Background: Aβ aggregation may be modulated by small lipid-like molecules.
Results: Activators induced β-structure and rapid aggregation, whereas inhibitors induced α-helical structure and small Aβ oligomers.
Conclusion: Small lipid-like molecules modulate Aβ secondary structure and self-association at stoichiometric levels.
Significance: Understanding the role of small molecules and lipids in Alzheimer disease is crucial for the development of effective therapeutic targets.
Keywords: Alzheimer Disease, Amyloid, Analytical Ultracentrifugation, Protein Aggregation, Small Molecules, Amyloid β, Thioflavin
Abstract
Amyloid fibril formation is associated with a number of debilitating systemic and neurodegenerative diseases. One of the most prominent is Alzheimer disease in which aggregation and deposition of the Aβ peptide occur. Aβ is widely considered to mediate the extensive neuronal loss observed in this disease through the formation of soluble oligomeric species, with the final fibrillar end product of the aggregation process being relatively inert. Factors that influence the aggregation of these amyloid-forming proteins are therefore very important. We have screened a library of 96 amphipathic molecules for effects on Aβ1–42 aggregation and self-association. We find, using thioflavin T fluorescence and electron microscopy assays, that 30 of the molecules inhibit the aggregation process, whereas 36 activate fibril formation. Several activators and inhibitors were subjected to further analysis using analytical ultracentrifugation and circular dichroism. Activators typically display a 1:10 peptide:detergent stoichiometry for maximal activation, whereas the inhibitors are effective at a 1:1 stoichiometry. Analytical ultracentrifugation and circular dichroism experiments show that activators promote a mixture of unfolded and β-sheet structures and rapidly form large aggregates, whereas inhibitors induce α-helical structures that form stable dimeric/trimeric oligomers. The results suggest that Aβ1–42 contains at least one small molecule binding site, which modulates the secondary structure and aggregation processes. Further studies of the binding of these compounds to Aβ may provide insight for developing therapeutic strategies aimed at stabilizing Aβ in a favorable conformation.
Introduction
Alzheimer disease is the leading cause of dementia in the elderly human population (1). This form of neurodegeneration is characterized by the formation of intracellular neurofibrillary tangles, neuronal and synaptic loss, and the extracellular aggregates of amyloid β (Aβ)2 peptide as plaque material (1). Despite extensive genetic (2) and animal model (3) evidence for a role of Aβ in the disease progression, the mechanism through which this peptide causes neurodegeneration is unclear. Early research suggested that the aggregation of Aβ into the classical fibrillar amyloid structure conferred a toxic effect (4, 5). However, more recent studies have shown that plaque load and levels of insoluble Aβ do not correlate with the progression of neuronal degeneration, whereas the soluble pool of Aβ does (6). These results indicate that a form of soluble oligomer is the toxic principle of this disease (for extensive review, see Ref. 7). Thus, factors that influence the formation of these oligomeric species are important in understanding the formation and role of small oligomers in Alzheimer disease.
One factor that can significantly alter the self-association and aggregation of Aβ is the presence of hydrophobic surfaces, such as the cell membrane or lipoprotein particles. The hypothesis that lipids and lipoproteins are important to the disease is supported by genetic studies, which show that the most important genetic risk factor is apolipoprotein E allele status. In addition, the Aβ peptide constitutes a portion of the transmembrane domain of amyloid precursor protein and is released from this precursor protein by secretases. γ-Secretase acts within the cell membrane, and it is likely that a large proportion of the released Aβ remains associated with the membrane after cleavage (8). Indeed, several studies have visualized extensive Aβ association with cell and synthetic membranes (9, 10). These surfaces have been shown either to increase the degree of amyloid fibril formation or to inhibit the process, based on the charge, curvature, and composition of the lipid surface (11–14). Given that the composition of the surface has a significant impact on the self-association and aggregation of Aβ, it is highly likely that individual lipid molecules can also impact on these processes. However, lipid molecules are not particularly amenable to biophysical studies as they spontaneously form aggregated structures such as micelles and bilayers at any appreciable concentration and thereby complicate any analysis of individual interactions.
One approach to avoid this issue is to use lipid mimetics, such as single chain phospholipids, detergents, and other amphipathic molecules to infer common features that may modulate the aggregation process. This approach was used successfully to explore the structural specificity of lipids and “lipid-like” mimetics as activators and inhibitors of amyloid fibril formation by apolipoprotein C-II (15–17). We have used a similar screen of the 96 lipid-like compounds used in the apoC-II study to investigate the effect of these amphipathic molecules on Aβ1–42 amyloid fibril formation and secondary structure with the aim of identifying compounds that may stabilize oligomeric forms of the peptide. Our results suggest a specific binding site, with comparison with the previously obtained apoC-II results showing a significantly different pattern of inhibition and activation.
