (Fig. 1E) and small cell (Fig. 1F) carcinomas exhibited UbcH10 nuclear positivity.
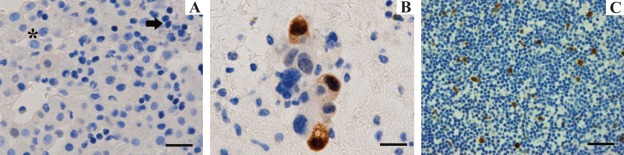
In the case of cell blocks prepared from cytologic specimens (Fig. 2), reactive mesothelial elements (asterisk) and lymphocytes (arrow) did not show appreciable UbcH10 expression (Fig. 2A), whereas cells of lung adenocarcinoma (Fig. 2B) and neoplastic mesothelial cells exhibited clear nuclear signal with no background staining (which is of critical importance in cytology) also when cells are scattered and isolated in an abundant, UbcH10-negative inflammatory infiltrate (Fig. 2C) (Table 2).
Figure 2.
UbcH10 expression in cell blocks of pleural fluid samples. (A) UbcH10-negative (unstained) reactive mesothelial elements (asterisk) and lymphocytes (arrow) (scale bar = 80 µm). (B) In pleural effusions from patients with primary lung adenocarcinomas, tumor cells stained positively for UbcH10 (scale bar = 40 µm). (C) Scattered UbcH10-positive mesothelial cells (brown reaction) in a mixed inflammatory cell background (scale bar = 80 µm).
Table 2.
Role of UbcH10 as a Potential Marker in an Immunocytochemical Panel for Pleural Effusions
| Marker | Reactive Mesothelia | Lung Adenocarcinoma | Mesothelioma | References |
|---|---|---|---|---|
| UbcH10 | − | + | + | |
| TTF-1 | − | + | − | Jiang et al. 2008; Agaimy and Wünsch 2006 |
| Calretinin | + | − | + | Simsir et al. 1999; Ko et al. 2001. |
Statistical Analysis
As shown in Table 3, no UbcH10 expression was observed in any normal lung tissues. UbcH10 positivity was detected in all types and grades of cancers analyzed in our series. Staining was present both in nuclei and cytoplasm in a variable percentage of cells. Statistical analysis (Table 4) suggested that UbcH10 expression correlated with tumor grade in lung adenocarcinoma and squamous cell carcinoma, but no significant difference was detectable among the main types of lung cancers.
Table 3.
Immunohistochemical Evaluation According to the Allred Score
| Tumor | Number of Cases | Mean | Standard Deviation |
|---|---|---|---|
| Adenocarcinoma | 57 | 4.12 | 1.13 |
| Adenocarcinoma grade I | 18 | 3.61 | 0.979 |
| Adenocarcinoma grade II | 20 | 4.05 | 0.945 |
| Adenocarcinoma grade III | 19 | 4.68 | 1.25 |
| Squamous cell carcinoma (SCC) | 54 | 4.26 | 1.46 |
| SCC grade I | 15 | 3.67 | 1.23 |
| SCC grade II | 22 | 3.91 | 1.27 |
| SCC grade III | 17 | 5.24 | 1.44 |
| Small cell carcinoma | 21 | 4.48 | 1.12 |
| Large cell carcinoma | 8 | 4.38 | 1.19 |
| Normal lung | 5 | 0 | 0 |
Table 4.
Statistical Analysis of the Correlation between UbcH10 Expression, Tumor Grade, and Histologic Type
| Parameter | Test | p-Value | Result |
|---|---|---|---|
| Between grades: adenocarcinomas | ANOVA | 0.013 | Significant |
| Between grades: SCC | ANOVA | 0.002 | Significant |
| Between histological types | ANOVA | 0.727 | Not significant |
ANOVA, analysis of variance; SCC, squamous cell carcinoma.
