Abstract
Hepatic fibrosis and cirrhosis are worldwide health care problems, especially in regions with a high rate of hepatitis infection. As these diseases affect a major part of the human population, the search for antifibrotic therapies has a high priority in medical research. Transforming growth factor β1 (TGF-β1) is one of the most powerful profibrotic cytokines. Thus, blocking TGF-β1 activity by natural inhibitors represents a valid and logical strategy to combat hepatic fibrosis. One of the natural inhibitors of TGF-β1 is decorin, a small leucine-rich proteoglycan that binds with high affinity to this cytokine and prevents its interaction with pro-fibrotic receptors. Recent evidence has shown that decorin has a protective role in liver fibrogenesis insofar as its genetic ablation in mice leads to enhanced matrix deposition, impaired matrix degradation, and “activation” of hepatic stellate cells, the main producers of fibrotic tissue. Moreover, TGF-β1 exerts a stronger effect when functional decorin is absent. These data provide robust genetic evidence for a direct role of endogenous decorin in preventing and retarding hepatic fibrosis. Thus, boosting the endogenous production of decorin or systemic delivery of recombinant decorin could represent an additional therapeutic modality against hepatic fibrosis.
Keywords: liver fibrosis, decorin, small leucine-rich proteoglycan, TGF-β1, extracellular matrix, hepatic stellate cells
Chronic liver diseases are among the major health problems worldwide. The liver is especially prone to fibrotic remodeling (Balsano et al. 2009) because this organ is extremely susceptible to viral injury, toxic insults like ethanol, as well as storage and autoimmune diseases (Epple et al. 1979; Friedman 2003; Fartoux and Serfaty 2005). Without eradication of the etiological agent, liver fibrosis normally progresses to cirrhosis and destroys the normal architecture of the liver culminating in parenchymal and vascular decompensation of the organ (Popper 1977; Desmet 1992). Cirrhosis is considered an irreversible disease in humans, and no curative treatment except liver transplantation is available so far (Albillos Martinez 2009; Garcia-Tsao and Lim 2009; Vallet-Pichard et al. 2009). Furthermore, increased incidence of liver cancer also threatens the cirrhotic patient’s life (Kato et al. 1992; La Vecchia et al. 1998). These facts have prompted physicians and scientists to seek curative interventions based on the growing awareness of the molecular mechanisms involved in the induction and development of the fibrotic process. Recent advances have culminated in the realization that cirrhosis can be reversible, and that effective antifibrotic therapy can significantly improve the management and prognosis of patients with liver disease (Friedman 2003).
Liver Fibrosis: The Role of Transforming Growth Factor β1
Fibrogenesis is characterized by excessive accumulation of extracellular matrix (ECM) as a result of an imbalance between its synthesis and degradation (Schnaper 1995). In response to liver injury, hepatic stellate cells (HSCs), also known as Ito cells or fat storing cells, become “activated” and are phenotypically modulated to resemble a proliferative “myofibroblast-like” phenotype. As such, these activated HSCs synthesize and secrete an excessive amount of ECM, which is deposited in hepatic interstitium leading to a frank fibrotic liver (Gressner 1996; Knittel et al. 1999; Shek and Benyon 2004).
Among factors implicated in the induction and maintenance of matrix overproduction, transforming growth factor β1 (TGF-β1) occupies a central position, as it was seen also in glomerulosclerosis and pulmonary fibrosis (Clouthier et al. 1997; De Bleser et al. 1997; El-Gamel et al. 1999; Kanzler et al. 1999; Wang et al. 2005; Schmidt et al. 2006; Gauldie et al. 2007). This peptide growth factor can activate fibroblasts and HSCs, inhibit their apoptosis and force them to synthesize excess amount of matrix proteins such as fibronectin; collagen types I, III, and IV; tenascin; elastin; osteonectin; biglycan; and decorin (Kanzler et al. 1999). Besides stimulating these cells to increase the synthesis of most matrix proteins, TGF-β1 also hinders the production of matrix-degrading proteases, and upregulates their inhibitors such as tissue inhibitor of metalloproteinase I and plasminogen activator inhibitor (Dudas et al. 2001). Moreover, TGF-β1 modulates the expression of integrins in a manner that results in increased cellular adhesion to the matrix. These complex effects exerted on the extracellular matrix reflect the versatile biological potential of the growth factor. TGF-β1 binds to proteoglycans embedded in the hepatic extracellular matrix or bound to the cell surface. Such binding may act as a signal to terminate the production of TGF-β1 after tissue repair is complete (Border and Noble 1994). The strategic role of TGF-β1 in fibrogenesis implies that inactivation of this growth factor could be an approach in the management of liver fibrosis. Indeed, blockade of TGF-β1 activity has proven to be an effective way of inhibiting the fibrotic response to injury in various organs (Shek and Benyon 2004).
