Abstract
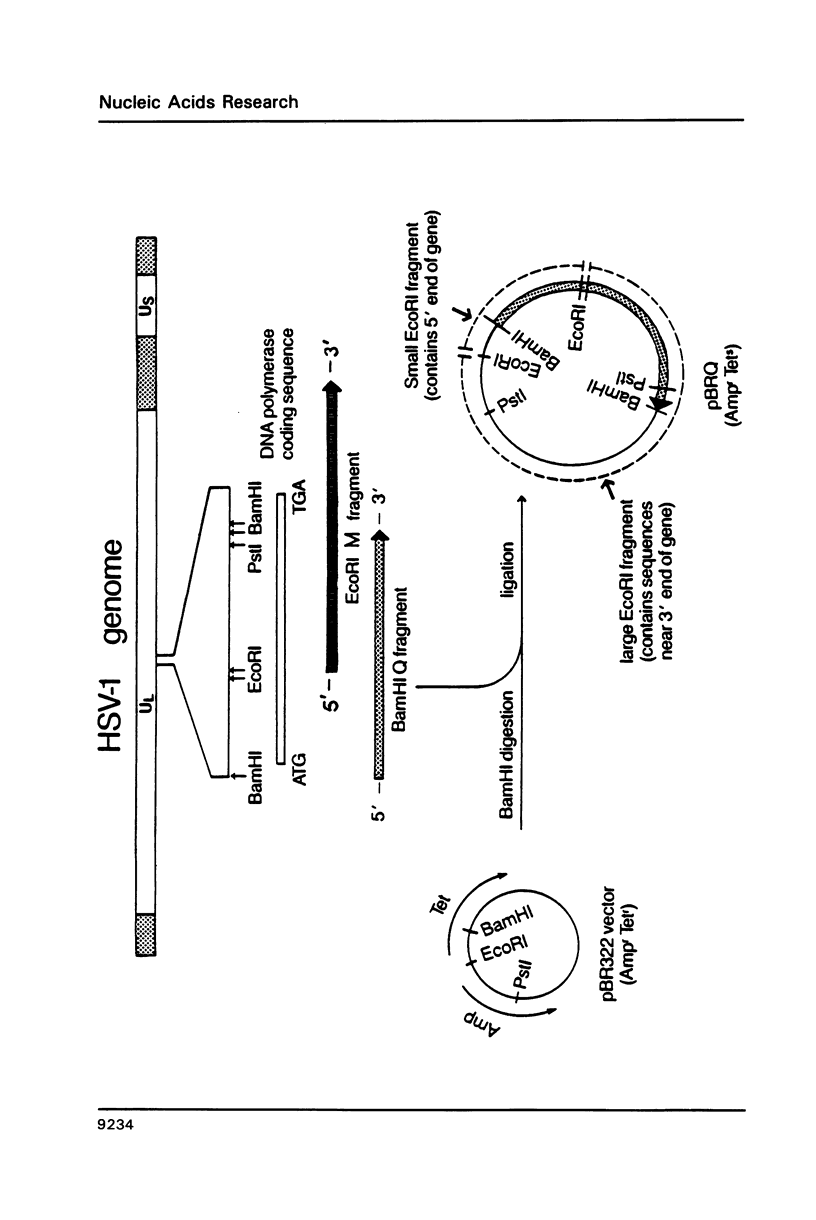
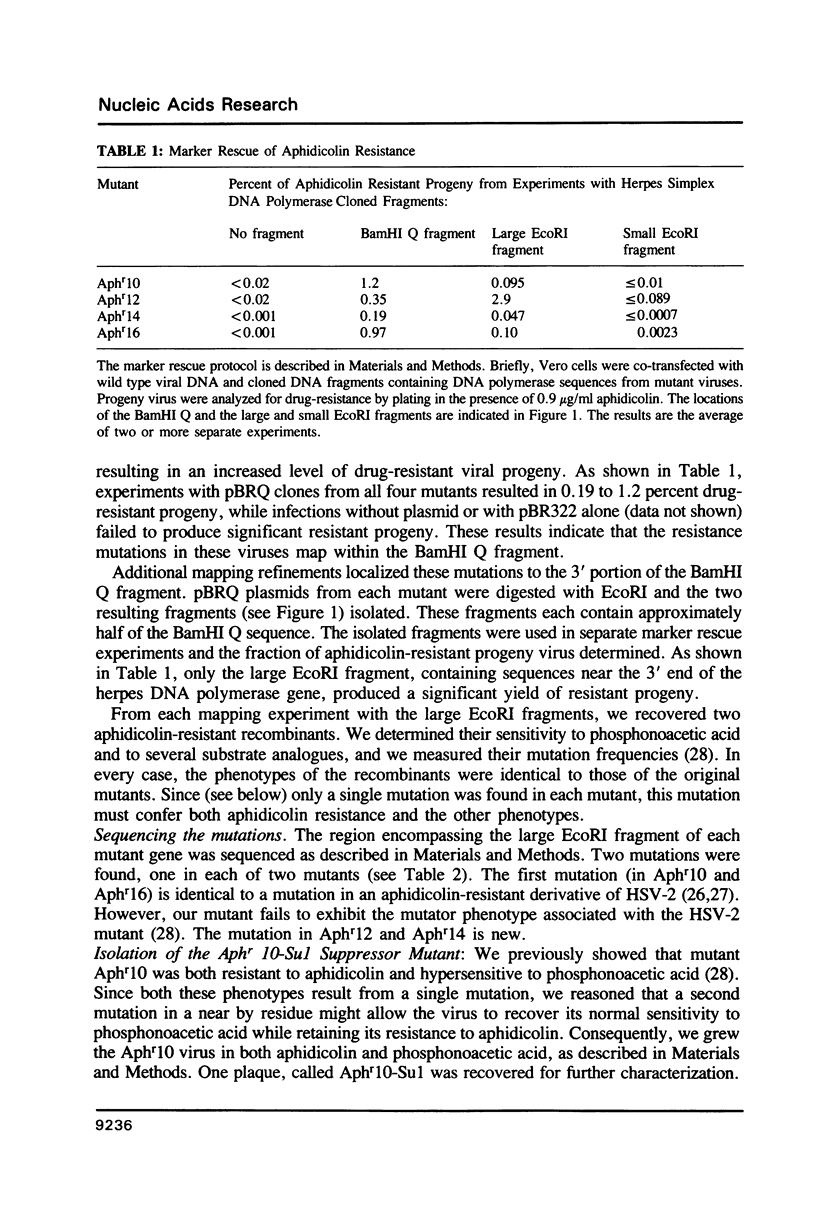
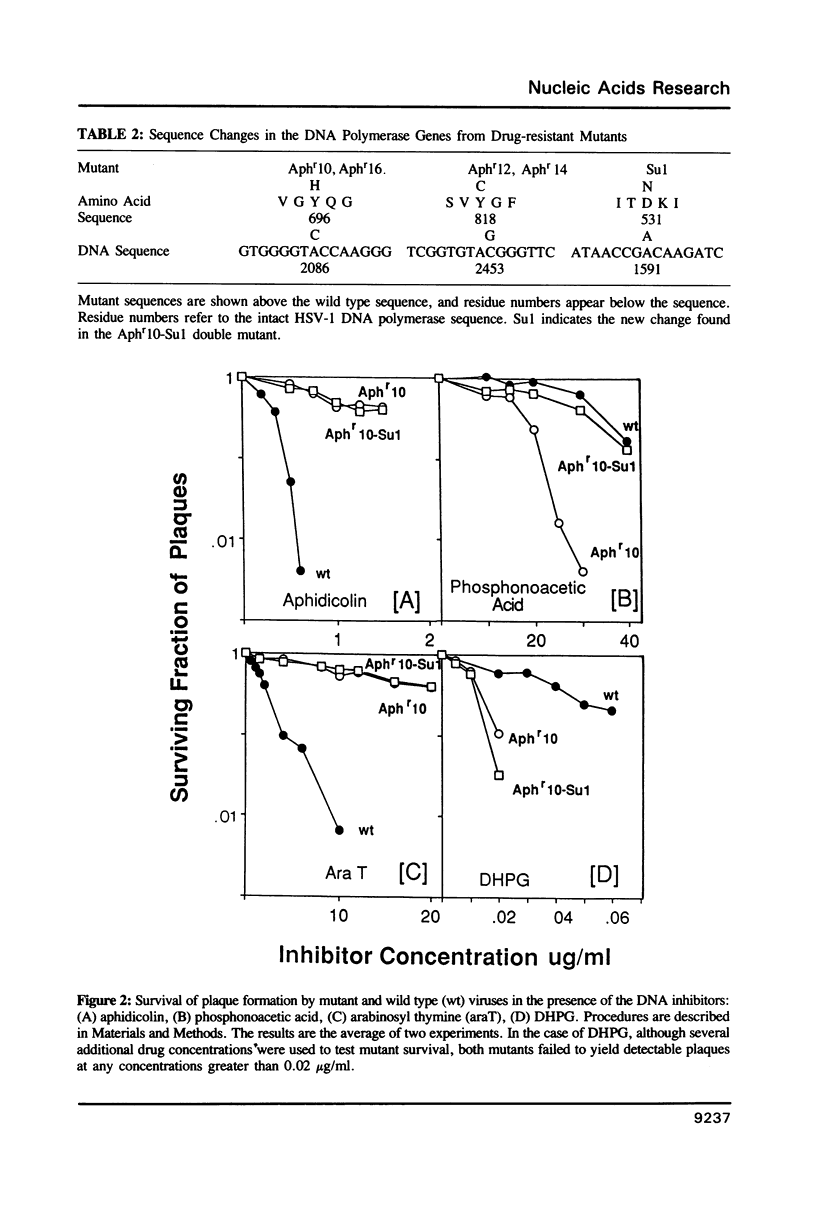
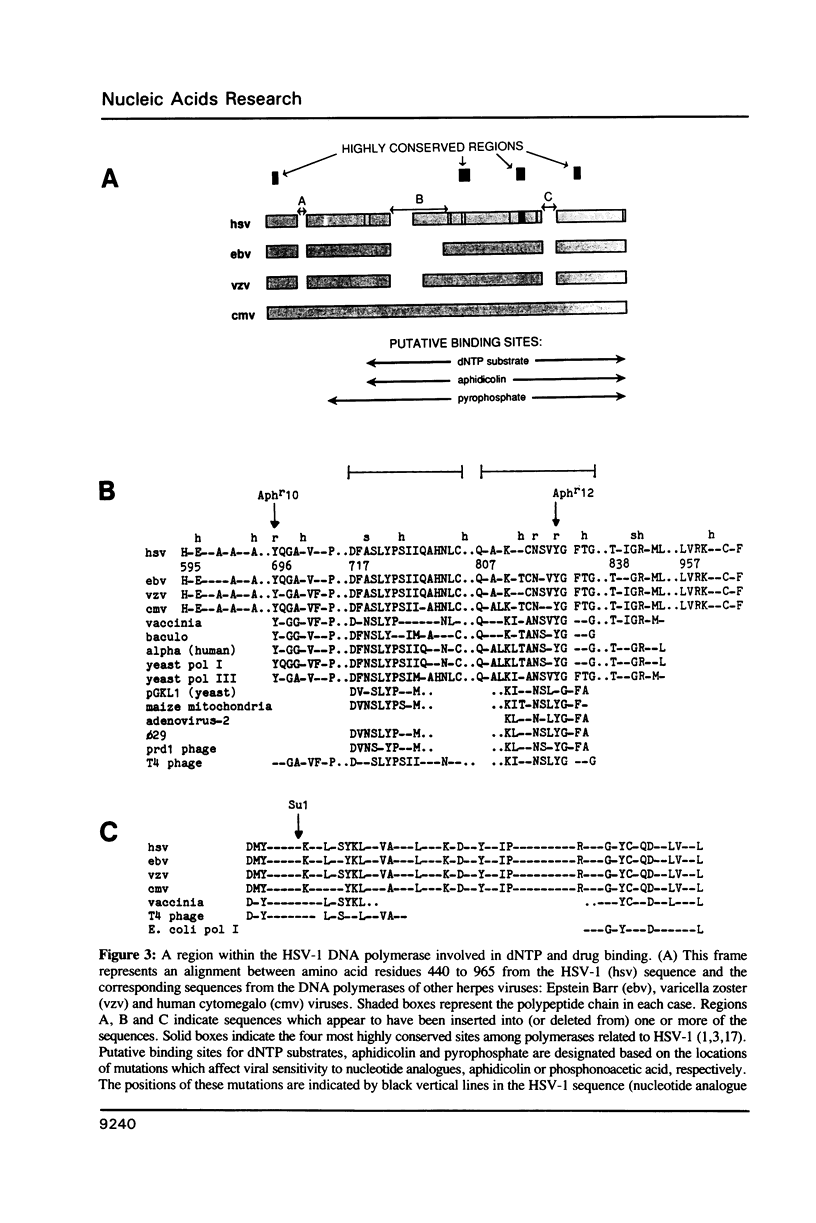
We describe the mapping and sequencing of mutations within the DNA polymerase gene of herpes simplex virus type 1 which confer resistance to aphidicolin, a DNA polymerase inhibitor. The mutations occur near two regions which are highly conserved among DNA polymerases related to the herpes simplex enzyme. They also occur near other herpes simplex mutations which affect the interactions between the polymerase and deoxyribonucleoside triphosphate substrates. Consequently, we argue in favor of the idea that the aphidicolin binding site overlaps the substrate binding site and that the near-by conserved regions are functionally required for substrate binding. Our mutants also exhibit abnormal sensitivity to another DNA polymerase inhibitor, phosphonoacetic acid. This drug is thought to bind as an analogue of pyrophosphate. A second-site mutation which suppresses the hypersensitivity of one mutant to phosphonoacetic acid (but not its aphidicolin resistance) is described. This second mutation may represent a new class of mutations, which specifically affects pyrophosphate, but not substrate, binding.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bernad A., Zaballos A., Salas M., Blanco L. Structural and functional relationships between prokaryotic and eukaryotic DNA polymerases. EMBO J. 1987 Dec 20;6(13):4219–4225. doi: 10.1002/j.1460-2075.1987.tb02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Effect of aphidicolin and nucleotide analogs on the phage phi 29 DNA polymerase. Virology. 1986 Sep;153(2):179–187. doi: 10.1016/0042-6822(86)90021-8. [DOI] [PubMed] [Google Scholar]
