Abstract
Micronuclei (MN) can be induced by different mutagenic substances. Even though this has been known for decades, it is still not clear which genetic content, especially which chromosomes, these MN are constituted of and if there are any influences on this content by the MN-inducing substance. Also, the interphase position, size, and gene density of a chromosome could influence its involvement in MN formation. To study some of these questions, fluorescence in situ hybridization using centromeric and whole-chromosome painting probes for chromosomes 3, 4, 6, 7, 9, 16, 17, 18, and X was applied in mitomycin C (MMC)–induced MN in human leukocytes. The obtained results showed that material from all studied chromosomes was present in MN. Also, there was no correlation between interphase position, size, and gene density of the studied chromosomes and their migration in MN. Interestingly, material derived from chromosomes 9 and 16 was overrepresented in MMC-induced MN. Finally, further studies using substances other than MMC are necessary to clarify if the MN-inducing mutagen has an influence on the chromosomal content of the MN.
Keywords: micronuclei, chromosome, fluorescence in situ hybridization, mitomycin C, human leukocytes
The micronucleus (MN) test is a standard procedure in screening for potential genotoxic compounds in vitro and in vivo. Micronuclei originate from chromosomal fragments or whole chromosomes that fail to engage with the mitotic spindle and therefore lag behind when the cell divides (Ying and Hickson 2011). The current MN test methodology is based on the cytokinesis block micronucleus (CBMN) assay in which the once-divided cells are recognized by their binucleate appearance after cytokinesis block with cytochalasin-B (Fenech 2000). By the MN test combined with fluorescence in situ hybridization (FISH), the genetic contents of the MN can be characterized. The application of FISH probes allows one to distinguish MN originating either from chromosome loss or breakage and to determine the involvement of specific chromosomes and chromosome fragments in MN formation. Using multicolor FISH, the relative contribution of all chromosomes in MN formation can be studied, but such studies are scarce (Leach and Jackson-Cook 2001; Norppa and Falck 2003; Hovhannisyan et al. 2008).
A non-random distribution of chromosome breaks has been observed in human cells after in vitro exposure to chemicals (Funes-Cravioto et al. 1974; Reeves and Margoles 1974; Morad and Zawahri 1977; Meyne et al. 1979; Savage and Reddy 1987) and after radiation exposure (Knehr et al. 1996; Braselmann et al. 2003). Also, there are indications that different chromosomes are included in micronuclei non-randomly (Fimognari et al. 1997; Leach and Jackson-Cook 2001; Chung et al. 2002; Norppa and Falck 2003). Some chromosomes are frequently included in spontaneous MN (Peace et al. 1999; Leach and Jackson-Cook 2001), whereas others can be overrepresented in induced MN (Fauth and Zankl 1999). Leach and Jackson-Cook (2001) demonstrated that all of the 23 different chromosomes could be present in spontaneous MN; overall, the X-chromosome was seen most frequently. For some agents assayed in vitro, there is evidence for a chromosome-specific effect. Chung et al. (2002) found that chromosome 8 was involved more frequently in 1,2,4-benzenetriol-induced MN than chromosome 7. Guttenbach and Schmid (1994) reported that chromosomes 1, 9, 15, 16, and Y were preferentially moved into MN after 5-azacytidine exposure. Migliore et al. (1995) reported that acrocentric chromosomes were preferentially involved in vanadium-induced MN. Analysis of mitomycin C (MMC)–induced MN revealed the preferential occurrence of chromosome 1 and 9 material in MN (Fauth et al. 2000). The results of Fimognari et al. (1997) support a random model of radiation-induced damage for chromosomes 1, 7, 11, 14, 17, and 21 in human lymphocytes exposed to ionizing radiation. Wuttke et al. (1997) also found no difference in the frequency of chromosome 2 or 7 loss into MN after exposure to X-rays, but chromosome 7 showed an elevated frequency after colchicine treatment. Therefore, the literature suggests that different chromosomes are involved in spontaneous and mutagen-induced MN with different probability.
