Abstract
OBJECTIVE:
The aim of this study was to evaluate the multisegmental static postural balance of active eutrophic and obese elderly women using a three-dimensional system under different sensory conditions.
METHODS:
A cross-sectional study was conducted on 31 elderly women (16 eutrophic and 15 obese) aged 65 to 75 years. The following anthropometric measurements were obtained: weight, height, waist and hip circumference, and handgrip strength. The physical activity level was evaluated using the International Physical Activity Questionnaire. Body composition was measured using the deuterium oxide dilution technique. The Polhemus® Patriot (three-dimensional) equipment was used to measure the parameters of postural balance along the anteroposterior and laterolateral axes. The data acquisition involved one trial of 60 s to test the limit of stability and four trials of 90 s each under the following conditions: (1) eyes open, stable surface; (2) eyes closed, stable surface; (3) eyes open, unstable surface; and (4) eyes closed, unstable surface.
RESULTS:
For the limit of stability, significant differences were observed in the maximum anteroposterior and laterolateral displacement (p<0.01) and in the parameter maximum anteroposterior displacement in the eyes closed stable surface condition (p<0.01) and maximum anteroposterior and laterolateral displacement in the eyes open unstable surface (p<0.01 and p = 0.03) and eyes closed unstable surface (p<0.01 and p<0.01) conditions.
CONCLUSIONS:
Obese elderly women exhibited a lower stability limit (lower sway area) compared with eutrophic women, leaving them more vulnerable to falls.
Keywords: Elderly, Obesity, Postural Balance, Sensory Deprivation, Three-Dimensional System
INTRODUCTION
Postural balance requires the integrity of the central nervous system (CNS) for the recognition of the position and movement of the head relative to the body and the environment. In addition, to maintain body stability, the CNS depends on the afferent information of the vestibular, visual, proprioceptive, and interoceptive systems, which promote the interaction of the body in space (1,2).
It is known that aging promotes a decrease in the sensory systems responsible for postural balance, identified as multisensory deficit, making it difficult for the elderly to maintain their balance (3). Impaired balance in the elderly has been shown to be an important factor that increases the risk of falls (4,5). Falls are a leading cause of disability, injury, and death among the elderly. Falls occur in over 30% of people over 65 years of age and in over 50% of the population older than 80 years (6,7). After the first fall, approximately 50% of elderly people are victims of another fall during the following year (7).
Few studies have analyzed balance in eutrophic and obese elderly people (8,9). Being overweight or obese is significantly associated with diseases such as dyslipidemia, endothelial dysfunction, stroke, cancer (endometrial, breast, prostate and colon cancer), osteoarthritis, sleep apnea, and respiratory problems that impair quality of life (10,11). These conditions also modify body geometry, increase the mass in different areas of the body (12,13) and, therefore, impose functional limitations in the biomechanics associated with postural balance control and daily living activities (14,15). In this context, obesity has been shown to be a cause of postural stability impairment and to increase the risk of falling, particularly when combined with low muscle mass in adults (16).
Until now, no study has analyzed the postural balance of eutrophic and obese elderly people using a three-dimensional (3D) system. The Polhemus three-dimensional electromagnetic sensor system has been shown to be an important tool in this area of study (17-19). Thus, the aim of this study was to evaluate the multisegmental static postural balance of active eutrophic and obese elderly women using a three-dimensional system under different sensory conditions.
METHODS
Study design, recruitment, and participants
A cross-sectional study was conducted on 31 healthy elderly women aged 65 to 75 years who were divided into two groups (16 eutrophic and 15 obese). The subjects were considered eutrophic if their body mass index (BMI) was less than 24.9 kg/m2 and obese if their BMI was greater than 30 kg/m2. The subjects were recruited at the Acquaintance Center of the Community of Ribeirão Preto at São Paulo, which provides a regular exercise program.
The volunteers were first administered a questionnaire by the examiner, who gathered information regarding demographic data, health status and physical activities. Exclusion criteria were the presence of vestibular, neurological, osteomuscular, cardiovascular, or psychiatric diseases and the presence of visual impairments without the use of corrective lenses. Elderly subjects with diabetes and hypertension that were under control were included in the study.