EXPERIMENTAL PROCEDURES
Materials
Human Aβ1–42 was synthesized by the W. M. Keck Laboratory (Yale University, New Haven, CT). This peptide batch was checked by mass spectrometry and found to be free of oxidation. A 96-well detergent screen (product HR2-406) was obtained from Hampton Research (a list of compounds can be found in supplemental Table S1). For complete data on the chemical properties of these molecules visit the Hampton Research web site. All other reagents were of analytical grade.
Aβ1–42 Solubilization
For amyloid fibril formation assays 1 mg of peptide was resuspended in 200 μl of 60 mm NaOH and incubated for 5 min at room temperature. This solution was diluted with 700 μl of distilled water and bath-sonicated at room temperature for a further 5 min. The sonicated solution was neutralized with 100 μl of 10× phosphate-buffered saline (PBS), pH 7, and centrifuged at 14,000 × g in a benchtop centrifuge. The optical density at 214 nm of the supernatant, containing the resolubilized Aβ, was determined with a Quartz microplate and a Flexstation plate reader (Molecular Devices, Sunnyvale, CA) equipped with absorbance optics. The concentration was calculated from the 214 nm absorbance value using an extinction coefficient for Aβ1–42 of 95,452 m−1 cm−1 (18). Recovery of the peptide was typically 70–80%.
Fibril Formation
The effect of test compounds on fibril formation by Aβ1–42 was measured using a continuous thioflavin T (ThT) fluorescence assay in a 96-well plate. Detergents were diluted 1:20 with 1× PBS containing 30 μm ThT, providing a final concentration equivalent to one half of the quoted critical micelle concentration. Aβ1–42 was added to a final concentration of 5 μm, and the plate was incubated at 37 °C, with shaking every 7 min for 3 s, prior to the measurement of the ThT fluorescence intensity (444-nm excitation and 485-nm emission), using a Flexstation Plate reader (Molecular Devices). Data were analyzed in a fashion similar to that described by Ryan et al. (16, 17) using Hill plots to estimate the time for half-maximal change (t50), which was inverted to obtain an estimate of the rate of fibril formation. For plotting purposes a value of 0 was assigned to Aβ/compound mixtures where no change in the ThT fluorescence was observed during the assay. These compounds are noted in supplemental Table S1 as nc, indicating that there was no change in ThT emission during the time course. For compounds where there was a change in ThT emission, this value was expressed relative to the rate of fibril formation by Aβ1–42 alone by dividing the rate by that of Aβ1–42 alone.
Electron Microscopy
Solutions from aggregation assays for selected activating and inhibiting compounds were vortexed to suspend particulate matter, and an aliquot was spotted onto carbon-coated copper grids (ProSciTech). The grids were washed several times in distilled water to remove excess phosphate and then allowed to air dry. The fibrils were negatively stained with 0.5% uranyl acetate for 3 min and then washed several times with water. The samples were analyzed on a Siemens ELMIS-KOP 102 electron microscope.
Circular Dichroism (CD) Measurements
CD spectra for Aβ1–42 (5 μm) in the presence of detergent (50 μm) were acquired using an Aviv model 62 DS CD spectrometer (Aviv Associates Inc., Lakewood, NJ) at 25 °C with a 1-mm pathlength quartz cuvette, a spectral bandwidth of 1 nm, a signal averaging time of 2 s, and a data interval of 0.5 nm. Data were collected after 5 min of incubation with detergent, a time that was sufficient for the sample to equilibrate, but insignificant in terms of fibril formation. Data were corrected by subtracting the spectra of a sample containing all components except the Aβ. Data were converted to mean residue ellipticity and analyzed using CDPro.
Sedimentation Velocity Experiments
Sedimentation experiments were conducted at a concentration of 50 μm Aβ1–42 using an XL-A analytical ultracentrifuge (Beckman Coulter Instruments). For sedimentation velocity measurement samples were centrifuged at 50,000 rpm and radial 214 nm absorbance scans acquired every 7 min. The data were analyzed using a c(s) model and the program SEDFIT 9.4 (19, 20).