Discussion
Ubiquitin-dependent proteolysis is closely related to diverse cellular processes, including cell cycle progression, signal transduction, and differentiation (Hershko and Ciechanover 1998; Joazeiro and Weissman 2000; Okamoto et al. 2003). In this system, substrate proteins are processed for degradation by three distinct enzymes, including the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3) (van Wijk and Timmers 2010). Among the E2 gene family, the expression levels of UbcH10 have been shown to be extremely low in many of the normal tissues but prominent in a variety of human and experimental tumors of the stomach, lung, uterus, and bladder (Okamoto et al. 2003). In this article, the expression profile of UbcH10 in human lung cancer has been studied for the first time in the scientific literature by using immunocytochemical techniques. The results of our examination strongly support the involvement of UbcH10 in lung adenocarcinoma and squamous cell carcinoma of human origin and extend these previous observations to the main types of lung tumors, both large cell and small cell lung cancer. In our series, no significant UbcH10 expression was observed in normal lung tissues, whereas neoplastic cells were positive for UbcH10 protein, in both histological and cytological preparations, that usually show a nucleus-restricted UbcH10 positivity associated with a moderate cytoplasmic labeling. Just a weak expression of UbcH10 was found in the well-differentiated variants of lung adenocarcinoma and squamous cell carcinoma, whereas a higher incidence of UbcH10 positivity was observed in the poorly differentiated types. These data provoked the hypothesis that an inverse relationship exists between UbcH10 expression and the histological grade of both adenocarcinoma and squamous cell carcinoma because high expression of UbcH10 correlates with poor differentiation of lung tumors.
Several reports have demonstrated that aberrant expression of UbcH10 can promote tumor formation by deregulating the normal progression of the cell cycle and/or by impairing the spindle assembly checkpoint, potentially inducing chromosomal instability (Townsley et al. 1997; Rape and Kirschner 2004; de Gramont et al. 2006; Reddy et al. 2007; Jiang et al. 2008; Fujita et al. 2009; Patel and McCance 2010). Because in our series, the intensity of UbcH10 immunoreactivity was significantly higher in poorly differentiated lung carcinomas as compared with the well-differentiated variants, we suggest that overexpression of UbcH10 may critically contribute to the growth and progression of the neoplasm rather than its initiation. Our observations are entirely compatible with a previous report by Wagner and coworkers showing a marked upregulation of UbcH10 transcript levels with increasing cellular grade in cancers of the breast, lung, bladder, and brain (Wagner et al. 2004). In addition, previous large-scale genetic studies have revealed that UbcH10 is one of the candidate molecules related to aggressive behavior of the tumors (Ma et al. 2003; Sotiriou et al. 2006). Therefore, our immunohistochemical analysis is in agreement with prior biological studies that have implicated UbcH10 as a predictor of the biological characteristics of cancer.
In the case of cytologic specimens prepared from pleural effusions, cellular changes provide very useful diagnostic parameters but do not always allow a differential diagnosis between a reactive and neoplastic proliferation, making immunocytochemistry the best technique to differentiate them and to detect specific molecules on the cells that act as markers.
In the present study, we have demonstrated that UbcH10 staining was always easily detectable in the nuclei of the neoplastic cells of pleural effusions from patients with malignant disease; conversely, lymphocytes and reactive, nonneoplastic mesothelial cells accompanied by malignant cells have been found to be completely free of labeling. To our knowledge, this is the first study to evaluate the role of UbcH10 in the field of cytology. Our data demonstrate that UbcH10 might increase the efficiency of cytology to discriminate between non-neoplastic reactive states and neoplastic lesions and even to diagnose some particularly characteristic tumors. For instance, in a classical diagnostic setting (lung adenocarcinoma vs. mesothelioma), we found positive immunoreactivity for UbcH10 in malignant mesothelioma but not in reactive mesothelial cells. In the case of lung adenocarcinoma, which lack specific mesothelial markers such as calretinin, UbcH10 should be used in conjunction with a range of other antibodies (i.e., thyroid transcription factor–1 [TTF-1]) to validate the lung origin.