All volunteers received detailed information regarding the study protocol and signed an informed consent form prior to participation. The study was approved by the local Human Research Ethics Committee (Protocol 244/2008).
Physical activity level
Physical activity level during a typical week was evaluated using the long version of the International Physical Activity Questionnaire (IPAQ) (20). The 2002 consensus between CELAFISCS and the Center for Disease Control (CDC, Atlanta, Georgia, USA) was used for physical activity classification regarding frequency and duration (21).
Anthropometric measurements
The following anthropometric measurements were obtained: weight, height, waist and hip circumferences, and handgrip strength. Body composition was measured using the deuterium oxide dilution technique (22).
Body weight was measured with a Filizola digital scale (maximum capacity of 300 kg) with subjects wearing shorts and a T-shirt, and height was measured using a stadiometer with an inextensible vertical bar graduated in 0.5-cm increments. The BMI was calculated from these measurements and used as inclusion and exclusion criteria to identify eutrophic and obese elderly subjects according to the World Health Organization (WHO) classification. The waist and hip circumferences were measured with a flexible and inextensible tape (Sanny, SP, Brazil), and the waist/hip ratio was calculated to analyze fat distribution. The handgrip strength of the dominant hand was assessed by standardized testing procedures according to the American Society of Hand Therapists (23) using a hand-held dynamometer (Jamar, Bolingbrook IL, USA), which is a method that has been established as a reliable measure of handgrip strength in community-dwelling older adults (24). The average of three trials was recorded in kgf. All measurements described above were performed by the same investigator according to the guidelines of the International Society for the Advancement of Kinanthropometry (25).
Body composition was measured using the deuterium oxide dilution (2H2O) technique. After an overnight (8-hour) fast, each volunteer received 1 ml/kg deuterium oxide (99.9% deuterium oxide, Cambridge Isotope, USA) diluted to 7%, followed by 50 ml of natural water for complete ingestion of the deuterium and cleansing of the mouth. Saliva samples were collected before and three hours after ingestion of the deuterium and were stored at -10°C until analysis. The deuterium enrichment of the saliva samples was determined by isotope-ratio mass spectrometry (Europa Scientific Hydra System, Cheshire, United Kingdom) after equilibration with 100% hydrogen by the platinum-aluminum catalyst method. Body composition was determined as described by Schoeler et al. (22).
Assessment of postural balance
The electromagnetic sensor system Patriot Polhemus was used to evaluate subject balance in three dimensions by recording the relative position between the receptor and the transmitter sensors (x, y, z coordinates and θ, φ, ρ Euler angles). This system, consisting of three perpendicular coils (22.9×28.3×15.2 mm) connected to an amplifier, is based on both the emission and detection of magnetic fields.
The data were transferred to a notebook at a rate of 100 samples per second through a USB interface and a LabView 8.0 environment with specially designed software. The data were processed to allow the visualization of the profile of voluntary oscillation in real-time through the graphic presentation of the three independent coordinates (x, y, z) of the record. These coordinates represent the movement in the anteroposterior, laterolateral, and craniocaudal axes. The surfaces employed for assessment were a wooden platform measuring 1×50×50 cm (stable surface) and a 30-kg/m3 foam platform measuring 5×50×50 cm (unstable surface) (26).
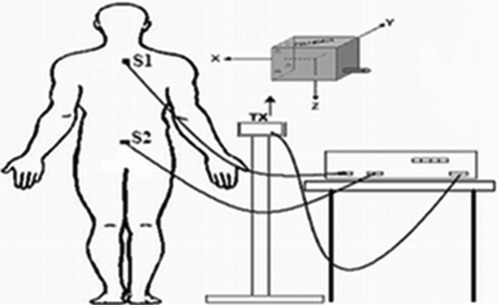
During data acquisition, the volunteers remained standing in the orthostatic position (barefoot and with arms at their sides) first on the wood platform (stable surface) and then on the foam platform (unstable surface) (26), with a distance of approximately 12 cm between their heels on both platforms. The magnetic sensors were placed on the skin and fixed with a bandage over the spinous process of the first thoracic vertebra (S1) and over the sacral region (S2). The magnetic transmitter bobbin was placed on a support approximately 40 cm away from the volunteer's body at approximately the height of the sensors (Figure 1). The system had a normal sensitivity with a range of 1 m (boostable to 3 m) (19).