RESULTS
Screen of Amphipathic Compounds against Aβ1–42 Fibril Formation
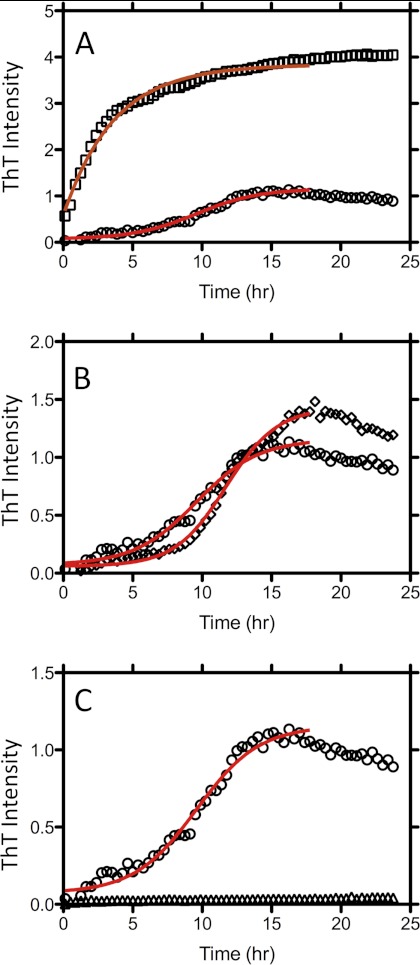
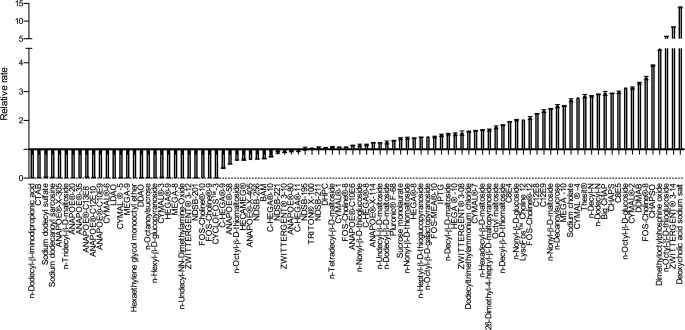
The potential for submicellar concentrations of the 96 compounds to modulate Aβ1–42 fibril formation was tested using a continuous ThT assay method. Compounds were assayed at final concentrations equivalent to half the critical micelle concentration (CMC) for each respective compound. Fibril formation was initiated by mixing Aβ1–42 with these solutions, and the change in ThT fluorescence was monitored over a 24-h period. Representative datasets for Aβ fibril formation in the presence of deoxycholate (CMC, 20 mm), NDSB-211, which does not form micelles, and LDAO (CMC, 2 mm) show 15-fold activation, no effect, and complete inhibition, respectively (Fig. 1). These datasets were fitted to sigmoidal Hill plots to obtain the time to half-maximal fluorescence change (t50) that, when inverted, provides a value for the rate of fibril formation. The rate of fibril formation was expressed relative to the rate of Aβ1–42 alone, hence, showing the extent of activation or inhibition by the compounds analyzed (Fig. 2). Compounds that displayed no change in ThT fluorescence over the time of the assay were assigned entered into supplemental Table S1 as nc and arbitrarily assigned a rate of zero. This analysis revealed 30 inhibitors and 36 activators of Aβ fibril formation, with the remaining 30 compounds showing no effect. Similar effects were observed with 10 μm Aβ (the data for the 5 and 10 μm Aβ are presented in a tabular form in supplemental Table S1).
FIGURE 1.
Effect of detergents on fibril formation by Aβ1–42. A, ThT fluorescence over time for Aβ1–42 in the absence (circles) and presence (squares) of the activating compound deoxycholate. B, ThT fluorescence over time for Aβ1–42 in the absence (circles) and presence (diamonds) of the compound NDSB-211, which has no effect on rate. C, ThT fluorescence over time for Aβ1–42 in the absence (circles) and presence (triangles) of the inhibiting compound LDAO. Solid lines represent the fit of the data to a sigmoidal Hill plot.
FIGURE 2.
Effects of the 96 detergents on the rate of Aβ1–42 fibril formation. The rate of fibril formation is expressed as the inverse of the time to half-maximal fluorescence relative to that of Aβ1–42 alone. Error bars are ± Standard deviation.
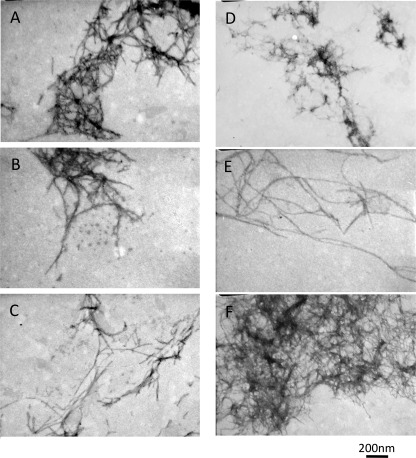
To confirm the results of the ThT screen we subjected Aβ1–42 incubated in the presence of the selected detergents for 48 h to electron microscopy (Fig. 3). Representative electron micrographs of Aβ1–42 in the presence of the activators showed the presence of fibrillar structures (Fig. 3), whereas those in the presence of the inhibitory compounds did not show any aggregates (supplemental Fig. S1). The fibers observed in Fig. 3 display some differences in morphology, suggesting that the compounds do act to modulate the fibril-forming pathway and the further self-association of the fibers.
