Abstract
Several classes of neurotransmitters exert modulatory effects on a broad and diverse population of neurons throughout the brain. Some of these neuromodulators, especially acetylcholine and dopamine, have long been implicated in the neural control of selective attention. We review recent evidence and evolving ideas about the importance of these neuromodulatory systems in attention, particularly visual selective attention. We conclude that, although our understanding of their role in the neural circuitry of selective attention remains rudimentary, recent research has begun to suggest unique contributions of neuromodulators to different forms of attention, such as bottom-up and top-down attention.
From correlates to causes
The majority of work on the neural mechanisms of selective attention, particularly visual selective attention (see Glossary), has focused on the changes in neural activity observed in epochs in which particular stimuli are either behaviorally relevant or irrelevant to a particular task at hand. Changes in neural activity, whether measured in the spiking activity of individual neurons (e.g., [1]) or populations of neurons (e.g., [2]), or in changes of blood-oxygenation-level-dependent (BOLD) responses in functional neuroimaging experiments (e.g., [3]), have generally demonstrated that the magnitude and fidelity of stimulus-related neural signals depend on the attentional focus (see [4] for a review). While the question of which component of neural signals (e.g. spike rate, synchronous activity) is the most informative about the underlying neural mechanism responsible for the perceptual benefits of attentional deployment remains unresolved (e.g., [2,5–7]), it is nonetheless clear that directing attention to a target stimulus involves alteration in the ‘gain’ of sensory representations that favor that target. Recently, there has been some progress in identifying the specific neural circuits causally involved in modulating the gain of sensory signals, particularly in the case of visuospatial attention (reviewed in [8]). These studies have implicated brain structures with established involvement in oculomotor control, specifically the saccadic system, as having a causal role in controlling attention [9–12] and generating correlates of attention within visual cortex [13–15].
Largely separate from these studies are studies that have addressed the long-suspected role of particular neuromodulators in attentional control in a variety of species, including humans, in both normal and clinical subjects [16–18]. Neuromodulators are classes of neurotransmitters that influence synaptic transmission broadly within neural circuits. In this paper, we review some of the progress made in understanding the role that neuromodulators play in attention, particularly visual selective attention. We discuss how that role can be integrated with evolving views on the underlying neural circuitry of attention, and what future research might be needed to identify the specific roles of particular neuromodulators. We focus on two neuromodulators most often implicated in the control of attention, namely acetylcholine (Ach) and dopamine (DA). These neuromodulators have several characteristics in common: i) they are all released primarily by neurons within specific brainstem or midbrain nuclei [19]; ii) these neuromodulating subcortical neurons project broadly to many subcortical and cortical structures. Projections to the cortex include both posterior sensory areas where correlates of selective attention are observed, as well as projections to the prefrontal cortex (PFC) where the control of selective attention is thought to originate; iii) each of the specific neuromodulatory nuclei also receives projections from areas within the PFC [20–22, but see 23], suggesting a means by which PFC control can exert network-wide attentional effects.
Acetylcholine
In the past twenty years, a number of studies using human and animal subjects have yielded evidence of a role of Ach in attention [24,25]. Alzheimer’s disease in humans is associated with reduced cortical cholinergic innervation [26] and patients with dementia exhibit deficits in the orienting of attention [27]. Systemic increases in Ach activity can enhance visual selective attention in normal human subjects [24,28]. Cholinergic receptors are generally broken into two classes: metabotropic muscarinic receptors (mAchRs) and ionotropic nicotinic receptors (nAchRs) [19]. Although much of the evidence for behavioral enhancement through cholinergic stimulation involves nAchRs (for instance, from smoking tobacco [29,30]), there is evidence for a role of both receptors in some aspects of attentional control. Studies in rodents suggest that the processing of sensory signals within posterior areas might be influenced by the interaction of PFC with ascending cholinergic projections [17] and this interaction appears to depend on nAchRs [31]. Within posterior areas, basal forebrain stimulation enhances sensory signals within somatosensory [32], auditory [33] and visual cortex [34], and in all cases the effects appear to involve mAChRs. However, Disney and colleagues [35] recently showed that within primary visual cortex (V1) it is nAchRs that are involved in gain control. Within V1, nAchRs are localized presynaptically at geniculocortical inputs to layer IVc neurons, where they enhance responsiveness and contrast sensitivity of thalamorecipient neurons.
