Abstract
A sensitive immunohistochemical procedure, the tyramide signal amplification (TSA) system, was applied to detect the localization of immunolabeled disease-associated prion protein (PrPSc) in cattle affected with bovine spongiform encephalopathy (BSE). In this procedure, immunolabeling could be visualized in the optic nerve and the adrenal medulla. In the optic nerve, the dual immunofluorescent technique showed that the granular PrPSc was occasionally detected in the astrocytes, microglia, and myelin sheath adjacent to the axon. Clustered PrPSc was also scattered in association with microglial cells and astrocytes of the optic nerve. In the adrenal gland, PrPSc immunolabeling was confined within the sympathetic nerve fibers and endings. The results suggest that (1) PrPSc might centrifugally spread within and between glial cells and/or the non-axonal (also known as ad-axonal) region of nerve fibers, rather than the axonal and/or extracellular space pathway in the optic nerve, and (2) the sympathetic innervations might be important for the trafficking of BSE agent in the adrenal glands of cattle. This study also suggests that tyramide-based immunochemical analysis should be performed to detect immunolabeled PrPSc in the extracerebral tissues of BSE-affected cattle.
Keywords: BSE, prion, tyramide amplification, TSA system, immunohistochemistry, optic nerve, adrenal gland
Cellular prion protein (PrPC) is expressed ubiquitously on the normal cell surfaces of nerve cells, lymphocytes, and follicular dendritic cells. Transmissible spongiform encephalopathy (TSE), or prion disease, is a neurodegenerative disorder characterized by the presence of an abnormal, protease-resistant isoform of the prion protein (PrPSc) (Prusiner 1991). Enzyme-linked immunosorbent assay, Western blotting, and immunohistochemical analysis have been performed for the diagnosis of prion diseases such as scrapie in sheep and goats, bovine spongiform encephalopathy (BSE) in cattle, chronic wasting disease (CWD) in deer, feline spongiform encephalopathy (FSE) in cheetah, and Creutzfeldt-Jakob disease (CJD) in humans. At present, formalin-fixed specimens are conventionally used for histopathological and immunohistochemical diagnosis of TSEs (Bodemer 1999).
A highly sensitive Western blotting technique using phosphotungstic acid precipitation has been used to detect PrPSc accumulation in the peripheral nerves and the adrenal glands of experimentally BSE-infected cattle with clinical signs of the disease (Masujin et al. 2007). Moreover, transgenic mice overexpressing bovine PrPC (Tgbov XV) have been used to detect infectivity in the brains; spinal cords; retinas; optic, facial, and sciatic nerves; and ilea of naturally infected cows in the terminal stage of BSE (Buschmann et al. 2006). Although the Western blotting technique has detected the presence of PrPSc in various tissues, few immunohistochemical studies have addressed the distribution of PrPSc in the peripheral tissues of cattle with naturally occurring BSE (Terry et al. 2003; Iwata et al. 2006; Vidal et al. 2006; Hoffmann et al. 2007; Okada et al. 2010), probably because of the low levels of PrPSc in these tissues. This suggests that a highly sensitive immunohistochemical procedure using biotinylated tyramide and including appropriate antigen retrieval is required to detect the presence of minimal quantities of PrPSc aggregates in tissues (Heggebø, González, et al. 2003; Heggebø, Press, et al. 2003; Monleón et al. 2004; Espenes et al. 2006).
The purpose of this study was to demonstrate the deposition of PrPSc in the optic nerves and adrenal glands of cattle infected with BSE by using the tyramide signal amplification (TSA) system, which greatly enhances the sensitivity of immunohistochemical procedures. The second objective of this study was to assess and identify the location of PrPSc in the optic nerve by using the sensitive double immunofluorescent labeling technique.
Materials and Methods
Samples
All the experiments were performed in accordance with the guidelines of the Animal Ethical Committee and the Animal Care and Use Committee of National Institute of Animal Health.
Immunohistochemical analysis was performed on the coronal brain and peripheral tissues, including the optic nerves, retinas, and adrenal glands from 5 naturally occurring BSE cases in Japan (BSE/JP15, 17, 21, 22, 26) (Okada, Iwamaru, et al. 2011), 9 cattle with experimentally induced BSE (codes 1479, 3217, 3728, 4394, 4437, 4612, 5087, 5426, 5523; Fukuda et al. 2012), and 2 non-infected control cattle. Experimental intracerebral transmission of BSE has been reported previously in cattle (Fukuda et al. 2009; Fukuda et al. 2012). In brief, 9 Holstein calves at 3 months of age were inoculated intracerebrally with 1 ml of 10% brainstem homogenate prepared from the brainstem of BSE-affected animals obtained from the United Kingdom (Yokoyama et al. 2007) and Japan (Iwata et al. 2006). Animals inoculated with BSE agent developed the disease and were euthanized after an incubation time of 665 ± 86 days (expressed as mean ± standard deviation [SD]).
At necropsy, the tissues were fixed in 10% neutral buffered formalin (pH 7.4) and then immersed in 98% formic acid for 60 min at room temperature to reduce infectivity (Taylor et al. 1997). The tissues were processed through a graded alcohol to xylene series and embedded by standard procedures in paraffin wax by using an automated processing machine (ETP-150C; Sakura Finetek Japan, Tokyo, Japan). Sections of 4-mm thickness were cut, mounted on silane-coated glass slides (Immuno-Coat; Muto Pure Chemicals, Tokyo, Japan), stained with hematoxylin and eosin, and used for immunohistochemical analysis.