FIGURE 3.
Electron microscope images of Aβ1–42 solutions containing activating detergents (deoxycholate (A), octyl-β-d-thioglucoside (B), FOS-choline-8 (C), CYMAL-4 (D), thesit (E), and n-decanoylsucrose (F)).
Concentration-dependent Effects of Selected Activators and Inhibitors
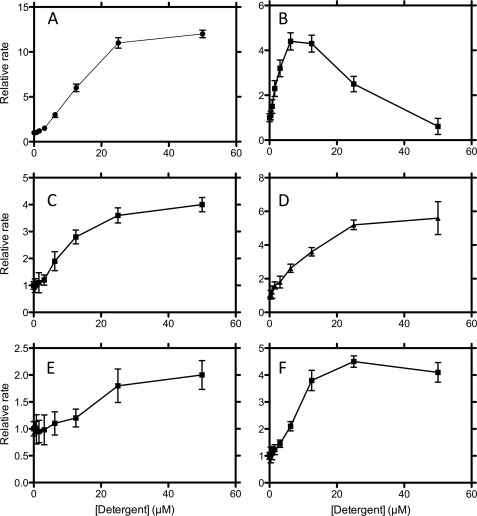
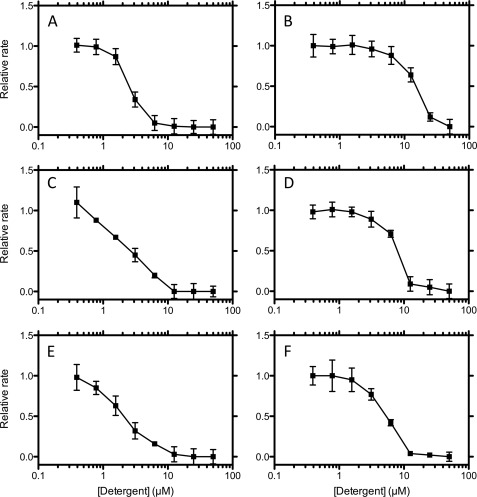
The concentration dependence of a selection of activators and inhibitors over the concentration range 0–50 μm are shown in Figs. 4 and 5, respectively. The activating detergents typically show maximal effect at about 25–50 μm corresponding to a 5:1 to 10: 1 stoichiometry of detergent to protein (Fig. 5A). Activators such as Thesit showed maximal activation at the lowest concentration, indicating that this compound is a potent activator of fibril formation. However, deoxycholate showed a far larger enhancement of the rate of fibril formation, suggesting that this molecule is by far a more aggressive activator. The concentration dependence of the inhibitors showed that complete inhibition for most compounds occurred at 5 μm, giving a 1:1 stoichiometry. This suggests that the mode of inhibition of these compounds is quite similar and that there is a specific stoichiometric binding site for these compounds (Fig. 5). These data for the inhibitory molecules can be used to obtain an IC50, which although not a quantitative measure of affinity, is indicative of the relative affinities of these compounds for Aβ (Table 1). A similar approach for the activators is inappropriate, due to the aggregation leading to an ill defined population of acceptors, which will have heterogeneous affinities for the compounds. A more extended concentration dependence encompassing the CMC indicates that whereas the activators are affected by the transition to micelles, which inhibit fibril formation, the inhibitors are unaffected by the formation of micelles (data not shown). This is unsurprising because it agrees with the average stoichiometry of one Aβ molecule/micelle.
FIGURE 4.
Concentration dependence of the effects of activating detergents (deoxycholate (A), octyl-β-d-thioglucoside (B), FOS-choline-8 (C), CYMAL-4 (D), thesit (E), and n-decanoylsucrose (F)). Error bars are ± Standard deviation.
FIGURE 5.
Concentration dependence of the effects of inhibiting detergents (LDAO (A), C13E8 (B), CYMAL-3 (C), MEGA-8 (D), FOS-choline-10 (E), and cycloFOS-choline-3 (F)). Error bars are ± Standard deviation.
TABLE 1.