Figure 1.
The location of the electromagnetic sensors. Tx: transmitter and representation of the three planes. S1 and S2: 1st thoracic vertebra and sacral region.
The limit of stability (LOS) test was conducted with the subjects standing upright on a wood platform with arms at their sides. The instructions were to keep the body rigid and to perform circles with the body in the extreme positions (maximum displacement that a subject is able to achieve forwards and backwards) for a period of 60 s. The dependent LOS variables included the maximum anteroposterior and laterolateral displacements. The LOS test applied to older adults who have fallen has been shown to be consistent and reliable (27). Subsequently, the data acquisition was performed during one trial for 90 s under each of the following four conditions: (1) eyes open, stable surface; (2) eyes closed, stable surface; (3) eyes open, unstable surface (foam), and (4) eyes closed, unstable surface (foam). The test described is known as the modified Clinical Test of Sensory Interaction on Balance (CTSIB-M). During the open-eye tests, the volunteers were asked to look at a wall-mounted object placed at a distance of 1 m at eye level.
Data analyses
The data provided by the sensor system were submitted to mathematical processing performed using specially designed software in a Lab-View 8.0 environment and transformed to values of maximum displacement and speed.
To determine whether sensor 1 and sensor 2 were in agreement, normalized cross-correlations with zero lag were calculated (28) from the MatLab program. The result lies between -1 (signals identical but opposite in phase) and +1 (signals strongly identical). The closer the correlation is to zero, the more the signals are different.
The posturographic data were not normalized according to the height of each volunteer because there was no significant difference in height between groups. The Shapiro-Wilk test was used to analyze whether the variables had a normal distribution. Initially, descriptive statistics were used to analyze the physical characteristics of the sample group. The Student's t-test was used to compare the physical characteristics between the groups and to compare cross-correlations of the S1 and S2 body segments under all sensory conditions. The Mann-Whitney test was used to compare the LOS (maximum a-p and l-l displacement) and postural (maximum displacement and speed on the a-p and l-l axes) parameters under different sensory conditions. The influence of body fat on a-p displacement was calculated in all elderly subjects by the Spearman rank correlation. The S2 data were used because they concern the sacral region, which is closer to the center of mass (5 cm in front of the second sacral vertebra) (29). The data were processed electronically using the Statistical Package for the Social Sciences (SPSS 16.0), and the Origin software (Mi-crocal origin ®, 6.0, USA) was used to draw the graphs. In all tests, the criterion for significance was two-tailed and set at α<0.05.
RESULTS
The physical characteristics of the groups are presented in Table 1. There were no significant differences in age, height, lean mass, and handgrip between the two groups. All subjects were active according to the IPAQ.
Table 1.
The physical characteristics of the subjects. The values are reported as the mean ± SD.
| Characteristics | Eutrophic elderly(n = 16) | Obese elderly(n = 15) | p-value |
| Age (years) | 68.3±2.7 | 69.1±2.7 | 0.41 |
| Body height (cm) | 158±0.05 | 153±0.04 | 0.19 |
| Body weight (kg) | 59.1±7.1 | 79.1±8.8 | <0.001 |
| BMI (kg/m2) | 23.4±1.6 | 33.5±3.0 | <0.001 |
| % fat | 35.1±5 | 48.2±4 | <0.001 |
| Lean mass (kg) | 38.1±3.3 | 39.9±3.0 | 0.12 |
| Body fat (kg) | 21.0±5.2 | 37.3±4.9 | <0.001 |
| Waist circumference (cm) | 78.6±5.3 | 97.2±9.4 | <0.001 |
| Hip circumference (cm) | 96.7±6.5 | 111.4±8.3 | <0.001 |
| Hip/waist circ. ratio (cm) | 0.81±0 | 0.87±0.1 | 0.03 |
| Handgrip (kgf) | 25.1±4.6 | 24.8±5.2 | 0.84 |
Abbreviations: BMI, body mass index.
