Abstract
OBJECTIVE:
Evaluation of myocardial histological changes in an experimental animal model of neonatal hypoxia-reoxygenation.
METHODS:
Normocapnic hypoxia was induced in 40 male Landrace/Large White piglets. Reoxygenation was initiated when the animals developed bradycardia (HR <60 beats/min) or severe hypotension (MAP <15 mmHg). The animals were divided into four groups based on the oxygen (O2) concentration used for reoxygenation; groups 1, 2, 3, and 4 received 18%, 21%, 40%, and 100% O2, respectively. The animals were further classified into five groups based on the time required for reoxygenation: A: fast recovery (<15 min); B: medium recovery (15-45 min); C: slow recovery (45-90 min); D: very slow recovery (>90 min), and E: nine deceased piglets.
RESULTS:
Histology revealed changes in all heart specimens. Interstitial edema, a wavy arrangement, hypereosinophilia and coagulative necrosis of cardiomyocytes were observed frequently. No differences in the incidence of changes were observed among groups 1-4, whereas marked differences regarding the frequency and the degree of changes were found among groups A-E. Coagulative necrosis was correlated with increased recovery time: this condition was detected post-asphyxia in 14%, 57%, and 100% of piglets with fast, medium, and slow or very slow recovery rates, respectively.
CONCLUSIONS:
The significant myocardial histological changes observed suggest that this experimental model might be a reliable model for investigating human neonatal cardiac hypoxia-related injury. No correlation was observed between the severity of histological changes and the fiO2 used during reoxygenation. Severe myocardial changes correlated strictly with recovery time, suggesting an unreported individual susceptibility of myocardiocytes to hypoxia, possibly leading to death after the typical time-sequence of events.
Keywords: Cardiomyocyte, Coagulative Necrosis, Hypoxia, Reoxygenation, Newborn Piglet
INTRODUCTION
Human gestation is a critical period during which the vulnerability of various organs to disease is determined. Acute, chronic, and intermittent hypoxia have been shown to induce various cardiovascular responses, including increased peripheral resistance and heart mass and hemodynamic modifications (1). In the chick embryo, acute hypoxia caused contraction of the femoral arteries in response to exogenous norepinephrine (2). In rats exposed to low oxygen levels alternated with normal oxygen concentration, right ventricular hypertrophy was reported (3). Fetal hypoxia-reperfusion injury in rats increased the susceptibility of the heart to ischemia-reperfusion injury in adult life (4). In fetal guinea pigs, acute non-lethal hypoxia in utero reduced the cardiac levels of ATP. Histological analysis revealed that the apoptotic index increased in the absence of other remarkable histological changes (5). Maternal hypoxia during pregnancy in rats led to early changes typical of atherogenesis in the offspring in adult life (6). Increased cardiomyocyte apoptosis was described in human newborns with documented chronic hypoxia in utero (7). Hypoxic stress early in life has been associated with a number of adverse consequences, including increased arterial wall thickness, metabolic alterations of cardiomyocytes, and cardiac remodeling (8,9). The hemodynamic fluctuations at baseline and during normocapnic hypoxia and reoxygenation in a neonatal piglet model were reported by our group to be similar to those observed in human neonates, suggesting that this model is reliable for the investigation of hypoxia (10).
Considering these data, which suggest an important impact of hypoxia on myocardial architecture, and considering that human perinatal hypoxia is associated with immediate and long-term mortality and morbidity, we aimed to investigate the effects of acute hypoxia-reoxygenation on newborn swine hearts.
MATERIALS AND METHODS
Forty male Landrace/Large White piglets, aged 1-4 days and weighing 2.3–3.8 kg, were included in the study. The animals were obtained from the same breeder (N. Validakis, Koropi, Greece) on the day of the experiment. They were transported to the laboratory (Experimental–Research Center ELPEN) in a temperature-controlled truck in pre-warmed padded boxes within half an hour; therefore, feeding was not required during transportation or at the research facility.
