Abstract
OBJECTIVE:
Echinophora platyloba DC is a widely used herbal medicine and food seasoning in Iran. It is claimed to exert antimicrobial, antifungal, and antispasmodic effects. Despite the prevalent use of this plant as a food and medicine, there are no reports on its possible toxic effects. To evaluate the safety of E. platyloba, we tested its acute and sub-chronic toxicity in male and female Wistar rats.
METHODS:
Rats were orally treated with four different single doses of E. platyloba total extract and screened for signs of toxicity two weeks after administration. In the sub-chronic toxicity study, E. platyloba was administered for 45 days. Mortality, clinical signs, body weight changes, hematological and biochemical parameters, gross findings, organ weights, and histological markers were monitored during the study.
RESULTS:
We found no mortality and no abnormality in clinical signs, body weight, or necropsy findings in any of the animals in the acute study. The results of the subchronic study showed no significant difference in hematological parameters in either sex. There was a significant increase in lactate dehydrogenase in the female groups. A significant increase in the relative lung weight of female rats was noted at 500 mg/kg. Histopathological examinations revealed intra-alveolar hemorrhage in the male rats (500 mg/kg). In the females, congestion of the alveolar capillaries (at 500 mg/kg) and liver bridging necrosis (at 200 mg/kg) were significantly increased.
CONCLUSION:
The no observed adverse effect level of E. platyloba was determined to be 200 and 50 mg/kg for male and female rats, respectively.
Keywords: Echinophora Platyloba; Rat; Acute Toxicity; Subchronic Toxicity
INTRODUCTION
The Apiaceae family is of particular interest in food and medicinal chemistry because it includes many commonly consumed plants, such as celery, carrot, fennel, caraway, and dill. Coumarins, polyacetylenes, flavonoids, sesquiterpenes, and phthalides are among the important chemical constituents of this family, along with biologically active essential oils (1-5). Iran's climate conditions are especially suitable for the growth of apiaceous plants, as demonstrated by the growth of 114 wild genera with 12 endemic genera (6), most of which are consumed daily as food or used in traditional medicine.
Echinophora is a ten-species genus of Apiaceae that contains four species native to Iran, including E. orientalis, E. sibthorpiana, E. cinerea, and E. platyloba (6). Called “Khousharizeh” or “Tigh Touragh” in Persian, E. platyloba is widely used in western and central Iran as a food seasoning and edible vegetable. Local people add the plant to pickles and tomato pastes as an antifungal and antimicrobial preservative (7). These effects have been demonstrated in several experiments. For example, alcoholic extracts of E. platyloba inhibited Candida albicans (7), Trichophyton spp., Microsporum (8), Pseudomonas, and Staphylococcus growth, but it was not effective against Aspergillus (9). Moreover, it could increase the effect of amphotericin B against C. albicans (10). E. platyloba is also traditionally used as an antispasmodic in dysmenorrhea, and its efficacy was proven in a clinical trial (11). Moreover, its hydroalcoholic extract and essential oil were shown to have acceptable antispasmodic effects in the rat ileum (12).
Despite the widespread folk uses and pharmacological effects of E. platyloba, there have been no toxicological studies on its safety. Therefore, in this study, we investigated the acute and repeated-dose (45 days) toxicity of E. platyloba in Wistar rats.
MATERIALS AND METHODS
Plant material and hydroalcoholic extract preparation
The aerial parts of the plant were collected from Charmahal-Bakhtiary, Iran, in April 2009 and dried away from direct sunlight. The plant was authenticated by M. K., and a voucher specimen (Registration Number 1030) was deposited in the Herbarium of the School of Pharmacy (Shaheed Beheshti University of Medical Sciences, Tehran, Iran). The plant samples were ground to fine powder and macerated with EtOH:H2O (80:20) three times. The extract was concentrated to dryness first via eliminating EtOH in a rotary evaporator and then removing the H2O by freeze drying.