Antibodies
All primary antibodies were diluted in phosphate-buffered saline (PBS, pH 7.4) containing 1% bovine serum albumin and 0.05% sodium azide. For the detection of PrPSc, the primary prion protein (PrP) antibody used in this study was either monoclonal antibody (mAb) F99/97.6.1 (VMRD; Pullman, WA), T1 (Shimizu et al. 2010), or polyclonal antibody (pAb) T4 (Takahashi et al. 1999) at a concentration of 1 mg/ml for the conventional polymer method and 0.2 mg/ml for the TSA system, respectively.
In addition, the following primary antibodies were used for immunohistochemical analysis or immunofluorescence: rabbit anti–glial fibrillary acid protein (GFAP; Dako, Carpinteria, CA; dilution 1:4500) for astrocytes, rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1; Wako Pure Chemical, Osaka, Japan; dilution 1:300) for microglia, rat anti–myelin basic protein (MBP, clone 12; Millipore, Billerica, MA; dilution 1:300) and mouse anti-2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase, clone 11–5B; Millipore; dilution 1:150) for the myelin sheath of oligodendrocytes, rabbit anti–protein gene product 9.5 (PGP9.5; Dako; dilution 1:300) for the axons of peripheral nerves, and rabbit anti-S100 (Dako; dilution 1:600) for peripheral nerves.
For the visualization of immunoreactions, a horseradish-conjugated polymer reagent (Histofine Simple Stain MAX-PO [M] or [R]) was purchased from Nichirei (Tokyo, Japan). The TSA systems (TSA biotin and TSA fluorescein systems), including blocking reagent, biotinylated anti-mouse or rabbit secondary antibody (dilution 1:1800), streptavidin–horseradish peroxidase (SA-HRP; dilution 1:100), biotinyl tyramide (dilution 1:50) for the single immunolabeling procedure, and fluorophore tyramide (FITC-TA; dilution 1:50) for double immunofluorescence, were purchased from PerkinElmer (Boston, MA). Secondary antibodies for dual immunofluorescence, Alexa Fluor 546–conjugated goat anti-rabbit immunoglobulin G (IgG), anti-mouse IgG, and anti-rat IgG were purchased from Molecular Probes (Portland, OR) and diluted (1:450).
Single Immunolabeling Procedure
Dewaxed sections were treated in 3% hydrogen peroxide followed by incubation with 10 mg/ml proteinase K (PK) in PBS containing 0.1% Triton-X100 (PBST) for 10 min at room temperature and then autoclaved for 3 min at 121C in 10 mM citrate buffer (pH 6.0) to enhance PrPSc immunolabeling (Brun et al. 2004). After pretreatment followed by rinsing in PBST, the sections were incubated with 10% normal goat serum for 10 min and then incubated with either monoclonal or polyclonal PrP antibody on different serial tissue sections for 1 hr. Immunoreactions were detected with the horseradish peroxidase–labeled polymer (Histofine Simple Stain MAX-PO) as the conventional method and the TSA biotin system, which was an improvement over the conventional immunohistochemical procedures of different manufacturers. After each step in the incubation with primary and secondary antibodies and reagents, the sections were washed three times (5 min for each washing) with PBST. Immunoreactions were visualized using 3,3′-diaminobenzedine tetrachloride (DAB) as the chromogen. Finally, the sections were counterstained with hematoxylin. As negative controls, the sections were incubated with non-immune mouse or rabbit IgG (1:30 dilution; Dako) and PBS instead of the primary antibody.
In the conventional polymer method, the sections were reacted with the horseradish-conjugated polymer reagent for 30 min after incubation with each primary antibody. All sections were then visualized with DAB.
With the TSA biotin system, after each pretreatment, sections were (1) treated for 30 min with a blocking buffer consisting of 0.1 M Tris-HCl (pH 7.5), 0.15 M NaCl, and 0.5% blocking reagent; (2) treated with each primary PrP antibody for 60 min; (3) exposed to biotinylated anti-mouse or rabbit secondary antibodies for 30 min; (4) treated with SA-HRP for 30 min; (5) treated with biotinyl tyramide as the amplification reagent for 5 min; and (6) treated with SA-HRP for 30 min before the visualization with DAB.
Image Analysis of Immunolabeled PrPSc by a Single Immunolabeling Procedure
Two serial sections of the dorsolateral nucleus of thalamus for each animal were incubated with mAb F99/97.6.1 and immunolabeled by two methods, the conventional method and TSA-biotin system, without hematoxylin counterstaining to avoid false-positive results for image analysis. The appearance of immunolabeled PrPSc in the optic nerves, retina, and adrenal glands was less common as compared with that in the brain, especially in the naturally occurring cases, and therefore image analysis of the optic nerves, retina, and adrenal glands was avoided in this study.
The images in the dorsolateral nucleus of the thalamus were captured randomly by selecting three microscope fields with a digital camera (Nikon DS-2Mv, Tokyo, Japan) with a 20× magnification objective lens and analyzed using the ImageJ software (National Institutes of Health; Bethesda, MD) to determine the immunostained areas occupied by PrPSc deposition. For each field (0.3 mm2), the total area occupied by immunolabeled areas was estimated by setting a “threshold” using the thresholding tool for selection of these areas. The highlighted area of immunolabeled PrPSc was calculated and divided by the total area, designated as the mean percentage of immunolabeled area (highlighted area/total area [%]). Statistical analysis was performed with the Student’s t-test using InStat3 software (GraphPad Software; La Jolla, CA). All data are expressed as means ± SD.