Different factors have been suggested to explain the non-random distribution of chromosome breakage and micronucleation, including chromosome size and gene density (Puerto et al. 1999), chromatin organization (Surrallés et al. 1998; Fauth et al. 2000), or lethality or loss of some chromosomes for cells (Chung et al. 2002). However, the recognition of the chromosome-specific contents of MN is still very incomplete, and mechanisms for micronucleation of different chromosomes are not clear. Three-dimensional organization of the nucleus (Cremer and Cremer 2001, 2010) and position of chromosomes (Manvelyan, Hunstig, Bhatt, et al. 2008) could also contribute to determining migration of chromosomes and their fragments in MN. To test this latter hypothesis, we applied here FISH using sequential centromeric and painting probes for chromosomes 3, 4, 6, 7, 9, 16, 17, 18, and X to determine the frequency of their contribution in MMC-induced MN formation in human leukocytes. We chose chromosomes with different central or peripheral localization in the human nucleus (Weierich et al. 2003; Manvelyan, Hunstig, and Bhatt, et al. 2008) and different length and gene density (Scherer 2010) to analyze input of these factors in their involvement in MN. Combined application of centromeric (CEP) and whole-chromosome painting (WCPs) probes was introduced to recognize MN with whole chromosomes and chromosome fragments and to distinguish clastogenic and aneugenic effects.
Materials and Methods
Peripheral blood was obtained from one female (F1) and two male donors (M1 and M2) aged 25, 23, and 21 years. The study was approved by the Ethics Committee of the Institute of Molecular Biology of National Academy of Sciences of RA (IRB # IORG 0002437), and informed consent was obtained from all study donors.
CBMN Technique
Heparinized whole blood was added to RPMI 1640 medium (1:10) containing 10% fetal bovine serum, 1% penicillin/streptomycin, and 10 µg/ml phytohemagglutinin. The CBMN test was performed according to Fenech (2000). After 22 hr of cultivation, MMC was added to the cell culture at a final concentration of 0.1 µg/ml. After 44 hr of cultivation, cultures were supplemented with cytochalasin B (3 µg/ml) to inhibit cytoplasmic division. In total, blood cultures were incubated for 72 hr at 37C. Hypotonic treatment was performed for 3 min in cold 0.075 M KCl at +4C. This procedure preserves the cytoplasm, which is required for the recognition of cell borders. Thus, MN can be assigned to their corresponding main nucleus. Fixation was done twice in ethanol/acetic acid (3:1). MN identification was done following DAPI staining according to the criteria of Fenech (2000) in binucleated cells.
FISH Technique
FISH was performed according to standard procedures (Liehr et al. 1995).
For F1, 1202 MN were hybridized and evaluated by a three-color FISH probe set consisting of CEPs (Abbott/Vysis, Abbott GmbH & Co. KG, Wiesbaden, Germany) for chromosomes 7 (SpectrumRed), 18 (SpectrumGreen), and X (Spectrum- Orange). For M1, 1732 MN were analyzed by a three-color FISH probe set consisting of CEPs (Abbott/Vysis) for chromosomes 6 (SpectrumOrange), 9 (SpectrumGreen), and 17 (diethylaminocoumarin). For M2, 1416 MN were analyzed by three-color FISH using CEPs (Abbott/Vysis) for chromosomes 3 (SpectrumOrange), 4 (SpectrumAqua), and 16 (SE 16 [D16Z2]; Poseidon Satellite Enumeration Probes KREATECH Diagnostics, Amsterdam, The Netherlands). The exact positions of MN on the evaluated slides were recorded for their further analysis by WCPs after a second round of hybridization.