Effects of Decorin on TGF-β1 Action in Liver Fibrosis
Decorin, a small leucine-rich proteoglycan (SLRP; Iozzo 1999; Ameye and Young 2002; Schaefer and Iozzo 2008) can hinder the proliferation of cells that are dependent on TGF-β1 for their in vitro growth (Yamaguchi et al. 1990). This observation has been exploited in various in vivo models of experimental glomerulonephritis and nephrosclerosis, as well as in scar formation models (Isaka et al. 1996; Zhang et al. 2007). On the other hand, there was no conclusive information on the role of decorin in the fibrotic remodeling of the liver (Dudas et al. 2001). Decorin, as a regulator of matrix assembly, not only targets TGF-β1, but it is also involved in the maturation of collagen fibrils (Weber et al. 1996; Danielson et al. 1997; Zhang et al. 2006). Furthermore, decorin functions as a ligand for growth factor receptors modulating signals initiated on EGFR, IGF-IR, and Met receptors (Iozzo et al. 1999; Csordas et al. 2000; Reed et al. 2002; Santra et al. 2002; Schonherr et al. 2005; Schaefer et al. 2007; Fiedler et al. 2008; Goldoni et al. 2009; Merline et al. 2009). A central question is whether decorin-evoked modulation of signal transduction is directly implicated in matrix production or is acting indirectly by controlling cell proliferation and decreasing the number of matrix-synthesizing cells.
Since the original discovery that decorin binds to this growth factor (Yamaguchi et al. 1990), it has been shown that decorin–TGF-β1 interaction can interfere with TGF-β1 bioactivity, both in vitro and in vivo (Huijun et al. 2005; Zhang et al. 2007). Collectively, the results of the aforementioned studies underline the efficacy of decorin in inhibiting not only matrix deposition, but also migration of fibroblasts, trophoblasts, and differentiation of myocytes (Fischer et al. 2001; Droguett et al. 2006). Using a cohort of liver pathologies including chronic hepatitis, fibrosis, and cirrhosis, it was previously discovered that high amounts of TGF-β1 colocalize with decorin within the fibrotic areas of the liver (Dudas et al. 2001). However, these findings could not distinguish between a decorin-mediated inhibition of TGF-β1 action and a bystander effect. Indeed, in contrast to dermal fibroblasts (Kahari et al. 1991), TGF-β1 upregulates decorin production in HSCs (Breitkopf et al. 2005). Thus, enhanced deposition of decorin could reflect the stimulatory effect of overproduced TGF-β1, without necessarily exerting a protective role against fibrosis.
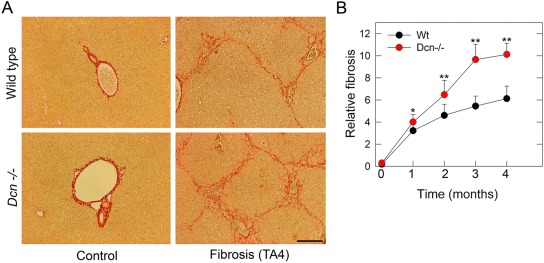
To circumvent these uncertainties, we compared the matrix production induced by thioacetamide (TA) in wild-type (Wt) and decorin-null (Dcn–/–) mouse livers. Moreover, to validate the events taking place on the liver tissue level, decorin was silenced in LX2 hepatic stellate cell line to determine the major biochemical changes (Baghy et al. 2011). By comparing the fibrogenic response in Wt and decorin-deficient livers, we provide strong evidence for a role of decorin in protection against fibrogenesis. The degree of TA-induced matrix deposition was higher in the liver from Dcn–/– mice; and this was observed as early as the first month of toxin exposure, lasting throughout the entire experimental period (Baghy et al. 2011; Fig. 1). This response correlated well with the enhancement of collagen I, III, and IV proteins in the decorin-deficient mice. Moreover the relative decrease in two major matrix metalloproteases, MMP-2 and MMP-9, and the concurrent increase in the hepatic levels of two major MMP inhibitors, TIMP-1 and PAI-1, indicated an impaired clearance of the deposited ECM (Baghy et al. 2011). An adenovirus-mediated direct stimulatory effect of decorin on metalloproteases has been previously described (Al Haj Zen et al. 2003; Dong et al. 2008). On the other hand, TIMP-1 as well as PAI-1 levels can also be stimulated by TGF-β1 (Kutz et al. 2001; Akool el et al. 2005). To answer whether the changes observed in our model relate to lack of decorin’s effect on TGF-β1, gene expression of TGF-β1-inducible early response gene (TIEG), a downstream effector of TGF-β1 activity, was measured. The increase in TIEG indicates that the lack of decorin in experimental hepatic fibrosis enhances its expression, thereby validating the results obtained with liver tissue. Thus, decorin exerts its effect, at least in part, via inactivation of TGF-β1.