- Boulet A., Simon M., Faye G., Bauer G. A., Burgers P. M. Structure and function of the Saccharomyces cerevisiae CDC2 gene encoding the large subunit of DNA polymerase III. EMBO J. 1989 Jun;8(6):1849–1854. doi: 10.1002/j.1460-2075.1989.tb03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigden D., Fiddian P., Rosling A. E., Ravenscroft T. Acyclovir - a review of the preclinical and early clinical data of a new antiherpes drug. Antiviral Res. 1981 Nov;1(4):203–212. doi: 10.1016/0166-3542(81)90011-5. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Beta-turns in proteins. J Mol Biol. 1977 Sep 15;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Derbyshire V., Freemont P. S., Sanderson M. R., Beese L., Friedman J. M., Joyce C. M., Steitz T. A. Genetic and crystallographic studies of the 3',5'-exonucleolytic site of DNA polymerase I. Science. 1988 Apr 8;240(4849):199–201. doi: 10.1126/science.2832946. [DOI] [PubMed] [Google Scholar]
- Derse D., Bastow K. F., Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982 Sep 10;257(17):10251–10260. [PubMed] [Google Scholar]
- Derse D., Cheng Y. C., Furman P. A., St Clair M. H., Elion G. B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981 Nov 25;256(22):11447–11451. [PubMed] [Google Scholar]
- Derse D., Cheng Y. C. Herpes simplex virus type I DNA polymerase. Kinetic properties of the associated 3'-5' exonuclease activity and its role in araAMP incorporation. J Biol Chem. 1981 Aug 25;256(16):8525–8530. [PubMed] [Google Scholar]
- Dicioccio R. A., Chadha K., Sahai Srivastava B. I. Inhibition of herpes simplex virus-induced DNA polymerase, cellular DNA polymerase alpha, and virus production by aphidicolin. Biochim Biophys Acta. 1980 Sep 19;609(2):224–231. doi: 10.1016/0005-2787(80)90233-6. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Jones E. V., Moss B. Homology between DNA polymerases of poxviruses, herpesviruses, and adenoviruses: nucleotide sequence of the vaccinia virus DNA polymerase gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3659–3663. doi: 10.1073/pnas.83.11.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Derse D. D., Bastow K. F., Cheng Y. C. Novel interaction of aphidicolin with herpes simplex virus DNA polymerase and polymerase-associated exonuclease. J Biol Chem. 1984 Nov 10;259(21):13282–13286. [PubMed] [Google Scholar]
