Abstract
Introduction
Perfusion (Q) single photon emission computerized tomography (SPECT) has been used to divert dose away from higher-functioning lung during radiation therapy (RT) planning. This study aimed to 1) study regional lung function through co-registered pulmonary ventilation/perfusion (V/Q) SPECT-CT, and 2) classify these defects for its potential value in radiation planning in patients with non-small cell lung cancer (NSCLC).
Methods
Patients with stages I-III NSCLC requiring radiation-based therapy were eligible for this prospective study. V/Q SPECT performed within 2 weeks prior to radiation start was interpreted by nuclear medicine physicians and then measured by a semi-quantitative score. The potential mechanism of V, Q defect was analyzed; the potential impact of V/Q SPECT over Q SPECT alone was completed through classified applications (high dose RT versus RT avoidance) during planning.
Results
Images of 51 consecutive patients were analyzed. The V, Q defects were matched, reverse mismatched (V- defect greater than Q-defect), and mismatched (Q-defect greater than V-defect) in 61%, 31% and 8% patients, respectively. Tumor was the leading cause of the defects of ipsilateral lung in 73% patients. The defect scores of the ipsilateral lung were greater in patients with central primaries than those with peripheral primaries for both V- (2.3±1.1 vs. 1.5±0.8, p=0.017) and Q-SPECT (2.2±0.8 vs. 1.4±0.6, p=0.000). The patients with chronic obstructive pulmonary disease had greater defect scores in contralateral lung for both V- (1.5±0.7 vs. 1.0±0.8, p=0.006) and Q- SPECT (1.4±0.6 vs. 1.0±0.4, p=0.010). On assessing the potential value of SPECT on RT plan, 39% patients could have their RT plan if applying V/Q SPECT rather than Q SPECT alone.
Conclusions
V/Q SPECT provides a more comprehensive functional assessment, may provide additional value over Q-SPECT alone in assessing local pulmonary function and guide RT plan decisions in patients with NSCLC.
Keywords: Non-small cell lung cancer, Ventilation, Perfusion, Single photon emission computerized tomography (SPECT), Radiotherapy
INTRODUCTION
Lung cancer is the leading cause of cancer death in the United States and worldwide (1). Approximately, 80–85% of lung cancer cases are non-small cell lung cancer (NSCLC); of these, over 60% will require radiation at least once during the course of the disease (2). Most patients with lung cancer have a history of cigarette smoking, which brings risks for other conditions such as chronic obstructive pulmonary disease (COPD) and coronary artery disease, which may increase treatment risks. Advanced-stage lung tumors can also obstruct the bronchus or bronchioles and lead to atelectasis or compress the large vessels and cause decreased perfusion (Q). Local functional lung assessment by ventilation (V) and Q single photon emission computed tomography (SPECT) provides estimates of patient tolerance to surgical procedures and local radiation therapy, and monitors lung toxicity induced by radiotherapy (3–7). Importantly, functional mapping guides radiotherapy (RT) planning to avoid or minimize exposure of functional lung (FL), with the potential of reducing pulmonary toxicity and allowing effective dose escalation in the patients with NSCLC (8, 9).
Q-SPECT has been used in RT planning to identify normal functioning lung tissue for avoidance and perform more targeted RT (10–14). However, both airflow and blood flow are the fundamental elements in the gas exchange process. Without sufficient V, Q is of no value in oxygen exchange. A few studies have suggested the utility of V SPECT for prediction of postoperative lung function and guiding radiation beam arrangement in NSCLC (5, 9, 15). We hypothesized that patients with lung cancer may also have V defects mismatched to Q defects and that V/Q SPECT would thus provide a more comprehensive pulmonary function assessment for their application in RT planning. This study aimed to 1) study regional functional distribution on V/Q SPECT and 2) classify these defects for its potential value in RT planning in patients with NSCLC.
MATERIAL AND METHODS
Study Population
The study population included consecutive patients enrolled in the institutional review board approved prospective studies (UMCC2003-376 & 2006-040) who had V/Q scan performed prior to and during the course of radiation therapy between 2003 and 2009. Adult patients with histologically confirmed stage I to III NSCLC (AJCC 2003) requiring definitive irradiation with or without chemotherapy were enrolled and RT deliveries were not modulated on the basis of V/Q scans. Patients with small cell lung cancer or mixed small cell/non-small cell histology, pericardial effusion, pregnant or lactating were excluded. Patients had to be able to lie flat for the duration of PET/CT and V/Q SPECT.