Figure 1.
Hepatic fibrosis is accentuated in decorin-null (Dcn–/–) mice. (A) Picrosirius-stained sections from the liver of wild-type (Wt) and Dcn–/– mice without treatment (first column), after 4 months of thioacetamide treatment (TA4, second column). Scale bar = 150 µm. (B) Quantification of hepatic fibrosis by morphometric analysis of livers from Wt and Dcn–/– animals as indicated. Values represent the mean (%) ± SEM of three animals. *p<0.05, **p<0.01.
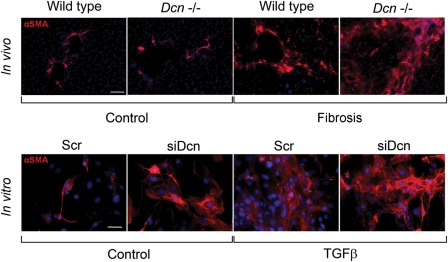
As a result of liver injury, HSCs undergo a process known as “activation,” during which the quiescent cells transform into proliferative, fibrogenic, and contractile myofibroblasts. Hence, α-smooth muscle actin, which is highly expressed in myofibroblasts, is used as a cellular marker for TGF-β1-activated HSCs. As the effect of TGF-β1 on HSC activation is well known, we hypothesized that if decorin had to interfere with the growth factor’s action, its lack would result in an increase in the number of activated HSCs. Indeed, decorin silencing caused increased activation of HSCs both in vivo and in vitro (Baghy et al. 2011; Fig. 2).
Figure 2.
Changes in α-smooth muscle actin (αSMA) in liver sections and in LX2 cells. In vivo: immunostaining of αSMA on sections of wild-type and decorin-null fibrotic livers. In vitro: immuncytochemistry of αSMA performed on cells transfected with scrambled small interfering RNA (siRNA) or with siRNA specific for decorin (siDcn) in the absence or presence of TGF-β1 for 48 hr. αSMA is shown as red, and nuclei are counterstained with DAPI. Scale bar = 100 µm for each picture.
To reveal the underlying cellular signaling, we measured the activation of Erk1/2 and Smad2/3 proteins, all known downstream effectors of the TGF-β1 signaling pathway (Fig. 3). In our study, no significant difference in P-Smad2 between Wt and decorin-deficient animals with cirrhosis or between LX2 cells with normal or silenced decorin content was detected after TGF-β1 exposure (Baghy et al. 2011). On the other hand, significant difference was seen in the activation of Smad3 in Dcn–/– livers after 4 months of fibrogenesis (Baghy et al. 2011). Silencing of decorin in immortalized HSCs resulted in Smad3 activation as well, further corroborating the effect of decorin (Baghy et al. 2011). These data suggest that the signaling pathway causing this increased matrix production in Dcn–/– animals and in LX2 cells might be related to the Smad pathway. Phosphorylation of Smad2/3 proteins in fibrotic diseases has been described many times previously (Border and Noble 1994; Massague 1998; Miyazono et al. 2000; Flanders 2004). Earlier, Shi and coworkers found a decrease of P-Smad2 level in LX2 stellate cells activated by TGF-β1 upon decorin treatment (Shi et al. 2006). The prominent role of Smad3 is well demonstrated by the experiment showing that targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice (Latella et al. 2009). Moreover, it is well documented that maximal expression of collagen type I in activated HSCs (i.e., the most fibrogenic cell type in liver) requires Smad3 rather than Smad2, both in vivo and in cultured cells (Schnabl et al. 2001).