IC50 values for inhibition of Aβ fibril formation by inhibitory compounds
The IC50 is defined as the concentration of compound required to produce 50% inhibition of fibril formation. Values are obtained using the inhibitory concentration algorithm provided by Prism 5. These values do not represent Kd values of the interaction of the compound with Aβ, but are estimates of the relative affinity of the compound for Aβ.
| Compound | IC50 |
|---|---|
| [μm (+/−)] | |
| LDAO | 2.38 (0.11) |
| C13E9 | 8.00 (0.22) |
| CycloFOS-3 | 2.69 (0.14) |
| MEGA 8 | 11.23 (0.23) |
| FOS-10 | 2.11 (0.19) |
| CYMAL-3 | 5.03 (0.16) |
Secondary and Tertiary Structural Changes of Aβ1–42 in Presence of Select Amphipathic Compounds
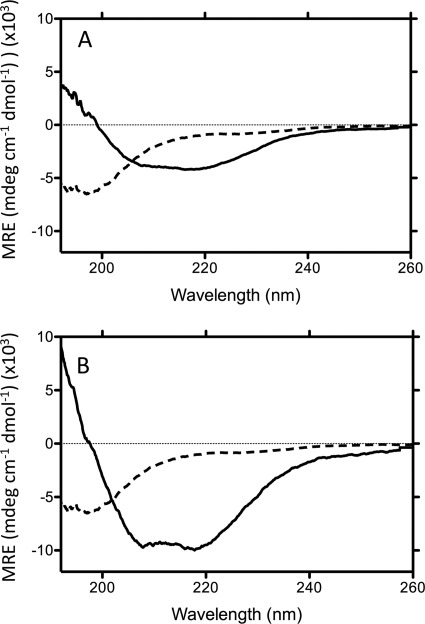
The secondary structure of Aβ in the presence of the selected compounds was investigated using CD spectroscopy. Fig. 6 shows representative CD spectra of Aβ1–42 alone and in the presence of the activating detergent deoxycholate or the inhibiting detergent LDAO, corrected for the contribution to ellipticity by these compounds. These detergents increased the secondary structure present in the Aβ as evidenced by the decrease in mean residue ellipticity between 200 and 250 nm. Singular value decomposition analysis of these datasets using CDPro CDSSTR and SELCON algorithms provided estimates of the percent secondary structure that was induced by these compounds (Table 2). Typically, the activators induce the formation of Aβ species containing increased β sheet over the absence of the compound and a very significant amount of random coil structure. The inhibitors, however, appear to induce a very high proportion of α-helical content (ca. 55%), and a significantly reduced proportion of random structure (Table 1).
FIGURE 6.
Example CD spectra of Aβ1–42 in the absence (dashed line in both panels) and presence of activating (deoxycholate (A), solid line) and inhibitory (LDAO (B), solid line) detergents.
TABLE 2.
Proportions of secondary structure induced by selected detergents
CD spectra were acquired using an Aviv model 62 DS CD spectrometer, buffer corrected, and the resulting spectra were analyzed using the SELCON and CDSSTR algorithms in the CDPro Suite of SVD tools. These algorithms provided the proportions of secondary structure that reconstituted the CD spectra observed in the presence of each compound (representative data in Fig. 6).
| Sample | α | β | Turn | Random |
|---|---|---|---|---|
| % | % | % | % | |
| Aβ1–42 alone | 7.8 | 22.4 | 20.6 | 49.2 |
| + Deoxycholatea | 0.1 | 56.4 | 16.4 | 27.1 |
| +Octyl Thioglucosidea | 0 | 53.7 | 17.1 | 29.2 |
| + Fos-Choline 8a | 3.8 | 47.4 | 21.1 | 27.7 |
| + Thesita | 15.2 | 33.6 | 19.9 | 31.3 |
| + N-Decanoyl sucrosea | 3.2 | 47.3 | 18.8 | 30.7 |
| + CYMAL 2a | 16.3 | 26.5 | 20.1 | 37.1 |
| + C13E8b | 58.1 | 5.4 | 20.1 | 16.4 |
| + LDAOb | 65.2 | 6.3 | 16.4 | 12.1 |
| + Fos-Choline 10b | 51.2 | 3.5 | 22.4 | 22.9 |
| + MEGA-8b | 55.3 | 3.9 | 15.6 | 25.2 |
| + CycloFos-Choline 3b | 54.2 | 4.7 | 19.1 | 32 |
| + CYMAL 3b | 45.3 | 15.3 | 19.2 | 20.2 |
a Indicates activating compounds.
b Indicates inhibitory compounds.