The experimental protocol was approved by the General Directorate of Veterinary Services (permit No. 404/21-04-09) and has been described previously (10). All of the animals were initially sedated with a single intramuscular injection of 10 mg/kg ketamine (Narketan, Vétoquinol UK Ltd, Buckingham, UK) and 0.5 mg/kg midazolam (Dormicum®, Roche, (Hellas) S.A., Maroussi, Greece). The marginal auricular vein was catheterized with a 24 G catheter (Jelco R., Smiths Medical, N. Papapostolou SA, Athens, Greece), and anesthesia was induced with 1 mg/kg propofol (Diprivan, AstraZeneca, Athens, Greece) followed by 10 µg/kg fentanyl (Fentanyl, Janssen-Cilag, Buckinghamshire, UK). The animals were intubated with a 3.0- or 3.5-mm endotracheal tube (Portex, PMSmiths Medical International Ltd, Ashford, Kent, UK), as appropriate. Correct endotracheal intubation was confirmed by auscultation and capnography (Datex Engstrom, Instrumentarium Corp., Helsinki, Finland). The infusion of 10 ml/kg/h of 0.9% normal saline and 5 ml/kg/h of 5% dextrose in water was initiated to prevent dehydration and hypoglycemia. Non-invasive continuous monitoring included the heart rate (HR), electrocardiograms (ECG), saturation of oxygen by pulse oximetry (SpO2), and rectal temperature measurements. Body temperature was maintained at 38±1°C with a table heating pad and an overhead heating lamp. The animals were administered an intravenous bolus of 20 µg/kg fentanyl and 0.2 mg/kg cis-atracurium (Nimbex, Abbott Laboratories (Hellas) S.A., Athens, Greece), after which they were connected to the mechanical ventilator (Soxil, Soxitronic, Felino, Italy). They were ventilated with a tidal volume of 10-15 ml/kg, pressure of 19 cm H2O and respiratory rate of 30–40 breaths/min, aiming at an end-tidal CO2 (ETCO2) of 35-45 mmHg. The fraction of inspired oxygen (fiO2) was adjusted between 0.21 and 0.25 to maintain SpO2 90-95%. The anesthesia was maintained by infusing 8-10 mg/kg/h propofol and boluses of 10 µg/kg fentanyl and 0.15 mg/kg cis-atracurium.
Subsequently, the right internal jugular vein and carotid artery were catheterized with single-lumen catheters (S1UVC5.0, NeoCare®; Klein-Baker Medical Co., San Antonio, TX, USA) via a paratracheal incision. The catheters were connected to external transducers (Transpac, Abbott Critical Care Systems, Salt Lake City, UT, USA) for the continuous monitoring of central venous pressure (CVP), systolic arterial pressure (SAP), and mean (MAP) and diastolic pressure (DAP) of the carotid artery. Following catheterization, the incision was sutured and covered with warm, sterile gauze to prevent heat loss. The animals were stabilized for 30 min prior to experimentation.
Hypoxia was induced by decreasing the inspired fiO2 to 0.06–0.08 while maintaining the other settings of ventilation. Close monitoring was conducted to detect either bradycardia (HR<60 beats per minute) or severe hypotension (MAP<15 mmHg). The time needed for these conditions to occur was recorded. As soon as hemodynamic compromise occurred, arterial blood samples were obtained to confirm hypoxemia (pO2 30-50 mmHg), and resuscitation efforts were initiated according to the Newborn Life Support (NLS) algorithm (1). The animals were resuscitated with different fiO2 according to their allocation into groups 1, 2, 3, and 4 (receiving 18%, 21%, 40%, and 100% O2, respectively) until HR and MAP returned to 90% of baseline levels. Resuscitation efforts were assessed every 30 sec. If the HR did not increase despite oxygen administration for 30 sec, oxygenation was administered for 30 more sec. If the HR did not increase after two cycles (30 sec/cycle) of oxygenation, chest compressions were applied at a rate of three compressions: one ventilation for 30 sec. If one cycle of chest compressions failed to increase HR, 10 mcg/kg adrenaline (1∶10,000 dilution) was administered via the auricular venous catheter. As soon as these hemodynamic parameters returned to baseline values, the piglets were ventilated under anesthesia for 30 min prior to collecting arterial blood for blood gas analysis. The endpoints of the experiment were either persisting asystole despite 10 min of cardiopulmonary resuscitation or a return of the hemodynamic parameters to baseline values. The surviving animals were euthanized by the slow intravenous infusion of 30 mg/kg sodium thiopental [Pentothal, Hospira Enterprises BV, Hoofddorp, The Netherlands]. Subsequently, necropsy was performed to identify possible injury or abnormality.