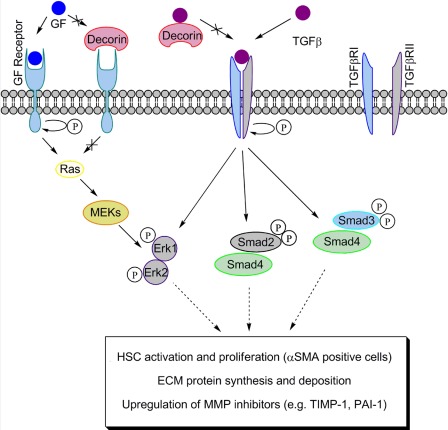
Figure 3.
Action of decorin on TGF-β signaling in liver fibrosis. Decorin may prevent TGF-β from binding to its receptors, resulting in an interference with the Smad and non-Smad pathways downstream of the TGF-β receptors. The changes in Erk1/2 upon the presence or absence of decorin can be an outcome of the crosstalk between several growth factor receptors, including TGF-β receptor, as well as EGFR, IGFR, or Met.
Activation of the Erk1/2 was also remarkably higher in Dcn–/– mice during the fibrotic period (Baghy et al. 2011). In harmony with this result, several studies have reported the importance of non-Smad pathways of TGF-β signaling (Massague 1998; Pannu et al. 2007; Zhang 2009), which culminates in elevated collagen production, transdifferentiation of HSCs into myofibroblasts (Pannu et al. 2007), and epithelial-mesenchymal transition (Zhang 2009). We do not know whether activation of Erk1/2 occurs through the TGF-β receptor or through crosstalk with different receptor tyrosine kinases. Notably, Erk1/2 is downstream of both EGFR and Met, and decorin is a known negative regulator of both these receptors. Thus, the lack of decorin might cause a protracted activation of one or both of these receptors, especially the Met receptor, which is highly expressed in hepatocytes (Fig. 3).
Conclusions
As liver cirrhosis is characterized by the deposition of decorin, its role in the matrix deposition has been enigmatic. Our experiments provide clear genetic and biochemical evidence supporting a key role for this proteoglycan and, perhaps, other SLRP members in attenuating TGF-β1 bioactivity during hepatic fibrogenesis. However, we do not know yet whether collagen-bound decorin, deposited into the matrix, is as effective as soluble decorin. At the present stage of our understanding, we suggest that soluble decorin (i.e., decorin that is not engaged with collagen I) could represent an endogenous TGF-β1-blocker active compound against fibrosis. Furthermore, it is conceivable that not only the absolute amount of decorin but also the ratio of decorin to active TGF-β1 might be important. This implies that besides appreciating the role of decorin as a natural inhibitor of liver fibrogenesis, several questions need to be addressed until we can utilize it in the treatment of human cirrhosis. Finally, although inhibition of TGF-β1 activity appears to be well tolerated in rodents during several weeks, lethality of knockout animals warns us of the necessity of this cytokine for the normal functioning of our bodies.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the Hungarian Scientific Research Fund, Grants 67925, 100904 and by the National Institutes of Health grant RO1 CA39481 (to RVI).
References
- Akool el S, Doller A, Muller R, Gutwein P, Xin C, Huwiler A, Pfeilschifter J, Eberhardt W. 2005. Nitric oxide induces TIMP-1 expression by activating the transforming growth factor beta-Smad signaling pathway. J Biol Chem. 280:39403–39416 [DOI] [PubMed] [Google Scholar]
- Albillos Martinez A. 2009. What is the treatment of choice in a patient with liver cirrhosis and esophageal varices that have bled to prevent rebleeding: beta-blockers, endoscopic ligation or both? Gastroenterol Hepatol. 32:124–125 [DOI] [PubMed] [Google Scholar]
- Al Haj Zen A, Lafont A, Durand E, Brasselet C, Lemarchand P, Godeau G, Gogly B. 2003. Effect of adenovirus-mediated overexpression of decorin on metalloproteinases, tissue inhibitors of metalloproteinases and cytokines secretion by human gingival fibroblasts. Matrix Biol. 22:251–258 [DOI] [PubMed] [Google Scholar]
- Ameye L, Young MF. 2002. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 12(9):107R–1016R [DOI] [PubMed] [Google Scholar]
- Baghy K, Dezso K, Laszlo V, Fullar A, Peterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV, Kovalszky I. 2011. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest. 91:439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsano C, Alisi A, Nobili V. 2009. Liver fibrosis and therapeutic strategies: the goal for improving metabolism. Curr Drug Targets. 10:505–512 [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. 1994. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 331:1286–1292 [DOI] [PubMed] [Google Scholar]
- Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. 2005. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 29:121S-131S [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Comerford SA, Hammer RE. 1997. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest. 100:697–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnoczky G, Iozzo RV. 2000. Sustained down-regulation of the epidermal growth factor receptor by decorin: a mechanism for controlling tumor growth in vivo. J Biol Chem. 275:32879–32887 [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. 1997. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 136:729–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bleser PJ, Niki T, Rogiers V, Geerts A. 1997. Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol. 26:886–893 [DOI] [PubMed] [Google Scholar]
- Desmet VJ, editor. 1992. Morphology of cell damage and inflammatory reaction. Molecular and cell biology of liver fibrogenesis. Dordrecht (NL): Kluwer Academic Publishers; p. 27–44 [Google Scholar]
- Dong FQ, Li H, Wu F, Yao HP. 2008. [Effects of overexpression of decorin on matrix metalloproteinases 2 and 9 in rat mesangial and tubular cells.] Zhonghua Yi Xue Za Zhi. 88:3444–3447 [PubMed] [Google Scholar]
- Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. 2006. Extracellular proteoglycans modify TGF-beta bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 25:332–341 [DOI] [PubMed] [Google Scholar]
- Dudas J, Kovalszky I, Gallai M, Nagy JO, Schaff Z, Knittel T, Mehde M, Neubauer K, Szalay F, Ramadori G. 2001. Expression of decorin, transforming growth factor-beta 1, tissue inhibitor metalloproteinase 1 and 2, and type IV collagenases in chronic hepatitis. Am J Clin Pathol. 115:725–735 [DOI] [PubMed] [Google Scholar]
- El-Gamel A, Awad MR, Hasleton PS, Yonan NA, Hutchinson JA, Campbell CS, Rahman AH, Deiraniya AK, Sinnott PJ, Hutchinson IV. 1999. Transforming growth factor-beta (TGF-beta1) genotype and lung allograft fibrosis. J Heart Lung Transplant. 18:517–523 [DOI] [PubMed] [Google Scholar]
- Epple A, Kuhn HA, Leveringhaus M, Liehr H. 1979. [Etiology, complications and prognosis of liver cirrhosis in 917 patients: II. Causes of death and prognosis in liver cirrhosis.] Lebensversicher Med. 31:117–122 [PubMed] [Google Scholar]
- Fartoux L, Serfaty L. 2005. [Liver cirrhosis in adults: etiology and specific treatments.] Rev Prat. 55:1539–1548 [PubMed] [Google Scholar]
- Fiedler LR, Schonherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, Eble JA. 2008. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J Biol Chem. 283:17406–17415 [DOI] [PubMed] [Google Scholar]
- Fischer JW, Kinsella MG, Levkau B, Clowes AW, Wight TN. 2001. Retroviral overexpression of decorin differentially affects the response of arterial smooth muscle cells to growth factors. Arterioscler Thromb Vasc Biol. 21:777–784 [DOI] [PubMed] [Google Scholar]
- Flanders KC. 2004. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 85:47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL. 2003. Liver fibrosis: from bench to bedside. J Hepatol. 38 Suppl 1:S38–S53 [DOI] [PubMed] [Google Scholar]
- Garcia-Tsao G, Lim JK. 2009. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 104:1894. [DOI] [PubMed] [Google Scholar]
- Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. 2007. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 35:661–664 [DOI] [PubMed] [Google Scholar]
- Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. 2009. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 185:743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner AM. 1996. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int. 54 Suppl:S39–S45 [PubMed] [Google Scholar]
- Huijun W, Long C, Zhigang Z, Feng J, Muyi G. 2005. Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis. Exp Mol Pathol. 78:17–24 [DOI] [PubMed] [Google Scholar]
- Iozzo RV. 1999. The biology of the small leucine-rich proteoglycans: functional network of interactive proteins. J Biol Chem. 274:18843–18846 [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. 1999. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 274:4489–4492 [DOI] [PubMed] [Google Scholar]
- Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. 1996. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 2:418–423 [DOI] [PubMed] [Google Scholar]
- Kahari VM, Larjava H, Uitto J. 1991. Differential regulation of extracellular matrix proteoglycan (PG) gene expression: transforming growth factor-beta 1 up-regulates biglycan (PGI) and versican (large fibroblast PG), but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 266:10608–10615 [PubMed] [Google Scholar]
- Kanzler S, Lohse AW, Keil A, Henninger J, Dienes HP, Schirmacher P, Rose-John S, zum Buschenfelde KH, Blessing M. 1999. TGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol. 276:G1059–G1068 [DOI] [PubMed] [Google Scholar]