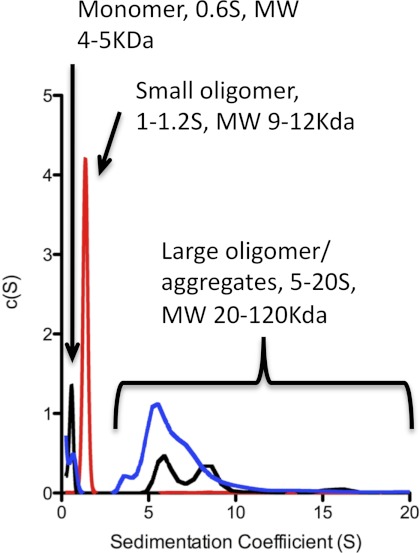
The solution state oligomerization of Aβ in the presence of the amphipathic molecules was investigated using sedimentation velocity experiments (Fig. 7). Sedimentation velocity data of Aβ alone indicates an ∼60:40 mix of monomeric Aβ (0.6 s) (∼5 kDa) and large aggregates with sedimentation coefficients of 5–100 s (∼60 kDa to >1000 kDa). Addition of activating compounds changed the ratio of monomer: aggregate to ∼40:60, whereas addition of the inhibitory compounds suppressed the formation of larger aggregates and induced the formation of a single 1.2 s species (10–15 kDa). These results demonstrate that the activating and inhibiting compounds have distinct effects on the conformation and aggregation of Aβ1–42. The increased structural stability and the appearance of a stable small oligomeric species for Aβ in the presence of inhibiting compounds suggests the mechanism by which these compounds inhibit Aβ fibril formation.
FIGURE 7.
Sedimentation coefficient distributions for Aβ1–42 in the absence (black line) and presence of an activating detergent (deoxycholate, blue) and an inhibiting detergent (LDAO, red).
DISCUSSION
Nonfibrillar compounds, such as lipids and glycans, are a major component of amyloid deposits. These molecules have been shown to have the ability to determine the rate of formation, stability, and morphology of amyloid fibrils by several different amyloidogenic proteins including medin (21), apoA-I (22), α-synuclein (23), islet amyloid polypeptide (24), β2-microglobulin (25), and prions (26). In addition, truncated and oxidized lipids, such as hydroxynonenol and a variety of cholesterol oxidation products, affect amyloid fibril formation by α-synuclein (27) and Aβ (28). Many studies have investigated the role of lipids in amyloid fibril formation by focusing on the effects of these molecules incorporated into lipid membranes and bilayers. This leads to the competing effects of electrostatic interactions between the lipid surface head groups, acting to enhance fibril formation, and strong hydrophobic interactions between the hydrophobic core and the protein acting to stabilize the aggregating protein (29). The strategy applied in this study avoids this issue by using lipid mimetics, which are assayed at concentrations below their respective critical micelle concentrations, permitting analysis of the effects of the individual molecules on Aβ amyloid fibril formation. This approach has been used previously to demonstrate the ability of specific amphiphiles to modulate apoC-II amyloid fibril formation (17) and in this study has effectively shown a similar ability of small amphipathic molecules to affect Aβ1–42 amyloid fibril formation.
The screen conducted in this study identified a large number of activators and inhibitors of Aβ amyloid fibril formation, which is consistent with the hydrophobic nature of the peptide. However, comparison of our results with those previously published by Ryan et al. (17) for apoC-II indicates a specific interaction, as molecules that were identified as activators or inhibitors of Aβ fibril formation differed from those observed to affect apoC-II amyloid fibril formation. Further support for the specificity of these detergent interactions is provided by another study of hexadecyl-N-methylpiperidinium bromide inhibition of Aβ aggregation, which indicates a 1:1 stoichiometry and a lack of effects on the aggregation of transthryretin and α-synuclein (30).
Similar to the previous study on apoC-II, these compounds show a distinct ability to stabilize secondary, tertiary, and quaternary structure; the inhibitors induced the formation of small oligomeric species of Aβ with an α-helical conformation, and the activators induced β-structured high molecular mass aggregates. Further inspection of the activating and inhibiting structures reveals common structural classes within these broad groups. One of the most active structural groups within the activating compounds is based on bile salts, such as deoxycholate and CHAPS. These molecules constitute some of the most effective activators of Aβ fibril in the screen, in some cases increasing the rate of fibril formation by >14-fold. This would suggest that cholesterol itself would be a potent activator of fibril formation. Indeed, the role of cholesterol in Alzheimer disease has garnered great attention in the literature, and there are several studies that indicate that cholesterol can modulate amyloid fibril formation and may be important in the pathogenesis of Alzheimer disease (31–37). The activation by several sterol-containing molecules suggests that there is a specific sterol binding site that enhances aggregation.
Another interesting structural observation could be made with NDSB-type detergents which are structurally similar to the compound 3-aminopropane-1-sulfonic acid (marketed as Tramiprosate or Alzhemed), which was shown to inhibit fibril formation, bind to soluble Aβ, and reduce plaque load in TgCRND8 mice (38). Interestingly, these compounds showed a distinct requirement for a ring structure for complete inhibition. In general, the remaining inhibitory molecules have structural similarity with fatty acids and small phospholipids, which have been shown to modulate aggregation of Aβ. Indeed, several published epidemiological studies suggest that high levels of docosahexanoic acid are associated with positive effects in Alzheimer disease sufferers (39, 40). Furthermore, ω-3 unsaturated fatty acids have a neuroprotective effect, and it has been suggested that a diet rich in these forms of fatty acids may aid in the prevention of Alzheimer disease (41).
