Background: Mammary stem cells require a specific Wnt signaling receptor, LRP5, despite co-expression of the (better studied) LRP6 receptor.
Results: Both LRP5 and LRP6 are required for mammary epithelial cell responsiveness to Wnt1/9b/10b (but not Wnt3a).
Conclusion: LRP5 co-expression with LRP6 can confer active Wnt signaling where either receptor alone cannot.
Significance: This explains aspects of stem cell regulation and tumorigenesis.
Keywords: Breast Cancer, Lipoprotein-like Receptor (LRP), Mammary gland, Stem Cells, Wnt Signaling
Abstract
A canonical Wnt signal maintains adult mammary ductal stem cell activity, and this signal requires the Wnt signaling reception, LRP5. However, previous data from our laboratory have shown that LRP5 and LRP6 are co-expressed in mammary basal cells and that LRP6 is active, leading us to question why LRP6 is insufficient to mediate canonical signaling in the absence of LRP5. Here, we show that at endogenous levels of LRP5 and LRP6 both receptors are required to signal in response to some Wnt ligands both in vitro (in mouse embryonic fibroblasts and mammary epithelial cells) and in vivo (in mammary outgrowths). This subgroup of canonical ligands includes Wnt1, Wnt9b, and Wnt10b; the latter two are expressed in mammary gland. In contrast, the ligand commonly used experimentally, Wnt3a, prefers LRP6 and requires just one receptor regardless of cellular context. When either LRP5 or LRP6 is overexpressed, signaling remains ligand-dependent, but the requirement for both receptors is abrogated (regardless of ligand type). We have documented an LRP5-6 heteromer using immiscible filtration assisted by surface tension (IFAST) immunoprecipitation. Together, our data imply that under physiological conditions some Wnt ligands require both receptors to be present to generate a canonical signal. We have designed a model to explain our results based on the resistance of LRP5-6 heteromers to a selective inhibitor of E1/2-binding Wnt-LRP6 interaction. These data have implications for stem cell biology and for the analysis of the oncogenicity of LRP receptors that are often overexpressed in breast tumors.
Introduction
The Wnt signaling pathway plays critical roles in embryonic development and tissue homeostasis and regulates processes as diverse as cell migration, cell polarity, and adhesion (1, 2). The β-catenin/TCF3 (so-called “canonical”) pathway is essential to the self-renewal, proliferation, and differentiation of stem and progenitor cells in a variety of contexts and is highly oncogenic when dysregulated (3, 4). Genetic and biochemical data suggest that this pathway is activated when Wnt ligands interact with members of two distinct families of cell surface receptors, the Frizzled (Fzd) receptors and the low density lipoprotein receptor-related proteins LRP5 and LRP6 (5, 6). This initiates a signaling cascade (associated with phosphorylation of LRP species) (7–9), resulting in translocation of β-catenin to the nucleus where it interacts with the LEF-1/TCF family of transcription factors to modulate transcription of Wnt target genes (10, 11). In vertebrates, there are several Wnt ligands and Fzd receptors (19 Wnt ligands and 10 Fzd homologues) but only two LRP species with defined roles in Wnt signaling (1). Although Wnt-Fzd interactions orchestrate the activation of both β-catenin/TCF-dependent and -independent pathways, the LRP family of Wnt receptors specifically mediates activation of the β-catenin/TCF arm of the pathway (12–16).
LRP5 and LRP6 are type I, single span transmembrane receptors with a large extracellular domain shown to bind several Wnt ligand species tested in vitro (14, 15, 17, 18). In addition to Wnt proteins, the extracellular regions of LRP5 and LRP6 also bind other agonists and antagonists of the Wnt pathway, including members of the Dkk family, Sclerostin, and Wise (17, 19–22). Presumably, the output of LRP receptor activation represents the sum total of these interactions. LRP5 and LRP6 exhibit a high degree of sequence homology, sharing 73 and 64% sequence identity in their extracellular and intracellular domains, respectively (15). This, coupled with extensive similarities in structural and biochemical properties, has led to the assumption of functional redundancy between the two receptors. However, in vivo studies show that the two receptors mediate unique functions. Although homozygous deletion of LRP6 in transgenic mice leads to perinatal lethality, LRP5 knock-out mice are viable and fertile (18, 23, 24). Early lethality of LRP6 knock-out mice has hindered systematic, comparative studies of the relative contributions of the two receptors to Wnt signaling in vivo. In this study, we evaluated the relative signaling potential of LRP5 and LRP6 when expressed at endogenous levels in contrast to many other molecular structure/function studies that have relied on ectopic overexpression of the two receptors (13, 25, 26). This revealed a novel, ligand-dependent restriction of their activities.
We have shown previously that lrp5−/− mammary glands exhibit depleted mammary stem cell activity and are protected from Wnt1-mediated tumorigenicity despite expression of LRP6 (24). The absence of LRP5, however, has no effect on Wnt3a-mediated transactivation of the canonical Wnt pathway in lrp5−/− mammary epithelial cells (MECs) (27). These data suggest that LRP5 and LRP6 serve different functions in the mouse mammary gland, although the mechanistic details remain unknown. One intriguing hypothesis to explain the distinct roles of LRP5 and LRP6 is that different Wnt ligands activate the canonical Wnt pathway by preferentially signaling through either LRP5 or LRP6.
To test this hypothesis, we used mouse embryonic fibroblasts (MEFs) harvested from wild type, lrp5−/−, and lrp6−/− embryos and treated them with different Wnt ligands. We observed that whereas Wnt3a requires LRP6 to activate the Wnt pathway another group of Wnt ligands, including Wnt1, -9b, and -10b, requires both LRP5 and LRP6 for optimal Wnt pathway activation in MEFs. Using a modified immunoprecipitation assay (IFAST), we obtained evidence that LRP5 and LRP6 exist in mixed heteromeric signaling complexes. Furthermore, we show that when these receptors are overexpressed either LRP5 or LRP6 can mediate Wnt1 class signals. The dual requirement for LRP5 and LRP6 was also observed in vivo for mammary gland outgrowth. Our studies illustrate an important regulatory mechanism operating in vivo with implications for specific ligand-receptor complexes functioning as mediators of various Wnt-dependent physiologies.
EXPERIMENTAL PROCEDURES
Cell Culture
MEFs were harvested from 13.5-day pregnant C57Bl/6 lrp5+/− or lrp6+/− mice (that were crossed to heterozygous males). Briefly, embryos were diced and trypsinized (0.05% trypsin, EDTA) for 10 min at 37 °C followed by resuspension in growth medium. The genotype of each individual embryo was identified (+/+, +/−, or −/−). MEFs and HEK293T cells were maintained in low glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (Harlan, Indianapolis, IN) and 100 units/ml penicillin/streptomycin (Invitrogen). MEFs were propagated in low oxygen chambers (1% O2 content) to extend the lifespan of the primary cells (28). HC11 cells (gift of Nancy Hynes, Friedrich Miescher Institute, Basel, Switzerland) were cultured in RPMI 1640 medium containing 10% FBS (Harlan), 5 μg/ml insulin (Sigma-Aldrich), and 10 ng/ml recombinant human EGF (R&D Systems, Minneapolis, MN). MECs harvested from wild type or lrp5−/− mammary glands were maintained as described previously (27).
Plasmids and Reagents
cDNA constructs encoding mouse Wnt3a and Wnt10b were generously provided by Bart Williams (Van Andel Research Institute, Grand Rapids, MI) and Ormond MacDougald (University of Michigan Medical School, Ann Arbor, MI) (29, 30), respectively. Wnt1 expression plasmid (31) was subcloned into the retroviral vector Pcmmp-MCS (multiple cloning site)-IRES-eGFP. An expression plasmid for mouse LRP5 (pCMV-SPORT6-LRP5) was purchased from Open Biosystems (Huntsville, AL), and an expression plasmid for mouse LRP6 was generated by subcloning the NotI-cut cDNA from pYX-Asc-LRP6 (Invitrogen) into pCDNA3 mammalian expression vector. The LRP5-myc and LRP6-myc tagged constructs were generously provided by Gail V. W. Johnson (University of Rochester, Rochester, NY) and Anthony M. C. Brown (Weill Cornell Medical College, New York, NY), respectively (12, 26). Lentiviral vectors designed to express either Wnt1 or Wnt3a were assembled using the backbone of a self-inactivating lentiviral vector (SIN) with an EF1α promoter (a kind gift from Dr. Robert G. Hawley, American Red Cross). To create a bicistronic vector, an IRES DNA fragment was inserted at the MCS followed by a human placental alkaline phosphatase (hPAP) cDNA construct (a gift from Dr. Deborah A. Brown, State University of New York, Albany, NY) (SIN-EF1α-MCS-IRES-hPAP). Recombinant mouse Wnt3a (100 ng/ml unless otherwise indicated), Wnt5a (40 ng/ml), and Wnt9b (400 ng/ml) were purchased from R&D Systems.