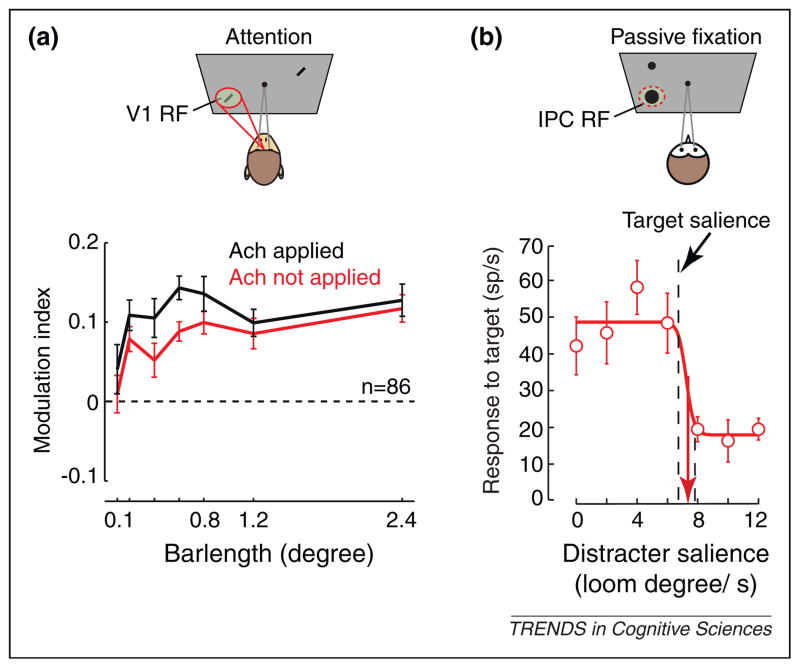
Studies in nonhuman primates have established that the deployment of covert attention to visual stimuli leads to corresponding changes in the visual responses of neurons throughout the visual system [4]. Thiele and colleagues [36] recently tested the role of Ach in this modulation. They recorded visual responses of V1 neurons in monkeys performing a covert attention task. Consistent with previous results (e.g., [37]), they found an increase in V1 responses when monkeys attend to receptive field (RF) stimuli compared to when they attend to non-RF stimuli (Figure 1a). Iontophoretic application of acetylcholine augmented the attentional modulation of V1 responses. Furthermore, application of scopolamine, a mAchR antagonist, reduced the attentional modulation, while application of the nAchR antagonist, mecamylamine, had no effect. These results demonstrate a robust interaction of attentional deployment and mAchR activity on the representation of stimuli within visual cortex.
Figure 1.
Cholinergic involvement in attentional selection. (a) Enhancement of attention effects in macaque V1 by Ach. The cartoon above depicts the behavioral task in which attention was directed covertly to a neuron’s RF stimulus (red spotlight) or to a stimulus outside of the RF (not shown) during fixation of a central spot (gray lines). The effect of spatial attention on the responses of V1 neurons to visual stimuli is quantified with a modulation index for stimuli of varying lengths. Positive indices indicate greater responses when attention is directed toward the stimulus within the neuron’s RF compared to when attention is elsewhere. Indices measured during control trials (red) and during iontophoretic application of acetylcholine (black) are shown. Adapted from [36]. (b) Cholinergic neurons in the owl’s IPC nucleus signal the physical salience of stimuli by a characteristic switch-response. The exemplar neuron responds almost invariantly to RF stimuli across a range of stimulus intensities as long as the stimulus is more physically salient than the other stimulus on the screen (distracter). When the RF stimulus is less salient, the neuron responds at a uniformly low rate. Salience is manipulated by varying the speed at which a given stimulus looms (target salience in this example is set at 7 degrees/second). IPC neurons were recorded in owls during passive viewing. Adapted from [46].