On the basis of previous work on various other small molecule modulators of Aβ aggregation, such as 8-hydroxyquinolines (42), oleuropein (43, 44), α-helix stabilizers (45), various chaperones (46, 47), anesthetics (48, 49), and gangliosides (50), two potential binding sites can be suggested. From these previous studies it is clear that Aβ contains a small molecule binding site near residues 10–20 and a second binding site in the C-terminal glycine zipper motif. From our results it is unclear whether the compounds identified bind to either of these potential sites, and further work, similar to the study by Bieschke et al. (51), will be required to define the exact binding site and hence the mechanistic basis for the activity of these compounds. Understanding the exact binding site of these molecules and the biological implications of the structures formed may lead to new therapeutic strategies for Alzheimer disease and related protein aggregation disorders.

This article contains supplemental Fig. S1 and Table S1.
- Aβ
- amyloid β
- CMC
- critical micelle concentration
- ThT
- thioflavin T
- LDAO
- lauryldimethylamine oxide.
REFERENCES
- 1. Breteler M. M., Claus J. J., van Duijn C. M., Launer L. J., Hofman A. (1992) Epidemiology of Alzheimer's disease. Epidemiol. Rev. 14, 59–82 [DOI] [PubMed] [Google Scholar]
- 2. Chai C. K. (2007) The genetics of Alzheimer's disease. Am. J. Alzheimers Dis. Other Demen. 22, 37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Götz J., Ittner L. M. (2008) Animal models of Alzheimer's disease and frontotemporal dementia. Nat. Rev. Neurosci. 9, 532–544 [DOI] [PubMed] [Google Scholar]
- 4. Lorenzo A., Yankner B. A. (1994) β-Amyloid neurotoxicity requires fibril formation and is inhibited by Congo Red. Proc. Natl. Acad. Sci. U.S.A. 91, 12243–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorenzo A., Yankner B. A. (1996) Amyloid fibril toxicity in Alzheimer's disease and diabetes. Ann. N.Y. Acad. Sci. 777, 89–95 [DOI] [PubMed] [Google Scholar]
- 6. McLean C. A., Cherny R. A., Fraser F. W., Fuller S. J., Smith M. J., Beyreuther K., Bush A. I., Masters C. L. (1999) Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann. Neurol. 46, 860–866 [DOI] [PubMed] [Google Scholar]
- 7. Haass C., Selkoe D. J. (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid-β peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 [DOI] [PubMed] [Google Scholar]
- 8. Marchesi V. T. (2005) An alternative interpretation of the amyloid Aβ hypothesis with regard to the pathogenesis of Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 102, 9093–9098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kokubo H., Saido T. C., Iwata N., Helms J. B., Shinohara R., Yamaguchi H. (2005) Part of membrane-bound Aβ exists in rafts within senile plaques in Tg2576 mouse brain. Neurobiol. Aging 26, 409–418 [DOI] [PubMed] [Google Scholar]
- 10. Widenbrant M. J., Rajadas J., Sutardja C., Fuller G. G. (2006) Lipid-induced β-amyloid peptide assemblage fragmentation. Biophys. J. 91, 4071–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong P. T., Schauerte J. A., Wisser K. C., Ding H., Lee E. L., Steel D. G., Gafni A. (2009) Amyloid-β membrane binding and permeabilization are distinct processes influenced separately by membrane charge and fluidity. J. Mol. Biol. 386, 81–96 [DOI] [PubMed] [Google Scholar]
- 12. Yoda M., Miura T., Takeuchi H. (2008) Non-electrostatic binding and self-association of amyloid-β peptide on the surface of tightly packed phosphatidylcholine membranes. Biochem. Biophys. Res. Commun. 376, 56–59 [DOI] [PubMed] [Google Scholar]
- 13. Ikeda K., Matsuzaki K. (2008) Driving force of binding of amyloid-β protein to lipid bilayers. Biochem. Biophys. Res. Commun. 370, 525–529 [DOI] [PubMed] [Google Scholar]
- 14. Chi E. Y., Frey S. L., Lee K. Y. (2007) Ganglioside GM1-mediated amyloid-β fibrillogenesis and membrane disruption. Biochemistry 46, 1913–1924 [DOI] [PubMed] [Google Scholar]