Table 2 shows the LOS values and the stabilometric variables of static balance under different sensory conditions. There was a significant difference in the maximum a-p and l-l displacements (p<0.01) of the LOS. In addition, there were significant differences in the maximum a-p displacement in the EOSS and ECSS conditions and in the maximum a-p and l-l displacements in the EOUS and ECUS conditions (p<0.05).
Table 2.
The parameters of postural oscillation recorded by sensor 2 under different conditions.
| Eutrophic Elderly | Obese Elderly | p-value | |
| LOS | mean±SD | mean±SD | |
| Max. displacement (a-p) cm | 32.02±11.92 | 17.35±6.47 | <0.01 |
| Max. displacement (l-l) cm | 23.59±8.01 | 14.49±5.5 | <0.01 |
| EOSS | |||
| Max. displacement (a-p) cm | 2.59±0.1 | 1.95±0.69 | 0.04 |
| Max. displacement (l-l) cm | 1.34±0.64 | 0.98±0.54 | 0.28 |
| Mean speed (a-p) cm/s | 0.96±0.21 | 1.23±0.58 | 0.32 |
| Mean speed (l-l) cm/s | 0.54±0.19 | 0.52±0.25 | 0.32 |
| ECSS | |||
| Max. displacement (a-p) cm | 2.84±1.27 | 1.83±0.50 | <0.01 |
| Max. displacement (l-l) cm | 1.42±1.08 | 1.04±0.59 | 0.38 |
| Mean speed (a-p) cm/s | 1.10±0.35 | 1.24±0.57 | 0.78 |
| Mean speed (l-l) cm/s | 0.57±0.16 | 0.55±0.33 | 0.12 |
| EOUS | |||
| Max. displacement (a-p) cm | 4.24±1.59 | 2.36±0.63 | <0.01 |
| Max. displacement (l-l) cm | 2.45±0.96 | 1.60±0.94 | 0.03 |
| Mean speed (a-p) cm/s | 1.17±0.24 | 1.43±0.63 | 0.51 |
| Mean speed (l-l) cm/s | 0.71±0.16 | 0.66±0.37 | 0.08 |
| ECUS | |||
| Max. displacement (a-p) cm | 4.88±1.90 | 2.59±0.68 | <0.01 |
| Max. displacement (l-l) cm | 3.05±1.34 | 1.69±0.71 | <0.01 |
| Mean speed (a-p) cm/s | 1.26±0.26 | 1.42±0.51 | 0.90 |
| Mean speed (l-l) cm/s | 0.74±0.25 | 0.66±0.27 | 0.14 |
EOSS, eyes open, stable surface; ECSS, eyes closed, stable surface; EOUS, eyes open, unstable surface; ECUS, eyes closed, unstable surface; a-p, anteroposterior axis; l-l, laterolateral axis; Max., maximum.
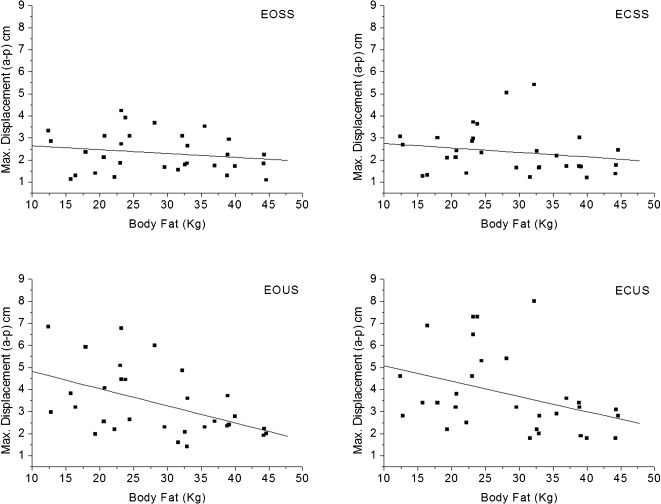
Figure 2) shows the correlation between the maximum displacement (a-p) of the postural oscillation acquired by sensor 2 (sacral region) and body fat under all sensory conditions: EOSS (r = -0.11; p = 0.52); ECSS (r = -0.21; p = 0.26); EOUS (r = -0.46; p<0.01); and ECUS (r = -0. 41; p<0.05). There was a small, negative correlation between the maximum displacement and body fat in the eyes open and closed conditions on the unstable surface.