Studies employing behavioral paradigms that manipulate bottom-up attentional orienting, for example by using spatial cues [38], have generally found that lesions of the basal cholinergic nuclei impair such orienting [39], while increased cholinergic activity (e.g., via nicotine) increases orienting [30,40]. Moreover, both systemic administration of the muscarinic antagonist scopolamine [41] and its local injection into posterior parietal cortex slows bottom-up orienting of attention in nonhuman primates [42], which suggests a role of Ach in the mechanism of bottom-up attention. In contrast to top-down attention, whereby selection among different sensory stimuli depends solely on the relevance of those stimuli to behavioral goals, bottom-up driven selection is based solely on the (physical) salience of stimuli. As with top-down attention, bottom-up salience enhances the responses of neurons within visual cortex [43–45]. Yet it remains unclear how Ach contributes to these effects. In a recent study employing an owl model, it was found that neurons in a cholinergic nucleus exhibit response characteristics consistent with a potential role in the selection of visual objects based on salience [46]. Neurons within the nucleus isthmi pars parvocellularis (IPC) of owls transmit cholinergic inputs to the tectum and respond to both auditory and visual stimuli. Interestingly, the visual responses of these neurons depend heavily on whether the stimulus in their RF is more salient than stimuli outside of their RF: the magnitude of their responses decreases sharply at the boundary where the relative salience of the RF stimulus falls below that of a stimulus outside of the RF (Figure 1b). Similar effects are observed within the owl optic tectum [47], which is reciprocally connected with IPC in a precisely topographic manner. These results suggest that salience-driven selection may originate in part from cholinergic inputs, or at least it involves those inputs.
Dopamine
Dopamine (DA) receptors are generally divided into two classes, D1 and D2 [46]. The D1 family includes D1 and D5 receptors, whereas D2, D3, and D4 receptors make up the D2 family [48,49]. Compared to other subtypes, D1 receptors (D1Rs) are more abundant in prefrontal cortex, which suggests a more prominent role in regulating the cognitive functions of the PFC [50–55]. Within the PFC, D1Rs exhibit a bilaminar pattern of expression, while D2Rs are less abundant and appear to be expressed primarily within infragranular layers [50,55]. The effect of DA on the activity of PFC neurons is rather complex. However, evidence from a variety of experimental approaches suggests that when acting via D1Rs, the effects of dopamine have two general properties. First, DA can alter the strength and reliability (efficacy) of converging excitatory (glutamatergic) synapses [49]. Second, DA’s modulatory influence can exhibit an inverted-U shaped property wherein ‘optimal’ DA levels lead to peak effects on synaptic efficacy, with reduced effects at higher or lower levels [49,56].
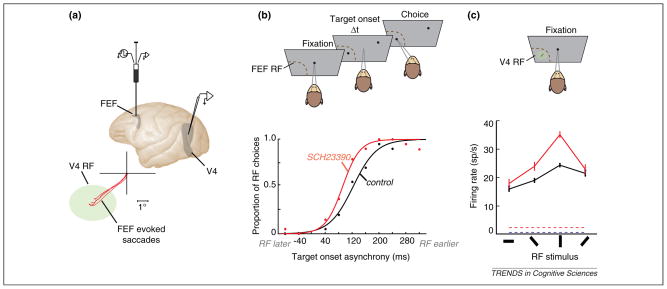
In spite of much evidence of a role of PFC DA in attention and strong evidence that attentional control is achieved in part by PFC modulation of signals within sensory cortices [57], these two lines of evidence remain largely separate. However, recent work suggests that PFC control of signals within visual cortex may rely on PFC D1Rs [58]. Noudoost and Moore [58] addressed the impact of manipulating D1R-mediated activity within the frontal eye field (FEF) on saccadic target selection and on visual responses of extrastriate area V4 neurons (Figure 2A). The FEF appears to be the part of the PFC from which modulation of visual cortical signals originates during spatially directed attention [7,13]. Thus, if DA plays a role in visuospatial attention then changes in dopaminergic activity within the FEF should alter signals within visual cortex. Manipulation of D1R-mediated FEF activity was achieved via volume injections [59] of a D1 antagonist (SCH23390) into sites within the FEF where neurons represented the same part of visual space as simultaneously recorded area V4 neurons. Following the D1R manipulation, visual targets presented within the affected part of space were more likely to be chosen by the monkey as targets for saccades than