V/Q SPECT imaging
V/Q-SPECTs (Siemens Symbia T6 SPECT-CT system, Siemens Medical Solutions, Hoffman Estates, IL) were performed prior to radiotherapy in the treatment pose using a flat thoracic positioning board. Each patient inhaled aerosolized Tc 99m DTPA from a 50 mCi reservoir for V scanning. SPECT imaging was then performed. Following the V scan, 5 mCi [Tc-99m] MAA was injected intravenously for Q imaging. SPECT projections were acquired at discrete 3- angular intervals with each camera head rotating through 180°. Low-dosage X-ray CT were then performed in the same position using low energy, high-resolution collimators and automatically co-registered with V/Q SPECT. The CT slice thickness was set at 5 mm and CT imaging did not employ oral or intravenous contrast administrations or diagnostic collimation. Scans were carried out at the end of exhale or at 75% vital capacity when patients were simulated and treated under active breathing control device.
V/Q SPECT image analysis
Co-registered V/Q SPECT were interpreted by experienced nuclear medicine physicians to determine whether the V, Q defects matched in distribution or intensity. V/Q SPECT imaging intensities were classified as follows: 0 - absence of activity (virtually invisible), 1- reduced activity (but still visible), 2 - activity within normal lung range. The potential etiologies of V/Q defects were determined based on comprehensive consideration of CT findings, PET/CT findings and other clinical data.
Volumetric assessment of pulmonary V/Q SPECT function was scored on a semi-quantitative scale (Table 1). The largest V/Q defect score (DS) in each lung was scored as follows: 0=no defect; 1, 2, 3, 4 = <25%, 25–49%, 50–74%, 75–100% of one lung. The DS of remaining lung was assessed for the degree of homogeneity of the tracer distribution outside the largest defect area of each lung as following: 1 = homogeneous, 2, 3, 4 = mild, moderate, marked heterogeneity, respectively. A total lung function score was tallied as ipsilateral lung DS plus contralateral lungs DS plus remaining lung DS; with a resulting range of 1–12. (Table 1) (7).
Table 1.
Lung V/Q SPECT image scoring system
| DS of the largest defect in each lung on SPECT |
| 0 = No defect |
| 1 = Defect <25% of one lung |
| 2 = Defect 25–49% of one lung |
| 3 = Defect 50–74% of one lung |
| 4 = Defect 75–100% of one lung
|
| Remaining lung DS on SPECT |
| 1 = Homogeneous |
| 2= Mild heterogeneity |
| 3 = Moderate heterogeneity |
| 4 = Marked heterogeneity
|
| DS of total lung = ipsilateral lung DS + contralateral lung DS + RLDS = 1–12 |
Abbreviations: ventilation=V; perfusion=Q; single photon emission computed tomography=SPECT; defect score=DS. RLDS=remaining lung defect score, the degree of homogeneity of the tracer distribution outside the largest defect area in both lungs.
This method is modified from Gayed et al. J Thorac Oncol. 2008; 3(8):858–64.
Pulmonary V/Q SPECT function was further classified according to age (≤70 or >70), history of COPD (Yes, or No), T stage (T1, T2, T3, T4), clinical stage (I, II, III), tumor localization. Central tumors were defined as ≤2 cm from the main bronchus, and peripheral tumors > 2 cm. If there were tumors in both central and peripheral locations, we classified the cases based on the lesion that was clearly larger. The main criterion for COPD is FEV1/FVC (forced expiratory volume in one second/ forced vital capacity) ratio <70%. Sub classification into mild (Stage I), moderate (Stage II), severe (Stage III) and very severe (Stage IV) disease is achieved by including various levels of FEV1 as percentage of predicted value postbronchodilator according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD).(16)
Classifications of local lung regions based on application of RT planning
Local lung regions were further classified according to the underlying etiology and their potential application in guiding RT.
Type A regions: functional defects corresponding to the location of tumor. These “bad” tumor occupying lung regions should be given as high RT dose as possible.
Type B1 regions: complete function defect induced by COPD or other unrecoverable diseases. These regions, with unrecoverable non-functioning “bad” lung, can be given high dose RT without causing change in the global lung function.
Type B2 regions: reduced lung function induced by COPD or other unrecoverable diseases. These regions, with unrecoverable low functioning lung, may be given high dose (if no worse lung available) without causing remarkable change in the global lung function.
Type B3 regions: temporarily dysfunctional lung due to tumor and other potentially reversible conditions. These regions could be “good”, if they improve during treatment, and would then be minimized for further RT or high dose RT; may be “bad”, if there is no improvement during treatment, can be given high dose without impairing the pulmonary function.
Type C regions: normal functioning “good” lung. The RT dose to such regions should be minimized to decrease functional or clinically significant sequelae.
The regional categories and their applications were compared between V/Q SPECT and Q SPECT alone.
Statistical Analysis
Descriptive statistics were used to summarize the functional mapping and the impact on RT planning. To study the potential mechanism of the defect, we used a nonparametric test to compare the mean values of patients with different clinical characteristics (such as with and without COPD). For all tests, P <0.05 was considered significant.