Evaluation of toxicity following a single-dose administration
Four-week-old Wistar rats of both sexes were purchased from Razi Research Institute (Hesarak, Karaj, Iran) and acclimated to holding facilities for two weeks prior to dosing. The animals were randomly assigned to control and four treatment groups (five rats per sex per group) and housed in clear plastic cages containing wood shavings for bedding. Each cage contained five rats of the same sex fed normal laboratory chow (Pars Co., Tehran, Iran) and tap water ad libitum throughout the study. Environmental conditions were maintained at 23±2 °C and a relative humidity of 40±10% with a 12 h light/dark cycle. At the onset of dosing, the males weighed 164±19 g, and the females weighed 142±15 g. The research was conducted in accordance with internationally accepted principles for laboratory animal use and care (NIH Publication no. 85–23, revised in 1985).
Administration
The animals were fasted for 4 h prior to dosing. The extract was administered by gavage at doses of 50, 500, 1,000, and 2,000 mg/kg body weight. The control rats received tap water by gavage in the same volume.
Observations
The rats were observed for clinical signs prior to dosing, at 1, 2, 3, 4, 5, 6, 7, and 8 h post-dosing and daily thereafter for 14 days. They were also monitored daily for mortality, any changes in food and water consumption, and any additional clinical or behavioral signs of toxicity. The animal body weights were measured prior to dosing and on days 7 and 14. The numbers of dead animals in each group at the end of the study were expressed as a percentage, and when possible, the LD50 value was established using the Probit method (13). All of the animals were sacrificed on day 15.
Evaluation of toxicity following subchronic treatment
Group assignment and treatment
The animals were randomly caged in clear plastic cages containing wood shavings for bedding. Dosing was initiated when the rats were 8 weeks old, at which point the males and females weighed 200±19 g and 161±10 g, respectively. The animals were divided into four dose groups (five rats per sex per group). The first group was given 1 ml normal saline and used as a control. The second, third, and fourth groups were given single doses of 50, 200, and 500 mg/kg of E. platyloba by gavage daily. All of the solutions were prepared just prior to dosing and were kept chilled and tightly capped.
In vivo observations
Observations of mortality and toxicological signs were made daily for 45 days. The onset, intensity, and duration of these symptoms, if any, were recorded. The weight of each rat was recorded on day 0 and at weekly intervals throughout the course of the study. Food and water consumption was measured three times per week.
Biochemical and hematology analysis
After 45 days, 12 h-fasted animals were anesthetized with the IP injection of a mixture containing ketamine (40 mg/kg) and xylazine (10 mg/kg). The jugular vein was exposed, and blood samples were obtained by jugular vein puncture (14). The following hematological parameters were determined with the Sysmex K-1000 fully automated hematology analyzer: erythrocyte (RBC), total and differential leukocyte (WBC), hematocrit (Hct), hemoglobin (Hb), platelet count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean platelet volume (MPV), platelet distribution width (PDW), and red distribution width (RDW). Blood samples for biochemical analyses were centrifuged at 3,000×g for 5 min, and the plasma was collected and analyzed for glucose, uric acid, creatinine, albumin, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, total protein, and lactate dehydrogenase (LDH) using a COBAS Mira S chemistry analyzer (Roche Diagnostic Systems, West Sussex, England).
Necropsy
Following blood collection, the rats were sacrificed by decapitation, and the organs identified in Table 3 were removed, weighed, and examined macroscopically. The organs were then preserved in 10% neutral buffered formalin and prepared for microscopic histopathological examinations.
Table 3.
Relative organ weight at termination of treatment with E. platyloba (g % body weight).
| Sex | Dose mg/kg | liver% | kidney% | Heart% | Lung% |
| Male | Control | 2.9±0.136 | 0.76±0.024 | 0.4±0.072 | 0.56±0.024 |
| 50 | 3.03±0.21 | 0.79±0.04 | 0.378±0.015 | 0.66±0.04 | |
| 200 | 2.86±0.15 | 0.79±0.066 | 0.34±0.024 | 0.71±0.08 | |
| 500 | 3.07±0.28 | 0.83±0.05 | 0.39±0.055 | 0.54±0.03 | |
| Female | Control | 4±0.2 | 0.79±0.1 | 0.38±0.009 | 0.59±0.036 |
| 50 | 3.1±0.25 | 0.822±0. 052 | 0.41±0.006 | 0.67±0. 03 | |
| 200 | 3.03±0.14 | 0.831±0.0052 | 0.37±0.031 | 063±0.056 | |
| 500 | 3.26±0.28 | 0.88±0.04 | 0.4±0.032 | 0.80±0.07* |
Data presented as mean±S.E.M. for n = 5. Significantly different from control: *p < 0.05.