In this second round of hybridization, the nuclei/MN from F1 were hybridized with WCPs for chromosomes 7 (SpectrumGreen), 18 (diethylaminocoumarine), and X (SpectrumOrange); from M1 with WCPs for chromosomes 6 (SpectrumOrange), 9 (SpectrumGreen), and 17 (Cy5); and from M2 with WCPs for chromosomes 3 (SpectrumOrange), 4 (Cy5), and 16 (SpectrumGreen). WCP probes were prepared as reported in Liehr and Claussen (2002). Only 916 of the 1202 from F1 and 996 of the 1732 MN from M1 were evaluated with the WCP probes. The remaining cells could not be relocated due to cell/MN loss on the slides during the second hybridization, since repeated treatment with pepsin and high temperature induces slight destruction of cell material. In total, 1416 MN of the third donor have been analyzed both at CEP and WCP hybridization. The cells with CEP signals lost during WCP hybridization have been excluded from the present study.
Image capturing and acquisition were processed with the Isis imaging system (MetaSystems, GmbH, Altlussheim, Germany).
Statistical Analysis
Statistical analysis was performed by Pearson’s correlation, two-way analysis of variance (ANOVA), and χ2 test using the statistical package SPSS version 19 (SPSS, Inc., an IBM Company, Chicago, IL).
Results
Number and Ratio of WCP and CEP Signals in MN
The results of MN-FISH analysis using WCP and CEP probes are presented in Table 1 and Fig. 1. MN mainly contained material of chromosomes 9 (31.60%), X (4.25%), and 16 (3.04%). Material from other chromosomes was found only in 1.27% to 2.21% of MN. WCP-positive MN were found about 2–10 times more often than corresponding CEP-positive MN.
Table 1.
Frequencies of Micronuclei with Centromeric and Whole-Chromosome Painting Signals for the Studied Chromosomes in Human Leukocytes Treated with MMC
| Chromosome Number | Number of MN Scored in CEP Experiments | Number (%) of MN with Centromeric Signals | Number of MN Scored in WCP Experiments | Number (%) of MN with Whole Chromosome Painting Signals |
|---|---|---|---|---|
| 3 | 1416 | 6 (0.42) | 1416 | 21 (1.48) |
| 4 | 1416 | 5 (0.35) | 1416 | 18 (1.27) |
| 6 | 1732 | 16 (0.92) | 994 | 22 (2.21) |
| 7 | 1202 | 9 (0.74) | 916 | 19 (2.07) |
| 9 | 1732 | 53 (3.06) | 994 | 314 (31.60) |
| 16 | 1416 | 3 (0.21) | 1416 | 43 (3.04) |
| 17 | 1732 | 8 (0.46) | 994 | 16 (1.61) |
| 18 | 1202 | 15 (1.24) | 916 | 19 (2.07) |
| X | 1202 | 26 (2.16) | 916 | 39 (4.25) |
CEP, centromeric; MMC, mitomycin C; MN, micronuclei; WCP, whole-chromosome painting.
Figure 1.
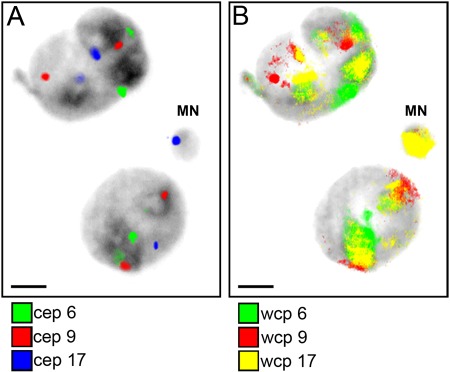
Example of binucleated cell in mitomycin C–treated human leukocytes with centromeric (CEP) (A) and whole-chromosome painting (WCP) (B) probes for chromosomes 6, 9, and 17. The micronucleus (MN) contains CEP- and WCP-derived material of chromosome 17. Bars = 5 µm.
Chromosomes Present in MN and Their Original Localization in Nucleus, Length, and Gene Density
The original chromosomal localization in the interphase nucleus is based on data of Weierich et al. (2003) for human lymphocytes and Manvelyan, Hunstig, Bhatt, et al. (2008) for human sperm since for most of the chromosomes, the distribution of the territories seems to be similar in sperm and lymphocytes apart from the acrocentric chromosomes (Manvelyan, Hunstig, Mrasek, et al. 2008). The data on length and gene density of human chromosomes were published in Scherer (2010). This information is summarized in Table 2.