Double Immunofluorescence Procedure
Selected sections of the optic nerves and adrenal glands were dewaxed and then treated with 10 mg/ml PK for 10 min at room temperature followed by autoclaving at 121C for 3 min in 10 mM citrate buffer (pH 6.0). After pretreatments, the sections were incubated for 60 min with anti-GFAP pAb, anti-Iba1 pAb, anti-PGP9.5 pAb, anti-S100 pAb, anti-MBP mAb, or anti-CNPase mAb as the primary antibody and then with Alexa Fluor 546 goat anti-rabbit IgG for GFAP, Iba1, PGP9.5, and S100; anti-mouse IgG for CNPase; or anti-rat IgG for MBP as the secondary antibody. Sections were then incubated with 0.5% blocking reagent for 30 min and with mAb T1, F99/97.6.1, or pAbT4 for 60 min. PrPSc immunofluorescence was visualized by TSA fluorescence in the system according to the manufacturer’s instructions. In brief, the sections were incubated with a biotinylated anti-mouse or anti-rabbit secondary antibody for 30 min, with SA-HRP for 30 min, and finally with FITC-TA as the amplification reagent for 5 min. Immunofluorescence images were evaluated using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany).
Results
Comparison of the PrPSc Immunolabeling Intensity by Using the Conventional Polymer Method and the TSA Biotin System
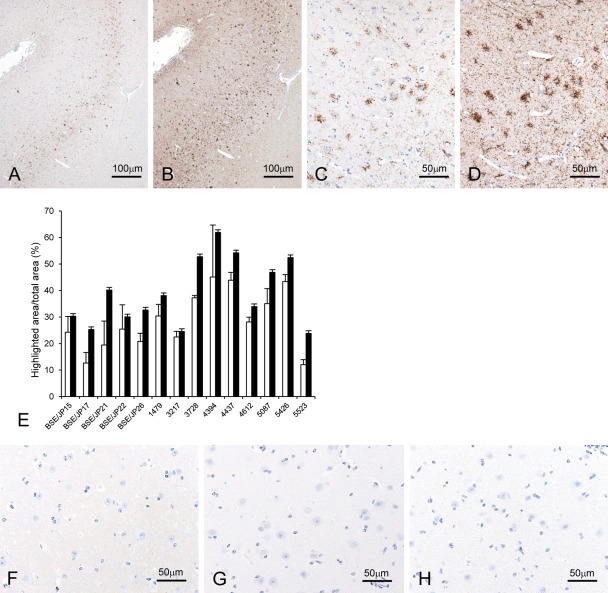
The various types of PrPSc deposition that are usually detected using standard immunohistochemical procedures, such as granular, linear, intraneuronal, perineuronal, stellate, or coalescing deposits, were also found in the brain by using the PrP antibody and detection systems. Both the intensity and topographical distribution of PrPSc in the brain were similar between experimental and natural BSE animals as described (Okada, Iwamaru, et al. 2011; Fukuda et al. 2012). Immunolabeled PrPSc had accumulated mainly in the brainstem (midbrain, pons, and medulla oblongata), thalamus, basal ganglia, cerebellar nucleus, and gray matter of the spinal cord, but it was less frequent in the cerebral and cerebellar cortices. The immunolabeling intensity with the TSA system was notably higher than that by the conventional polymer method (Fig. 1A–D), but no statistically significant differences in the ratio of PrPSc-immunolabeling area to total area was present in the dorsolateral nucleus of the thalamus between the two immunodetection methods (Fig. 1E). To compare the intensity of PrPSc immunolabeling using the three primary antibodies, we had all antibodies react with various types of PrPSc, and no evident difference in results intensity was observed among the three primary antibodies used in this study as previously described (Okada, Sato, et al. 2011). In addition, PrPSc deposition was detected in the optic nerves and adrenal glands with the TSA system but not by using the conventional polymer method. The weak background staining was detected in the brains, retinas, optic nerves, and adrenal glands in both infected and uninfected animals by using the TSA biotin system but not the conventional polymer method when the primary PrP-antibodies were applied to the sections (Fig. 1F,G). However, no specific background immunolabeling was present in any sections by both methods when non-immune mouse and rabbit IgG or PBS was applied to the sections instead of the primary PrP-antibodies (Fig. 1H).
Figure 1.
Comparison of immunolabeling intensity by the conventional polymer method and the tyramide signal amplification (TSA) biotin system. Localization of the disease-associated prion protein (PrPSc) in the dorsolateral prefrontal cortex along the superior frontal sulcus of bovine spongiform encephalopathy (BSE)–affected cattle (case 4437) by using mAb F99/97.6.1 (A–D). The graph illustrates the mean percentage of the ratio of PrPSc-immunolabeled area to total area (highlighted area/total area [%]) for each animal analyzed by using ImageJ (E). No statistically significant differences were determined between TSA (filled bars) and conventional polymer (empty bars) methods (Student’s t-test). Data expressed as the means ± standard deviation. Weak background immunostaining is present with the TSA system (F) but not the conventional polymer method (G) in the cerebral cortex of an uninfected control animal immunolabeled with mAb F99/97.6.1. No specific background immunolabeling is detected in the cerebral cortex of BSE cattle (case 4394) immunostained with phosphate-buffered saline instead of the primary prion protein (PrP)–primary antibody using the TSA system (H).