Figure 2.
The correlation between maximum a-p displacement and body fat under the different sensory conditions.
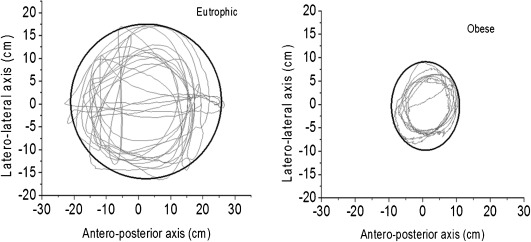
Figure 3) represents the LOS in eutrophic and obese elderly women with similar ages and heights. It shows a lower stability limit (smaller sway area) of the obese elderly compared with eutrophic elderly women.
Figure 3.
The representative limit of stability of a eutrophic and an obese elderly woman (registered by sensor 2).
There was a strong cross-correlation between the sensors (r>0.99; p<0.001) in both groups under the different sensory conditions (Table 3. There were no significant differences in cross-correlations between eutrophic and obese elderly subjects.
Table 3.
The cross-correlations of S1 and S2 under the different sensory conditions and a comparison of the cross-correlations between groups (Mann-Whitney U test).
| Eutrophic Elderlymean±SD | Obese Elderlymean±SD | p-value | |
| EOSS | 0.99±0.06 | 0.99±0.01 | 0.32 |
| ECSS | 0.99±0.06 | 0.99±0.20 | 0.55 |
| EOUS | 0.99±0.01 | 0.99±0.00 | 0.50 |
| ECUS | 0.99±0.01 | 0.99±0.00 | 0.30 |
EOSS, eyes open, stable surface; ECSS, eyes closed, stable surface; EOUS, eyes open, unstable surface; ECUS, eyes closed, unstable surface; SD, standard deviation.
DISCUSSION
To our knowledge, this was the first study to analyze the multisegmental static postural balance of active eutrophic and obese elderly people using a three-dimensional system under different sensory conditions.
As expected, significant differences were observed between groups regarding most of the physical variables evaluated, with obese women exhibiting higher anthropometric values than eutrophic women. Similar values of handgrip strength and lean muscle mass were found in the two groups, a fact possibly explained by the similar physical activity level of the groups, which were considered active according to the IPAQ classification. Apovian et al. (30) did not observe differences in BMI or muscle strength between eutrophic and obese elderly people, in agreement with the results of the present study.
Postural balance is traditionally evaluated by the Clinical Test of Sensory Interaction on Balance (CTSIB), which consists of the combination of visual sensory conditions (eyes open, eyes closed, and visual conflict) and the supporting surface (normal and imprecise orientations). The six resulting sensory conditions help identify both the sensory information on which the individual primarily relies for his/her spatial orientation and the situations of sensory conflict that provoke instability (31). In the present study, we used the modified Clinical Test of Sensory Interaction on Balance (CTSIB-M), which, according to Rosa et al. (32), provides global evidence of sensory function related to body balance but does not provide specific information related to each system separately (visual, somatosensory and vestibular systems).
When the parameters of postural sway were compared between groups, significant differences were observed only in the maximal a-p displacement (stable surface) with eyes open and closed and in the a-p and l-l displacements (unstable surface) with eyes open and closed, with obese women exhibiting lower displacements than eutrophic women. These lower maximal a-p and l-l displacements of sway in obese women may be a preventive mechanism to control static postural balance and reduce the risk of falling. Because obese women had a lower limit of stability, they may be more susceptible to falls due to the smaller sway area and because they needed more muscle strength to reestablish their balance. When body fat evaluated by the deuterium oxide technique was correlated with maximum a-p displacement, no correlation was observed on the stable surface for individuals with eyes open and closed, whereas on the unstable surface, there was a small negative correlation for individuals with eyes open and closed. These results demonstrate that in more challenging situations, the greater the body mass, the lower the maximum displacement, a fact that may intensify the risk of falling if an external perturbation occurs.