Table 2.
Original Localization in the Interphase Nucleus, Length, and Gene Density of the Studied Chromosomes
| Chromosome Number | Chromosome Localizationa | Chromosome Lengthb (Mb) | Gene Density (Genes per Mb)b |
|---|---|---|---|
| 3 | Peripheral | 198.0 (long) | 5.20 (low density of genes) |
| 4 | Peripheral | 191.2 (long) | 3.77 (low density of genes) |
| 6 | Central | 171.1 (long) | 5.86 (low density of genes) |
| 7 | Peripheral | 159.1 (long) | 5.37 (low density of genes) |
| 9 | Peripheral | 141.2 (long) | 5.30 (low density of genes) |
| 16 | Central | 90.4 (short) | 8.67 (high density of genes) |
| 17 | Central | 81.2 (short) | 13.68 (high density of genes) |
| 18 | Peripheral | 78.1 (short) | 3.29 (low density of genes) |
| X | Peripheral | 155.3 (long) | 5.19 (low density of genes) |
Data from Manvelyan, Hunstig, Bhatt, et al. (2008).
Data from Scherer (2010).
The results of Pearson’s correlation analysis indicate no significant correlations between number of WCP-positive MN and original chromosome localization (peripheral vs. central) in the interphase nucleus (r = 0.246), chromosome length (r = −0.011), or gene density (r = −0.117) (p>0.05 for all cases). No significant correlations (p>0.05) were also found between number of CEP-positive MN and chromosome localization (r = 0.416), length (r = 0.006), and gene density (r = −0.303). However, comparison of frequencies of involvement of chromosomes investigated in MN shows that chromosomes with higher gene density tend to be included in MN less often than chromosomes with lower gene density, and chromosomes with peripheral localization and their fragments tend to be included in MN more frequently than chromosomes with central localization.
The two-way ANOVA was applied to measure the simultaneous effects of combinations of two factors (localization in nucleus and chromosome length, localization in nucleus and gene density, chromosome length and gene density) on the number of CEP and WCP signals in MMC-induced MN. Three p-values were generated, one for each parameter independently, and one measuring the interaction between the two parameters.
There was no interaction between localization and length of chromosomes in experiments with CEP (F(1, 5) = 0.073, p=0.798) and WCP (F(1, 5) = 0.102, p=0.763). For other combinations of factors, the analysis was not possible (details not shown).
Overall, Pearson’s correlation analysis and ANOVA test did not reveal any main effect of nuclear position (p>0.05), size (p>0.05), and gene density (p>0.05) of chromosomes investigated on the levels of CEP and WCP signals detected.
Chromosomes Present in MN and Their Size
The χ2 test was used to determine whether the observed number of MN with WCP signals was proportional to chromosome length. The numbers of expected WCP signal-positive MN were calculated for the total number of MN scored using a model of random breakage or DNA proportionality (Scherer 2010). χ2 values were determined for each chromosome investigated (Table 3).
Table 3.
Observed and Expected Frequencies of Migration of the Studied Chromosomes in Mitomycin C–Induced Micronuclei (MN)
| Chromosome Number | Observed MN+ | Expected MN+ | Observed MN– | Expected MN– | χ2 | Total MN |
|---|---|---|---|---|---|---|
| 3 | 21** | 91 | 1395 | 1325 | 57.54 | 1416 |
| 4 | 18** | 88 | 1398 | 1328 | 59.37 | 1416 |
| 6 | 22** | 55 | 972 | 939 | 20.96 | 994 |
| 7 | 19** | 47 | 897 | 869 | 17.58 | 916 |
| 9 | 314** | 45 | 680 | 949 | 1684.27 | 994 |
| 16 | 43 | 41 | 1373 | 1375 | 0.09 | 1416 |
| 17 | 16* | 26 | 978 | 968 | 3.95 | 994 |
| 18 | 19 | 23 | 897 | 893 | 0.72 | 916 |
| X | 39 | 46 | 877 | 870 | 1.12 | 916 |
The number of expected signal-positive (MN+) and signal-negative (MN–) micronuclei was calculated for the number of MN scored according to a model of random chromosome breakage. The expected level of chromosome involvement in MN was calculated as the proportion of investigated chromosome length with respect to total genome.
p<0.05 and **p<0.001 when compared with expected value (χ2 test).