- Kato I, Tominaga S, Ikari A. 1992. The risk and predictive factors for developing liver cancer among patients with decompensated liver cirrhosis. Jpn J Clin Oncol. 22:278–285 [PubMed] [Google Scholar]
- Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G. 1999. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 117:1205–1221 [DOI] [PubMed] [Google Scholar]
- Kutz SM, Hordines J, McKeown-Longo PJ, Higgins PJ. 2001. TGF-beta1-induced PAI-1 gene expression requires MEK activity and cell-to-substrate adhesion. J Cell Sci. 114:3905–3914 [DOI] [PubMed] [Google Scholar]
- Latella G, Vetuschi A, Sferra R, Catitti V, D’Angelo A, Zanninelli G, Flanders KC, Gaudio E. 2009. Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int. 29:997–1009 [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Negri E, Cavalieri d’Oro L, Franceschi S. 1998. Liver cirrhosis and the risk of primary liver cancer. Eur J Cancer Prev. 7:315–320 [DOI] [PubMed] [Google Scholar]
- Massague J. 1998. TGF-beta signal transduction. Annu Rev Biochem. 67:753–791 [DOI] [PubMed] [Google Scholar]
- Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. 2009. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol. 60 Suppl 4:5–13 [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, ten Dijke P, Heldin CH. 2000. TGF-beta signaling by Smad proteins. Adv Immunol. 75:115–157 [DOI] [PubMed] [Google Scholar]
- Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M. 2007. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 282:10405–10413 [DOI] [PubMed] [Google Scholar]
- Popper H. 1977. Pathologic aspects of cirrhosis: a review. Am J Pathol. 87:228–264 [PMC free article] [PubMed] [Google Scholar]
- Reed CC, Gauldie J, Iozzo RV. 2002. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 21:3688–3695 [DOI] [PubMed] [Google Scholar]
- Santra M, Reed CC, Iozzo RV. 2002. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem. 277:35671–35681 [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. 2008. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 283:21305–21309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, Grone HJ, Reinhardt DP, Pfeilschifter J, Iozzo RV, et al. 2007. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. Am J Pathol. 170: 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Lorkowski S, Seidler D, Breithardt G, Buddecke E. 2006. TGF-beta1 generates a specific multicomponent extracellular matrix in human coronary SMC. Eur J Clin Invest. 36:473–482 [DOI] [PubMed] [Google Scholar]
- Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. 2001. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 34:89–100 [DOI] [PubMed] [Google Scholar]
- Schnaper HW. 1995. Balance between matrix synthesis and degradation: a determinant of glomerulosclerosis. Pediatr Nephrol. 9:104–111 [DOI] [PubMed] [Google Scholar]
- Schonherr E, Sunderkotter C, Iozzo RV, Schaefer L. 2005. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 280:15767–15772 [DOI] [PubMed] [Google Scholar]
- Shek FW, Benyon RC. 2004. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur J Gastroenterol Hepatol. 16:123–126 [DOI] [PubMed] [Google Scholar]
- Shi YF, Zhang Q, Cheung PY, Shi L, Fong CC, Zhang Y, Tzang CH, Chan BP, Fong WF, Chun J, et al. 2006. Effects of rhDecorin on TGF-beta1 induced human hepatic stellate cells LX-2 activation. Biochim Biophys Acta. 1760:1587–1595 [DOI] [PubMed] [Google Scholar]
- Vallet-Pichard A, Mallet V, Costentin CE, Pol S. 2009. Treatment of HBV-related cirrhosis. Expert Rev Anti Infect Ther. 7:527–535 [DOI] [PubMed] [Google Scholar]
- Wang H, Mengsteab S, Tag CG, Gao CF, Hellerbrand C, Lammert F, Gressner AM, Weiskirchen R. 2005. Transforming growth factor-beta1 gene polymorphisms are associated with progression of liver fibrosis in Caucasians with chronic hepatitis C infection. World J Gastroenterol. 11:1929–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber IT, Harrison RW, Iozzo RV. 1996. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem. 271:31767–31770 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. 1990. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 346:281–284 [DOI] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. 2006. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 98:1436–1449 [DOI] [PubMed] [Google Scholar]
- Zhang YE. 2009. Non-Smad pathways in TGF-beta signaling. Cell Res. 19:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. 2007. Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns. 33:634–641 [DOI] [PubMed] [Google Scholar]