Recent studies have investigated the contribution of body mass and/or BMI to the postural balance of adults with and without vision, evaluated with a strength platform, and showed that the greater the body mass and/or BMI, the greater the postural instability (15,33,34).
According to Hue et al. (15), there are at least two reasons why balance is strongly correlated with body mass. The first reason is related to the mechanoreceptors in the plantar region, which are responsible for the cutaneous sensation used to control balance. Obese people have a greater area and pressure of plantar contact, which may reduce the quality and/or quantity of information received by the mechanoreceptors (35,36) when the eyes are open and closed. The second explanation is that in the erect posture, the human body is frequently compared to an inverted pendulum, with movements rotating around the ankle joint. In obese people, due to the greater mass concentration in the abdominal region, the mass center is dislocated forward, thus requiring greater control of the hip and increased torque in the ankle joint to reestablish balance (15,37). This fact has been cited as one of the main reasons for the high risk of falling in obese elderly people during daily physical activity (15,37,38).
In the present study, body fat was not a determining factor for the increased velocity of postural sway in the presence or absence of vision. However, there was a reduction in the maximum a-p and l-l displacements both in the stability limit test (Figure 2) and under different sensory situations, possibly reflecting a greater postural rigidity and a potentially increased risk of falls. Although these data are in contrast to results reported in other studies (which showed an increase in sway), most of the previous studies included only adults under the eyes open and eyes closed conditions and used the strength platform as a tool to assess postural balance (15,16,37,38).
To determine whether eutrophic and obese elderly women use the ankle or the hip to control their balance in multisegmental posturography, we used cross-correlation analysis between sensors 1 and 2. The values obtained show a strong cross-correlation between the sensors (r>0.99) in both groups under the different sensory conditions. No significant differences were observed between eutrophic and obese elderly women who exhibited an inverted pendulum sway (ankle strategy).
In the present study, the use of a three-dimensional electromagnetic system reduced the possibility of a comparison with studies using the strength platform. However, it has been previously shown that the system used here is a tool with which to assess postural sway in an effective and efficient manner under different conditions (17-19). Furthermore, this system is being increasingly applied in the area of posturography because it can be used to measure the position and spatial orientation of an object in three dimensions in real-time (17-19). A limitation of the use of this system is the limitations in the site used for data acquisition because it is necessary to avoid areas with a considerable amount of metal or that may induce a magnetic field capable of directly interfering with data collection. However, one of its greatest advantages is that it can be transported to different locations (17-18).
In addition to sample size, another possible limiting factor of the present study is that all of the elderly women investigated, whether eutrophic or obese, were physically active. Elderly subjects who practice regular physical activity can obtain various health benefits, such as better muscle strength, postural balance and ability to walk, and fall prevention (39,40).
In the present study, obesity was found to cause lower maximum a-p and l-l displacements and a lower stability limit (smaller sway area), with the obese women being more rigid and having a higher risk of falls than eutrophic women. Multisegmental posturographic analysis revealed a strong correlation between sensors and demonstrated an inverted pendulum sway (ankle strategy) in both groups. Further investigation is needed to determine the influence of obesity on multisegmental posturography of active elderly compared to sedentary elderly people.
ACKNOWLEDGMENTS
Support for this work was provided in part by FAEPA and CAPES, which we gratefully acknowledge.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Duarte M, Freitas SMSF. Revision of posturography based on force plate for balance evaluation. Brazilian Journal of physical therapy. 2010;14(3):183–92. [PubMed] [Google Scholar]
- 2.Mittelstaedt H. Origin and processing of postural information. Neurosci Biobehav Rev. 1998;22(4):473–8. doi: 10.1016/s0149-7634(97)00032-8. [DOI] [PubMed] [Google Scholar]
- 3.Shumaway – Cook A, Woollacott MH. Motor Control: Theory and Practical Applications 2a edição. 2003.