- Freemont P. S., Friedman J. M., Beese L. S., Sanderson M. R., Steitz T. A. Cocrystal structure of an editing complex of Klenow fragment with DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8924–8928. doi: 10.1073/pnas.85.23.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Bastow K. F., Cheng Y. C., Coen D. M. Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6672–6676. doi: 10.1073/pnas.85.18.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Hall J. D., Mount D. W., Retondo M. J., Weller S. K., Coen D. M. Sequence and mapping analyses of the herpes simplex virus DNA polymerase gene predict a C-terminal substrate binding domain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7969–7973. doi: 10.1073/pnas.82.23.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Habara A., Kano K., Nagano H., Mano Y., Ikegami S., Yamashita T. Inhibition of DNA synthesis in the adenovirus DNA replication complex by aphidicolin and 2',3'-dideoxythymidine triphosphate. Biochem Biophys Res Commun. 1980 Jan 15;92(1):8–12. doi: 10.1016/0006-291x(80)91511-9. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Coen D. M., Fisher B. L., Weisslitz M., Randall S., Almy R. E., Gelep P. T., Schaffer P. A. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology. 1984 Jan 15;132(1):26–37. doi: 10.1016/0042-6822(84)90088-6. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Furman P. A., St Clair M. H., Knopf C. W. Reduced in vivo mutagenesis by mutant herpes simplex DNA polymerase involves improved nucleotide selection. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3889–3893. doi: 10.1073/pnas.82.11.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. D., Gibbs J. S., Coen D. M., Mount D. W. Structural organization and unusual codon usage in the DNA polymerase gene from herpes simplex virus type 1. DNA. 1986 Aug;5(4):281–288. doi: 10.1089/dna.1986.5.281. [DOI] [PubMed] [Google Scholar]