RESULTS
Patient Characteristics
From 2003 to 2009, 51 patients with stages I-III NSCLC requiring radiation based therapy were enrolled. All patients had V/Q SPECT within 1–2 weeks prior to radiation start. Patient characteristics are shown in Table 2. Twenty-one (41%) patients had medically inoperable stage I–II disease and other 30 (59%) patients had locally advanced stage IIIA–B disease. Twenty-six (51%) patients had central type primary tumors and 25 (49%) patients had peripheral type primary tumors. Twenty-six (51%) patients had COPD, including 4 GOLD stage I-II, 22 GOLD stage III-IV.
Table 2.
Patient characteristics
| Variable | Patient # |
|---|---|
| Gender | |
| Male | 42 |
| Female | 9 |
| Age | |
| ≤70 | 25 |
| > 70 | 26 |
| T stage | |
| T1 | 12 |
| T2 | 9 |
| T3 | 14 |
| T4 | 16 |
| Clinical Stage | |
| I | 11 |
| II | 9 |
| III | 31 |
| Tumor localization | |
| Central type | 26 |
| Peripheral type | 25 |
| COPD | |
| Without COPD | 25 |
| With COPD | 26 |
| Stage I-II | 4 |
| Stage III-IV | 22 |
Abbreviation: chronic obstructive pulmonary disease=COPD.
Overall V/Q SPECT Mapping in Patients with NSCLC
Co-registered SPECT and CT images demonstrated that 100% of these 51 patients had functional defects (V, or Q or both) at the CT-defined tumor with or without additional involvement of adjacent lung. V/Q defects were matched (V defect on the same order of magnitude as Q), reverse mismatched (V defect greater than Q), and mismatched (Q defect greater than V) in 61% (31/51), 31% (16/51) and 8% (4/51) patients, respectively. The V/Q relationship classifications and applications in RT are summarized in Table 3 and examples for V/Q relationship are shown in Figures 1–3.
Table 3.
Pulmonary V/Q relationship and potential application on RT planning
| V/Q defect relationship | Potential etiology (CT based findings) | Activity degree | Application types in guiding RT | No of patients (%) | ||
|---|---|---|---|---|---|---|
| V | Q | Q SPECT | V/Q SPECT | |||
| Matched | 31 (61%) | |||||
| Mass effect | 0 | 0 | A | A | 19 | |
| Hilar mass compression to both large vessels & airway | 0 | 0 | B3 | B3 | 3 | |
| Collapsed lung | 0 | 0 | B3 | B3 | 2 | |
| Stage I-II COPD | 1 | 1 | B2 | B2 | 4 | |
| Stage III COPD | 1 | 1 | B2 | B2 | 2 | |
| Interstitial lung disease | 1 | 1 | B2 | B2 | 1 | |
| Reverse mismatched | 16 (31%) | |||||
| Tumor induced endobronchial obstruction | 0 | 1 | C | B3 | 9 | |
| Stage III-IV COPD | 0 | 1 | B2 | B1 | 7 | |
| Mismatched | 4 (8%) | |||||
| Tumor induced extrinsic compression of the pulmonary artery or its branches | 2 | 0 | B3 | C | 4 | |
(1) Abbreviation: V=ventilation; Q=perfusion; RT=radiotherapy; COPD=chronic obstructive pulmonary disease.
(2) According to V/Q SPECT imaging intensity, the activity degree of pulmonary ventilation, perfusion defect was defined, respectively, as 0 if activity absent (virtually invisible), 1 if activity reduced, but still visible, 2 if activity in normal lung range. The potential etiologies of V/Q defects were determined based on CT findings, PET/CT findings and other clinical data.
- Type A regions: functional defects corresponding to the location of tumor. These “bad” tumor occupying lung regions, should be given as high RT dose as possible;
- Type B1 regions: complete function defect induced by COPD or other unrecoverable diseases. These regions, with unrecoverable non-functioning “bad” lung, can be given high dose RT without causing change in the global lung function.
- Type B2 regions: reduced lung function induced by COPD or other unrecoverable diseases. These regions, with unrecoverable low functioning lung, may be given high dose (if no worse lung available) without causing remarkable change in the global lung function.
- Type B3 regions: temporarily dysfunctional lung induced by tumor and other potentially recoverable diseases. These regions could be “good”, if they improve during treatment, and would then be minimized for further RT or high dose RT; may be “bad”, if there is no improvement during treatment, can be given high dose as indicated.
- Type C regions: normal functioning “good” lung. The RT dose to such regions should be minimized to decrease functional or clinically significant sequalae.
Figure 1. Matched V/Q defect.