Retina
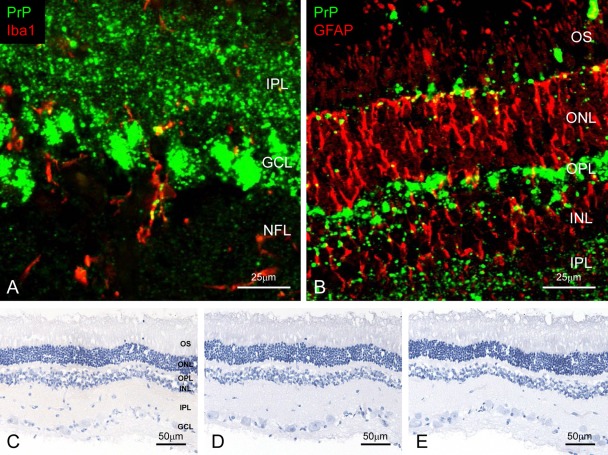
In contrast to the brain, the appearance and intensity of immunolabeled PrPSc in the retina varied between experimental and naturally occurring BSE animals with both immunodetection systems. In experimental animals, PrPSc was distributed prominently throughout all layers of the retina, especially in the inner and outer plexiforms and the ganglion cell layers (Fig. 2A,B). In addition, granular PrPSc deposits were detected in the cytoplasm of activated microglia and astrocytes. In naturally occurring cases, however, PrPSc accumulation was noted in the outer part of the inner plexiform layer and less frequently in the outer plexiform layer. Intracytoplasmic PrPSc immunolabeling was rarely present within the ganglion cells. No immunolabeled PrPSc was detected in any layers of retina of control animals (Fig. 2C–E).
Figure 2.
Detection of disease-associated prion protein (PrPSc) in the retina. Dual immunofluorescence labeled with T1 for PrPSc (green; A and B) and Iba1 (red; A, case 5426) or glial fibrillary acid protein (GFAP; red; B, case 5523). PrPSc accumulates throughout the retina. Granular PrPSc deposits are present in the cytoplasm of the microglia (A) and astrocytes (B). Weak background immunostaining is present with the tyramide signal amplification (TSA) system (C) but not the conventional polymer method (D) in the retina of an uninfected control animal immunolabeled with mAb F99/97.6.1. No specific background immunolabeling is detected in the retina of a control animal immunostained with phosphate-buffered saline instead of the primary prion protein (PrP)–primary antibody using the TSA system (E). NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nucleus layer; OPL, outer plexiform layer; ONL, outer nucleus layer; OS, outer segments.
Optic Nerve
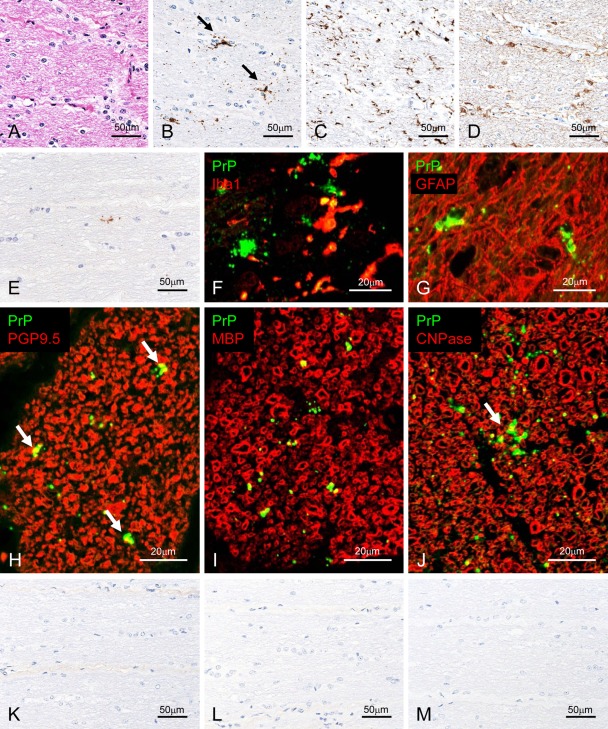
Two different types of PrPSc deposits were detected in the optic nerves in all the nine experimentally BSE-challenged cases (Fig. 3). One type was characterized by a single or a few small granules; the other consisted of a patchy distribution of multiple or small granules (Fig. 3B). The severity of PrPSc deposits in naturally occurring BSE cases was much lower as compared with that in experimentally BSE-challenged cases. All naturally occurring BSE cases showed only faintly granular immunolabeling (Fig. 3E). In addition, the images of semi-serial sections did reveal whether PrPSc was present in the cells.
Figure 3.
Detection of disease-associated prion protein (PrPSc) in the optic nerve. Semi-serial sections of experimental bovine spongiform encephalopathy (BSE) cattle (case 4437) are stained with hematoxylin and eosin (A) and immunolabeled with mAb F99/97.6.1 (B; tyramide signal amplification [TSA] system), Iba1 (C), or glial fibrillary acid protein (GFAP; D). Patchy (arrows in B) and particulate PrPSc deposits are detected in the optic nerve. Weak positive signal was detected in a naturally occurring case (BSE/JP21) with mAb F99/97.6.1 using the TSA system (E). Dual immunofluorescence labeled with mAb T1 (green; F and G) and Iba1 (red; F) or GFAP (red; G) in an experimental BSE animal (case 4612). PrPSc granules are localized in the cytoplasm of microglial cells (F) and astrocytes (G). In addition, periastrocytic deposition of PrPSc is noted (yellow; G). Dual immunofluorescence labeled with F99/97.6.1 (green) and PGP9.5 (red; H) in an experimental BSE animal (case 4437). Peri-axonal localization of PrPSc (arrows) is noted. Dual immunofluorescence labeled with T4 (green; I and J) and MBP (red; I) or CNPase (red; J) in an experimental BSE animal (case 4437). Granular PrPSc is colocalized on the myelin sheath (yellow) and patchy PrPSc (arrow) is located at the periphery of the myelin sheath (yellow). Weak background immunostaining is present in the connective tissue with the TSA system (K) but not the conventional polymer method (L) in the optic nerve of an uninfected control animal immunolabeled with mAb F99/97.6.1. No specific background immunolabeling is detected in the optic nerve of a control animal immunostained with phosphate-buffered saline instead of the primary prion protein (PrP)–primary antibody using the TSA system (M).