- Hall J. D. Modeling functional sites in DNA polymerases. Trends Genet. 1988 Feb;4(2):42–46. doi: 10.1016/0168-9525(88)90065-0. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Myers E. W. A software tool for finding locally optimal alignments in protein and nucleic acid sequences. Comput Appl Biosci. 1988 Mar;4(1):35–40. doi: 10.1093/bioinformatics/4.1.35. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Woodward S. Aphidicolin resistance in herpes simplex virus type 1 appears to alter substrate specificity in the DNA polymerase. J Virol. 1989 Jun;63(6):2874–2876. doi: 10.1128/jvi.63.6.2874-2876.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Joyce C. M., Kelley W. S., Grindley N. D. Nucleotide sequence of the Escherichia coli polA gene and primary structure of DNA polymerase I. J Biol Chem. 1982 Feb 25;257(4):1958–1964. [PubMed] [Google Scholar]
- Jung G. H., Leavitt M. C., Hsieh J. C., Ito J. Bacteriophage PRD1 DNA polymerase: evolution of DNA polymerases. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8287–8291. doi: 10.1073/pnas.84.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G. Susceptibility to other antiherpes drugs of pathogenic variants of herpes simplex virus selected for resistance to acyclovir. Antimicrob Agents Chemother. 1986 May;29(5):894–898. doi: 10.1128/aac.29.5.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Liu P. K., Chang C. C., Trosko J. E., Dube D. K., Martin G. M., Loeb L. A. Mammalian mutator mutant with an aphidicolin-resistant DNA polymerase alpha. Proc Natl Acad Sci U S A. 1983 Feb;80(3):797–801. doi: 10.1073/pnas.80.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Korn D., Wang T. S. The evolutionary conservation of DNA polymerase alpha. Nucleic Acids Res. 1988 Aug 25;16(16):7961–7973. doi: 10.1093/nar/16.16.