during control trials. Thus, the manipulation increased saccadic target selection (Figure 2B). In addition, the responses of area V4 neurons with RFs within the part of space affected by the D1R manipulation were measured and an enhancement in the gain of visual signals during passive fixation was observed (Figure 2C). The responses of V4 neurons were altered in three important ways. First, there was an enhancement in the magnitude of responses to visual stimulation. Second, the visual responses became more selective to stimulus orientation. Third, the visual responses became less variable across trials. Importantly, all three changes in V4 visual activity have also been observed in monkeys trained to covertly attend to RF stimuli [60–62]. Thus, manipulation of D1R-mediated FEF activity increased not only saccadic target selection but also the magnitude, selectivity and reliability of V4 visual responses within the corresponding part of space. The manipulation effectively elicited correlates of covert attention within extrastriate cortex in the absence of a behavioral task. Interestingly, injection of a D2 agonist into FEF sites resulted in equivalent target selection effects as the D1 antagonist. However, only the latter produced attention-like effects within area V4. Thus, in addition to being dissociable at the level of functional subclasses of FEF neurons [63], the control of attention and target selection appear to be dissociable at the level of dopamine receptors.
Figure 2.
Dopamine-mediated FEF control of saccadic target selection and visual cortical processing. (a) Local manipulation of D1R-mediated activity within the FEF during single neuron electrophysiology in area V4. Lateral view of the macaque brain depicts the location of a recording microinjectrode within the FEF and of recording sites within area V4. Bottom diagram shows saccades evoked via electrical microstimulation at the infusion site (red traces) and the RF (green ellipse) of a recorded V4 neuron in an example experiment. (b) Free-choice saccade task used to measure the monkey’s tendency to make saccades to a target within the FEF RF vs. one at an opposite location. In the task, two targets appear at varying temporal onset asynchronies. The RF target can appear earlier or later than a target outside of the RF. The monkey’s bias toward either target is measured as the asynchrony at which the monkey chooses the target with equal probability. The bottom plot shows the leftward shift in the asynchrony curve (indicating more RF choices), following manipulation of D1R mediated FEF activity. (c) Visual responses of a V4 neuron with a RF that overlapped the FEF RF measured during passive fixation. The plot shows mean ± standard error of the mean (SEM) visual responses to bar stimuli presented at varying orientations and the baseline firing rate in the absence of visual stimulation (dashed lines) before (black) and after (red) the FEF D1R manipulation. Adapted from [58].
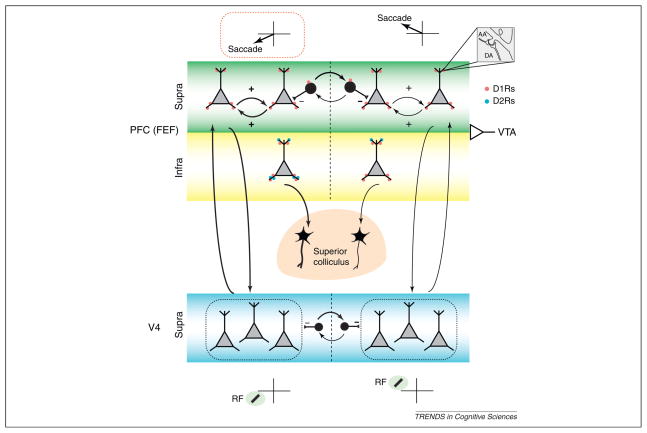
The effect of manipulating D1R-mediated FEF activity on responses of V4 neurons shows that changes in FEF neuronal activity are sufficient to exert a long-range influence on representations within visual cortex, an influence suggested, but not demonstrated, by previous studies [7,13]. In addition, the above effects show that dopamine, acting via D1Rs, is involved in the FEF’s influence on visual cortical signals as well as its influence on saccadic preparation. As there is a wealth of evidence implicating D1Rs in the neural mechanisms of spatial working memory, specifically in regulating the persistent activity of neurons within dorsolateral prefrontal cortex (dlPFC) [56,64], the above results suggest that D1Rs are part of a common mechanism underlying spatial attention and spatial working memory [58]. Like dlPFC neurons, FEF neurons also exhibit persistent, delay-period activity, even in tasks not involving saccades [65]. It has been suggested that persistent activity within the PFC is generated by recurrent glutamatergic connections between prefrontal pyramidal neurons [66]. Dopaminergic modulation of persistent activity within the PFC appears to be achieved by the influence of D1Rs on these recurrent connections [67]. The above results suggest a model in which D1Rs contribute to signatures of attention