- 4.Overstall PW, Exton-Smith AN, Imms FJ, Jonhson AL. Falls in the elderly related to postural imbalance. Br Med J. 1977;29(6056):261–4. doi: 10.1136/bmj.1.6056.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stalenhoef PA, Diederiks JP, Knottnerus JA, Kester AD, Crebolder HF. A risk model for the prediction of recurrent falls in community-dwelling elderly: a prospective cohort study. J Clin Epidemiol. 2002;55(11):1088–94. doi: 10.1016/s0895-4356(02)00502-4. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Speechley M, Ginter SF. RisK factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–7. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 7.Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, Fentem PH, Bassey EJ. Falls by elderly people at home prevalence and associated factors. Age Ageing. 1988;17(6):365–72. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- 8.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obesity Res. 2004;12(6):913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 9.Lang IA, Llewellyn DJ, Alexander K, Melzer D. Obesity, Physical Function, and Mortality in Older Adults. J Am Geriatric Soc. 2008;56(8):1474–8. doi: 10.1111/j.1532-5415.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 10.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 11.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282(26):1519–22. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 12.Rodacki AL, Fowler NE, Provensi CL, Rodacki Cde L, Dezan VH. Body mass as a factor in stature change. Clin Biomech. 2005;20(8):799–805. doi: 10.1016/j.clinbiomech.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Souza SAF, Faintuch J, Valezi AC, SantAnna AF, Gama-Rodrigues JJ, Fonseca ICB, et al. Postural changes in morbidly obese patients. Obes Surg. 2005;15(7):1013–6. doi: 10.1381/0960892054621224. [DOI] [PubMed] [Google Scholar]
- 14.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. The biomechanics of restricted movement in adult obesity. Obest Rev. 2006;7(1):13–24. doi: 10.1111/j.1467-789X.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 15.Hue O, Simoneau M, Marcotte J, Berrigan F, Doré J, Marceau P, Marceau S, Tremblay A, Teasdale N. Body weight is strong predictor of postural stability. Gait Posture. 2007;26(1):32–8. doi: 10.1016/j.gaitpost.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Teasdale N, Hue O, Marcotte J, Berrigan F, Simoneau M, Doré J, et al. Reducing weight increases postural stability in obese and morbid obese men. Int J Obes. 2007;31(1):153–60. doi: 10.1038/sj.ijo.0803360. [DOI] [PubMed] [Google Scholar]
- 17.Melo PS, Ferreira TP, Santos-Pontelli TEG, Carneiro JAO, Carneiro AAO, Colafêmina JF. Comparing static sitting postural sway of healthy young and older adults. Brazilian Journal of physical therapy. 2009;13(6):549–54. [Google Scholar]
- 18.Carneiro JAO, Santos-Pontelli TEG, Colafêmina JF, Carneiro AAO, Ferriolli E. Analysis of static postural balance using a tridimensional electromagnetic system. Braz. J. Otorhinolaryngol. 2010;76(6):783–8. doi: 10.1590/S1808-86942010000600018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Accornero N, Capozza M, Rinalduzzi S, Manfredi GW. Clinical multisegmental posturography: Age-related changes in stance control. Electroencephalography and Clinical Neurographysiology. 1997;105(3):213–9. doi: 10.1016/s0924-980x(97)96567-x. [DOI] [PubMed] [Google Scholar]
- 20.Matsudo S, Araújo T, Matsudo V, Andrade D, Andrade E, Oliveira LC, Braggion G. International Physical Activity Questionnaire (IPAQ): Study of validity and reliability in Brazil. Revista Brasileira de Atividade Física e Saúde. 2001;6:5–18. [Google Scholar]
- 21.Matsudo S, Matsudo V, Araújo T, Andrade D, Andrade E, Oliveira LC, Braggion G. Physical activity level of São Paulo State population: an analysis based on gender, age, socio-economic status, demographics and knowledge. Rev. Bras. Ciên. e Mov. 2002;10(4):41–50. [Google Scholar]