Statistics
The mean±SEM was calculated for body weights, food consumption, organ/body weight ratios, and hematological and biochemistry factors. The difference between dose groups and controls was separately evaluated for males and females with a one-way analysis of variance (ANOVA) followed by Tukey's test. p-values of 0.05 or less were considered to be significant. Because no treatment-related animal deaths were observed, the LD50 values were not measured.
RESULTS
Acute study
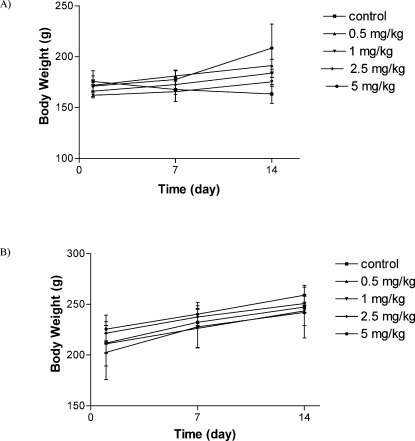
All of the rats treated with different concentrations of total extracts of E. platyloba were alive for all 14 days of observation. Normal body weight gains were observed in the males and females of all of the dose groups. No abnormal gross findings were observed in any of the animals. The oral acute toxicity of E. platyloba total extract (LD50) was therefore considered to be unclassified; doses up to 2,000 g/kg did not induce death or toxic symptoms.
Subchronic study
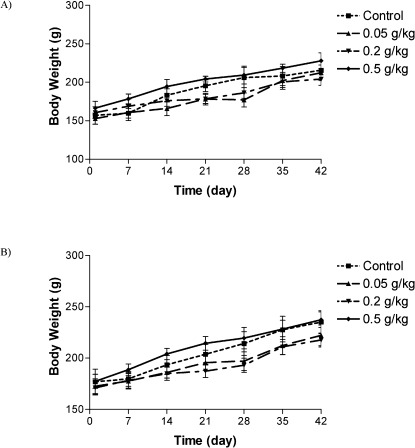
There was no significant difference in body weights between the control and treatment groups (Figures 1 and 2). There was no significant difference between the food consumption of E. platyloba-treated animals and controls. No death was found in any of the groups throughout the experimental period, and no abnormal gross findings were observed in any of the animals. The results of the hematological study are shown in Table 1. No treatment-related changes in hematological parameters were observed during the study period. Biochemical parameter evaluation showed an increase in LDH levels in the 200 and 500 mg/kg female groups (Table 2). No significant differences were observed between the vehicle control and E. platyloba treatment groups in the other biochemical parameters, such as ALT, AST, creatinine, and/or urea. Organ weights measured at necropsy showed increases in the relative lung weights of the 500 mg/kg-treated female rats (Table 3). Histopathological examinations showed intra-alveolar hemorrhage in the male rats (500 mg/kg). In the female rats, congestion of the alveolar capillaries (at 500 mg/kg) and liver bridging necrosis (at 200 mg/kg) were significantly increased compared with the control group. No changes were observed in any of the other parameters evaluated, including alveolar collapse, septal thickness, interstitial infiltrate, intra-alveolar neutrophil counts, alveolar edema, and fatty changes in the lung, hepatocyte regeneration/degeneration, architectural distortion, necrosis, interface hepatitis, portal infiltration, and obstruction/dilatation of the bile duct in the liver. No histological findings in the kidney or heart could be attributed to the E. platyloba treatment.
Figure 1.
Changes in male (A) and female (B) rats body weight with duration of acute treatment. Each point represents mean±SEM, n = 5.
Figure 2.
Changes in male (A) and female (B) rats body weight with duration of subchronic treatment. Each point represents mean±SEM, n = 5.
Table 1.