Generation of Lentiviral Particles
The two series of lentiviral vectors used for this study, SIN-EF1α expression vectors that encode Wnt1 or Wnt3a (or the control MCS plasmid) and vesicular stomatitis virus G and viral polymerase/core protein constructs, were transfected into HEK293T cells using Lipofectamine LTX (Invitrogen) according to the manufacturer's instructions, and lentiviral particles were harvested from the cell supernatant 48–72 h later. Commercially available expression vectors (pLKO.1) from Open Biosystems were used to express shRNA constructs targeting lrp5 (catalog number RMM4534-NM_008513) or lrp6 (catalog number RMM4534-NM_008514) and packaged using Lipofectamine LTX (including a scrambled control). Cells were infected with the viral supernatant with 8 μg/ml Polybrene (Sigma) and maintained in medium with puromycin (1 μg/ml for MEFs and 6 μg/ml for HC11 cells as determined from puromycin kill curves).
Transient Transfections/Viral Transductions
All transient transfections in MEFs and HC11 cells were performed using Lipofectamine LTX reagent (Invitrogen) by following the manufacturer's protocol designed for MEFs. Briefly, 0.02 × 104 cells were plated in 24-well plates, and a total amount of 0.5 μg of plasmid was added to each well. Transfections were scaled up to 6-well plates/60-mm dishes when performed for quantitative PCR or Western blot analysis.
Methods from Welm et al. (32) and Britt et al. (33) were adapted for viral transductions of MECs in suspension. 0.5 × 106 wild type or lrp5−/− MECs were plated in 24-well low attachment plates and infected with viral supernatant for 16 h (32, 33). The cells were collected, spun down at 450 × g for 5 min, and used for in vivo transplantation assays or plated in 6-well plates for evaluation of transduction efficiency and for qRT-PCR analysis. To assay for transduction efficiency of Wnt1- and Wnt3a-expressing viruses, cells were stained for human placental alkaline phosphatase activity; transduction efficiency was similar (approximately 60%).
Fat Pad Assays of Outgrowth Potential in Vivo
Mammary glands of 3-week-old C57Bl/6 virgin mice were cleared of endogenous epithelium. MECs transduced with different lentiviral constructs were resuspended in DMEM containing 5 μg/ml Matrigel and loading dye (5% glycerol, 0.5% trypan blue, 25 mm HEPES, pH 7.2). 1-μl volumes containing 50,000 cells were injected into cleared fat pads, and outgrowths were harvested 8 weeks post-transplantation as described previously (27).
Quantitative Real Time PCR Analysis
RNA isolation, cDNA generation, and amplification by real time PCR were performed as described previously (27). Relative transcript levels were calculated using the comparative Ct method and normalized to housekeeping genes, ywhaz and hprt1. The 5′ to 3′ sequences of the primer pairs are detailed in Ref. 34.
Western Blot Analysis
Cells were solubilized in lysis buffer (25 mm HEPES, pH 7.4, 300 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA, 50 mm glycerophosphate, 0.5% Triton X-100) with freshly added protease and phosphatase inhibitors (Thermo Fisher Scientific). Western blotting analysis was performed as described previously (27). Membranes were incubated (overnight at 4 °C) with the following primary antibodies at the dilutions indicated: anti-LRP5, D5G4, 1:1000 (Cell Signaling Technology catalog number 5440); anti-LRP6 antibodies, either 1C10, 1:1000 (Abcam catalog number ab75358) or C47E12, 1:1000 (Cell Signaling Technology catalog number 3395); anti-phospho-LRP (Ser-1490), 1:1000 (Cell Signaling Technology catalog number 2568); anti-β-Actin, 1:5000 (Sigma); and anti-myc, 71D10, 1:1000 (Cell Signaling Technology catalog number 2278). To quantify the bands obtained via Western blotting, ImageJ (version 1.43u) software-based analysis was applied.
To determine the relative signal from mouse LRP5 and LRP6 proteins after Western blotting, HEK293T cells were transfected with LRP-myc constructs, and a dilution curve of total protein was loaded for SDS-PAGE. To quantify relative myc-tagged protein amounts, the Western blotting procedure and exposure times were strictly standardized. Replicated pixel numbers for each dilution of each antigen (myc, LRP5, and LRP6) were combined to assess linearity of response, and the relative signal for the specific (LRP5 and LRP6) antigens was determined with respect to the myc tag on each protein.
Dual-Luciferase Assay
To measure canonical Wnt signaling activity using the superTOP-FLASH reporter assay, MEFs or HC11 cells were transfected with the various expression vectors as indicated in the experiments together with superTOP-FLASH reporter plasmid (0.1 μg) and Renilla luciferase gene (0.01 μg) using Lipofectamine LTX (Invitrogen). The total transfected DNA amount was kept constant at 0.5 μg by transfection with the Pcmmp-MCS-IRES-eGFP expression plasmid, and the reporter assays were performed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) as described previously (31). To normalize for transfection efficiency, firefly luciferase activity was divided by Renilla luciferase activity. The value was expressed as relative luminescence units, and the average relative luminescence units were calculated for triplicate samples.
IFAST Immunoprecipitation
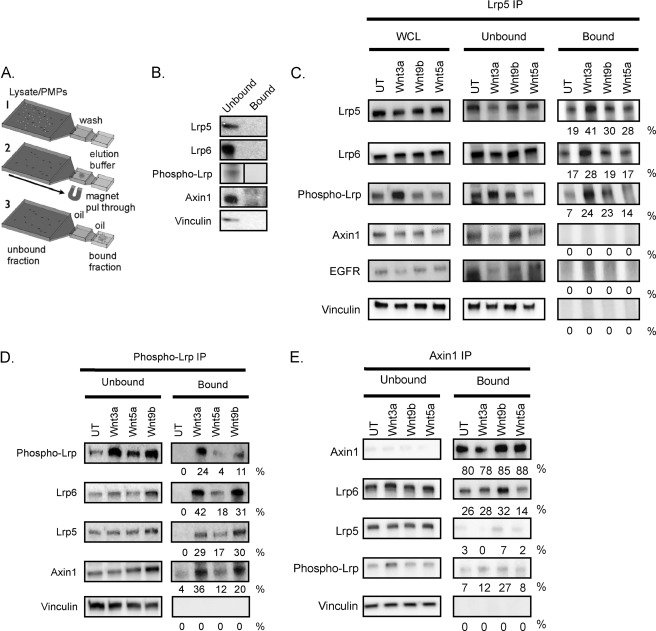
Wild type MEFs were treated for 2 h with Wnt3a (20 ng/ml), Wnt9b (400 ng/ml), Wnt5a (40 ng/ml), or nothing. Cells were lysed in immunoprecipitation (IP) lysis buffer (10 mm sodium phosphate, pH 7.2, 150 mm NaCl, 1% Nonidet P-40, 1× protease inhibitor mixture, 1× phosphatase inhibitor mixture) as described previously (35). Approximately 300 μg of lysate (1.5 μg/ml) was incubated with either 1.2 μg of anti-LRP5 (Cell Signaling, catalog no. 5731), 130 ng of anti-Axin1 (Cell Signaling, catalog no. 3323), 450 ng of anti-Phospho-LRP (Cell Signaling, catalog no. 2568), or 1.2 μg rabbit IgG antibody (Sigma, catalog no. I5006), for 1 h while rotating at 4 °C. 5 μl of prewashed protein G Dynabeads (Invitrogen catalog number 100-03D) were added to each tube, and lysates were rotated overnight with antibody and beads at 4 °C. IP complexes were purified using the IFAST method (illustrated in Fig. 7A) (36). Immune complexes were eluted in the output well in PBS containing 0.01% Tween 20 (17 μl). Prior to the IP reaction, whole cell lysate was withdrawn for analysis. Gels were loaded with 10 μg of whole cell lysate, 10 μg of unbound protein (defined as the proteins remaining in the lysate after pull-through, which exceeded 95% of whole cell lysate, measured by protein assay) and 2 μl (out of 17 μl total) of the bound protein fraction. LRP5, LRP6, phospho-LRP, and Axin1 proteins were detected by incubating blots with the respective antibodies listed previously. To determine the specificity of the interactions, blots were incubated with anti-vinculin (Chemicon catalog number CBL233) at a 1:5000 dilution and/or anti-EGF receptor antibody (Cell Signaling Technology catalog number 2232) at a 1:1000 dilution. To calculate the efficiency of binding and pull-through of immune complexes, band intensities from the unbound and bound fractions were quantified and corrected for background using the UVP ChemiDoc-It Imaging System with Visionworks software. The total protein amount for each fraction was calculated as the product of intensities and total volumes. The percent extraction efficiency for each protein was approximated by dividing the signal equivalent to the bound protein by the signal from total (unbound plus bound) protein fractions.
FIGURE 7.