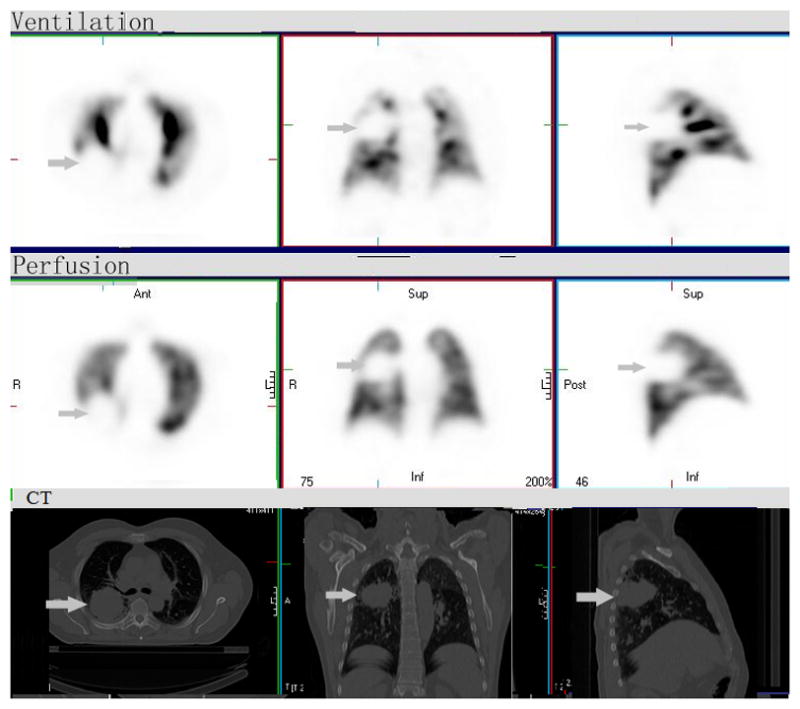
There is a large matched ventilation (V) and perfusion (Q) defect in the right mid-posterior lung. The corresponding CT demonstrates a large mass in this region (arrow). This “bad” tumor occupying lung region should be given as high RT dose as possible, categorized as “type A region” based on either V/Q SPECT alone or Q SPECT. V/Q SPECT have no additional value over Q SPECT alone in this V, Q matched case.
Figure 3. Mismatched V/Q defect.
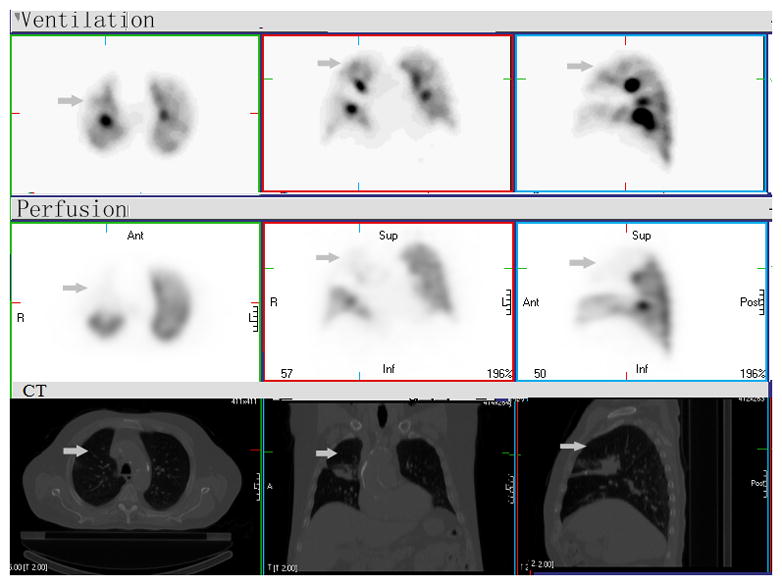
There is a large mismatched defect with significantly reduced perfusion (Q) and normal V (arrow) in right middle and upper lobes, consistent with extrinsic compression of the pulmonary artery by the mass in hilum and mediastinum. If based on Q-SPECT only, this mismatched region would be considered as defect (“type B3” region) and potential for high dose radiation. Combined V/Q SPECT-CT can define and apply these regions as “type C region”, normal functioning “good” lung. The RT dose to such region should be minimized to decrease functional or clinically significant sequalae.
Factors Associated with V/Q Defects
Overall, tumor is the leading cause of defect (V, or Q or both) of ipsilateral lung in 73 %( 37/51) patients. Among the 31 patients with matched V/Q defects, tumor was the overwhelming etiology for main defects in 24 patients including lung mass effect, hilar mass compression to both large vessels & airway, and collapsed lung. COPD was the second most important reason for main matched V/Q defect in 6 patients. Interstitial lung disease was the cause of matched V/Q defects in 1 patient. In the 16 patients with reversed mismatched defects, tumor related endobronchial obstruction was the main etiology in 9 patients, and COPD was the main etiology in the remaining 7 patients. Additionally, 4 patients had mismatched V/Q (Q worse than V), consistent with extrinsic tumor mass compression of the pulmonary artery or its branches (Table 3).