To identify any association between histological changes and the duration of hypoxia, the piglets were further divided into five groups based on recovery time: Group A: fast recovery (<15 min); group B: medium (15-45 min); group C: slow (45-90 min); group D: very slow recovery (>90 min), and group E: deceased piglets.
Multiple cardiac samples were collected in toto, reduced, fixed in 10% formalin, processed, and paraffin-embedded; the initial block was cut into 6-7 blocks, 2-3 mm wide, that were subsequently deparaffinized and hydrated. The blocks were then colored with hematoxylin for 15 min, washed in running tap water for 20 min and counterstained with eosin from 15 sec to 2 min. Finally, the slides were dehydrated in 95% alcohol and cleared in xylene. All hematoxylin-eosin (H&E) slices were assessed by a pathologist blinded to the animal outcome.
The variables were first tested for normality using the Kolmogorov-Smirnov test. The normal variables are expressed as the means±standard deviation (SD). The qualitative variables are expressed as absolute and relative frequencies. Repeated-measurement analysis of variance (ANOVA) was used to evaluate any possible differences among the baseline, hypoxia, and reoxygenation time-points. To control for type I error, post-hoc analysis with a Bonferroni correction was used. The p-values reported are two-tailed. The statistical significance was set at 0.05, and the analysis was conducted using SPSS statistical software (version 17.0).
RESULTS
In total, 13 out of 40 piglets required chest compressions and the use of adrenaline. More specifically, five animals from group 1, two animals from group 2, three animals from group 3 and three animals from group 4 required more aggressive resuscitation.
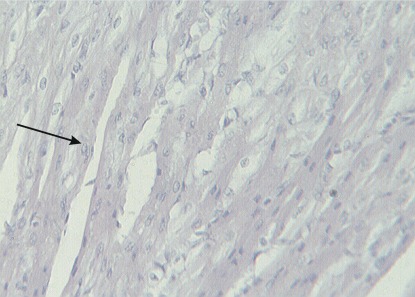
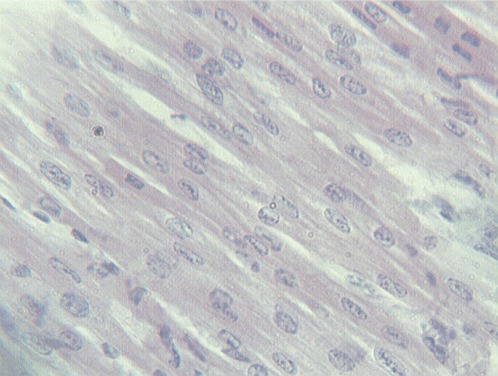
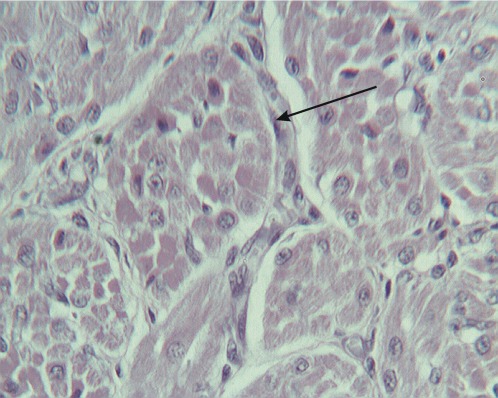
The histological examination of H&E-stained sections revealed pathological changes in all of the specimens. Interstitial edema was observed in 37/40 cases (92.5%); the degree of edema varied among the cases. In four cases (one in group 1, two in group 2 and one in group 3), the edema was severe and diffuse, leading to disarrangement of the myocardial architecture (Figure 1). In the majority of cases, the interstitial edema was mild and focal, primarily localized around small, dilated vessels. A wavy