Evidence for LRP5-LRP6 complexes. A, the IFAST method for immunoprecipitation (36). Microchannel devices contain 3 wells connected by oil. Antibody, paramagnetic particles (PMPs), and cell lysate are combined in the input well (left-hand side), and complexes are pulled through into the wash well and the final elution well (right-hand side) by means of a magnet (according to the “Experimental Procedures”). B, specificity control. Cell lysate (300 μg; wild type MEFs) was incubated with non-immune IgG and paramagnetic particles and processed. Lanes are labeled “Unbound” (input after pull-through of paramagnetic particles) and “Bound” (output after elution). C, evaluation of co-immunoprecipitation with LRP5. MEFs were treated with no ligand (UT), Wnt3a (20 ng/ml), Wnt9b (400 ng/ml), or Wnt5a (40 ng/ml) for 2 h, lysed, and immunoprecipitated with anti-LRP5 (1.2 μg) and paramagnetic particles. The analysis of whole cell lysate (WCL; before IP) is shown on the left-hand side together with unbound and bound fractions. The efficiency of pull-through was calculated as described under “Experimental Procedures” (and presented here as a percentage). The same or parallel blots were probed for LRP6, phospho-LRP6, Axin1, and two specificity controls, EGF receptor (EGFR) and vinculin. D, evaluation of co-immunoprecipitation with Axin1. E, evaluation of co-immunoprecipitation with phospho-LRP. Results shown are representative of three separate MEF batches.
RESULTS
Composition and Amount of LRP Species Are Similar in MEFs and MECs
We have shown previously that LRP5 is key to Wnt1-dependent tumor development in the murine mammary tumor virus-Wnt1 transgenic mouse model despite expression of LRP6, and that Wnt3a-dependent transactivation of the canonical Wnt pathway is not significantly affected in lrp5−/− MECs (24, 27). To understand the molecular basis for this apparent paradox, we turned to MEFs as a potential culture model. (lrp6−/− MECs are not readily available given the perinatal lethality of lrp6−/− mice (23)). We derived six types of MEFs from embryos harvested from interbred lrp+/− heterozygotes (wild type, lrp+/−, and lrp−/− from C57Bl/6 strains). The results we show here are derived from comparisons of wild type and knock-out MEFs (several assays also included heterozygous strains and show predictable, dose-dependent phenotypes; data not shown). MEF cultures were maintained in low oxygen (allowing survival over many generations) (28), and key results were repeated with separate batches of MEFs. The LRP5/LRP6 genotype of the MEF cultures did not grossly affect their morphology or growth characteristics (data not shown).
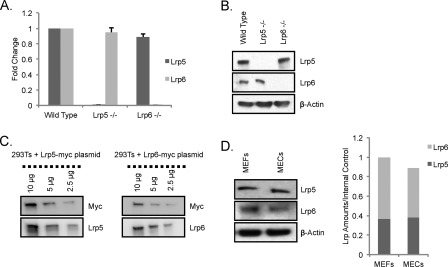
To examine the characteristics of this model, we evaluated the amounts of LRP5 and LRP6 in MEFs by various means. qRT-PCR and Western blot analyses showed that both LRP5 and LRP6 are present in cultured MEFs (Fig. 1, A and B). As expected, LRP5 and LRP6 transcripts were undetectable in RNA isolated from lrp5−/− and lrp6−/− MEFs, respectively (Fig. 1A). Western blot analysis using lrp5−/− and lrp6−/− MEFs confirmed the specificity of the LRP5 and LRP6 antibodies (Fig. 1B). Importantly, there was no compensatory up-regulation of lrp6 mRNA or protein in the lrp5−/− MEFs (or vice versa).
FIGURE 1.
LRP5 and LRP6 expression in MEFs and MECs. A, lrp5 and lrp6 mRNA expression in wild type, lrp5−/−, and lrp6−/− MEFs. To test for compensatory expression of alternate LRP species in knock-out cells, lrp5 and lrp6 mRNA was quantified using qRT-PCR analysis of RNA harvested from wild type, lrp5−/−, and lrp6−/− MEFs. Values shown represent -fold changes compared with wild type MEFs (set to 1) (after normalization to housekeeping genes). B, LRP5 and LRP6 protein expression in wild type, lrp5−/−, and lrp6−/− MEFs. Western blots of protein lysates prepared from wild type, lrp5−/−, and lrp6−/− MEFs after probing with anti-LRP5 or -LRP6 (and β-actin) antibodies are shown. C, comparison of the relative signal per molecule for anti-LRP5 and anti-LRP6 antibodies. HEK293T cells were transfected with LRP5-myc and LRP6-myc plasmids, and doubling dilutions of total protein (10, 5, and 2.5 μg) were probed with anti-myc antibody and with anti-LRP5 or -LRP6 antibody to determine the relative signal with respect to the myc tag (image analysis is described under “Experimental Procedures”). D, determination of relative LRP5 and LRP6 protein expression in MEFs and MECs. Protein extracts prepared from MEFs and MECs were analyzed by SDS-PAGE, and the relative amounts of LRP5 and LRP6 expressed by the two cell types were determined. Error bars show standard deviations.
Very few studies have evaluated the function of endogenous levels of LRP5 and LRP6 as we do here. We compared the expression levels of these two receptors in MECs (the cell type of interest in vivo) and in MEFs (the principal assay model). To determine the relative amounts of LRP5 and LRP6, we assessed the relative detection efficiencies of LRP5 and LRP6 proteins by their cognate antibodies. Constructs encoding LRP5 and LRP6 receptors with a carboxyl-terminal myc tag were transfected into HEK293T cells. Using the internal myc tag, the relative number of LRP5 or LRP6 protein molecules could be compared (Fig. 1C). Specific detection with anti-LRP5 and anti-LRP6 antibodies revealed that the LRP5 antibody is ∼60% more efficient at detecting its target protein than the anti-LRP6 antibody. Using this information, we calculated the ratio of endogenous LRP5 to LRP6 protein in MEFs to be 1.5. We also compared the relative expression of LRP5 and LRP6 in MECs and MEFs and found that the ratio of LRP5:LRP6 is similar in these two cell types (Fig. 1D).
LRP6 Is Principal Transducer of Wnt3a-mediated Canonical Wnt Signaling in MEFs
Wnt3a is by far the most commonly used Wnt ligand for in vitro studies of Wnt signaling due to its solubility and availability. To test Wnt3a-mediated responses, MEFs were transfected with a plasmid encoding Wnt3a, and canonical Wnt signaling activity was measured using the β-catenin/TCF-dependent reporter assay (TOP-FLASH assay) 36 h post-transfection (31) (see supplemental Fig. S1A, time course of Wnt reporter activity). These responses are specific, and no activation was observed with the control construct, FOP-FLASH (data not shown).
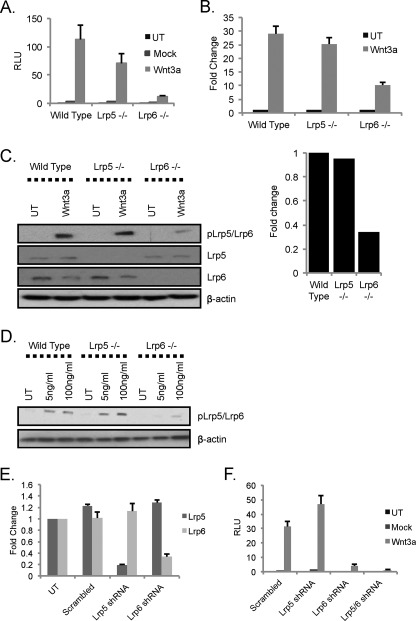
As we showed previously for lrp5−/− MECs, lrp5−/− MEFs do not show dramatic differences in Wnt3a-induced β-catenin/TCF transactivation compared with wild type MEFs (TOP-FLASH was reduced by approximately 30%; Fig. 2A). In contrast, absence of LRP6 resulted in reduction of Wnt signaling activity by 80% (Fig. 2A). To confirm this result, we also assayed expression of an endogenous Wnt reporter, Axin2 (a consistent and robust Wnt reporter across many cell types (27, 37)), using qRT-PCR. In agreement with the results from the TOP-FLASH assay, Wnt3a-dependent induction of Axin2 mRNA was attenuated (∼70%) in lrp6−/− MEFs and was independent of LRP5 status (Fig. 2B).
FIGURE 2.