The deviation of obtained values from expected ones arose from chromosomes 3 (χ2 = 57.54; p<0.001), 4 (χ2 = 59.37; p<0.001), 6 (χ2 = 20.96; p<0.001), 7 (χ2 = 17.58; p<0.001), and 17 (χ2 = 3.95; p<0.05), which were underrepresented, and 9 (χ2 = 1684.27; p<0.001), which was overrepresented, in signal-positive MN. It can be seen that the highest signal-positive MN frequencies were obtained with the experiments using paint for chromosome 9. Chromosomes 16, 18, and X were included in MN 1.05 times more and 1.22 and 1.18 times less than expected, respectively; however, these differences are not statistically significant (χ2 = 0.09, χ2 = 0.72, and χ2 = 1.12, respectively; p>0.05 for all cases).
Overall, chromosomes 3, 4, 6, 7, 17, 18, and X were found to be damaged less often, and chromosomes 9 and 16 more often, than expected. Chromosomes 9 and 16 were involved in the formation of MN 6.93 and 1.05 times more frequently than expected on the base of DNA content.
Discussion
FISH using CEP and WCP probes for chromosomes 3, 4, 6, 7, 9, 16, 17, 18, and X was applied to determine the presence of specific chromosomal material in MMC-induced MN in human leukocytes. No significant correlation between involvement of chromosomes in MN and their localization in nuclei, length, and gene density was found. However, gene density and chromosome position in nuclei have a tendency of slightly influencing MMC-induced MN formation. The explanation for this tendency can be the exceptional high contribution in MN formation of chromosome 9 with low gene density and peripheral location in nuclei, caused by its high sensitivity to ММС (Fauth et al. 2000). However, further studies are certainly needed to clarify the mechanisms of MN formation.
Chromosomes 3, 4, 6, 7, 17, 18, and X were found to be damaged less often, and chromosomes 9 and 16 more often, than expected according to DNA-proportional distribution. The abundance of material from chromosomes 9 and 16 in MMC-treated cells can be interpreted together with data of Cohen and Shaw (1964), Nowell (1964), Morad et al. (1973), Brogger (1977), Abdel-Halim et al. (2005), and Sontakke and Fulzele (2009), which showed that MMC induces undercondensation and breakage mainly in the pericentromeric heterochromatin blocks of chromosomes 1, 9, and 16. The highest level of MN containing DNA from chromosome 9 compared with all other chromosomes in MMC-treated lymphocytes was shown as well by Fauth et al. (2000). Even taking into account that the number of MN investigated in our experiments was higher (994–1416 vs. 50–100), it is noteworthy that both studies confirmed a high involvement of chromosome 9 in MN formation. However, our study revealed that chromosome 9 is involved in around one-third (31.60%) of MMC-induced MN only. The data of Fauth et al. suggested that around two-thirds (62%–69%) can arise from application of five times higher doses of MMC and/or individual susceptibility of donors to MMC treatment and the higher number of evaluated MN. Fauth et al. (2000) also showed that the heterochromatin blocks of chromosomes 9q12, 1q12, and 16q12 are specific targets for MMC-induced undercondensation in metaphases and preferential micronucleation. Preferential breakage at these heterochromatic blocks and their incorporation in MN also has been seen in cells treated with 5-azacytidine (Guttenbach and Schmid 1994; Fauth et al. 1998), 2,6-diaminopurine (Smith et al. 1998), and idoxuridine (Fauth and Zankl 1999). In these cases, the structure of chromatin seems a major determinant of non-random chromosome micronucleation.