arrangement of cardiomyocytes was detected in all but nine cases (77.5%). In 19 hearts, the wavy fibers were focal, whereas in the remaining specimens, the wavy arrangement was diffuse (Figure 1). Cardiomyocyte hypereosinophilia was observed in 28/40 cases (70%). This condition was characterized by cardiomyocyte cytoplasm with a homogeneous appearance, which was associated with an increase in the intensity of eosin staining (Figure 2). Hypereosinophilic fibers were mainly detected in small foci. Coagulative necrosis was detected in 26/40 hearts (65%). The degree of coagulative necrosis varied significantly among cases. In 13 hearts, this condition was mild and focal; in 12, it appeared in large foci; and in one case, coagulative necrosis diffused to large areas of the heart specimen examined (Figure 3).
Figure 1.
Interstitial edema and wavy fibers.
Figure 2.
Hypereosinophilia of cardiomyocytes.
Figure 3.
Coagulative necrosis.
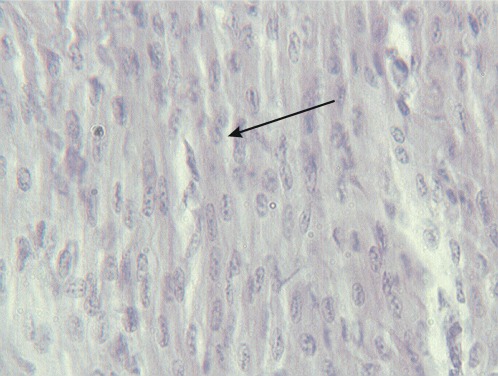
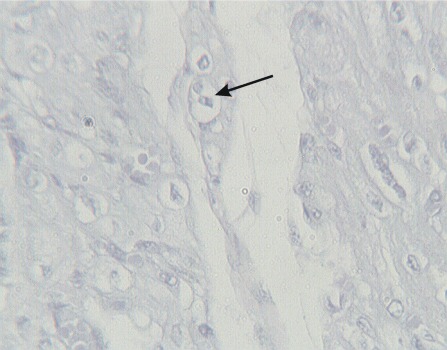
Apoptotic cells, characterized by the detachment of scattered cardiomyocytes from neighboring cells with the formation of roundish eosinophilic globules (Figure 4), were found in 3/40 cases (7.5%). This apoptotic cell death was mild and focal in five cases, whereas in one case, apoptotic globules were frequent and had diffused to all the heart regions sampled. Cytoplasmic vacuoles were found in 15/40 hearts (37.5%); vacuolization most often affected small groups of cardiomyocytes (Figure 5), whereas in one case, it was observed in the majority of cardiac cells.
Figure 4.
Nuclear polymorphism and apoptosis.
Figure 5.
Cytoplasmic vacuolization of the cardiomyocytes.
Contraction changes were rarely observed, and when present, the lesion was always focal. In contrast, nuclear lysis (30/40) and cytoplasmic homogenization (24/40) were observed in the majority of cases; the nuclear loss typically appeared as a focal lesion, although in four cases, it was diffuse. Neutrophilic infiltrate was detected in 16/40 cases (40%). The foci of neutrophils were always mild, mainly located around blood vessels and always in association with interstitial edema. Small foci of hemorrhage were observed in 3/40 cases (7.5%); these features were always very small and were detected in proximity to the dilated vessels. Perinuclear halos were detected in only one deceased animal.