LRP6 is principal transducer of Wnt3a-mediated canonical Wnt activity in MEFs. A, assay of Wnt3a-induced TOP-FLASH Wnt reporter activity. Wild type, lrp5−/−, and lrp6−/− MEFs were transfected with the Wnt reporter superTOP-FLASH along with a mock plasmid or a plasmid encoding mWnt3a and a transfection control (Renilla luciferase). 36 h post-transfection, expression of the reporter plasmid was assayed (normalized to Renilla luciferase). Results are shown for triplicate wells, and the results are representative of three different sets of wild type, lrp5−/−, and lrp6−/− MEFs. B, assay of Wnt3a-induced activation of the endogenous Wnt reporter gene, Axin2. Wild type, lrp5−/−, and lrp6−/− MEFs were transfected with the mWnt3a expression construct, and 36 h later, RNA was analyzed by qRT-PCR analysis for Axin2 mRNA expression. Values show -fold change over untreated samples (set to 1) following normalization to housekeeping genes ywhaz and hprt1. C, proximal readout of Wnt3a-mediated LRP activation in MEFs. Wild type, lrp5−/−, and lrp6−/− MEFs were treated with rmWnt3A (100 ng/ml) for 16 h. Protein lysates were analyzed by immunoblotting with the indicated antibodies. Quantification of band intensities was performed using ImageJ analysis and expressed as the -fold difference in Wnt3a-mediated phospho-LRP5/6 induction levels in lrp5−/− and lrp6−/− MEFs relative to wild type MEFs. D, effect of LRP repertoire on receptor activation at a low dose of rmWnt3a. Wild type, lrp5−/−, and lrp6−/− MEFs were treated with rmWnt3A (100 or 5 ng/ml) and processed as described in C. E, efficiency of RNAi-mediated knockdown of LRP5 and LRP6. Wild type MEFs were transduced with lentiviral shRNA constructs targeting lrp5, lrp6, or a scrambled control. RNA was isolated 36 h later, and relative knockdown and specificity were evaluated by qRT-PCR analysis. F, Wnt3a signaling in MEFs following shRNA-mediated knockdown of lrp5 or lrp6. The MEFs described in D were assayed for Wnt responsiveness 36 h after co-transfection with superTOP-FLASH, mock plasmid, or mWnt3a and Renilla luciferase (processed as for A). UT, untransduced; RLU, relative luminescence units. Error bars show standard deviations.
Binding of a Wnt ligand to its cognate receptor(s) activates a signal transduction cascade that results in phosphorylation of the LRP receptors (7–9). Using an antibody shown previously to detect phosphorylation of LRP6 at Ser-1490 in response to Wnt treatment (38), we detected a band of the expected size (210 kDa) in lrp6−/− MEFs. This suggests that the antibody is not specific to LRP6 but also detects phospho-LRP5 phosphorylated at the corresponding residue (Ser-1493). Exposure to rmWnt3a (for 16 h) resulted in robust phosphorylation of LRP5/LRP6 in wild type and lrp5−/− MEFs but a 70% reduction in lrp6−/− MEFs (Fig. 2C). To test whether this selectivity is related to ligand concentration, we assayed lower concentrations of rmWnt3a. At 5 ng/ml, a concentration 20× less than that commonly used, reporter expression was induced 50× (compared with 400× for 100 ng/ml; supplemental Fig. S1B). Even at 5 ng/ml, LRP5 was not required for Wnt3a responses, and LRP6 was the predominant signaling receptor (Fig. 2D).
To rule out the possibility that our results from knock-out MEFs are due to chronic adaptation to the absence of LRP5 or LRP6 expression, we used an RNAi-mediated approach to test acute effects of LRP knockdown. MEFs were transduced with lentiviral shRNA constructs specifically targeting either lrp5 or lrp6 mRNAs (showing approximately 80 and 70% knockdown respectively; Fig. 2E), and Wnt3a-dependent TOP-FLASH activation was assayed (Fig. 2F). This approach confirmed that LRP5 has little role in transmitting signals from Wnt3a, whereas LRP6 knockdown reduced trans-activation by at least 90%. The double knockdown of LRP5 and LRP6 confirmed that any TOP-FLASH signal above background requires the presence of LRP (LRP5/LRP6 double knock-out MEFs are not available because this genotype results in gastrulation defects (15)). Taken together, these multiple assays support the identification of LRP6 as the principal transducer of Wnt3a-dependent canonical signaling. This result is supported by the recent results of MacDonald et al. (39), who attributed this enhanced signaling to the presence of a specific gap sequence between the phosphorylatable PPPSPXP motifs of the carboxyl-terminal domain of LRP6.
LRP5 and LRP6 Are Both Required for Efficient Wnt1-mediated Canonical Wnt Signaling in MEFs
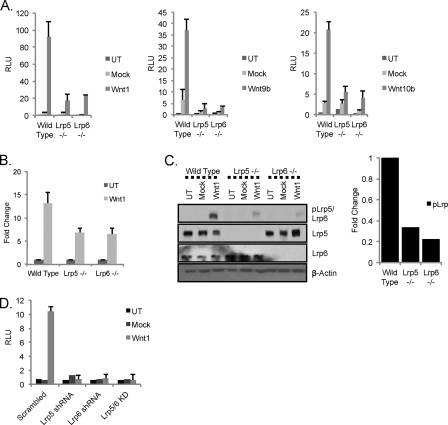
Wnt3a is not often used in vivo to study gain of function in mice, and indeed our data for the unique functionality of LRP5 were derived from murine mammary tumor virus-Wnt1 transgenic mice. Therefore, we turned next to the study of Wnt1. The same experimental series performed for Fig. 2 were used to evaluate the roles of LRP5 and LRP6 in Wnt1-induced signaling (Fig. 3). A dramatic loss of canonical Wnt activity in response to Wnt1 was observed in the absence of either LRP5 or LRP6 (approximately 80%; Fig. 3A). Recently, structure/function analyses have proposed different binding sites for Wnt ligands on LRP6 receptors. Specifically, these studies have provided evidence that Wnt3a belongs to a class of ligands that binds the E3-E4 domain, whereas Wnt1, Wnt9b, and Wnt10b belong to a different class that binds the E1-E2 domain of LRP6 (40, 41). Using this information and the expression of Wnt ligands in mouse mammary gland to guide our choices, we tested whether Wnt9b and Wnt10b also require both LRP receptors and found that they too show a dual requirement for the LRP receptors (Fig. 3A).
FIGURE 3.
LRP5 and LRP6 are both required for efficient signaling in response to Wnt1. A, absence of either LRP5 or LRP6 results in loss of canonical Wnt activity in response to Wnt1. Wild type, lrp5−/−, and lrp6−/− MEFs were transfected with the Wnt reporter superTOP-FLASH (and Renilla luciferase) along with plasmids encoding mWnt1 or mWnt10b (left- and right-hand side, respectively) (or without the ligand expression plasmids; mock) or with ectopic addition of rmWnt9b (400 ng/ml; middle panel). Cells were processed as described for Fig. 2A. B, Wnt1-dependent transcriptional activation of Axin2. Wild type, lrp5−/−, and lrp6−/− MEFs were plated, transfected with mWnt1 plasmid, and processed for analysis of Axin2 mRNA levels (as per Fig. 2B). C, Wnt1-mediated proximal canonical Wnt signaling. Wild type, lrp5−/−, and lrp6−/− MEFs were transduced with lentiviral constructs expressing Wnt1 or the vector backbone (Mock) or untransduced (UT). 36 h later, proteins were analyzed by Western blotting as described for Fig. 2C. Relative phospho-LRP activation was quantified (right-hand side). D, Wnt1 signaling in MEFs following shRNA-mediated knockdown of lrp5 or lrp6. Wild type MEFs were transduced with lentiviral shRNA constructs targeting lrp5, lrp6, or both mRNAs. 24 h later, cells were transfected with TOP-FLASH, Renilla luciferase, and mWnt1 expression construct and processed as for Fig. 2D. RLU, relative luminescence units; KD, knockdown. Error bars show standard deviations.
The requirement for both LRP receptors is also evident from measurement of endogenous Axin2 mRNA expression levels (reduced equivalently by 50% in both lrp5−/− and lrp6−/− MEFs; Fig. 3B) and by assay of activated phospho-LRP (reduced by at least 70% in each knock-out cell strain; Fig. 3C). We compared results from knock-out cell strains with those derived from cells subjected to shRNA-mediated knockdown of lrp5 and lrp6 mRNAs (Fig. 3D). Loss of either LRP species resulted in the loss of 90% of Wnt signaling activity as measured by the TOP-FLASH reporter. We conclude that, for the Wnt1 class of ligands (defined here to include Wnt9b and -10b and to exclude Wnt3a), there is a functional interaction between LRP5 and LRP6 receptors, resulting in the requirement for both receptors to be present to generate an active Wnt signaling complex.
Exogenous Expression of LRP5 or LRP6 Eliminates Requirements for Specific Wnt-LRP Complexes
We tested whether the requirement for both receptor species was also true when LRP species are overexpressed for the following two reasons. First, most previous studies have used overexpression to test for ligand-receptor signaling activity, and none have demonstrated the dual requirement for LRP receptors observed in our study. Second, LRP overexpression has been linked to the pathogenesis of breast tumors (42, 43), and this condition is therefore likely to be important physiologically.