within visual cortex by a mechanism similar to their influence on persistent activity, namely by modulating long-range, recurrent connections between the FEF and visual cortex (Figure 3). Consistent with this idea is the finding that FEF neurons exhibiting persistent activity tend to show greater attentional modulation than those without [65]. In this model, attention (and/or saccadic preparation) is directed toward particular locations according to the pattern of activity across the map of visual space within the FEF, similar to what has been proposed for the lateral intraparietal area (LIP) [12]. Cortical columns with greater activity would correspond to locations of greater attentional deployment (and/or saccadic preparation) and consequently higher ‘gain’ of visual cortical signals for stimuli in that location compared to other stimuli. A possible role of dopamine would be to control the extent of the FEF gain modulation, effectively setting its dynamic range. Thus, optimum DA levels would translate into larger differences between attended and unattended stimuli while suboptimal DA would mean small differences and perhaps a less stable attentional focus. At least superficially, such a role of DA in attentional deployment would be consistent with the perceptual deficits of ADHD patients [68], who generally exhibit abnormal PFC DA [69].
Figure 3.
Possible influence of D1Rs on recurrent networks within the PFC (specifically FEF) and between the PFC and V4. The diagram depicts two adjacent FEF or V4 columns representing different, but adjacent, locations in saccadic or visual space, respectively. The columns are assumed to interact competitively (black inhibitory neurons). Positive arrows between FEF neurons within the same column depict the recurrent excitatory connections thought to underlie the persistence of spatial signals during remembered saccades or locations. Recurrence between the FEF and V4 is proposed to underlie the influence of FEF on the gain of visual inputs within V4. Dopaminergic input from the ventral tegmental area (VTA, input at right) to the PFC may modulate recurrence both within the FEF and between FEF and V4 through D1Rs and to influence competition between spatial representations. For example, increases in recurrence in a particular column while remembering or attending to a corresponding location (thicker arrows at left) can be modulated by the level of dopamine. Biases in competitive interactions between columns within visual cortex can also be achieved by experimental manipulation of D1R-mediated FEF activity, as the results of [58] suggest. Also shown are the projections from infragranular FEF neurons to the superior colliculus (SC). Other anatomical details are omitted for simplicity. Red circles represent D1Rs and blue circles D2Rs. Note the localization of D2Rs primarily in infragranular, SC-projecting layers [50,55] which is consistent with the observation that changes in D2R-mediated FEF activity only affects target selection, and not visual cortical activity [58]. The inset at the upper right depicts the involvement of DA inputs in ‘synaptic triads’, in which those inputs coincide with glutamatergic (AA) ones [52].
Future studies will need to focus on the specific neural circuitry underlying the role of DA in the PFC’s control of visual cortical signals. Details such as which functional classes of FEF neurons project to extrastriate areas or which classes express D1Rs are particularly critical to uncover. Equally important is the question of how dopamine’s apparent role in visual attention relates to its well-established role in reward signaling [70]. Several recent studies have noted the inherent difficulty in dissociating the neural mechanisms of reward and attention [71,72], given that effects of both on sensory responses can be, and perhaps should be, of a similar nature. Thus, future studies might also look for concomitant effects of changing tonic and phasic endogenous dopamine on sensory representations and reward value. Lastly, we note that the parallel role of dopamine in reward signaling would seem consistent with a contribution of dopamine specifically to top-down, rather than bottom-up, attention. Reward value is after all determined more by task-relevance and endogenous factors than by the salience of a particular stimulus.