The mean lung DSs of V function were significantly greater than those of Q (4.8±1.8 vs. 4.3±1.3, p=0.006). Stratified analysis showed that the DSs of ipsilateral lung in patients with central primaries were significantly greater than those with peripheral primaries in both V-scan (2.3±1.1 vs. 1.5±0.8, p=0.017) and Q-scan (2.2±0.8 vs. 1.4±0.6, p=0.000) (Table-4).
Table 4.
Pulmonary V/Q defect score in patients stratified by tumor location
| DS | Central tumor, Mean ± SD | Peripheral tumor, Mean ± SD | p value |
|---|---|---|---|
| Ventilation | |||
| Ipsilateral lung | 2.3±1.1 | 1.6±0.7 | 0.017 |
| Contralateral lung | 1.4±0.8 | 1.3±0.6 | 0.435 |
| Remaining lung | 1.5±0.9 | 1.8±1.1 | 0.370 |
| Total lung | 5.0±1.9 | 4.6±1.7 | 0.440 |
| Perfusion | |||
| Ipsilateral lung | 2.2±0.8 | 1.4±0.6 | 0.000 |
| Contralateral lung | 1.1±0.3 | 1.3±0.6 | 0.156 |
| Remaining lung | 1.3±0.5 | 1.6±0.9 | 0.384 |
| Total lung | 4.5±1.1 | 4.2±1.5 | 0.110 |
The patients with COPD had greater contralateral lung DS in both V-scan (1.5±0.7 vs. 1.0±0.8, p=0.006) and Q-scan (1.4±0.6 vs. 1.0±0.4, p=0.010), but only greater total lung Q DS (4.8±1.4 vs. 3.8±1.0, p=0.019), than those without COPD (Table-5).
Table 5.
Pulmonary V/Q defect score in patients stratified by COPD
| DS | Patients with COPD, Mean ± SD | Patients without COPD, Mean ± SD | p value |
|---|---|---|---|
| Ventilation | |||
| Ipsilateral lung | 1.9±0.9 | 2.0±1.1 | 0.865 |
| Contralateral lung | 1.5±0.7 | 1.0±0.8 | 0.006 |
| Remaining lung | 1.7±0.8 | 1.6±1.1 | 0.248 |
| Total lung | 5.1±1.64 | 4. 6±1.9 | 0.167 |
| Perfusion | |||
| Ipsilateral lung | 1.8±0.8 | 1.7±0.8 | 0.640 |
| Contralateral lung | 1.4±0.6 | 1.0±0.4 | 0.010 |
| Remaining lung | 1.6±0.8 | 1.2±0.6 | 0.017 |
| Total lung | 4.8±1.4 | 3.8±1.0 | 0.019 |
For other stratified factors, only T stage was significant for ipsilateral lung Q DS. The DSs of T1, 2, 3, 4 were 1.3±0.4, 1.6±0.7, 1.8±0.8 and 2.3±0.8, respectively. (Among groups, p=0.002, T1 vs. T2, p= 0.295; T1 vs. T3, p= 0.075; T1 vs. T4, p=0.000; T2 vs. T3, p=0.557; T2 vs. T4, p=0.037; T3 vs. T4, p=0.110). Age and clinical stage were not significantly correlated with V, Q defects.
Classification of V/Q SPECT on its potential impact on RT planning
Table-3 shows the potential application of SPECT on RT planning. In this study, 61% (31/51) had matched V, Q distribution and 39% (20/51) patients had V/Q discrepancy (16 reverse mismatches and 4 mismatches). To guide RT planning/beam arrangement or optimization, V/Q SPECT would have no additional value over Q SPECT alone if patients have only matched defects. V/Q SPECT would provide additional value over Q-SPECT alone to guide RT planning in patients with mismatched or reverse matched defects. In the 16 patients with reverse mismatched defects, 9 had tumor related endobronchial obstruction as the main etiology. In this group, Q SPECT would classify type C region for RT planning, while V/Q SPECT would suggest a type B3 region. The remaining 7 patients showed reduced Q activity and severely reduced V throughout both lungs from advanced COPD (GOLD Stage III-IV). For this group, Q-SPECT alone classified type B2 while V/Q SPECT to type B1 region. In 4 patients with mismatched defect, SPECT application in RT planning designated type B3 under Q-SPECT, but changed to type C under V/Q-SPECT. In summary, V/Q SPECT could have altered the SPECT guided RT plan in a total of 39% (20/51) of patients from that of Q SPECT guided RT plan.