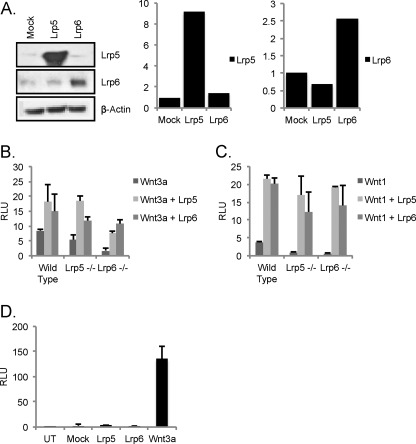
Wild type MEFs were transfected with expression constructs for LRP5 and LRP6, producing 9× and 2.5× overexpression, respectively (Fig. 4A). To test whether cells overexpressing LRP receptors to the levels reported in Fig. 4A are still ligand-dependent for Wnt transactivation, these cells were transfected with the TOP-FLASH Wnt reporter (Fig. 4B). Signaling was undetectable (over background) without the introduction of Wnt ligands. To evaluate the properties of cells expressing higher levels of just one LRP species, lrp5−/− and lrp6−/− MEFs were transfected with LRP6 and LRP5, respectively (and with LRP5 and LRP6 for comparison). LRP6 expression in lrp6−/− MEFs produced the (approximately proportional) rescue of Wnt3a-dependent responses predicted (Fig. 4C). Interestingly, overexpression of LRP5 was also able to rescue Wnt3a-dependent responses (despite being largely ineffective at transducing Wnt3a signals at endogenous levels/ratios). Most surprising was the result of testing for Wnt1 responses when LRP5 was overexpressed in lrp6−/− MEFs (high levels of LRP5 present). In this case, LRP5 alone was sufficient, and the dual requirement was eliminated. In fact, LRP5 and LRP6 were equally effective at rescuing responses to both Wnt ligand types (Fig. 4D).
FIGURE 4.
Ectopic expression of either LRP5 or LRP6 eliminates requirement for both LRP5 and LRP6. A, overexpression of LRP5 and LRP6 in wild type MEFs. Wild type MEFs were transfected with constructs encoding full-length mLRP5, mLRP6, or a control construct (GFP plasmid; Mock). 36 h later, protein extracts were probed with anti-LRP antibodies as indicated, and the relative amount of expression was quantified. B–D, assay of ligand dependence of signaling in MEFs overexpressing LRP species. To test whether MEFs overexpressing LRP receptors to the levels reported in A were still ligand-dependent for Wnt transactivation, cells were transfected with the TOP-FLASH Wnt reporter (B), and luciferase activity was measured 36 h later. Signaling was undetectable (over background) without the introduction of Wnt ligands (D). To test the signaling properties of single receptor species in knock-out backgrounds, wild type, lrp5−/−, and lrp6−/− MEFs were transfected with the Wnt reporter superTOP-FLASH (and the transfection control Renilla luciferase) together with mWnt3a (B) or mWnt1 (C) and either LRP5 or LRP6 receptor. 36 h post-transfection, cells were lysed, and Wnt signaling activity was measured. UT, untransduced; RLU, relative luminescence units. Error bars show standard deviations.
Not All Cell Types Show Ligand-specific Receptor Requirements
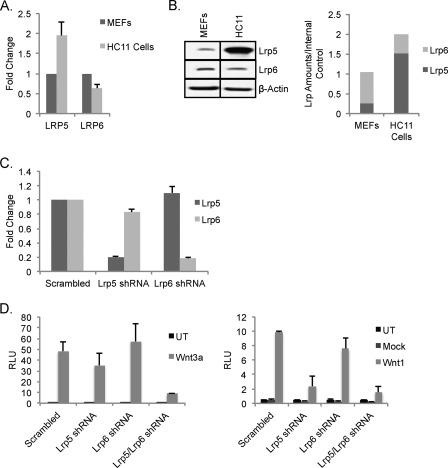
To further develop the idea that endogenous levels of LRP5 and LRP6 modulate ligand-receptor specific signaling, we identified cells with endogenous LRP5 and LRP6 expression levels that are different from MECs and MEFs. Thus, the HC11 mouse mammary epithelial cell line, a clonal derivative of the COMMA-1D cell line, that has been used previously as an in vitro model for Wnt signaling in the mammary gland (44, 45) was examined. Evaluation of mRNA levels of LRP5 and LRP6 showed that HC11 cells express both LRP5 and LRP6 but have 2× higher lrp5 mRNA and 4× higher LRP5 protein (Fig. 5, A and B). The overall amount of LRP5/6 protein is 2-fold higher in MEFs, and the ratio of LRP5:LRP6 protein is ∼0.25 for MEFs compared with ∼3.0 for HC11 cells. Knocking down LRP5 or LRP6 alone (Fig. 5C) had broadly similar effects on Wnt3a and Wnt1 responses. Knockdown of LRP5 was more effective than knockdown of LRP6, but neither alone produced a loss of function that was comparable with the knockdown of both species (Fig. 5, D and E). Given the relative receptor species concentration, this result was predictable. There is therefore little evidence from HC11 cells to suggest that loss of function of one receptor impacts the function of the remaining receptor species.
FIGURE 5.
Mammary epithelial cells with endogenously high expression levels of LRP5 compared with MEFs do not require LRP6 for Wnt1-responsiveness. A, evaluation of LRP expression patterns in HC11 cells. The relative mRNA expression of LRP5 and LRP6 was compared for MEFs and HC11 cells using qRT-PCR analysis (normalized to housekeeping genes YWHAZ and hypoxanthine-guanine phosphoribosyltransferase). B, relative LRP expression in HC11 cells. Wild type MEFs and HC11 cells were harvested and analyzed for their LRP expression (using β-actin as a loading control) as for Fig. 1. C, RNAi-mediated knockdown of LRP5 and LRP6. HC11 cells were transduced with lentiviral shRNA constructs targeting lrp5, lrp6, or a scrambled control (as for Fig. 2E). qRT-PCR analysis shows the specificity and efficiency of the shRNA constructs. D, evaluation of the requirement for LRP5 and/or LRP6 in response to Wnt3a and Wnt1 in HC11 cells. Knockdown cells (prepared as for C) were transfected with superTOP-FLASH, Renilla luciferase, and either mWnt3a or mWnt3a (D). Cells were lysed 36 h post-transfection, and superTOP-FLASH reporter activity was measured using a luciferase assay. Results are shown from triplicate wells and are representative of three independent experiments. UT, untransduced; RLU, relative luminescence units. Error bars show standard deviations.
Dual Requirement for Both LRP5 and LRP6 for Wnt1-dependent Responses Is Also Observed in Vivo
We considered the possibility that the dual requirement revealed by MEFs could be associated with a regulatory mechanism unique to this model system. To test whether the dual dependence applies to other cell types and to determine the importance of this for specific Wnt responses, we evaluated a Wnt-dependent preneoplastic physiology in vivo.
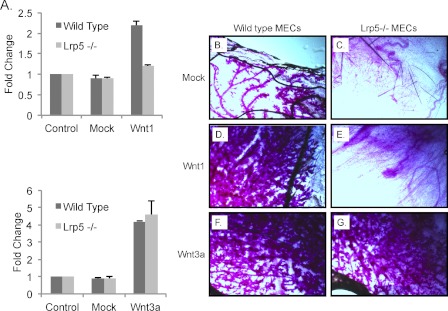
Wild type and lrp5−/− MECs were transduced with lentiviral constructs encoding Wnt1 or Wnt3a (in suspension; see “Experimental Procedures”), transferred to cleared mammary fat pads of wild type mice, and allowed to develop in vivo for 8 weeks. Some of these cells were transferred to primary culture to evaluate their infection efficiency and to test their Wnt signaling response (by qRT-PCR analysis of Axin2 mRNA expression). Consistent with our previous findings from mWnt3a-treated MECs (27), the absence of LRP5 did not affect the robust induction of Axin2 in response to Wnt3a. However, the response to Wnt1 was ablated in the absence of LRP5 (Fig. 6A).
FIGURE 6.
Wnt1 class of ligands requires LRP5 to induce hyperplasia in vivo in mammary glands, whereas Wnt3a does not. A, Wnt1- and Wnt3a-induced transcriptional activation of Axin2 expression. Wild type and lrp5−/− MECs were transduced with lentiviral expression vectors for mWnt1 or mWnt3a (or a mock lentiviral vector) in suspension. For evaluation of expression, some cells were transferred to culture, and RNA was assayed for Axin2 mRNA expression 48 h later (as for Fig. 2B). B–G, analysis of ductal hyperplasia in outgrowths from wild type and lrp5−/− MECs expressing mWnt1 or mWnt3a. MECs (50,000) transduced with mock or mWnt1- or mWnt3a-expressing lentiviral constructs were transplanted to cleared fat pads (see “Experimental Procedures”), and development of mammary trees was evaluated 8 weeks later. Fat pads were harvested and stained with carmine red (trees are dark against the clear fat pad background; n = 3). Error bars show standard deviations.