Hematologic parameters in Wistar rats after 45 days treatment with E. platyloba.
| Sex | Group | WBC(/µl) | RBC(106/mm3) | Hb(g%) | HCT(%) |
| Male | Control | 11200±521 | 9.22±0.1 | 16.4±0.24 | 48.17±0.46 |
| 50 | 9900±1121.45 | 8.21±0.3 | 16.01±0.1 | 46.2±0.45 | |
| 200 | 9300.23±645.23 | 8.28±0.5 | 14.6±0.09 | 44.4±0.42 | |
| 500 | 8700.65±1117.89 | 7.66±0.9 | 15.8±0.6 | 44.7±0.8 | |
| Female | Control | 7700.89±189.49 | 7.46±0.1 | 14.77±0.1 | 39.1±0.6 |
| 50 | 8700±425.25 | 7.1±0.07 | 14.36±0.02 | 37.46±0.2 | |
| 200 | 7300±202.45 | 7±0.2 | 13.6±0.1 | 36.45±0.4 | |
| 500 | 7700±1128.06 | 7.5±0.1 | 13.3±0.07 | 36.4±1.1 |
| Sex | Group | MCV(fi) | MCH(pg) | MCHC(%) | MPV(fi) |
| Male | Control | 53±0.06 | 18±0 | 34.3±0.25 | 7.2±0.25 |
| 50 | 52.6±0.3 | 18±0 | 35.25±0.25 | 7.4±0.23 | |
| 200 | 58.62±0.4 | 18±0.05 | 34.75±0.25 | 6.7±0.18 | |
| 500 | 50.66±0.06 | 21.3±2.5 | 35.75±0.9 | 9.3±0.89 | |
| Female | Control | 52.75±0.06 | 20.3±0.25 | 38.3±0.25 | 6.7±0.1 |
| 50 | 52.25±0.45 | 20±2.3 | 37.75±0.05 | 6.66±0.05 | |
| 200 | 52±0.8 | 21.41±3.6 | 37±0 | 6.65±0.1 | |
| 500 | 51.75±0.9 | 18.1±2.37 | 36.5±1.1 | 6.5±0.1 |
| Sex | Group | Platelets(1000/µl) | RDW(%) | PDW(%) |
| Male | Control | 819.58±142 | 13.3±0.2 | 8.65±1 |
| 50 | 841.98±56.32 | 13.1±0.08 | 8.4±0.1 | |
| 200 | 875.6±69.22 | 13.1±0.34 | 7.63±0.2 | |
| 500 | 800.36±98.3 | 13.05±0.3 | 7.6±0.2 | |
| Female | Control | 935±52 | 12.25±0.21 | 7.62±0.1 |
| 50 | 854.23±25.4 | 12.1±0.3 | 7.52±0.2 | |
| 200 | 921.89±89.6 | 12±0.31 | 7.4±0.05 | |
| 500 | 732.6±124.3 | 11.9±0.1 | 7.17±0.2 |
Data presented as mean±S.E.M. for N = 5.
Table 2.
Biochemical parameters of Wistar rats after 45 days treatment with E.platyloba.
| Sex | Dose mg/kg | blood sugar(mg/dl) | Creatinine (mg/dl) | Uric Acid (mg/dl) | Triglycerides(mg/dl) |
| Male | Control | 162±10.19 | 0.42±0.02 | 2.96±0.9 | 55.2±7.13 |
| 50 | 202.6±23.02 | 0.42±0.04 | 2.5±0.54 | 48±7.34 | |
| 200 | 219.65±23.75 | 0.54±0.05 | 3.92±0.73 | 68.8±6.7 | |
| 500 | 379.5±4.05 | 0.57±0.09 | 2.97±0.35 | 72.8±17.6 | |
| Female | Control | 291.3±28.34 | 0.5±0.05 | 5±0.65 | 82.2±15. 5 |
| 50 | 151.4±11.9* | 0.44±0.05 | 4.14±0.84 | 66.6±11.25 | |
| 200 | 148.2±2.7* | 0.5±0.0 | 3.78±0.0.51 | 52±8.24 | |
| 500 | 177.6±23.64 | 0.46±0.06 | 3.08±0.99 | 62±6.1 |
| Sex | Dose mg/kg | Cholesterol(mg/dl) | HDL(mg/dl) | LDL(mg/dl) | VLDL(mg/dl) |
| Male | Control | 72±7.17 | 38.4±4.67 | 22.4±3.3 | 11.2±1.3 |
| 50 | 84.2±4.93 | 48.4±7.6 | 26.2±6.5 | 9.6±7.4 | |
| 200 | 76.4±4.26 | 34.6±4.2 | 27.4±5.7 | 14.4±1.63 | |
| 500 | 92.25±4.75 | 43±6.67 | 36.25±7.6 | 13±0.7 | |
| Female | Control | 112.4±9.6 | 69.5±6.7 | 21.5±3.8 | 16.6±1.69 |
| 50 | 91±3.8 | 91±3.8 | 34.6±5.6 | 13.2±2.1 | |
| 200 | 88.6±6.67 | 89.2±6.4 | 28±7.7 | 10.4±1.72 | |
| 500 | 98.2±7.37 | 92±5.14 | 33.8±6.2 | 12.4±1.28 |
| Sex | Dose mg/kg | Urea(g %) | SGOT(IU/L) | SGPT(IU/L) | LDH(U/L) |
| Male | Control | 41.6±1.91 | 130.8±16.25 | 48±4.84 | 942.8±138.7 |
| 50 | 45±4.48 | 144±19.45 | 58±6.39 | 1293±189.13 | |
| 200 | 50±3.59 | 170.4±16.7 | 61.25±10.2 | 2036.6±117.98* | |
| 500 | 52.5±1.65 | 191±15.02 | 71.25±7.2 | 2043±122.88* | |
| Female | Control | 60±6.15 | 184.2±26.02 | 66.8±9.84 | 2126±137.12 |