DISCUSSION
This study demonstrates that lung functional defects are present in all patients with NSCLC. Tumor was the most common cause of defect, COPD was the second. While many defects are matched and can be detected by Q-SPECT alone, 39% (20/51) patients had V/Q discrepancy including 31% reversed mismatch (V worse than Q) and 8% mismatch (Q worse than V) in (4/51). In patients with V/Q discrepancy, V/Q SPECT provides an additional value over Q SPECT alone in the local pulmonary function before treatment, which may provide better guidance for the RT planning. Additionally, patients with central primaries had greater ipsilateral lung defects than those with peripheral primaries, while patients with COPD had greater defects in contralateral lung than those without COPD in both V and Q.
It is not a new finding that patients with lung cancer have poor pulmonary function. The incidence of 39% in V/Q discrepancy based on V/Q-SPECT-CT analysis in current study is similar to that of 35% incidence from report of Narabayashi et al on planar scintigraphy analysis in patients with lung cancer (17). The finding that tumor is the major cause of V/Q discrepancy is in agreement with some previous reports (18, 19). The current study differs from previous studies in 1) semi-quantification and anatomic mapping of the defect (ipsilateral vs. contralateral sides); 2) defining the potential mechanism of defects including tumor associated etiology; and 3) classification or individualization of their potential impact in RT planning of V/Q SPECT. Nine patients had tumor induced endobronchial obstruction resulting in reverse V/Q mismatches, with almost complete absence of V but visible Q activity. If based on Q-SPECT alone, these reverse mismatched regions would be considered as “type C region”, functioning lung to minimize radiation. Combined V-and Q-SPECT and CT can define and apply these regions as “type B3 region”, temporarily dysfunctional lung, potentially functional, could be “good” if they improve during treatment, should minimize further RT; or “bad”, if no change during-RT, ok for high dose as indicated for tumor. The V/Q mismatches (Q DS greater than V DS), seen in 4 cases induced by extrinsic compression of the pulmonary artery or its branches by the tumor. If based on Q-SPECT only, these mismatched regions would have been considered as defective suitable for high dose radiation. Combined V/Q SPECT-CT analysis can be helpful to understand the underlying etiology and categorize these regions appropriately for radiation plan. The fact of an overall higher V-DS than Q-DS (4.82 vs. 4.31, p=0.004) also indicates that V-SPECT may add information to Q SPECT related to pulmonary function impairment; further suggesting the additional value of co-registered V/Q SPECT over Q SPECT alone in the local pulmonary function imaging.
Among the factors associated with V/Q defect, tumor location is an important one. Patients with central primaries have worse ipsilateral lung function than those with peripheral primaries in both V and Q SPECTs. This may be due to the mechanical effects of tumors in different location. Central tumors may cause the endobronchial obstruction of main stem bronchus or lobe bronchus and extrinsic compression of the pulmonary artery relating to large area of abnormalities in V and Q, while peripheral tumors only have lung mass effect, obstruction of segmental bronchus and related small vessels, resulted in a small area of abnormalities. This finding was in accordance with Narabayashi’s report (17). The significance of T stage on ipsilateral lung Q DS further supports the role of tumor on pulmonary function. The value of such defects on guiding radiation planning should be individualized as described on table-3, because poorly perfused lung due to vessel compressions should also be spared.
COPD can also contribute to V/Q SPECT discrepancy. However, one should note that most patients with COPD had a central airway aerosol deposit from the V tracer, Tc-99m DTPA, resulting in very hot regional images and these regions were excluded for V-Q discrepancy analysis in current study. Of note, only 30% (7/26) of COPD patients had COPD induced reverse mismatched defects. These patients all had advanced (stage III-IV) COPD which obstructed V tracer [Tc-99m] DTPA from alveoli, while the majority of V/Q matched COPD patients were diagnosed as mild to moderate COPD. Thus, reverse mismatched COPD patients may have worse pulmonary reserve than matched COPD patients, while reverse mismatched regions may have worse local function reserve than other regions in the lung with COPD. Rodriguez-Roisin et al (20) have also reported V-Q imbalance was associated with COPD severity. Hence, COPD induced V/Q SPECT mismatch is useful in grading COPD patients and their regional lung function, as well as guiding RT beam arrangement, even if it maybe not absolutely equal to V-Q discrepancy.