The outgrowth of MECs in vivo in cleared fat pads in 3-week-old recipient mice relies on ductal stem cell activity, which in turn depends on LRP5-dependent Wnt signaling activity (24, 27, 46, 47). Thus, lrp5−/− MECs (50,000) did not show mammary gland repopulating activity when compared with MECs from wild type mice (Fig. 6, B and C). MECs expressing Wnt1 from the lentiviral expression vector developed into hyperplastic ductal outgrowths that are also typical of glands from murine mammary tumor virus-Wnt1 transgenic mice (Fig. 6D and Ref. 32). This colonization and outgrowth activity were dependent on expression of LRP5 (Fig. 6E). This is consistent with our previous results from crossing murine mammary tumor virus-Wnt1 mice into the lrp5−/− background in which subsequent tumor development also depends upon LRP5 expression (24). In contrast, we found that Wnt3a expression induced hyperplasia in vivo regardless of expression of LRP5 (Fig. 6, F and G), matching the results in vitro from the analysis of MEFs.
Do LRP5 and LRP6 Physically Interact?
Given the functional co-dependence of LRP5 and LRP6 we have shown here, we hypothesized that these two proteins physically interact. We anticipated that evaluating this hypothesis would not be technically simple given that no prior studies have shown interactions of either of these receptors at endogenous levels (prior analyses have focused on overexpressed receptor interactions). Therefore, we used the “IFAST” adaptation of the immunoprecipitation technique because this technique eliminates typical dilutive wash steps present in traditional purification methods. We suspected it could be better at capturing low affinity and short lived complex components. This technique also dramatically reduces the purification time (36).4 A scheme is shown in Fig. 7A. Pull-through of magnetic beads coated with nonspecific IgG illustrates the specificity of this technique (Fig. 7B). Using lysates from MEFs treated under various conditions, LRP5 immunoprecipitates were probed for the presence of other Wnt signaling components, including LRP6, phospho-LRP, and Axin1 (Fig. 7C). LRP5 and LRP6 were pulled through together irrespective of Wnt treatment, whereas specificity controls (vinculin and EGF receptor) were not. Phospho-LRP was highly induced by Wnt3a, less induced by Wnt9b, and pulled through in both LRP5-associated fractions.
Interestingly, Axin1 did not pull through with LRP5, suggesting that LRP5-6 complexes did not contain this signaling molecule (irrespective of Wnt induction). Anti-Axin1 immunoprecipitation almost quantitatively retrieved Axin1 from the bound fraction (Fig. 7D). Interestingly, Axin1 immunoprecipitates contained LRP6 regardless of Wnt treatment (i.e. these are constitutively complexed), and there was no LRP5 present in that fraction, confirming the result from LRP5 immunoprecipitation. Anti-phospho-LRP antibody pulled through LRP6/Axin1 and also LRP6/LRP5 (Fig. 7E).
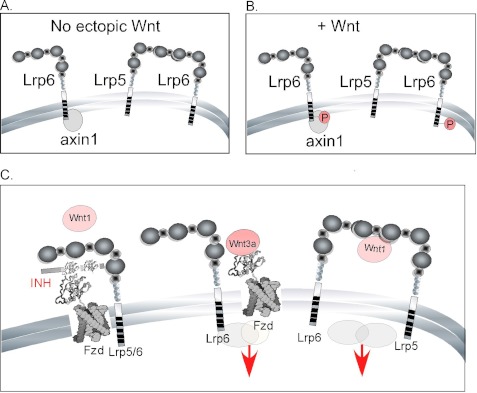
These data provide evidence in support of several close range interactions, namely LRP6-Axin1 and LRP5-LRP6 in untreated cells and phospho-LRP6-Axin1 and phospho-LRP6-LRP5 in Wnt-treated cells. Furthermore, these data suggest that binding of Axin1 to LRP6 does not require treatment with an exogenous canonical Wnt ligand (data are summarized in Fig. 8, A and B).
FIGURE 8.
Model of LRP interactions. A and B, evidence for interactions derived from IFAST IP. Proteins associated into complexes are shown in the uninduced (basal level) MEFs (A) and for MEFs treated with Wnt3a (B). C, a model depicting LRP signaling activities in response to different Wnt ligands. Left-hand side, binding of Wnt1/Wnt9b ligands is not effective when LRP5 or LRP6 are present alone. Shown here is one potential explanation: the presence of an inhibitor (INH) in the adult mammary gland/MEF culture that blocks assembly of LRP-Fzd complexes by E1-E2 ligands. Under the same conditions, Wnt3a (shown here binding at a separate domain, E3-E4) can effectively signal via LRP6 (or LRP5; center). Signaling by the Wnt1 class of ligands requires complexation of LRP5 and LRP6 (right-hand side). This complex is proposed to be inhibitor-resistant and may have different co-receptor/signaling component associations.
DISCUSSION
In this study, we aimed to resolve a paradox that arose from our analysis of the phenotype of lrp5−/− mouse mammary glands. Here, we show that both LRP5 and LRP6 are required to respond to a group of Wnt ligands that includes Wnt1, Wnt9b, and Wnt10b. For MEFs and MECs, unless both LRP5 and LRP6 are present, the signal generated is low. In contrast, Wnt3a can signal through either receptor alone, although LRP6 is more efficient (39). The concentrations of Wnt3a and Wnt9b used for some of these studies were chosen to generate similar Wnt signaling responses (TOP-FLASH/Axin2 transactivation). When Wnt3a was used at 20 ng/ml and Wnt9b was used at 400 ng/ml, these outputs matched. By this means, we hoped to reduce the effects of variable ligand affinity and stability on functional receptor interactions. Furthermore, the gland hyperplasia induced by Wnt1 or Wnt3a (expressed by the same vector construct) was approximately similar, suggesting that the potency issues that plague Wnt studies in vitro are unlikely to underlie the requirement for two instead of one LRP species.
A dual requirement explains why lrp5−/− mice are resistant to Wnt1-mediated tumorigenesis (24). Also, because ductal mammary stem cell activity is dependent upon Wnt signaling and specifically upon LRP5 (despite expression of LRP6), we propose that the Wnt ligand normally responsible for maintaining mammary stem cells falls into this LRP5-LRP6 dual-dependent group of ligands. Wnt ligands have classically been characterized into canonical and non-canonical ligands based on their functionality and ability to activate the canonical Wnt pathway (48–50). There are 10 Wnt ligands expressed in the mammary gland (Wnts 2, 4, 5a, 5b, 6, 7b, 9a, 9b, 10b, and 16), and several are specifically associated with either basal or luminal mammary epithelial cells or with stromal cell types (51). We tested Wnt9b and -10b in this study and showed that they had the same dual LRP dependency as Wnt1. Wnt1 and Wnt3a are not usually expressed in the mouse mammary gland, although they were identified as oncogenic loci for mouse mammary glands using a retroviral integration screen (52). Interestingly, Wnt10b also emerged from this unbiased screen. This information may also be helpful for studies of Wnt-dependent stem cells in vitro. Published work from another group showed that Wnt3a (identified as “pan-active” irrespective of LRP expression by our work) was successfully used to maintain stem cell function in vitro (200 ng/ml changed daily) (53). Another culture system devised to maintain intestinal stem cells included a Wnt relative, an R-Spondin (RSpo1), at 500 ng/ml (54). There are no data yet to describe whether selective LRP species are required by Lgr-RSpo complexes in mammary glands.
We assumed that results of functional receptor testing derived from the study of MEFs could be unique to only this cell type. Therefore, we corroborated key data obtained from cultured MEFs for MECs, which are our principal cell type of interest. Wnt1 required both LRP5 and LRP6 in vitro (by Axin2 expression analysis) and in vivo in an MEC outgrowth assay (whereas Wnt3a induced outgrowth and hyperplasia regardless of the absence of LRP5). The outgrowth assay typically depends upon ductal stem cell activity; thus, there was no outgrowth of the stem cell-deficient MEC populations extracted from lrp5−/− mammary glands (Fig. 6C). Interestingly, providing Wnt3a ectopically to lrp5−/− MECs generated hyperplastic outgrowth following transplantation in vivo (Fig. 6G). This observation suggests that stemness can be induced de novo by Wnt exposure.