Concluding remarks
Evidence thus far has provided solid evidence of an involvement of the cholinergic and dopaminergic systems in selective attention. In comparison, there is considerably less evidence for a contribution of other neuromodulators, such as serotonin or norepinephrine (NE) (see Box 1). However, establishing a role for neuromodulators in attention is one thing, whereas understanding those roles is entirely another. The fact that both Ach and DA seem to play a role in selective attention prompts the question of how these two systems might, or might not, uniquely contribute to attentional control. As it is known that different neuromodulatory systems interact with one another [73–75], including within PFC [76], the contributions of Ach and DA could be highly complex. However, as suggested above, one possibility is that they contribute differently to different forms of attention. Evidence to date suggests, for example, that Ach may serve a more unique role in bottom-up attention than it does in top-down attention, whereas the reverse may be true for DA. Studies of the neural correlates of attention have thus far yielded evidence of dissociable underlying neural circuits of these two varieties of attention (e.g. [77]), and it may turn out that the modulatory effects within those circuits differentially depend on DA and Ach. Future experiments might seek to test this possibility by manipulating cholinergic or dopaminergic signals during bottom-up and top-down attention tasks in the same animals, perhaps while also measuring neural correlates of either form of attention within sensory areas. For example, one might hypothesize that inactivation of the VTA might dramatically reduce visual search performance for ‘conjunction’ targets, but not for ‘popout’ targets [45], with correlative effects exhibited by parietal and prefrontal neurons [77]. In addition, there could be similar dissociations to be found between spatial and feature/object-based attention, cross-modal attention, and other varieties [8]. We suggest that testing such dissociations in future studies might be among the most important steps in understanding how neuromodulators contribute to attentional control. We stress, however, that these future studies will need to involve more rigorous behavioral and psychophysical measures than has been typical of past studies. Indeed, in many animal studies to date, how attention is specifically involved in the behavioral tasks employed is somewhat ambiguous. Thus, one major goal of precisely defining the contribution of neuromodulators to attentional control should be to establish behavioral paradigms in model organisms that clearly isolate the particular varieties of attention observable in human subjects or impaired in neurologic patients. One might argue that understanding the specific role of neuromodulators in attention will require leveraging the greater genetic and neurophysiological tractability of some model systems with the more rigorous behavioral and psychophysicalparadigms of other systems (see also Box 2).
Box 1. Norepinephrine: attention or arousal?
In contrast to the more extensive range of studies on the roles of Ach and DA in selective attention, less is understood about the role of NE. NE has classically been associated with mediating behavioral arousal rather than selective attention [78]. Similar to neurons within cholinergic nuclei [46], noradrenergic neurons within the locus coeruleus (LC) respond selectively to salient sensory stimuli [79,80]. Also consistent with a role in arousal is the observation that stimulation of the LC can modulate sensory responses in awake animals [81]. However, other studies indicate that LC activity does not simply reflect stimulus-driven salience, but depends heavily on the task relevance of stimuli. For example, LC neurons respond with robust phasic burst to the presentation of learned targets, but only weakly when non-targets are presented [82]. Thus, noradrenergic modulation may contribute to more than just mediating the influence of arousal state on sensory responses. For example, it has been suggested that NE serves to optimize performance through phasic activation of LC neurons [83]. This view may be consistent with the known benefits of noradrenergic drugs in attention-deficit hyperactivity disorder (ADHD) [84] and the finding that blockade of α2A NE receptors impairs response inhibition performance and increases hyperactivity in monkeys [85,86].
Box 2. Questions for future research.
Do different neuromodulators contribute separately to different forms of attention, e.g., top-down vs. bottom-up or spatial vs. feature-based?
Given the recent evidence of an involvement of prefrontal DA in attention, and the well-established role of DA in reward signaling (e.g. [70]), how do reward and attention mechanisms interact to guide behavior?
How spatially specific are projections of dopaminergic and cholinergic nuclei to the target cortical areas where attentional modulation is observed?
Which neuromodulators and receptor subtypes are principally involved in the influence of prefrontal cortex on sensory cortex?
What is the relationship between the influence of particular neuromodulators on arousal state and their influence on selective attention?
Acknowledgments
This work was supported by grants NIH EY014924 and NSF IOB-0546891 to T.M.