V/Q SPECT and V/Q relationship interpretations of this study have important clinical significances beyond Q SPECT alone. 1) V/Q discrepancy contributes to gas exchange inefficiency because low V regions contribute blood with low contents of O2 and produce hypoxemia, while V without Q equals dead air space (21). Hence, V/Q SPECT provides more comprehensive assessment of pulmonary function than Q SPECT alone and potentially useful in the treatment design and prognosis. 2) Using the V/Q SPECT as a direct correlation to V and Q, and thus a more accurate pulmonary function grading and mapping than Q SPECT alone, enables regions with different functionality to be identified and incorporated into the RT plan. Thus, V/Q SPECT guided RT is potentially better than Q SPECT alone guided RT to reduce dose delivered to high functional lung and decrease radiation induced lung toxicity. In this study, V/Q SPECT seems to provide additional information over Q SPECT alone, which may alter RT planning (mainly beam arrangement) in 39% (20/51) patients. This was an important step in improving pulmonary function imaging guided radiotherapy. The semi-quantitative method may not be able to identify some small defects. More accurate quantitative analysis of the regional lung function based on registered V, Q and CT by FIAT (function imaging analysis tool) is currently ongoing in our institution. RT planning comparison based on V/Q, Q alone, or CT alone is also ongoing in our institution and we hope to learn the full extent of the applicable value of these findings. However, the additional value of V/Q SPECT over Q alone in RT planning still can not be approved until a clinical trial can demonstrate that RT delivery based on V/Q SPECT data yields superior results compared to conventional RT based on Q SPECT data alone. We hope such a trial with RT delivery based on V/Q SPECT can yield less pulmonary toxicity with equal tumor control or similar toxicity with dose escalation and better tumor control.
CONCLUSION
This study has classified and semi-quantified regional V/Q function in a prospective series of patients with NSCLC, and demonstrated that 1) tumor is the major cause of lung functional defect; and 2) that more than one third of the studied patients with NSCLC had defect associated with V/Q discrepancy. These results suggest that there may be an added value associated with using co-registered V/Q SPECT images over Q-SPECT alone in the assessment of local pulmonary function and application in guiding RT.
Figure 2. Reverse mismatched V/Q defect.
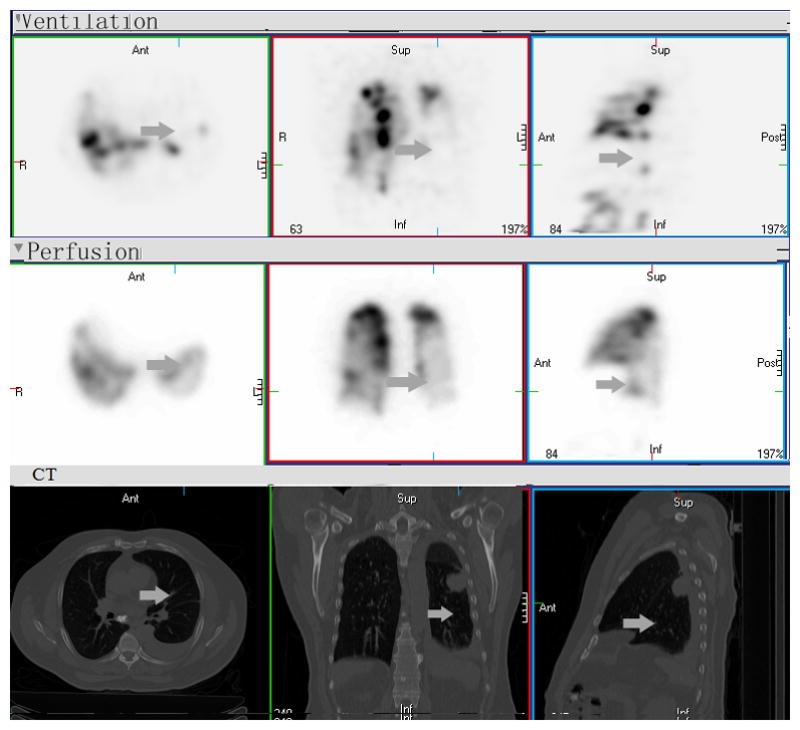
There are widespread matched V and Q defects (categorized as “type B2 region” based on either V/Q SPECT alone or Q SPECT) and central airway V tracer deposits consistent with COPD, large left pleural effusion and mass in superior segment of the lower lobe (categorized as “type A region” based on either V/Q SPECT alone or Q SPECT), and reverse mismatch (Q better than V) throughout the left lower lobe (arrow). If based on Q-SPECT only, this reverse mismatched region would be considered as “type C region”, functioning lung to minimize radiation. With combined V/Q SPECT-CT, however, these regions are classified as “type B3 region”, temporarily dysfunctional lung, potential functioning, could be “good” if they improve during treatment, should minimize further RT. It could also be “bad”, if no change during-RT, can be given high dose without clinical impact to the patient.
Acknowledgments
This study was supported in part by ASCO-CDA and R21CA127057, P01CA059827, SDWS-2007QW036 and BS2009YY012. The funding source had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit this article for publication. We are grateful to Chrissy Lockhart, many clinical physicists and nuclear medicine staff at University of Michigan and Ann Arbor VA and many for ensuring the position the V/Q SPECT scan. We also appreciate critical review and edits by Paul Stanton and Mary Davis (University of Michigan).