When histological findings were analyzed in the four groups of animals, based on the O2 concentration used for resuscitation, no significant differences were observed among the four groups regarding the following features: a) interstitial edema; b) wavy arrangement; c) hypereosinophilia; d) vacuolization; or e) contraction. Marked interindividual differences were observed within each group regarding the severity of the edema, which ranged from focal and mild to marked and diffuse. The wavy arrangement was found to be mild or moderate and always focal.
The histological changes observed in the four groups were as follows:
apoptotic bodies were observed in 30% of animals from groups 1 and 4, whereas they were not detected in groups 2 and 3
the percentage of positive cases for neutrophil infiltrate varied among the 4 groups, ranging from 20% in groups 2 and 3 up to 50% in groups 1 and 4.
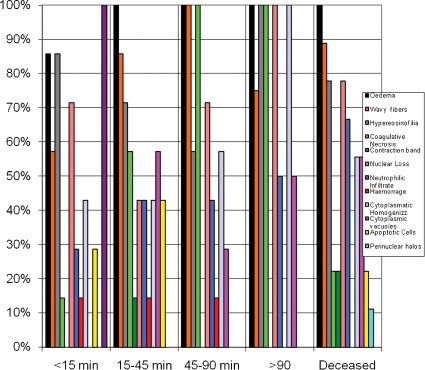
When histological changes were examined in the five groups (A-E) formed based on the time required for recovery, two histological changes exhibited a progressive enhancement associated with recovery time: coagulative necrosis and cytoplasmic vacuolization. Coagulative necrosis was present in 14.3% of the animals in group A, 57.1% of the animals in group B and 100% of the animals in groups C and D. Regarding cytoplasmic vacuoles in the tubular cells, they were absent in all of the animals in group A, with levels reaching 57% in the piglets in group B. Figure 6) elucidates the changes in cardiomyocytes with respect to recovery time.
Figure 6.
Changes in cardiomyocytes with respect to recovery time.
DISCUSSION
Fetuses and newborns are very susceptible to hypoxia and have a number of immediate adaptive mechanisms, such as hyperventilation and blood redistribution, which serve to protect vital organs and reduce tissue O2 needs and subsequent hypoxia (11-15). Despite the activation of these protective mechanisms, it was shown that hypoxia modifies the NMDA receptor/ion channel complex (16) and increases protein tyrosine kinase activity in cortical cell membranes (17), whereas hypoxia decreases the activity of nitric oxide synthase in every region of the brain except the cortex (18).
In the heart, perinatal hypoxia was reported to induce permanent changes in the structure and function of the left ventricle (19,20). Moreover, significant changes in left ventricular gene expression were reported in newborns, with physiologic implications for the adult myocardium (21).
Rodents, sheep, and primates have been used as animal models of perinatal hypoxia (22-24). The pig is used more frequently because its heart is anatomically and physiologically similar to that of the human (25,26). The coronary circulation and heart conduction system are similar in humans and swine (27,28). Consequently, humans and pigs have comparable histology under normal and pathologic conditions (29,30).
Thirteen animals required more aggressive resuscitation with adrenaline and chest compressions. Although adrenaline may enhance myocardial damage due to its beta-adrenergic effects (31), the fact that adrenaline was administered in a subset of animals in all of the groups makes a possible association between adrenaline and the observed cardiac findings unlikely. To the best of the authors' knowledge, chest compressions have never been associated with cardiac damage (32).
Histological examination of the heart specimens of our asphyxiated piglets revealed pathological changes in all of the specimens. The most important changes were interstitial edema leading to disarrangement of the cardiac architecture, the wavy arrangement of cardiomyocytes and hypereosinophilia/coagulative necrosis among the cardiomyocytes.
Although myocardial histological changes were observed in all four groups and observed to correlate with the fiO2 used for resuscitation, differences were not found between the groups but were detected among piglets within each group. In addition, the extreme O2 concentrations (18% and 100%) appear to be associated with higher apoptosis, whereas room air (21%) and moderate (40%) O2 concentrations appear to protect against apoptosis. These findings, taken together, suggest the existence of an individual susceptibility to hypoxia, allowing each piglet to organize a personal adaptive response to oxygen deprivation, tailored to its biochemical and enzymatic profile. Moreover, reoxygenation and, in particular, the concentration of O2 used, do not represent key factors in the determination of cardiac injury in our experimental model.