Glossary
- Acetylcholine (Ach)
an ester of acetic acid and choline, Ach is used both in the peripheral and central nervous system, generally as a neurotransmitter in the former and a neuromodulator in the latter
- Catecholamine
a tyrosine-derived amine that acts as a hormone or a neurotransmitter. Dopamine and norepinephrine are two catecholamines involved in inter-neuronal signaling in the central nervous system
- Dopamine (DA)
a catecholamine neurotransmitter and neuromodulator produced in several subcortical nuclei including the dopaminergic neurons in substantia nigra and ventral tegmental area
- Norepinephrine/Noradrenaline (NE)
a catecholamine neurotransmitter and neuromodulator produced in subcortical nuclei including the noradrenergic neurons in the locus coeruleus
- Visual selective attention
the selective processing of some visual stimuli (targets) in favor of others (distracters), according to their component features, identity, location within visual space or physical salience
- Covert attention
selective attention that does not involve an orienting movement (e.g. eye movement) toward the target of interest. Covert attention is distinguished from overt attention, which involves orienting movements
- Top-down and bottom-up attention
two major forms of attention distinguished by their stimulus or goal-driven cues. Bottom-up attention (also referred to as ‘exogenous’ or ‘stimulus-driven’ attention) refers to attention that is directed to a target by virtue of the target’s physical characteristics (e.g., high contrast). Top-down attention (also referred to as ‘endogenous’ or ‘task-driven’ attention) refers to attention that is directed to a target by virtue of the target’s relevance to a subject’s goals
- Feature/object-based attention & spatial attention
two major forms of attention distinguished by the parameters selectively processed. Target stimuli can be selectively processed (relative to distracters) by virtue of their component features or object identity (feature-based or object-based) or by virtue of their location within space (spatial)
References
- 1.Luck SJ, et al. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastner S, et al. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 4.Noudoost B, et al. Top-down control of visual attention. Curr Opin Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell JF, et al. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fries P, et al. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 7.Gregoriou GG, et al. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore T. The neurobiology of visual attention: finding sources. Curr Opin Neurobiol. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13:261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisley JW. The neural basis of visual attention. J Physiol. 2011;589:49–57. doi: 10.1113/jphysiol.2010.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 14.Ruff CC, et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: Evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekstrom LB, et al. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 17.Sarter M, et al. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Brennan AR, Arnsten AFT. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper JR, et al. The biochemical basis of neuropharmacology. Oxford University Press; 2003. [Google Scholar]
- 20.Arnsten AFT, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus-monkey. Brain Res. 1984;106:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- 21.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103:593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- 23.Frankle WG, et al. Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology. 2006;31:1627–1636. doi: 10.1038/sj.npp.1300990. [DOI] [PubMed] [Google Scholar]
- 24.Warburton DM, Rusted JM. cholinergic control of cognitive resources. Neuropsychobiology. 1993;28:43–46. doi: 10.1159/000118998. [DOI] [PubMed] [Google Scholar]
- 25.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 26.Mesulam MM. The systems-level organization of cholinergic innervation in the human cerebral cortex and its alterations in Alzheimer’s disease. Prog Brain Res. 1996;109:285–297. doi: 10.1016/s0079-6123(08)62112-3. [DOI] [PubMed] [Google Scholar]
- 27.Parasuraman R, et al. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- 28.Furey ML, et al. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warburton DM. Nicotine as a cognitive enhancer. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:181–191. doi: 10.1016/0278-5846(92)90069-q. [DOI] [PubMed] [Google Scholar]
- 30.Witte EA, et al. Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology. 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- 31.Guillem K, et al. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay N, et al. Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. II Cortical neurons excited by somatic stimuli. J Neurophysiol. 1990;64:1212–1222. doi: 10.1152/jn.1990.64.4.1212. [DOI] [PubMed] [Google Scholar]
- 33.Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory-cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- 34.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Disney AA, et al. Gain modulation by nicotine in macaque V1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrero JL, et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 39.Voytko ML, et al. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy FC, Klein RM. The effects of nicotineon spatial and non-spatial expectancies in a covert orienting task. Neuropsychologia. 1998;36:1103–1114. doi: 10.1016/s0028-3932(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 41.Davidson MC, et al. Scopolamine slows the orienting of attention in primates to cued visual targets. Psychopharmacology. 1999;142:1–8. doi: 10.1007/s002130050855. [DOI] [PubMed] [Google Scholar]