Using the dual immunofluorescence technique, small granular PrPSc immunolabeling corresponded mostly with Iba1-positive cytoplasmic processes (Fig. 3F). However, patchy PrPSc immunolabeling appeared to correspond to GFAP-positive cell bodies (Fig. 3G). Individual small PrPSc granules were located occasionally at the periphery of the axon in the transverse and cross sections (Fig. 3H–J). The myelinated fibers and round cell bodies in the brains and optic nerves were labeled with both MBP and CNPase (Fig. 3I, J). Granular PrPSc was localized at the periphery of axons, suggesting an ad-axonal location (Fig. 3H–J). In contrast, patchy PrPSc was occasionally colocalized with the optic nerve sheath labeled with MBP and CNPase in the merged images (Fig. 3I, J). However, no PrPSc labeling was detectable in MBP- and CNPase-positive round cells and in the optic nerve of control animals (Fig. 3K–M).
Adrenal Glands
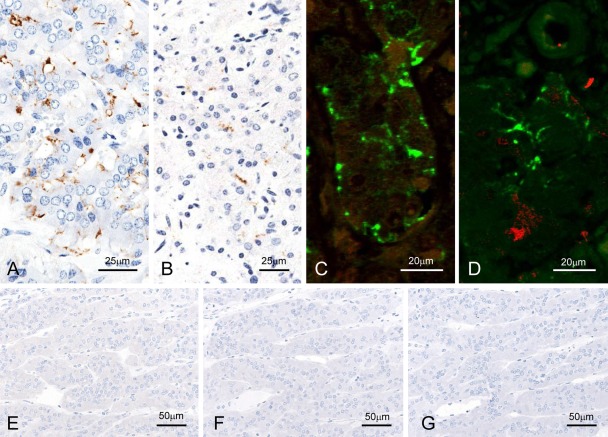
In the adrenal glands of all animals with naturally occurring and experimentally induced BSE, immunolabeled PrPSc was obvious in the outer layer of the adrenal medulla, as shown in the intercellular fine processes between paraganglionic cells (Fig. 4A,B). However, the nerve fibers and endings between chromaffin cells were negative for PGP9.5 and S100 (Fig. 4C,D). No positive signal was detected in the sections of control animals (Fig. 4E–G).
Figure 4.
Detection of disease-associated prion protein (PrPSc) in the adrenal glands. F99/97.6.1mAb-labeled nerve fibers and nerve endings between chromaffin cells of experimental (A, C, and D; case 5087) and naturally occurring (B; BSE/JP17) bovine spongiform encephalopathy (BSE) cases with the tyramide signal amplification (TSA) method (A and B) and dual immunofluorescence with F99/97.6.1 (green; C and D) and PGP9.5 (N) or S100 (red; O) in case 5087. No background immunostaining is present in the optic nerve of an uninfected control animal immunolabeled with mAb F99/97.6.1 using both TSA (E) and conventional polymer (F) methods. No specific background immunolabeling is detected in the optic nerve of a control animal immunostained with phosphate-buffered saline instead of the primary prion protein (PrP)–primary antibody using the TSA system (M).
Discussion
To the best of our knowledge, the present work is the first description of immunolabeled PrPSc in the optic nerves and adrenal glands of cattle affected with BSE. The optic nerve is the second of the 12-paired cranial nerves, but it is considered a part of the central nervous system. It has three major non-neuronal glial components: oligodendrocytes, astrocytes, and microglia (Chow and Lang 2001). In this study, the intensity of immunolabeling as well as the quantity of PrPSc was significantly higher in the retina than in the optic nerve. The granular pattern of immunolabeling in the cytoplasm of microglia and astrocytes seemed to be suggestive of lysosomes (Jeffrey et al. 2000). Only weak immunolabeling has been detected in the optic nerves of sheep and goats with naturally occurring scrapie (Valdez et al. 2003; Hortells et al. 2006) and patients with sporadic and variant CJD (vCJD) (Head et al. 2003), and in these instances, it seemed to be associated with oligodendrocytes or astrocytes. Although PrPSc immunolabeling in the optic nerve appears to be associated with axon and secondary myelin loss in astrocytosis (Head et al. 2003), axonal changes were undetectable in the present study. The presence of PrPSc in the optic nerve may also result from centrifugal trafficking from the brain, presumably through the optic nerve to the retina, rather than the propagation of the protein (Buyukmihci et al. 1980; Head et al. 2003; Kercher et al. 2004; Hortells et al. 2006). However, the transport mechanism of PrPSc within the peripheral nervous system (PNS) is still unclear. Within the PNS, two different pathways are conceivable: (1) axonal or (2) non-axonal (ad-axonal) transport (Glatzel and Aguzzi 2000). PrPC expression was detected at the cell surface and in the cytoplasm of Schwann cells but not in the myelin sheath (Follet et al. 2002). Localization of PrPSc was observed at the cell surface of Schwann cells on the peripheral nerves (Groschup et al. 1999; Hainfellner and Budka 1999; Lezmi et al. 2003; Herzog et al. 2004), suggesting that a non-axonal transport pathway may participate in PrPSc distribution in the peripheral tissues (Glatzel and Aguzzi 2000; Follet et al. 2002; Kovács et al. 2005). Alternatively, the spread of PrPSc from the brain to the retina may occur via cell-to-cell contact in epineural glial cells (Fraser 1982) or centrifugally along the subarachnoid space around the optic nerve (Bradbury and Cole 1980). Together with these findings, our results may suggest that PrPSc spreads centrifugally from the brain, presumably within and between glial cells or via an ad-axonal route along the optic nerve to the retina, or both, rather than through the extracellular space.