Involvement of the X chromosome in MN is a topic of special interest in the literature. The X chromosome was clearly overrepresented in spontaneous MN (Leach and Jackson-Cook 2001). Some authors have found that the special participation of the X chromosome in MN formation is caused by the inactive X chromosome (Tucker et al. 1996), whereas other studies have shown that active and inactive X chromosomes micronucleated equally (Surrallés et al. 1996). The reason for the extremely high spontaneous X micronucleation is still unknown. Earlier we found that the obvious difference between male- and female-derived MN contained WCP X chromosome–positive material (0.99% vs. 4.25%) (Hovhannisyan et al. 2008). We speculated about a preferred inclusion of the inactive female X chromosome in MMC-induced MN.
Compared with a previous molecular cytogenetic study of MN with application of chromosome painting (Fauth et al. 2000), in our work, the contents of MN were analyzed using sequential centromeric and painting probes. This approach permits the discrimination of MN with whole chromosomes and chromosome fragments and the estimation of the aneugenic and clastogenic potential of mutagens. At the same time, it does not permit distinguishing the whole chromosome from centromere-containing chromosome fragments. Earlier, the combination of CEP and WCP probes was used only to examine the contents of radiation-induced MN (Fimognari et al. 1997). However, this approach is especially critical for analysis of MN, induced by chemical mutagens. The results of the present study showed that MMC-induced MN comprise both chromosome fragments and whole chromosomes. WCP signals that reveal both whole chromosomes and acentric fragments always exceed the number of CEP signals that reveal only whole chromosomes. Due to the strong clastogenic effect of MMC, MN are predominantly composed of acentric fragments. However, MMC has an aneugenic potential, besides its strong clastogenic activity, because of its ability to detach kinetochores (Renzi et al. 1996). In addition, breakage in the centromere would be another explanation for MMC-induced centromere-positive MN.
In summary, our data have allowed preliminary estimation of the involvement of nine chromosomes in MN. However, the results obtained have to be confirmed on a larger group of donors and for all human chromosomes. Overall, the MN assay and FISH using CEP and WCP probes are considered a useful screening test to determine the origin and contents of micronuclei. This kind of MN-FISH has a high potential to identify chromosomal targets of different mutagens for elucidating mechanisms of mutagen action and developing biomarkers of mutagenic treatment.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported in part by the DFG (grant number LI 820/13–1), DAAD (grant number A/10/02362), and State Committee of Science of RA (grant number 11–1s-0160).
References
- Abdel-Halim HI, Natarajan AT, Mullenders LHF, Boei JJWA. 2005. Mitomycin C–induced pairing of heterochromatin reflects initiation of DNA repair and chromatid exchange formation. J Cell Sci. 118:1757–1767 [DOI] [PubMed] [Google Scholar]
- Braselmann H, Kulka U, Huber R, Figel HM, Zitzelsberger H. 2003. Distribution of radiation-induced exchange aberrations in all human chromosomes. Int J Radiat Biol. 79:393–403 [DOI] [PubMed] [Google Scholar]
- Brogger A. 1977. Non-random localization of chromosome damage in human cells and targets for clastogenic action. Chromosomes Today. 6:297–306 [Google Scholar]
- Chung HW, Kang SJ, Kim SY. 2002. A combination of the micronucleus assay and a FISH technique for evaluation of the genotoxicity of 1,2,4-benzenetriol. Mutat Res. 516:49–56 [DOI] [PubMed] [Google Scholar]