- 22.Schoeller DA Mass spectrometry calculations. J Clin Pharmacol. 1986;26(6):396–9. doi: 10.1002/j.1552-4604.1986.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 23. Fess EE.Grip strength Casanova JS, editor Clinical Assessment Recommendations. 2nd ed. Chicago: American Society of Hand Therapists; 1992 [Google Scholar]
- 24.Bohannon R, Schaubert Test-retest reliability of grip strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18(4):426–7, quiz 428. doi: 10.1197/j.jht.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 25. Hawes MR, Martin AD.Human Body Composition En:Eston R, Reilly T, Kinanthropometry and exercise physiology laboratory manual. London: Routledge; 20017–46. [Google Scholar]
- 26.Guerraz M, Shallo-Hoffmann J, Yarrow K, Thilo KV, Bronstein AM, Gresty MA. Visual Control of Postural orientation and equilibrium in congenital nystagmus. Invest Ophthalmol Vis Sci. 2000;41(12):3798–804. [PubMed] [Google Scholar]
- 27.Newton R. Validity of the multi-directional reach test: a practical measure for limits of stability in older adults. J Gerontol A Biol Sci Med Sci. 2001;56(4):M248–52. doi: 10.1093/gerona/56.4.m248. [DOI] [PubMed] [Google Scholar]
- 28.Termoz N, Halliday SE, Winter DA, Frank JS, Patla AE, Prince F. The control of upright stance in young, elderly and persons with Parkinson's disease. Gait Posture. 2008;27(3):463–70. doi: 10.1016/j.gaitpost.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 29. Smith LK, Weiss EL, Lehmkuhl LD.Cinesiologia clínica de Brunnstrom. 5a ed.São Paulo: Manole; 1997 [Google Scholar]
- 30.Apovian CM, Carolin MF, Wood GC, Rogers JZ, Still CD, Jensen GL. Body mass index and physical function in older women. Obs Res. 2002;10(8):740–7. doi: 10.1038/oby.2002.101. [DOI] [PubMed] [Google Scholar]
- 31.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance. Phys Ther. 1986;66(10):1548–50. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 32.Rosa JLS, Perracini MR, Ganança FF. Stabilometry in patients with ménière.s disease. Acta ORL. 2006;24:232–8. [Google Scholar]
- 33.Greve J, Bordini ACPG, Camanho GL. Correlation between body mass index and postural balance. Clinics. 2007;62(6):717–20. doi: 10.1590/s1807-59322007000600010. [DOI] [PubMed] [Google Scholar]
- 34.Menegoni F, Galli M, Tacchini E, Vismara L, Cavigioli M, Capodaglio P. Gender-specific Effect of Obesity on Balance. Obesity. 2009;17(10):1951–6. doi: 10.1038/oby.2009.82. [DOI] [PubMed] [Google Scholar]
- 35.Maki BE, Perry SD, Norrie RG, Mcilroy WE. Effect of facilitation of sensation from plantar foot-surface boundaries on postural stabilization in young and older adults. Journal of Gerontology: Biological Sciences. 1999;54(10):M281–7. doi: 10.1093/gerona/54.6.m281. [DOI] [PubMed] [Google Scholar]
- 36.Meyer PF, Oddsson LI, DE Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Experimental Brain Research. 2004;156(4):505–12. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- 37.Corbeil P, Simoneau M, Rancourt D, Tremblay A, Teasdale N. Increased risk for falling associated with obesity: mathematical modeling postural control. IEEE Trans Neural Syst Rehabil Eng. 2001;9(2):126–36. doi: 10.1109/7333.928572. [DOI] [PubMed] [Google Scholar]
- 38.Gazzola JM, Perracini MR, Ganança MM, Ganança FF. Functional balance associated factors in the elderly with chronic vestibular disorder. Braz. J. Otorhinolaryngol. 2006;72(5):683–90. doi: 10.1016/S1808-8694(15)31026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson MC, Devlin N, Gardner MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomized controlled trial. . BMJ. 2001;322(7288):1–6. doi: 10.1136/bmj.322.7288.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–14. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]