In conclusion, our results suggest that UbcH10 overexpression might chiefly represent a feature of the poorly differentiated carcinomas and propose UbcH10 as a novel and promising diagnostic marker for lung cancer with the potential to improve diagnostic accuracy in biopsy interpretation. In cytopathology, UbcH10 might be used as part of a tumor marker panel to discriminate neoplastic mesothelial cells from their benign, reactive counterparts, as well as when malignant cells are isolated and scattered in a background made of an abundant inflammatory infiltrate. Our results might encourage the perspective of a therapy of lung cancer based on the suppression of the UbcH10 synthesis and/or function. In such a perspective, the immunohistochemical examination of UbcH10 in lung neoplasms might become a predictive marker for therapy with proteasome inhibitors.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Agaimy A, Wünsch PH. 2006. Unexpected and potentially misleading TTF-1 expression: a word of caution. Virchows Arch. 449:603–605 [DOI] [PubMed] [Google Scholar]
- Allred DC, Harvey JM, Berardo M, Clark GM. 1998. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 11:155–168 [PubMed] [Google Scholar]
- Beasley MB, Brambilla E, Travis WD. 2005. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 40:90–97 [DOI] [PubMed] [Google Scholar]
- Berlingieri MT, Pallante P, Sboner A, Barbareschi M, Bianco M, Ferraro A, Mansueto G, Borbone E, Guerriero E, Troncone G, et al. 2007. UbcH10 is overexpressed in malignant breast carcinomas. Eur J Cancer. 43:2729–2735 [DOI] [PubMed] [Google Scholar]
- Berlingieri MT, Pallante P, Guida M, Nappi C, Maciullo V, Scambia G, Ferraro A, Leone V, Sboner A, Barbareschi M, et al. 2007. UbcH10 expression is a useful tool in the prognosis of ovarian carcinomas. Oncogene. 26:2136–2140 [DOI] [PubMed] [Google Scholar]
- Bunn PA., Jr. 2004. The potential role of proteasome inhibitors in the treatment of lung cancer. Clin Cancer Res. 10:4263s-4265s; [DOI] [PubMed] [Google Scholar]
- Crawford LJ, Walker B, Irvine AE. 2011. Proteasome inhibitors in cancer therapy. J Cell Commun Signal. 5:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gramont A, Ganier O, Cohen-Fix O. 2006. Before and after the spindle assembly checkpoint-an APC/C point of view. Cell Cycle. 5:2168–2171 [DOI] [PubMed] [Google Scholar]
- Donato G, Iofrida G, Lavano A, Volpentesta G, Signorelli F, Pallante PL, Berlingieri MT, Pierantoni MG, Palmieri D, Conforti F, et al. 2008. Analysis of UbcH10 expression represents a useful tool for the diagnosis and therapy of astrocytic tumors. Clin Neuropathol. 27:219–223 [DOI] [PubMed] [Google Scholar]
- Escobar M, Velez M, Belalcazar A, Santos ES, Raez LE. 2011. The role of proteasome inhibition in nonsmall cell lung cancer. J Biomed Biotechnol. 2011:806506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Ikeda H, Taira N, Hatoh S, Naito M, Doihara H. 2009. Overexpression of UbcH10 alternates the cell cycle profile and accelerate the tumor proliferation in colon cancer. BMC Cancer. 9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Jackson EB, Helm J, Strosberg J, Nasir NA, Yeatman TJ, Kvols LK, Coppola D, Nasir A. 2011. Palladin is a marker of liver metastasis in primary pancreatic endocrine carcinomas. Anticancer Res. 31:2957–2962 [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. 1998. The ubiquitin system. Annu Rev Biochem. 67:425–479 [DOI] [PubMed] [Google Scholar]
- Jiang L, Huang CG, Lu YC, Luo C, Hu GH, Liu HM, Chen JX, Han HX. 2008. Expression of ubiquitin-conjugating enzyme E2C/UbcH10 in astrocytic tumors. Brain Res. 1201:161–166 [DOI] [PubMed] [Google Scholar]
- Joazeiro CAP, Weissman AM. 2000. Ring finger proteins: mediators of ubiquitin ligase activity. Cell. 102:549–552 [DOI] [PubMed] [Google Scholar]