The immunolabeling pattern of PrPSc in the adrenal medulla, which consists of highly innervated tissue, is suggestive of (and may be associated with) fine nerve ending processes and fibers (Jeffrey et al. 2001; Lezmi et al. 2003; Herzog et al. 2004). PrPSc in the adrenal gland was detected by Western blotting in humans with vCJD (Wadsworth et al. 2001) and in BSE-affected cattle (Iwamaru et al. 2005; Masujin et al. 2007), and the infectivity of BSE-affected adrenal glands was demonstrated with a bioassay using Tgbov XV mice (Buschmann and Groschup 2005). In sheep scrapie (Jeffrey et al. 2001; Lezmi et al. 2003) and deer CWD (Sigurdson et al. 2001), immunolabeling was present in the adrenal medulla and/or sympathetic nerve fibers. In BSE primates and in a cheetah with FSE, a positive signal was detected in the capsule nerve fiber and in the fascicular and reticular zones of the adrenal gland, respectively. These discrepancies in the PrPSc distribution pattern in the adrenal gland might be related to differences in the host species, the prion strain, or both or may simply reflect variation in the spreading routes of the agent within the organ (Lezmi et al. 2003). The pattern of PrPSc immunostaining in the adrenal medulla of cheetah (Lezmi et al. 2003) and sheep (Jeffrey et al. 2001) showed fine processes related to sympathetic nerve endings. The adrenal gland has a rich sympathetic supply associated with the capsule and the medulla that allows it to be infected primarily or secondarily via the splanchnic nerves. PrPSc accumulation in the adrenal gland may result from the primary routing of the BSE agent to the CNS via sympathetic and parasympathetic circuits or a secondary spread from the CNS via the splanchnic pathway. In both the naturally occurring and experimentally induced BSE cases in the present study, it seemed likely that PrPSc was first detected in the CNS, and subsequent PrPSc transport was derived from the spinal cord via the splanchnic nerves (Lezmi et al. 2003; Masujin et al. 2007).
In a comparison of the signal intensity by Western blotting analysis, the quantities of PrPSc in the adrenal glands of BSE cattle with clinical signs of disease (Masujin et al. 2007) and in the glands of primates with BSE and vCJD (Herzog et al. 2005) were estimated to be 120 and 2500–10,000 times less, respectively, than the amounts present in brains. However, the proximal optic nerve of a vCJD patient contained extremely high levels of PrPSc—only four times less than that found in the brain (Wadsworth et al. 2001).
There is no doubt that a highly sensitive immunohistochemical analysis is an essential and practical tool for detecting the minimal accumulation of immunolabeled PrPSc and for investigating the pathogenesis of TSEs (Heggebø et al. 2000; Sigurdson et al. 2002; Heggebø, González, et al. 2003; Monleón et al. 2004; Espenes et al. 2006; Austbø et al. 2007). No noticeable differences in the immunolabeling intensity have been found between the two different visualization systems (the avidin-biotin complex system and polymer-based visualization methods such as EnVision+ [Dako]) that are frequently used in laboratories (Monleón et al. 2004). Although the TSA system actually improves the signal intensity, this technique requires multiple steps and thus may be unsuitable for routine immunopathological diagnosis. However, biotinyl tyramine-based immunostaining without modification yielded highly intense background staining, but it is evident that the background staining is not induced by the biotinyl tyramine itself but also by the secondary antibody and SA-HRP (Kim et al. 2003).
In summary, the present study elucidated (1) a highly sensitive immunohistochemical procedure that might generate clear PrPSc immunolabeling in BSE cases and (2) the trafficking and localization of PrPSc in the optic nerves and the adrenal glands of BSE-affected cattle. These results are in agreement with the findings of other experimental and natural TSEs and may also help explain the pathogenesis of BSE in cattle.
Acknowledgments
We thank Dr. Yuichi Tagawa, National Institute of Animal Health, for providing anti-prion mAb T1 and Ms. Mutsumi Sakurai, Junko Endo, Miyo Kakizaki, Noriko Amagai, Tomoko Murata, and Naomi Furuya for their expert technical assistance.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for research and/or authorship of this article: This research was supported by a Grant-in-Aid from the BSE and other Prion Disease Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan, and from the BSE research of the Ministry of Health, Labor, and Welfare of Japan.