Additional interesting data on histological myocardial changes were obtained when the piglets were grouped according to resuscitation time. The analysis of the pathological changes observed in group A animals allowed us to identify early and late lesions. Edema and hypereosinophilia were the typical early lesions, observed in animals with a very short recovery time. On the other extreme of the spectrum of recovery time, we found that cytoplasmic vacuoles were absent in all animals in group A; each animal also exhibited coagulative necrosis, hemorrhage, apoptosis, and neutrophil infiltrates, which are signs of mature lesions. The coagulative necrosis of cardiomyocytes deserves particular attention, as this severe heart lesion was very rare in animals in group A; its incidence progressively increased to 57% in animals with a medium recovery time, reaching 100% in piglets with slow and very slow recovery times. The association between the incidence of coagulative necrosis and recovery time post-asphyxia clearly indicates coagulative necrosis to be the most important pathological change occurring in the hearts of newborn piglets following hypoxia. The low incidence of coagulative necrosis in deceased animals might be attributed to the short survival time, as coagulative necrosis requires more than 15 min.
Another lesion worth mentioning is the presence of vacuoles in the cardiomyocyte cytoplasm. Heart cell vacuolization was not detected in any of the animals in the very fast recovery group, whereas cytoplasmic vacuoles were present in cardiomyocytes in 57% of the animals in group B. This finding suggests that cardiac cell vacuolization may be a marker of hypoxic insult lasting more than 15 min. This finding apparently runs counter to data obtained from the investigation of renal lesions in the same animals (unpublished data), which demonstrate the presence of vacuoles in proximal and/or distal tubular cells in more than 50% of the animals in group A, indicating renal cell vacuolization as the cause of the kidney lesions that develop soon after acute hypoxia. This difference may be attributed to the adaptive mechanisms of mammals following hypoxic insults, a situation in which a life support system should be adopted. These decisions must be made quickly and prioritize the heart over the kidney.
In conclusion, our data clearly indicate Landrace/Large White piglets as a favorable swine breed for experimentation on neonatal hypoxia. The hemodynamic fluctuations at baseline, as reported by our group, during normocapnic hypoxia and reoxygenation in this animal model resulted in important myocardial histological changes, indicating the reliability of this model for the study of human neonatal hypoxia (10). Cardiac lesions appear to play an important role in asphyxiated newborns, with coagulative necrosis being the principal and most severe lesion that is strictly associated with recovery time, which differs widely among animals in the same group. This study suggests an inter-individual variability in the response of cardiac cells to asphyxia that is independent from the fiO2 used during resuscitation. Further studies are needed in the field of hypoxia-reoxygenation before the application of these findings in clinical practice.