| 50 | 57.8±6.03 | 206.2±20.63 | 71±9.18 | 1992.4 ±243.18 | |
| 200 | 46.6±3.57 | 122.8±5.21 | 41±1.5 | 1743.3±37.6 | |
| 500 | 56.4±4.83 | 126.8±14.51 | 52.4±2 | 1555.25±86.96 |
| Sex | Dose mg/kg | Albumin(gr/dl) | Total Protein(mg/dl) |
| Male | Control | 3.98±0.15 | 9.12±0.56 |
| 50 | 3.59±0.1 | 8.97±0.39 | |
| 200 | 3.25±0.16 | 8.56±0.48 | |
| 500 | 3.73±0.19 | 8.75±0.72 | |
| Female | Control | 3.32±0.26 | 7.86±0.6 |
| 50 | 2.84±0.17 | 7.69±0.52 | |
| 200 | 3.13±0.33 | 8.14±0.69 | |
| 500 | 3.76±0.18 | 7.39±0.49 |
Data presented as mean±S.E.M. for N = 5. Significantly different from control: *p < 0.05.
DISCUSSION
Complementary and alternative medicines (CAMs), such as herbal remedies, require thorough safety and efficacy evaluation due to their growing use all over the world (15). Although many traditional herbal remedies are available and some have been verified by clinical trials, their safety is often still questioned by consumers.
E. platyloba DC is native to Iran and is used in traditional medicine and consumed as a vegetable (7,16).
Despite preliminary evidence of its therapeutic benefits, no toxicology studies have been performed on E. platyloba extracts. In the present study, the acute and subchronic toxicity of E. platyloba DC was evaluated in Wistar rats. The oral LD50 value in this study suggests that E. platyloba DC is a relatively nontoxic plant (17).
Generally, reductions in body weight gain and internal organ weights are considered to be simple and sensitive indices of toxicity after exposure to toxic substances (18). Oral gavage of E. platyloba at up to 500 mg/kg in male and female Wistar rats was not associated with any mortality or abnormality in their general condition, behavior, growth, food and water consumption, or body weight. Additionally, treatment with E. platyloba extract did not produce any statistically significant difference in hematological parameters. The observed elevation in LDH, an initial indicator of myopathy, in females indicates the potential for muscular damage. LDH levels can be diagnostic of myocardial or skeletal muscle injuries (14), but there were no treatment-related heart histopathological findings. Thus, the potential effects of E. platyloba treatment on cardiac muscle should be investigated further.
A significant increase in relative lung weight was also observed. This phenotype may have been caused by the congestion of the alveolar capillaries by retaining blood in the lung. Moreover, the intra-alveolar hemorrhage observed in the male rats (500 mg/kg) indicates that E. platyloba DC may have some effects on this organ. The liver bridging necrosis observed in female rats (200 mg/kg) was not dose–dependent and therefore was not considered to be a treatment-related effect. Additionally, no indicators of liver injury, such as ALT and AST, were observed.
Based on the results of this study, the no observed adverse effect level (NOAEL) of total E. platyloba extract was considered to be 200 and 50 mg/kg/day for male and female rats, respectively. This result indicates that gender is one factor that could influence the response to E. platyloba, and females are more sensitive than males to its subchronic toxicity.