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y., Suzuki S., Yamauchi M., Maeno K., Yoshida S. Characterization of an aphidicolin-resistant mutant of herpes simplex virus type 2 which induces an altered viral DNA polymerase. Virology. 1984 May;135(1):87–96. doi: 10.1016/0042-6822(84)90119-3. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Yoshida S., Tsurumi T., Yamamoto N., Maeno K. Drug-resistant mutants of herpes simplex virus type 2 with a mutator or antimutator phenotype. Microbiol Immunol. 1985;29(4):377–381. doi: 10.1111/j.1348-0421.1985.tb00837.x. [DOI] [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Aranucleosides and aranucleotides in viral chemotherapy. Pharmacol Ther. 1979;4(1):81–108. doi: 10.1016/0163-7258(79)90016-0. [DOI] [PubMed] [Google Scholar]
- Orberg P. K., Schaffer P. A. Expression of herpes simplex virus type 1 major DNA-binding protein, ICP8, in transformed cell lines: complementation of deletion mutants and inhibition of wild-type virus. J Virol. 1987 Apr;61(4):1136–1146. doi: 10.1128/jvi.61.4.1136-1146.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Paillard M., Sederoff R. R., Levings C. S. Nucleotide sequence of the S-1 mitochondrial DNA from the S cytoplasm of maize. EMBO J. 1985 May;4(5):1125–1128. doi: 10.1002/j.1460-2075.1985.tb03749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Dixon R. A., Schaffer P. A. Physical mapping of herpes simplex virus type 1 ts mutants by marker rescue: correlation of the physical and genetic maps. Virology. 1980 Jan 30;100(2):275–287. doi: 10.1016/0042-6822(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli A., Valsasnini P., Plevani P., Lucchini G. DNA polymerase I gene of Saccharomyces cerevisiae: nucleotide sequence, mapping of a temperature-sensitive mutation, and protein homology with other DNA polymerases. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3772–3776. doi: 10.1073/pnas.85.11.