References
- Austbø L, Espenes A, Olsaker I, Press CM, Skretting G. 2007. Increased PrP mRNA expression in lymphoid follicles of the ileal Peyer’s patch of sheep experimentally exposed to the scrapie agent. J Gen Virol. 88:2083–2090 [DOI] [PubMed] [Google Scholar]
- Bodemer W. 1999. The use of monoclonal antibodies in human prion disease. Naturwissenschaften. 86:212–220 [DOI] [PubMed] [Google Scholar]
- Bradbury MW, Cole DF. 1980. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol. 299:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Castilla J, Ramirez MA, Prager K, Parra B, Salguero FJ, Shiveral D, Sanchez C, Sanchez-Vizcaino JM, Douglas A, et al. 2004. Proteinase K enhanced immunoreactivity of the prion protein-specific monoclonal antibody 2A11. Neurosci Res. 48:75–83 [DOI] [PubMed] [Google Scholar]
- Buschmann A, Gretzschel A, Biacabe AG, Schiebel K, Corona C, Hoffmann C, Eiden M, Baron T, Casalone C, Groschup MH. 2006. Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet Microbiol. 117:103–116 [DOI] [PubMed] [Google Scholar]
- Buschmann A, Groschup MH. 2005. Highly bovine spongiform encephalopathy–sensitive transgenic mice confirm the essential restriction of infectivity to the nervous system in clinically diseased cattle. J Infect Dis. 192:934–942 [DOI] [PubMed] [Google Scholar]
- Buyukmihci N, Rorvik M, Marsh RF. 1980. Replication of the scrapie agent in ocular neural tissues. Proc Natl Acad Sci U S A. 77:1169–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. 2001. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 17:255–296 [DOI] [PubMed] [Google Scholar]
- Espenes A, Press CM, Landsverk T, Tranulis MA, Aleksandersen M, Gunnes G, Benestad SL, Fuglestveit R, Ulvund MJ. 2006. Detection of PrP(Sc) in rectal biopsy and necropsy samples from sheep with experimental scrapie. J Comp Pathol. 134:115–125 [DOI] [PubMed] [Google Scholar]
- Follet J, Lemaire-Vieille C, Blanquet-Grossard F, Podevin-Dimster V, Lehmann S, Chauvin JP, Decavel JP, Varea R, Grassi J, Fontes M, et al. 2002. PrP expression and replication by Schwann cells: implications in prion spreading. J Virol. 76:2434–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H. 1982. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature. 295:149–150 [DOI] [PubMed] [Google Scholar]
- Fukuda S, Iwamaru Y, Imamura M, Masujin K, Shimizu Y, Matsuura Y, Shu Y, Kurachi M, Kasai K, Murayama Y, et al. 2009. Intraspecies transmission of L-type-like bovine spongiform encephalopathy detected in Japan. Microbiol Immunol. 53:704–707 [DOI] [PubMed] [Google Scholar]
- Fukuda S, Onoe S, Nikaido S, Fujii K, Kageyama S, Iwamaru Y, Imamura M, Masujin K, Matsuura Y, Shimizu Y, et al. 2012. Neuroanatomical distribution of disease-associated prion protein in experimental bovine spongiform encephalopathy in cattle after intracerebral inoculation. Jpn J Infect Dis. 65:37–44 [PubMed] [Google Scholar]
- Glatzel M, Aguzzi A. 2000. Peripheral pathogenesis of prion diseases. Microbes Infect. 2:613–619 [DOI] [PubMed] [Google Scholar]
- Groschup MH, Beekes M, McBride PA, Hardt M, Hainfellner JA, Budka H. 1999. Deposition of disease-associated prion protein involves the peripheral nervous system in experimental scrapie. Acta Neuropathol. 98:453–457 [DOI] [PubMed] [Google Scholar]
- Hainfellner JA, Budka H. 1999. Disease associated prion protein may deposit in the peripheral nervous system in human transmissible spongiform encephalopathies. Acta Neuropathol. 98:458–460 [DOI] [PubMed] [Google Scholar]
- Head MW, Northcott V, Rennison K, Ritchie D, McCardle L, Bunn TJ, McLennan NF, Ironside JW, Tullo AB, Bonshek RE. 2003. Prion protein accumulation in eyes of patients with sporadic and variant Creutzfeldt-Jakob disease. Invest Ophthalmol Vis Sci. 44:342–346 [DOI] [PubMed] [Google Scholar]
- Heggebø R, González L, Press CM, Gunnes G, Espenes A, Jeffrey M. 2003. Disease-associated PrP in the enteric nervous system of scrapie-affected Suffolk sheep. J Gen Virol. 84:1327–1338 [DOI] [PubMed] [Google Scholar]
- Heggebø R, Press CM, Gunnes G, Lie KI, Tranulis MA, Ulvund M, Groschup MH, Landsverk T. 2000. Distribution of prion protein in the ileal Peyer’s patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. J Gen Virol. 81:2327–2337 [DOI] [PubMed] [Google Scholar]
- Heggebø R, Press CM, Gunnes G, Ulvund MJ, Tranulis MA, Lsverk T. 2003. Detection of PrPSc in lymphoid tissues of lambs experimentally exposed to the scrapie agent. J Comp Pathol. 128:172–181 [DOI] [PubMed] [Google Scholar]
- Herzog C, Riviere J, Lescoutra-Etchegaray N, Charbonnier A, Leblanc V, Sales N, Deslys JP, Lasmezas CI. 2005. PrPTSE distribution in a primate model of variant, sporadic, and iatrogenic Creutzfeldt-Jakob disease. J Virol. 79:14339–14345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C, Sales N, Etchegaray N, Charbonnier A, Freire S, Dormont D, Deslys JP, Lasmezas CI. 2004. Tissue distribution of bovine spongiform encephalopathy agent in primates after intravenous or oral infection. Lancet. 363:422–428 [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Ziegler U, Buschmann A, Weber A, Kupfer L, Oelschlegel A, Hammerschmidt B, Groschup MH. 2007. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. J Gen Virol. 88:1048–1055 [DOI] [PubMed] [Google Scholar]
- Hortells P, Monzón M, Monleón E, Acín C, Vargas A, Bolea R, Luján L, Badiola JJ. 2006. Pathological findings in retina and visual pathways associated to natural scrapie in sheep. Brain Res. 1108:188–194 [DOI] [PubMed] [Google Scholar]
- Iwamaru Y, Ookubo Y, Ikeda T, Hayashi H, Imamura M, Yokoyama T, Shinagawa M. 2005. PrPSc distribution of a natural case of bovine spongiform encephalopathy. Tokyo: Springer [Google Scholar]