- 42.Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J Neurophysiol. 2000;83:1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- 43.Knierim JJ, Van Essen DC. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J Neurophysiol. 1992;67:961–980. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- 44.Lamme VAF. The neurophysiology of figure ground segregation in primary visual-cortex. J Neurosci. 1995;15:1605–1615. doi: 10.1523/JNEUROSCI.15-02-01605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burrows BE, Moore T. Influence and limitations of popout in the selection of salient visual stimuli by area V4 neurons. J Neurosci. 2009;29:15169–15177. doi: 10.1523/JNEUROSCI.3710-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asadollahi A, et al. Stimulus-driven competition in a cholinergic midbrain nucleus. Nat Neurosci. 2010;13:889–895. doi: 10.1038/nn.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mysore SP, Knudsen EI. Flexible categorization of relative stimulus strength by the optic tectum. J Neurosci. 2011;31:7745–7752. doi: 10.1523/JNEUROSCI.5425-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Missale C, et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 49.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–57. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Lidow MS, et al. Distribution of dopaminergic receptors in the primate cerebral-cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]Sch23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 51.Lidow MS, et al. Layer V neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 1998;28:10–20. doi: 10.1002/(SICI)1098-2396(199801)28:1<10::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 52.Goldman-Rakic PS, et al. The anatomy of dopamine in monkey and human prefrontal cortex. J Neural Transm Suppl. 1992:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- 53.Farde L, et al. PET analysis of human dopamine receptor subtypes using C-11 Sch 23390 and C-11 raclopride. Psychopharmacology. 1987;92:278–284. doi: 10.1007/BF00210831. [DOI] [PubMed] [Google Scholar]
- 54.Gaspar P, et al. D1 and D2 receptor gene-expression in the rat frontal cortex: cellular-localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 55.Santana N, et al. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- 56.Vijayraghavan S, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 57.Barcelo F, et al. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 58.Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noudoost B, Moore T. A reliable microinjectrode system for use in behaving monkeys. J Neurosci Methods. 2011;194:218–223. doi: 10.1016/j.jneumeth.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 61.McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell JF, et al. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Thompson KG, et al. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol. 2005;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 65.Armstrong KM, et al. Selection and maintenance of spatial information by frontal eye field neurons. J Neurosci. 2009;29:15621–15629. doi: 10.1523/JNEUROSCI.4465-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldman-Rakic PS. Cellular basis of working-memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 67.Gao WJ, et al. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mason DJ, et al. Exploring selective attention in ADHD: visual search through space and time. J Child Psychol Psychiatry. 2003;44:1158–1176. doi: 10.1111/1469-7610.00204. [DOI] [PubMed] [Google Scholar]
- 69.Ernst M, et al. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 71.Maunsell JHR. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Peck CJ, et al. Reward modulates attention independently of action value in posterior parietal cortex. J Neurosci. 2009;29:11182–11191. doi: 10.1523/JNEUROSCI.1929-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antelman SM, Caggiula AR. Norepinephrine-dopamine interactions and behavior. Science. 1977;195:646–653. doi: 10.1126/science.841304. [DOI] [PubMed] [Google Scholar]
- 74.Gotti C, et al. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnsten AFT. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:E89–E99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 78.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 79.Foote SL, et al. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grant SJ, et al. Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res Bull. 1988;21:401–410. doi: 10.1016/0361-9230(88)90152-9. [DOI] [PubMed] [Google Scholar]
- 81.Devilbiss DM, et al. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 2006;26:9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aston-Jones G, et al. Locus-coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 84.Arnsten AFT, et al. Alpha-2 adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol. 2007;17:393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- 85.Ma CL, et al. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha(2)-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 86.Ma CL, et al. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha(2)-adrenoceptors in monkeys. Biol Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]