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., McGeoch D. J. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 1985 Nov 25;13(22):8143–8163. doi: 10.1093/nar/13.22.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savilahti H., Bamford D. H. The complete nucleotide sequence of the left very early region of Escherichia coli bacteriophage PRD1 coding for the terminal protein and the DNA polymerase. Gene. 1987;57(1):121–130. doi: 10.1016/0378-1119(87)90183-1. [DOI] [PubMed] [Google Scholar]
- Spicer E. K., Rush J., Fung C., Reha-Krantz L. J., Karam J. D., Konigsberg W. H. Primary structure of T4 DNA polymerase. Evolutionary relatedness to eucaryotic and other procaryotic DNA polymerases. J Biol Chem. 1988 Jun 5;263(16):7478–7486. [PubMed] [Google Scholar]
- Stark M. J., Mileham A. J., Romanos M. A., Boyd A. Nucleotide sequence and transcription analysis of a linear DNA plasmid associated with the killer character of the yeast Kluyveromyces lactis. Nucleic Acids Res. 1984 Aug 10;12(15):6011–6030. doi: 10.1093/nar/12.15.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski M. D., Wu J. G., Miller L. K. The location, sequence, transcription, and regulation of a baculovirus DNA polymerase gene. Virology. 1988 Dec;167(2):591–600. [PubMed] [Google Scholar]
- Toneguzzo F., Glynn S., Levi E., Mjolsness S., Hayday A. Use of a chemically modified T7 DNA polymerase for manual and automated sequencing of supercoiled DNA. Biotechniques. 1988 May;6(5):460–469. [PubMed] [Google Scholar]
- Tsurumi T., Maeno K., Nishiyama Y. A single-base change within the DNA polymerase locus of herpes simplex virus type 2 can confer resistance to aphidicolin. J Virol. 1987 Feb;61(2):388–394. doi: 10.1128/jvi.61.2.388-394.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Ito J. Nucleotide sequence of the major early region of bacteriophage phi 29. Gene. 1982 Mar;17(3):323–335. doi: 10.1016/0378-1119(82)90149-4. [DOI] [PubMed] [Google Scholar]