- Ko EC, Jhala NC, Shultz JJ, Chhieng DC. 2001. Use of a panel of markers in the differential diagnosis of adenocarcinoma and reactive mesothelial cells in fluid cytology. Am J Clin Pathol. 116:709–715 [DOI] [PubMed] [Google Scholar]
- Li T, Ho L, Piperdi B, Elrafei T, Camacho FJ, Rigas JR, Perez-Soler R, Gucalp R. 2010. Phase II study of the proteasome inhibitor bortezomib (PS-341, Velcade) in chemotherapy-naive patients with advanced stage non-small cell lung cancer (NSCLC). Lung Cancer. 68:89–93 [DOI] [PubMed] [Google Scholar]
- Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou YX, et al. 2003. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 100:5974–5979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, Roanh LD, To TV, Zho Q, Love RR, Allred DC. 2004. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol. 17:1545–1554 [DOI] [PubMed] [Google Scholar]
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. 2008. Non–small cell lung cancer: epidemiology, risk factors, treatment and survivorship. Mayo Clin Proc. 83:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Ozaki T, Miyazaki K, Aoyama M, Miyazaki M, Nakagawara A. 2003. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 63:4167–4173 [PubMed] [Google Scholar]
- Orlowski RZ, Kuhn DJ. 2008. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 14:1649–1657 [DOI] [PubMed] [Google Scholar]
- Pallante P, Berlingieri MT, Troncone G, Kruhoffer M, Orntoft TF, Biglietto G, Caleo A, Migliaccio I, Decaussin-Petrucci M, Santoro M, et al. 2005. UbcH10 overexpression May represent a marker of anaplastic thyroid carcinomas. Br J Cancer. 93:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, McCance DJ. 2010. Compromised spindle assembly checkpoint due to altered expression of Ubch10 and Cdc20 in human papillomavirus type 16 E6- and E7-expressing keratinocytes. J Virol. 84:10956–10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape M, Kirschner MW. 2004. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature. 432:588–595 [DOI] [PubMed] [Google Scholar]
- Reddy SK, Rape M, Margansky WA, Kirschner MW. 2007. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 446:921–925 [DOI] [PubMed] [Google Scholar]
- Rossi M, Oberst A, Emre Sayan A, Salomoni P. 2005. Proteasome inhibitors in cancer therapy: death by indigestion. Cell Death Differ. 12:1255–1257 [Google Scholar]
- Simsir A, Fetsch P, Mehta D, Zakowski M, Abati A. 1999. E- cadherin, N-cadherin, and calretinin in pleural effusions: the good, the bad, the worthless. Diagn Cytopathol. 20:125–130 [DOI] [PubMed] [Google Scholar]
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, et al. 2006. Gene expression profiling in breast cancer: understanding the molecular basis of histological grade to improve prognosis. J Natl Cancer Inst. 98:262–272 [DOI] [PubMed] [Google Scholar]
- Suster S, Moran C. Cancer grading manual. 2007. In: Damjanov I, Fan F, editors. Tumors of the lungs and pleura. New York: Springer; p. 23–30 [Google Scholar]
- Testa U. 2009. Proteasome inhibitors in cancer therapy. Curr Drug Targets. 10:968–981 [DOI] [PubMed] [Google Scholar]
- Townsley FM, Aristarkhov A, Beck S, Hershko A, Ruderman JV. 1997. Dominant negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Proc Natl Acad USA. 94:2362–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree JH, Jeganathan KB, Malureanu L, van Deursen JM. 2010. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J Cell Biol. 188:83–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk SJ, Timmers HT. 2010. The family of ubiquitin- conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 24:981–993 [DOI] [PubMed] [Google Scholar]
- Wagner KW, Sapinoso LM, El-Rifai W, Frierson HF, Butz N, Mestan J, Hofmann F, Deveraux Q, Hampton GM. 2004. Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene. 23:6621–6629 [DOI] [PubMed] [Google Scholar]