The currently accepted model of receptor activation is shown in Fig. 8C. Typically, Wnt ligands are proposed to associate with an LRP and Fzd species to generate a β-catenin/TCF signal (5, 6). The fact that both LRP5 and LRP6 are required to respond to the Wnt1 class of ligands shows that these receptors are not functionally equivalent. It implies that they may physically interact through heterodimerization. Earlier studies of receptor activation showed that overexpressed LRP6 receptors homodimerize and polymerize into “signalosomes” (16, 55, 56). More recent data support a role for the homodimerization of endogenous LRP6 in augmenting canonical Wnt activity (40). Using a novel immunoprecipitation assay, we provide data for the heteropolymerization of LRP5 and LRP6. Thus, LRP6 was pulled through with LRP5 with high efficiency in the IFAST assay (Fig. 7C), and this complex was present regardless of the presence of ectopic Wnt ligand. Interestingly, LRP5-6 was not pulled through with Axin1, whereas LRP6 alone was. In fact, Axin1 complexation with LRP6 was measured by this assay regardless of Wnt ligand treatment, whereas most studies would suggest that this complex forms as a result of receptor activation (9). However, none of the previous studies were able to study the receptor complexes at endogenous levels, and this may explain why our results differ. Furthermore, even when cells were Wnt-treated, the LRP5-6 heteromer did not associate with Axin1, and neither did Axin1 associate with LRP5. Axin1 association with LRP5 (overexpressed human LRP5 with a carboxyl-terminal domain tag) has been shown to occur only when an inhibitory interaction from the extracellular domain is relieved (58). This association is therefore known to be highly context-dependent. Although it can happen in vitro, perhaps it only rarely happens in vivo. MacDonald et al. (39) have recently shown that the phosphorylation and Axin1 binding properties of LRP6 can be mimicked in the low Axin1-binding LRP5 if gap sequences were introduced between the canonical phosphorylation sites (PPSPXP). Our data suggest that Axin1 association is not required for activation of the heteromer, or perhaps LRP5-Axin1 is so different from the better characterized LRP6-Axin1 complex that it cannot be measured by the same assays. The interactions that were demonstrated by the IFAST experiments are summarized in Fig. 8, A and B.
A model is presented as Fig. 8C to summarize our conclusions and propose an explanation for our data points as follows. Overexpression of either LRP5 or LRP6 overcomes the need for both receptors to be present (Figs. 4 and 5) either in normal mammary epithelial cell lines or in MEFs with LRP species overexpressed. Thus, this dual requirement does not reflect binding of ligand to a specific receptor or an obligate requirement for both to be present to generate a response. Instead, we propose that there is a cell type-specific restriction of Wnt ligand efficacy most easily explained if there is a limiting amount of an inhibitor present that is out-competed by higher concentrations of receptor. The identification of an inhibitor-resistant LRP5 species (Δ666–809) as a common mutation arising in breast cancer points to the importance of constitutively expressed Wnt inhibitors as a physiological suppressor for Wnt signaling (59). Along similar lines, Wnt inhibitors such as secreted Frizzled-related proteins (sFRPs) (60) and Wnt inhibitory factor (WIF1) (61) have been shown to be epigenetically silenced in tumors.
Interestingly, our description of two groups of Wnt ligands based on this functional assay correlates with recently published subgroups of Wnt ligands distinguished by their interactions with specific ligand binding regions on the LRP6 receptor (40, 41). Thus, Wnt1 was shown to interact with the E1-E2 domains of LRP6, whereas Wnt3a interacts with the E3-E4 domains. We have incorporated this into the model to propose that if an inhibitor effectively competes for E1-E2 Wnt ligand binding sites on the receptor (but not E3-E4) in ternary complexes of Fzd species then Wnt3a activity would not be restricted. Using this model, an E1-E2 ligand could only induce a β-catenin/TCF signal when both receptor types are present to form an inhibitor-resistant heteromer of LRP5 and LRP6 (Fig. 8C). The implication is that there is an extracellular inhibitor in MECs and MEFs that restricts Wnt1/9b/10b ligand activity unless both receptors are present. Various Wnt inhibitors have been found in mammary gland (51, 60, 61), and we are currently working to test this model. An obvious candidate is Dkk1 given recent structural data mapping the binding domain to E1 of LRP6 (62, 63). We have no information yet about the role of Fzd in these complexes.
We propose that overexpression of LRP receptors overcomes the physiological regulation and restrictions of β-catenin/TCF signaling by decreasing the effectiveness of secreted inhibitors, allowing all Wnt ligands in the extracellular milieu to signal effectively and expanding the responsive cells to include those that do not express both LRP species. This model also predicts that overexpression of LRP receptors would create a highly effective gain of function for Wnt signaling during tumorigenesis. Indeed, this has been observed in triple negative breast tumor cohorts (that include the majority of basaloid tumors), which were shown to express 2–5× higher levels of lrp6 mRNA (42, 43). In parallel with this, one-third of basaloid breast tumors show significant activation of a Wnt signaling reporter (nuclear β-catenin) (64). Furthermore, inhibition of LRP6 in the basaloid breast cancer cell line MDA-MB231 inhibits TOP-FLASH Wnt reporter activity (10–100×) together with the endogenous Axin2 reporter (2×) and tumorigenic physiologies such as growth (2×), colony formation (5×), and tumor growth in vivo (65). LRP receptor levels appear to be limiting to Wnt signaling, and overexpression of LRP6 induces significant hyperplasia in mouse mammary glands (57). These data all support LRP6 as a viable target for breast cancer therapy and provide rationale for the development of extracellular inhibitors of Wnt signaling aimed at inhibiting LRP6 function (40, 41).
In summary, we have shown that several key Wnt ligands require expression of both LRP5 and LRP6 receptors to generate a β-catenin/TCF Wnt signal. For normal mammary glands, Wnt responder activity is limited to the basal cell subpopulation, consistent with their co-expression of LRP5 and LRP6 (27). For a basaloid breast tumor model, tumor stem cell activity is associated with LRP5-expressing cells (34). The study presented here describes why LRP5 can act as a gatekeeper for Wnt responses in both stem cells and tumor cells, enabling Wnt responsiveness under conditions where LRP6 alone is not effective.
Supplementary Material
Acknowledgment
We thank laboratory members for continuous help and discussion.
This work was supported by Era of Hope Scholar Grant W81XWH-06-1-0491 (to C. M. A.) and a scholarship from the Kuwait Ministry of Higher Education (to S. F.).

This article contains supplemental Fig. S1A and S1B.
S. Goel, E. N. Chin, S. A. Fakhraldeen, S. M. Berry, D. J. Beebe, and C. M. Alexander, unpublished data.
- TCF
- T cell factor
- LRP
- lipoprotein receptor-related protein
- IFAST
- immiscible filtration assisted by surface tension
- Fzd
- Frizzled
- MEC
- mammary epithelial cell
- MEF
- mouse embryonic fibroblast
- MCS
- multiple cloning site
- IRES
- internal ribosome entry site
- qRT-PCR
- quantitative RT-PCR
- IP
- immunoprecipitation
- rmWnt
- recombinant mouse Wnt
- mWnt
- mouse Wnt
- mLRP
- mouse LRP.