- Iwata N, Sato Y, Higuchi Y, Nohtomi K, Nagata N, Hasegawa H, Tobiume M, Nakamura Y, Hagiwara K, Furuoka H, et al. 2006. Distribution of PrP(Sc) in cattle with bovine spongiform encephalopathy slaughtered at abattoirs in Japan. Jpn J Infect Dis. 59:100–107 [PubMed] [Google Scholar]
- Jeffrey M, Martin S, Thomson JR, Dingwall WS, Begara-McGorum I, Gonzalez L. 2001. Onset and distribution of tissue prp accumulation in scrapie-affected suffolk sheep as demonstrated by sequential necropsies and tonsillar biopsies. J Comp Pathol. 125:48–57 [DOI] [PubMed] [Google Scholar]
- Jeffrey M, McGovern G, Goodsir CM, Brown KL, Bruce ME. 2000. Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy. J Pathol. 191:323–332 [DOI] [PubMed] [Google Scholar]
- Kercher L, Favara C, Chan CC, Race R, Chesebro B. 2004. Differences in scrapie-induced pathology of the retina and brain in transgenic mice that express hamster prion protein in neurons, astrocytes, or multiple cell types. Am J Pathol. 165:2055–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Shin YK, Lee KM, Lee JS, Yun JH, Lee SM. 2003. An improved protocol of biotinylated tyramine-based immunohistochemistry minimizing nonspecific background staining. J Histochem Cytochem. 51:129–132 [DOI] [PubMed] [Google Scholar]
- Kovács GG, Preusser M, Strohschneider M, Budka H. 2005. Subcellular localization of disease-associated prion protein in the human brain. Am J Pathol. 166:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezmi S, Bencsik A, Monks E, Petit T, Baron T. 2003. First case of feline spongiform encephalopathy in a captive cheetah born in France: PrP(sc) analysis in various tissues revealed unexpected targeting of kidney and adrenal gland. Histochem Cell Biol. 119:415–422 [DOI] [PubMed] [Google Scholar]
- Masujin K, Matthews D, Wells GA, Mohri S, Yokoyama T. 2007. Prions in the peripheral nerves of bovine spongiform encephalopathy–affected cattle. J Gen Virol. 88:1850–1858 [DOI] [PubMed] [Google Scholar]
- Monleón E, Monzón M, Hortells P, Vargas A, Acín C, Badiola JJ. 2004. Detection of PrPsc on lymphoid tissues from naturally affected scrapie animals: comparison of three visualization systems. J Histochem Cytochem. 52:145–151 [DOI] [PubMed] [Google Scholar]
- Okada H, Iwamaru Y, Imamura M, Masujin K, Matsuura Y, Shimizu Y, Kasai K, Takata M, Fukuda S, Nikaido S, et al. 2011. Neuroanatomical distribution of disease-associated prion protein in cases of bovine spongiform encephalopathy detected by fallen stock surveillance in Japan. J Vet Med Sci. 73:1465–1471 [DOI] [PubMed] [Google Scholar]
- Okada H, Iwamaru Y, Imamura M, Masujin K, Yokoyama T, Mohri S. 2010. Immunohistochemical detection of disease-associated prion protein in the intestine of cattle naturally affected with bovine spongiform encephalopathy by using an alkaline-based chemical antigen retrieval method. J Vet Med Sci. 72:1423–1429 [DOI] [PubMed] [Google Scholar]
- Okada H, Sato Y, Sata T, Sakurai M, Endo J, Yokoyama T, Mohri S. 2011. Antigen retrieval using sodium hydroxide for prion immunohistochemistry in bovine spongiform encephalopathy and scrapie. J Comp Pathol. 144:251–256 [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 1991. Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle. Dev Biol Stand. 75:55–74 [PubMed] [Google Scholar]
- Shimizu Y, Kaku-Ushiki Y, Iwamaru Y, Muramoto T, Kitamoto T, Yokoyama T, Mohri S, Tagawa Y. 2010. A novel anti-prion protein monoclonal antibody and its single-chain fragment variable derivative with ability to inhibit abnormal prion protein accumulation in cultured cells. Microbiol Immunol. 54:112–121 [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Barillas-Mury C, Miller MW, Oesch B, van Keulen LJ, Langeveld JP, Hoover EA. 2002. PrP(CWD) lymphoid cell targets in early and advanced chronic wasting disease of mule deer. J Gen Virol. 83:2617–2628 [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. 2001. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol. 82:2327–2334 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Takahashi RH, Hasegawa H, Horiuchi M, Shinagawa M, Yokoyama T, Kimura K, Haritani M, Kurata T, Nagashima K. 1999. Characterization of antibodies raised against bovine-PrP-peptides. J Neurovirol. 5:300–307 [DOI] [PubMed] [Google Scholar]
- Taylor DM, Brown JM, Fernie K, McConnell I. 1997. The effect of formic acid on BSE and scrapie infectivity in fixed and unfixed brain-tissue. Vet Microbiol. 58:167–174 [DOI] [PubMed] [Google Scholar]
- Terry LA, Marsh S, Ryder SJ, Hawkins SA, Wells GA, Spencer YI. 2003. Detection of disease-specific PrP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet Rec. 152:387–392 [DOI] [PubMed] [Google Scholar]
- Valdez RA, Rock MJ, Anderson AK, O’Rourke KI. 2003. Immunohistochemical detection and distribution of prion protein in a goat with natural scrapie. J Vet Diagn Invest. 15:157–162 [DOI] [PubMed] [Google Scholar]
- Vidal E, Marquez M, Tortosa R, Costa C, Serafin A, Pumarola M. 2006. Immunohistochemical approach to the pathogenesis of bovine spongiform encephalopathy in its early stages. J Virol Methods. 134:15–29 [DOI] [PubMed] [Google Scholar]
- Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. 2001. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 358:171–180 [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Masujin K, Yamakawa Y, Sata T, Murayama Y, Shu Y, Okada H, Mohri S, Shinagawa M. 2007. Experimental transmission of two young and one suspended bovine spongiform encephalopathy (BSE) cases to bovinized transgenic mice. Jpn J Infect Dis. 60:317–320 [PubMed] [Google Scholar]