ACKNOWLEDGMENTS
The authors would like to thank Miss. Patrizia Baire and Mr. Ignazio Ferru for secretarial assistance. The technical work of Mrs. Sandra Serra is also acknowledged. The financial support of “Fondazione Banco di Sardegna” (Cagliari, Italy) and ELPEN Research-Experimental Center (Athens, Greece) is kindly acknowledged.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Banchero N. Cardiovascular responses to chronic hypoxia. Ann Rev Physiol. 1997;49:465–76. doi: 10.1146/annurev.ph.49.030187.002341. [DOI] [PubMed] [Google Scholar]
- 2.Ruijtenbeek K, Kessels CG, Villamor E, Blanco CE, De Mey JG. Direct effects of acute hypoxia on the reactivity of peripheral arteries of the chicken embryo. Am J Physiol. Regul Integr Comp Physiol. 2002;283(2):R331–8. doi: 10.1152/ajpregu.00675.2001. [DOI] [PubMed] [Google Scholar]
- 3.Mc Guire M, Bradford A. Chronic intermittent hypoxia increases haematocrit and causes right ventricular hypertrophy in the rat. Respir Physiol. 1999;117(1):53–8. doi: 10.1016/s0034-5687(99)00047-x. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Xiao Y, Estrella LJ, Ducsay CA, Gilbert RD, Zhang L. Effects of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10(5):265–74. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 5.Powell SR, Mantell LL, Garramone V, Drexler S, Teichberg S. Acute effects of non lethal in utero hypoxia on fetal guinea pig heart and lack of persistent cardiac or cerebral effects in the neonate. Biol Neonate. 2004;86(4):240–6. doi: 10.1159/000079933. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Huang Z, Lu G, Lin L, Ferrari M. Hypoxia during pregnancy in rats leads to early morphological changes of atherosclerosis in adult offspring. Am J Physiol Heart Circ Physiol. 2009;296(5):H1321–8. doi: 10.1152/ajpheart.00440.2008. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12(1):2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.De Luca D. NICE guidelines on neonatal jaundice: at risk of being too nice. Lancet. 2010;376(9743):771. doi: 10.1016/S0140-6736(10)61376-1. [DOI] [PubMed] [Google Scholar]
- 9.Lemler MS, Bies RD, Frid MG, Sastravaha A, Zisman LS, Bohlmeyer T, et al. Myocyte cytoscheletal disorganization and right heart failure in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2000;279(3):H1365–76. doi: 10.1152/ajpheart.2000.279.3.H1365. [DOI] [PubMed] [Google Scholar]
- 10.Aroni F, Xanthos T, Varsami M, Argyri I, Alexaki A, Stroumpoulis K, et al. An experimental model of neonatal normocapnic hypoxia and resuscitation in Landrace/Large white piglets. doi: 10.3109/14767058.2012.663823. J Matern Fetal Neonatal Med. (In press). [DOI] [PubMed] [Google Scholar]
- 11.Arbeille P, Maulik D, Fignon A, Stale H, Berson M, Bodard S, et al. Assessment of the fetal PO2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med Biol. 1995;21(7):861–70. doi: 10.1016/0301-5629(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 12.Richardson BS, Bocking AD. Metabolic and Circulatory Adaptations to Chronic Hypoxia in the Fetus. Comp Biochem Physiol A Mol Integr Physiol. Part A: Molecular & Integrative Physiology. 1998;119(3):717–23. doi: 10.1016/s1095-6433(98)01010-1. [DOI] [PubMed] [Google Scholar]
- 13.Kopelman AE, Matthew OP. Common Respiratory Disorders of the Newborrn. Pediatr Rev. 1995;16:209–17. doi: 10.1542/pir.16-6-209. [DOI] [PubMed] [Google Scholar]
- 14.Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116(2-3):95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Spilsbury M, Mota-Rojas D, Villanueva-García D. Perinatal asphyxia pathophysiology in pig and human: a review. Anim Reprod Sci. 2005;90(1-2):1–30. doi: 10.1016/j.anireprosci.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Fritz KI, Groenendaal F, McGowan JE, Mishra OP, Delivoria-Papadopoulos M. Effect of cerebral hypoxia on NMDA receptor binding characteristics after treatment with 3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid (CPP) in newborn piglets. Brain Research. 1996;729(1):66–74. [PubMed] [Google Scholar]