- Cohen MM, Shaw MW. 1964. Effects of mitomycin C on human chromosomes. J Cell Biol. 23:386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T, Cremer C. 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2:292–301 [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer M. 2010. Chromosome territories. Cold Spring Harb Perspect Biol. 2(3):a003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth E, Scherthan H, Zankl H. 1998. Frequencies of occurrence of all human chromosomes in micronuclei from normal and 5-azacytidine-treated lymphocytes as revealed by chromosome painting. Mutagenesis. 13:235–241 [DOI] [PubMed] [Google Scholar]
- Fauth E, Scherthan H, Zankl H. 2000. Chromosome painting reveals specific patterns of chromosome occurrence in mitomycin C– and diethylstilboestrol-induced micronuclei. Mutagenesis. 15:459–467 [DOI] [PubMed] [Google Scholar]
- Fauth E, Zankl H. 1999. Comparison of spontaneous and idoxuridine-induced micronuclei by chromosome painting. Mutat Res. 440:147–156 [DOI] [PubMed] [Google Scholar]
- Fenech M. 2000. The in vitro micronucleus technique. Mutat Res. 455:81–95 [DOI] [PubMed] [Google Scholar]
- Fimognari C, Sauer-Nehls S, Braselmann H, Nüsse M. 1997. Analysis of radiation-induced micronuclei by FISH using a combination of painting and centromeric DNA probes. Mutagenesis. 12:91–95 [DOI] [PubMed] [Google Scholar]
- Funes-Cravioto F, Yakovienko KN, Kuleshov NP, Zhurkov VS. 1974. Localization of chemically induced breaks in chromosomes of human leucocytes. Mutat Res. 23:87–105 [DOI] [PubMed] [Google Scholar]
- Guttenbach M, Schmid M. 1994. Exclusion of specific human chromosomes into micronuclei by 5-azacytidine treatment of lymphocyte cultures. Exp Cell Res. 211:127–132 [DOI] [PubMed] [Google Scholar]
- Hovhannisyan G, Mkrtchyan H, Liehr T, Aroutiounian R. 2008. Involvement of chromosomes 7, 18 and X in mitomycin C–induced micronuclei. Balkan J Med Genet. 11:45–49 [Google Scholar]
- Knehr S, Zitzelsberger H, Braselmann H, Nahrstedt U, Bauchinger M. 1996. Chromosome analysis by fluorescence in situ hybridization: further indications for a non-DNA-proportional involvement of single chromosomes in radiation-induced structural aberrations. Int J Radiat Biol. 70:385–392 [DOI] [PubMed] [Google Scholar]
- Leach NT, Jackson-Cook C. 2001. The application of spectral karyotyping (SKY) and fluorescent in situ hybridization (FISH) technology to determine the chromosomal content(s) of micronuclei. Mutat Res. 495:11–19 [DOI] [PubMed] [Google Scholar]
- Liehr T, Claussen U. 2002. Current developments in human molecular cytogenetic techniques. Curr Mol Med. 2:283–297 [DOI] [PubMed] [Google Scholar]
- Liehr T, Thoma K, Kammler K, Gehring C, Ekici A, Bathke KD, Grehl H, Rautenstrauss B. 1995. Direct preparation of uncultured EDTA-treated or heparinized blood for interphase FISH analysis. Appl Cytogenet. 21:185–188 [Google Scholar]
- Manvelyan M, Hunstig F, Bhatt S, Mrasek K, Pellestor F, Weise A, Simonyan I, Aroutiounian R, Liehr T. 2008. Chromosome distribution in human sperm—a 3D multicolour banding-study. Mol Cytogenet. 1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvelyan M, Hunstig F, Mrasek K, Bhatt S, Pellestor F, Weise A, Liehr T. 2008. Position of chromosomes 18, 19, 21 and 22 in 3D-preserved interphase nuclei of human and gorilla and white hand gibbon. Mol Cytogenet. 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyne J, Lockhart LH, Arrighi FE. 1979. Nonrandom distribution of chromosomal aberrations induced by three chemicals. Mutat Res. 63:201–209 [DOI] [PubMed] [Google Scholar]