ACKNOWLEDGMENTS
This study was financially supported by the Research Council of Tehran University of Medical Sciences, Iran.
Footnotes
No potential conflict of interest was reported.
REFRENCES
- 1.Christensen L, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: occurrence, bioactivity and analysis. J Pharm Biomed Anal. 2006;41(3):683–93. doi: 10.1016/j.jpba.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 2.Iranshahy M, Iranshahi M. Traditional uses; phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-A review. J Ethnopharmacol. 2011;134(1):1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 3.Nazari ZE, Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res. 2011;25(3):315–23. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 4.Sajjadi SE, Zeinvand H, Shokoohinia Y. Isolation and identification of osthol from the fruits and essential oil composition of the leaves of Prangos asperula Boiss. Res Pharm Sci (RPS) 2009;4(1):19–23. [Google Scholar]
- 5.Shokoohinia Y, Sajjadi SE, MehrAmiri P. Isolation of 3-butyliden-4,5-dihydrophthalide and steroids from Kelussia odoratissima, a Persian food seasoning. Planta Med. 2010;76:P328. [Google Scholar]
- 6. Mozaffarian V.A Dictionary of Iranian Plant Names Tehran: Farhang Moaser Publications; 1996 [Google Scholar]
- 7.Avizhgan M, Hafizi M, Saadat M. Anti-fungal effect of hydroalcoholic extract of Echinophora platyloba on Candida albicans. Iranian Journal of Medicinal and Aromatic Plants Winter. 2006;21(4(30):545–52. [Google Scholar]
- 8.Avizhgan M, Saadat M, Nilfrooshzadeh MR, Hafizi M. Anti fungal effect of Echinophora platyloba extract on some common dermathophytes. J Med Plants. 2006;5(18):10–6. [Google Scholar]
- 9.Entezari M, Hashemi M, Ashki M, Ebrahimian S, Bayat M, Saraji ARA, et al. Studying the effect Echinophora platyloba extract on bactira (Staphilococus aureus and Pseudomonas aeroginosa) and fungi (Candidia albicans, Aspergilus flavus and Aspergilus niger) in vitro. World J Med Sci. 2009;4(2):89–92. [Google Scholar]
- 10.Mahboubi M, Avijgan M, Darabi M, Kasaiyan N. Anticandidal activity of Echinophora platyloba against Candida albicans and comparison with amphotericin. J Med Plants. 2009;8(30):36–43. [Google Scholar]
- 11.Delaram M, Sadeghiyan Z. The effect of Echinophora platyloba extract on primary dysmenorrhea. Arak Med Univer J. 2010;13(3):61–7. [Google Scholar]
- 12. Sadraei H, Asghari G, Yaghobei K.Study of the effect of hydro-alcoholic and essential oil of Echinophora platyloba on rat isolated ileum contractions in vitro J Res Med Sci (JRMS). 2003;7(Supplement 2)150–5. [Google Scholar]
- 13.de Angelis Pereira MC, Carvalho JCT, Lima LM, Caputo LRG, Ferreira LR, Fiorini JE, et al. Toxicity of a subchronic treatment with hydroalcoholic crude extract from Solanum grandiflorum (Ruiz et Pav) in rats. J Ethnopharmacol. 2003;89(1):97–9. doi: 10.1016/s0378-8741(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 14.Rasekh HR, Nazari P, Kamli-Nejad M, Hosseinzadeh L. Acute and subchronic oral toxicity of Galega officinalis in rats. J Ethnopharmacol. 2008;116(1):21–6. doi: 10.1016/j.jep.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. Evid-Based Compl Alt Med. 2007;4(11):37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avijgan M, Mahboubi M, Darabi M, Saadat M, Sarikhani S, Kassaiyan N. Overview on Echinophora platyloba, a synergistic antifungal agent candidate. J Yeast Fungal Res. 2010;22(1):88–94. [Google Scholar]
- 17.Lu FC, Kacew S.Lu's Basic Toxicology: Fundamentals, target organs and risk assessment. 5 ed New York: Informa Healthcare; 2009 [Google Scholar]
- 18.Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA. Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharmac. 2002;70(2):135–45. [Google Scholar]