- 17.Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on protein tyrosine kinase activity in cortical membranes of newborn piglets—the role of nitric oxide. Neurosci Lett. 2004;372(1-2):114–8. doi: 10.1016/j.neulet.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Jiang K, Kim S, Murphy S, Song D, Pastuszko A. Effect of hypoxia and reoxygenation on regional activity of nitric oxide synthase in brain of newborn piglets. Neurosci Lett. 1996;206(2-3):199–203. doi: 10.1016/s0304-3940(96)12466-6. [DOI] [PubMed] [Google Scholar]
- 19.Del Duca D, Tadevosyan A, Karbassi F. Hypoxia in early life is associated with lasting changes in left ventricular structure and function at maturity in the rat. Int J Cardiol. 156(2):165–73. doi: 10.1016/j.ijcard.2010.10.135. [DOI] [PubMed] [Google Scholar]
- 20.Parer JT, Dijkstra HR, Vredebregt PPM, Harris,JL, Krueger TR. Increased fetal heart rate variability with acute hypoxia in chronically instrumented sheep. Eur JObstet Gynecol Reprod Biol. 1980;10(6):393–9. doi: 10.1016/0028-2243(80)90025-8. [DOI] [PubMed] [Google Scholar]
- 21.Del Duca D, Wong G, Trieu P. Association of neonatal hypoxia with lasting changes in left ventricular gene expression: An animal model. The Journal of Thoracic and cardiovascular Surgery. 2009;138(3):538–46. doi: 10.1016/j.jtcvs.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Northington FJ. Brief update on animal models of hypoxic-ischemic encephalopathy and neonatal stroke. ILAR J. 2006;47(1):32–8. doi: 10.1093/ilar.47.1.32. [DOI] [PubMed] [Google Scholar]
- 23.Papazisis G, Pourzitaki C, Sardeli C, Lallas A, Amaniti E, Kouvelas D. Deferoxamine decreases the excitatory amino acid levels and improves the histological outcome in the hippocampus of neonatal rats after hypoxia-ischemia. Pharmacol Res. 2008;57(1):73–8. doi: 10.1016/j.phrs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Blaikley JB, Gibberd GF. Asphyxia neonatorum: it's treatment by tracheal intubation. Lancet. 1935;1:736–9. [Google Scholar]
- 25. Duke EC, Vincent KOH, Wen C, Maced B.Studies in the physiology of cardiopulmonary bypass using a swine model In: Swindle MM, Moddy DC, Phillips LC, editors. Swine as models in biomedical research Medical University of South Carolina: Charleston (SC)1992. pp187–97. [Google Scholar]
- 26.Swindle MM, Horneffer PJ, Gardner TJ, Gott VL, Hall TS, Stuart RS, et al. Anatomic and anesthetic considerations in experimental cardiopulmonary surgery in swine. Lab Anim Sci. 1986;36(4):357–61. [PubMed] [Google Scholar]
- 27.Stepanek J, Klocke D, Malvin G, Parisi J, Tazelaar H. The piglet as an animal model for hypobaric hypoxia. Wilderness Environ Med. 1998;9(1):8–13. doi: 10.1580/1080-6032(1998)009[0008:tpaaam]2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Xanthos T, Lelovas P, Vlachos I, Tsirikos-Karapanos N, Kouskouni E, Perrea D, et al. Cardiopulmonary arrest and resuscitation in Landrace/Large White swine: a research model. Lab Anim. 2007;41(3):353–62. doi: 10.1258/002367707781282820. [DOI] [PubMed] [Google Scholar]
- 29.Swindle MM, Smith AC, Laber-Laird K, Dungan L. Swine in biomedical research: management and models. ILAR News. 1994;36(1):1–5. [Google Scholar]
- 30. Gal D, Isner JM.Atherosclerotic Yucatan microswine as a model for novel cardiovascular interventions and imaging Swindle MM. (ed.), Swine as Models in Biomedical Research, Ames, IA: Iowa State University Press, 1992. pp.118–40. [Google Scholar]
- 31.Xanthos T, Pantazopoulos I, Demestiha T, Stroumpoulis K. Epinephrine in ventricular fibrillation: friend or foe. A review for the Emergency Nurse. J Emerg Nurs. 37(4):408–12;quiz 425-6. doi: 10.1016/j.jen.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Xanthos T, Karatzas T, Stroumpoulis K, Lelovas P, Simitsis P, Vlachos I, et al. Continuous chest compressions improve survival and neurologic outcome in a swine model of prolonged ventricular fibrillation. Am J Emerg Med. 2011 Dec 26; doi: 10.1016/j.ajem.2011.10.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]