Footnotes
CONFLICTS OF INTEREST STATEMENT
All authors have approved this manuscript and no any actual or potential conflicts of interest exist.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Tyldesley S, Boyd C, Schulze K, et al. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973–985. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 3.Mariani G, Bruselli L, Kuwert T, et al. A review on the clinical uses of SPECT/CT. Eur J Nucl Med Mol Imaging. 2010 doi: 10.1007/s00259-010-1390-8. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimoto K, Nomori H, Mori T, et al. Quantification of the impact of segmentectomy on pulmonary function by perfusion single-photon-emission computed tomography and multidetector computed tomography. J Thorac Cardiovasc Surg. 2009;137:1200–1205. doi: 10.1016/j.jtcvs.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Ohno Y, Koyama H, Takenaka D, et al. Coregistered ventilation and perfusion SPECT using krypton-81m and Tc-99m-labeled macroaggregated albumin with multislice CT utility for prediction of postoperative lung function in non-small cell lung cancer patients. Acad Radiol. 2007;14:830–838. doi: 10.1016/j.acra.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Ma J, Zhou S, et al. Radiation-Induced Reductions in Regional Lung Perfusion: 0.1–12 Year Data from a Prospective Clinical Study. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Gayed IW, Chang J, Kim EE, et al. Lung perfusion imaging can risk stratify lung cancer patients for the development of pulmonary complications after chemoradiation. J Thorac Oncol. 2008;3:858–864. doi: 10.1097/JTO.0b013e31818020d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates EL, Bragg CM, Wild JM, et al. Functional image-based radiotherapy planning for non-small cell lung cancer: A simulation study. Radiother Oncol. 2009;93:32–36. doi: 10.1016/j.radonc.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munawar I, Yaremko BP, Craig J, et al. Intensity modulated radiotherapy of non-small-cell lung cancer incorporating SPECT ventilation imaging. Med Phys. 2010;37:1863–1872. doi: 10.1118/1.3358128. [DOI] [PubMed] [Google Scholar]
- 10.Yin Y, Chen JH, Li BS, et al. Protection of lung function by introducing single photon emission computed tomography lung perfusion image into radiotherapy plan of lung cancer. Chin Med J (Engl) 2009;122:509–513. [PubMed] [Google Scholar]
- 11.Zhang G, Dilling TJ, Stevens CW, et al. Functional lung imaging in thoracic cancer radiotherapy. Cancer Control. 2008;15:112–119. doi: 10.1177/107327480801500203. [DOI] [PubMed] [Google Scholar]
- 12.Lavrenkov K, Christian JA, Partridge M, et al. A potential to reduce pulmonary toxicity: the use of perfusion SPECT with IMRT for functional lung avoidance in radiotherapy of non-small cell lung cancer. Radiother Oncol. 2007;83:156–162. doi: 10.1016/j.radonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.McGuire SM, Zhou S, Marks LB, et al. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol Biol Phys. 2006;66:1543–1552. doi: 10.1016/j.ijrobp.2006.07.1377. [DOI] [PubMed] [Google Scholar]
- 14.Christian JA, Partridge M, Nioutsikou E, et al. The incorporation of SPECT functional lung imaging into inverse radiotherapy planning for non-small cell lung cancer. Radiother Oncol. 2005;77:271–277. doi: 10.1016/j.radonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Palmer J, Bitzen U, Jonson B, et al. Comprehensive ventilation/perfusion SPECT. J Nucl Med. 2001;42:1288–1294. [PubMed] [Google Scholar]
- 16.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 17.Narabayashi I, Otsuka N. Pulmonary ventilation and perfusion studies in lung cancer. Clin Nucl Med. 1984;9:97–102. doi: 10.1097/00003072-198402000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Achong DM. Ventilation-perfusion mismatch caused by extrinsic compression of the pulmonary artery. Correlative imaging. Clin Nucl Med. 1994;19:61–63. doi: 10.1097/00003072-199401000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Wartski M, Zerbib E, Regnard JF, et al. Reverse ventilation-perfusion mismatch in lung cancer suggests intrapulmonary functional shunting. J Nucl Med. 1998;39:1986–1989. [PubMed] [Google Scholar]
- 20.Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol. 2009;106:1902–1908. doi: 10.1152/japplphysiol.00085.2009. [DOI] [PubMed] [Google Scholar]
- 21.Glenny RW. Teaching ventilation/perfusion relationships in the lung. Adv Physiol Educ. 2008;32:192–195. doi: 10.1152/advan.90147.2008. [DOI] [PubMed] [Google Scholar]