- Migliore L, Scarpato R, Falco P. 1995. The use of fluorescence in situ hybridisation with a beta-satellite DNA probe for the detection of acrocentric chromosomes in vanadium-induced micronuclei. Cytogenet Cell Genet. 69:215–219 [DOI] [PubMed] [Google Scholar]
- Morad M, El Zawahri M. 1977. Non-random distribution of cyclophosphamide-induced chromosome breaks. Mutat Res. 42:125–130 [DOI] [PubMed] [Google Scholar]
- Morad M, Jonasson J, Lindsten J. 1973. Distribution of mitomycin C induced breaks on human chromosomes. Hereditas. 74:273–282 [DOI] [PubMed] [Google Scholar]
- Norppa H, Falck GC. 2003. What do human micronuclei contain? Mutagenesis. 18:221–233 [DOI] [PubMed] [Google Scholar]
- Nowell PC. 1964. Mitotic inhibition and chromosome damage by mitomycin in human leukocyte cultures. Exp Cell Res. 33:445–449 [DOI] [PubMed] [Google Scholar]
- Peace BE, Livingston G, Silberstein EB, Loper JC. 1999. A case of elevated spontaneous micronucleus frequency derived from chromosome 2. Mutat Res. 430:109–119 [DOI] [PubMed] [Google Scholar]
- Puerto S, Surrallés J, Ramírez MJ, Creus A, Marcos R. 1999. Equal induction and persistence of chromosome aberrations involving chromosomes with heterogeneous lengths and gene densities. Cytogenet Cell Genet. 87:62–68 [DOI] [PubMed] [Google Scholar]
- Reeves BR, Margoles C. 1974. Preferential location of chlorambucil-induced breakage in the chromosomes of normal human lymphocytes. Mutat Res. 26:205–208 [DOI] [PubMed] [Google Scholar]
- Renzi L, Pacchierotti F, Russo A. 1996. The centromere as a target for the induction of chromosome damage in resting and proliferating mammalian cells: assessment of mitomycin C–induced genetic damage at kinetochores and centromeres by a micronucleus test in mouse splenocytes. Mutagenesis. 11:133–138 [DOI] [PubMed] [Google Scholar]
- Savage JR, Reddy KS. 1987. On the localization of mitomycin C–induced aberrations in normal human and Fanconi’s anaemia cells. Mutat Res. 178:65–71 [DOI] [PubMed] [Google Scholar]
- Scherer S. 2010. Guide to Human Genome. http://www.cshlp.org/ghg5_all/section/dna.shtml
- Smith LE, Parks PP, Hasegawa LS, Eastmond DA, Grosovsky AJ. 1998. Targeted breakage of paracentromeric heterochromatin induces chromosomal instability. Mutagenesis. 13:435–443 [DOI] [PubMed] [Google Scholar]
- Sontakke YA, Fulzele RR. 2009. Cytogenetic study on genotoxicity of antitumor-antibiotic Mitomycin C. Biomed Res. 20:40–44 [Google Scholar]
- Surrallés J, Jeppesen P, Morrison H, Natarajan AT. 1996. Analysis of loss of inactive X chromosomes in interphase cells. Am J Hum Genet. 59:1091–1096 [PMC free article] [PubMed] [Google Scholar]
- Surrallés J, Puerto S, Ramírez MJ, Creus A, Marcos R, Mullenders LH, Natarajan AT. 1998. Links between chromatin structure, DNA repair and chromosome fragility. Mutat Res. 404:39–44 [DOI] [PubMed] [Google Scholar]
- Tucker JD, Nath J, Hando JC. 1996. Activation status of the X chromosome in human micronucleated lymphocytes. Hum Genet. 97:471–475 [DOI] [PubMed] [Google Scholar]
- Weierich C, Brero A, Stein S, von Hase J, Cremer C, Cremer T, Solovei I. 2003. Three-dimensional arrangements of centromeres and telomeres in nuclei of human and murine lymphocytes. Chromosome Res. 11:485–502 [DOI] [PubMed] [Google Scholar]
- Wuttke K, Streffer C, Muller WU. 1997. Detection of chromosome 2 and chromosome 7 within X-ray of cholchicine-induced micronuclei by fluorescence in situ hybridisation. Mutagenesis. 12:55–59 [DOI] [PubMed] [Google Scholar]
- Ying S, Hickson ID. 2011. Fanconi anaemia proteins are associated with sister chromatid bridging in mitosis. Int J Hematol. 93:440–445 [DOI] [PubMed] [Google Scholar]