REFERENCES
- 1. van Amerongen R., Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 [DOI] [PubMed] [Google Scholar]
- 2. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 3. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 4. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schweizer L., Varmus H. (2003) Wnt/Wingless signaling through β-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeng X., Huang H., Tamai K., Zhang X., Harada Y., Yokota C., Almeida K., Wang J., Doble B., Woodgett J., Wynshaw-Boris A., Hsieh J. C., He X. (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacDonald B. T., Yokota C., Tamai K., Zeng X., He X. (2008) Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J. Biol. Chem. 283, 16115–16123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. (2004) A mechanism for Wnt coreceptor activation. Mol. Cell 13, 149–156 [DOI] [PubMed] [Google Scholar]
- 9. Niehrs C., Shen J. (2010) Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. 67, 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bienz M., Clevers H. (2003) Armadillo/β-catenin signals in the nucleus—proof beyond a reasonable doubt? Nat. Cell Biol. 5, 179–182 [DOI] [PubMed] [Google Scholar]
- 11. Arce L., Yokoyama N. N., Waterman M. L. (2006) Diversity of LEF/TCF action in development and disease. Oncogene 25, 7492–7504 [DOI] [PubMed] [Google Scholar]
- 12. Brennan K., Gonzalez-Sancho J. M., Castelo-Soccio L. A., Howe L. R., Brown A. M. (2004) Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize β-catenin independently of Frizzled proteins. Oncogene 23, 4873–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. González-Sancho J. M., Brennan K. R., Castelo-Soccio L. A., Brown A. M. (2004) Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize β-catenin. Mol. Cell. Biol. 24, 4757–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., He X. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 [DOI] [PubMed] [Google Scholar]
- 15. He X., Semenov M., Tamai K., Zeng X. (2004) LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131, 1663–1677 [DOI] [PubMed] [Google Scholar]
- 16. Cong F., Schweizer L., Varmus H. (2004) Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131, 5103–5115 [DOI] [PubMed] [Google Scholar]
- 17. Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. (2001) LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325 [DOI] [PubMed] [Google Scholar]
- 18. Kato M., Patel M. S., Levasseur R., Lobov I., Chang B. H., Glass D. A., 2nd, Hartmann C., Li L., Hwang T. H., Brayton C. F., Lang R. A., Karsenty G., Chan L. (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei Q., Yokota C., Semenov M. V., Doble B., Woodgett J., He X. (2007) R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and β-catenin signaling. J. Biol. Chem. 282, 15903–15911 [DOI] [PubMed] [Google Scholar]
- 20. Hendrickx M., Leyns L. (2008) Non-conventional Frizzled ligands and Wnt receptors. Dev. Growth Differ. 50, 229–243 [DOI] [PubMed] [Google Scholar]
- 21. Mason J. J., Williams B. O. (2010) SOST and DKK: antagonists of LRP family signaling as targets for treating bone disease. J. Osteoporos. 2010, pii: 460120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Itasaki N., Jones C. M., Mercurio S., Rowe A., Domingos P. M., Smith J. C., Krumlauf R. (2003) Wise, a context-dependent activator and inhibitor of Wnt signalling. Development 130, 4295–4305 [DOI] [PubMed] [Google Scholar]
- 23. Pinson K. I., Brennan J., Monkley S., Avery B. J., Skarnes W. C. (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538 [DOI] [PubMed] [Google Scholar]
- 24. Lindvall C., Evans N. C., Zylstra C. R., Li Y., Alexander C. M., Williams B. O. (2006) The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J. Biol. Chem. 281, 35081–35087 [DOI] [PubMed] [Google Scholar]
- 25. Brennan K. R., Brown A. M. (2004) Wnt proteins in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 9, 119–131 [DOI] [PubMed] [Google Scholar]
- 26. Mi K., Johnson G. V. (2005) Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J. Cell. Biochem. 95, 328–338 [DOI] [PubMed] [Google Scholar]
- 27. Badders N. M., Goel S., Clark R. J., Klos K. S., Kim S., Bafico A., Lindvall C., Williams B. O., Alexander C. M. (2009) The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One 4, e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wright W. E., Shay J. W. (2006) Inexpensive low-oxygen incubators. Nat. Protoc. 1, 2088–2090 [DOI] [PubMed] [Google Scholar]
- 29. Holmen S. L., Salic A., Zylstra C. R., Kirschner M. W., Williams B. O. (2002) A novel set of Wnt-Frizzled fusion proteins identifies receptor components that activate β-catenin-dependent signaling. J. Biol. Chem. 277, 34727–34735 [DOI] [PubMed] [Google Scholar]
- 30. Bennett C. N., Hodge C. L., MacDougald O. A., Schwartz J. (2003) Role of Wnt10b and C/EBPα in spontaneous adipogenesis of 243 cells. Biochem. Biophys. Res. Commun. 302, 12–16 [DOI] [PubMed] [Google Scholar]
- 31. Kim Y. C., Clark R. J., Pelegri F., Alexander C. M. (2009) Wnt4 is not sufficient to induce lobuloalveolar mammary development. BMC Dev. Biol. 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Welm B. E., Dijkgraaf G. J., Bledau A. S., Welm A. L., Werb Z. (2008) Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell 2, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Britt K. L., Kendrick H., Regan J. L., Molyneux G., Magnay F. A., Ashworth A., Smalley M. J. (2009) Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells. Breast Cancer Res. 11, R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim S., Goel S., Alexander C. M. (2011) Differentiation generates paracrine cell pairs that maintain basaloid mouse mammary tumors: proof of concept. PLoS One 6, e19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khan Z., Vijayakumar S., de la Torre T. V., Rotolo S., Bafico A. (2007) Analysis of endogenous LRP6 function reveals a novel feedback mechanism by which Wnt negatively regulates its receptor. Mol. Cell. Biol. 27, 7291–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berry S. M., Alarid E. T., Beebe D. J. (2011) One-step purification of nucleic acid for gene expression analysis via immiscible filtration assisted by surface tension (IFAST). Lab Chip 11, 1747–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim Y. C., Clark R. J., Ranheim E. A., Alexander C. M. (2008) Wnt1 expression induces short-range and long-range cell recruitments that modify mammary tumor development and are not induced by a cell-autonomous β-catenin effector. Cancer Res. 68, 10145–10153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bryja V., Andersson E. R., Schambony A., Esner M., Bryjová L., Biris K. K., Hall A. C., Kraft B., Cajanek L., Yamaguchi T. P., Buckingham M., Arenas E. (2009) The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Mol. Biol. Cell 20, 924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. MacDonald B. T., Semenov M. V., Huang H., He X. (2011) Dissecting molecular differences between Wnt coreceptors LRP5 and LRP6. PLoS One 6, e23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ettenberg S. A., Charlat O., Daley M. P., Liu S., Vincent K. J., Stuart D. D., Schuller A. G., Yuan J., Ospina B., Green J., Yu Q., Walsh R., Li S., Schmitz R., Heine H., Bilic S., Ostrom L., Mosher R., Hartlepp K. F., Zhu Z., Fawell S., Yao Y. M., Stover D., Finan P. M., Porter J. A., Sellers W. R., Klagge I. M., Cong F. (2010) Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc. Natl. Acad. Sci. U.S.A. 107, 15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gong Y., Bourhis E., Chiu C., Stawicki S., DeAlmeida V. I., Liu B. Y., Phamluong K., Cao T. C., Carano R. A., Ernst J. A., Solloway M., Rubinfeld B., Hannoush R. N., Wu Y., Polakis P., Costa M. (2010) Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One 5, e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindvall C., Zylstra C. R., Evans N., West R. A., Dykema K., Furge K. A., Williams B. O. (2009) The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One 4, e5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu C. C., Prior J., Piwnica-Worms D., Bu G. (2010) LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc. Natl. Acad. Sci. U.S.A. 107, 5136–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Humphreys R. C., Rosen J. M. (1997) Stably transfected HC11 cells provide an in vitro and in vivo model system for studying Wnt gene function. Cell Growth Differ. 8, 839–849 [PubMed] [Google Scholar]
- 45. Schlange T., Matsuda Y., Lienhard S., Huber A., Hynes N. E. (2007) Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 9, R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shackleton M., Vaillant F., Simpson K. J., Stingl J., Smyth G. K., Asselin-Labat M. L., Wu L., Lindeman G. J., Visvader J. E. (2006) Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 [DOI] [PubMed] [Google Scholar]
- 47. Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H. I., Eaves C. J. (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 [DOI] [PubMed] [Google Scholar]
- 48. Shimizu H., Julius M. A., Giarré M., Zheng Z., Brown A. M., Kitajewski J. (1997) Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 8, 1349–1358 [PubMed] [Google Scholar]
- 49. Wong G. T., Gavin B. J., McMahon A. P. (1994) Differential transformation of mammary epithelial cells by Wnt genes. Mol. Cell. Biol. 14, 6278–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Du S. J., Purcell S. M., Christian J. L., McGrew L. L., Moon R. T. (1995) Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol. 15, 2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alexander C. M., Goel S., Fakhraldeen S. A., Kim S. (2012) Cold Spring Harb. Perspect. Biol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Callahan R., Smith G. H. (2008) Common integration sites for MMTV in viral induced mouse mammary tumors. J. Mammary Gland Biol. Neoplasia 13, 309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeng Y. A., Nusse R. (2010) Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6, 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., Clevers H. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 55. Liu G., Bafico A., Harris V. K., Aaronson S. A. (2003) A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol. Cell. Biol. 23, 5825–5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., Bienz M., Niehrs C. (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619–1622 [DOI] [PubMed] [Google Scholar]
- 57. Zhang J., Li Y., Liu Q., Lu W., Bu G. (2010) Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene 29, 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mao J., Wang J., Liu B., Pan W., Farr G. H., 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., Wu D. (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 59. Björklund P., Svedlund J., Olsson A. K., Akerström G., Westin G. (2009) The internally truncated LRP5 receptor presents a therapeutic target in breast cancer. PLoS One 4, e4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suzuki H., Toyota M., Carraway H., Gabrielson E., Ohmura T., Fujikane T., Nishikawa N., Sogabe Y., Nojima M., Sonoda T., Mori M., Hirata K., Imai K., Shinomura Y., Baylin S. B., Tokino T. (2008) Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br. J. Cancer 98, 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ai L., Tao Q., Zhong S., Fields C. R., Kim W. J., Lee M. W., Cui Y., Brown K. D., Robertson K. D. (2006) Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis 27, 1341–1348 [DOI] [PubMed] [Google Scholar]
- 62. Bourhis E., Wang W., Tam C., Hwang J., Zhang Y., Spittler D., Huang O. W., Gong Y., Estevez A., Zilberleyb I., Rouge L., Chiu C., Wu Y., Costa M., Hannoush R. N., Franke Y., Cochran A. G. (2011) Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure 19, 1433–1442 [DOI] [PubMed] [Google Scholar]
- 63. Chen S., Bubeck D., MacDonald B. T., Liang W. X., Mao J. H., Malinauskas T., Llorca O., Aricescu A. R., Siebold C., He X., Jones E. Y. (2011) Structural and functional studies of LRP6 ectodomain reveal a platform for Wnt signaling. Dev. Cell 21, 848–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Geyer F. C., Lacroix-Triki M., Savage K., Arnedos M., Lambros M. B., MacKay A., Natrajan R., Reis-Filho J. S. (2011) β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 24, 209–231 [DOI] [PubMed] [Google Scholar]
- 65. Matsuda Y., Schlange T., Oakeley E. J., Boulay A., Hynes N. E. (2009) WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 11, R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.