- 15. Ryan T. M., Howlett G. J., Bailey M. F. (2008) Fluorescence detection of a lipid-induced tetrameric intermediate in amyloid fibril formation by apolipoprotein C-II. J. Biol. Chem. 283, 35118–35128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryan T. M., Teoh C. L., Griffin M. D., Bailey M. F., Schuck P., Howlett G. J. (2010) Phospholipids enhance nucleation but not elongation of apolipoprotein C-II amyloid fibrils. J. Mol. Biol. 399, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan T. M., Griffin M. D., Teoh C. L., Ooi J., Howlett G. J. (2011) High-affinity amphipathic modulators of amyloid fibril nucleation and elongation. J. Mol. Biol. 406, 416–429 [DOI] [PubMed] [Google Scholar]
- 18. McColl G., Roberts B. R., Gunn A. P., Perez K. A., Tew D. J., Masters C. L., Barnham K. J., Cherny R. A., Bush A. I. (2009) The Caenorhabditis elegans Aβ1–42 model of Alzheimer disease predominantly expresses Aβ3–42. J. Biol. Chem. 284, 22697–22702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schuck P. (2000) Size distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuck P. (2003) On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 320, 104–124 [DOI] [PubMed] [Google Scholar]
- 21. Olofsson A., Borowik T., Gröbner G., Sauer-Eriksson A. E. (2007) Negatively charged phospholipid membranes induce amyloid formation of medin via an α-helical intermediate. J. Mol. Biol. 374, 186–194 [DOI] [PubMed] [Google Scholar]
- 22. Andreola A., Bellotti V., Giorgetti S., Mangione P., Obici L., Stoppini M., Torres J., Monzani E., Merlini G., Sunde M. (2003) Conformational switching and fibrillogenesis in the amyloidogenic fragment of apolipoprotein A-I. J. Biol. Chem. 278, 2444–2451 [DOI] [PubMed] [Google Scholar]
- 23. Stöckl M., Fischer P., Wanker E., Herrmann A. (2008) α-Synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J. Mol. Biol. 375, 1394–1404 [DOI] [PubMed] [Google Scholar]
- 24. Knight J. D., Miranker A. D. (2004) Phospholipid catalysis of diabetic amyloid assembly. J. Mol. Biol. 341, 1175–1187 [DOI] [PubMed] [Google Scholar]
- 25. Pál-Gabor H., Gombos L., Micsonai A., Kovács E., Petrik E., Kovács J., Gráf L., Fidy J., Naiki H., Goto Y., Liliom K., Kardos J. (2009) Mechanism of lysophosphatidic acid-induced amyloid fibril formation of β2-microglobulin in vitro under physiological conditions. Biochemistry 48, 5689–5699 [DOI] [PubMed] [Google Scholar]
- 26. Deleault N. R., Harris B. T., Rees J. R., Supattapone S. (2007) Formation of native prions from minimal components in vitro. Proc. Natl. Acad. Sci. U.S.A. 104, 9741–9746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Näsström T., Fagerqvist T., Barbu M., Karlsson M., Nikolajeff F., Kasrayan A., Ekberg M., Lannfelt L., Ingelsson M., Bergström J. (2011) The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of α-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radic. Biol. Med. 50, 428–437 [DOI] [PubMed] [Google Scholar]
- 28. Siegel S. J., Bieschke J., Powers E. T., Kelly J. W. (2007) The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 46, 1503–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bokvist M., Lindström F., Watts A., Gröbner G. (2004) Two types of Alzheimer's β-amyloid1–40 peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J. Mol. Biol. 335, 1039–1049 [DOI] [PubMed] [Google Scholar]
- 30. Wood S. J., MacKenzie L., Maleeff B., Hurle M. R., Wetzel R. (1996) Selective inhibition of Aβ fibril formation. J. Biol. Chem. 271, 4086–4092 [DOI] [PubMed] [Google Scholar]
- 31. Arispe N., Doh M. (2002) Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AβP1–40 and 1–42 peptides. FASEB J. 16, 1526–1536 [DOI] [PubMed] [Google Scholar]
- 32. Avdulov N. A., Chochina S. V., Igbavboa U., Warden C. S., Vassiliev A. V., Wood W. G. (1997) Lipid binding to amyloid-β peptide aggregates: preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J. Neurochem. 69, 1746–1752 [DOI] [PubMed] [Google Scholar]
- 33. Yip C. M., Elton E. A., Darabie A. A., Morrison M. R., McLaurin J. (2001) Cholesterol, a modulator of membrane-associated Aβ-fibrillogenesis and neurotoxicity. J. Mol. Biol. 311, 723–734 [DOI] [PubMed] [Google Scholar]
- 34. Gibson Wood W., Eckert G. P., Igbavboa U., Muller W. E. (2003) Amyloid-β protein interactions with membranes and cholesterol: causes or casualties of Alzheimer's disease. Biochim. Biophys. Acta 1610, 281–290 [DOI] [PubMed] [Google Scholar]
- 35. Hane F., Drolle E., Leonenko Z. (2010) Effect of cholesterol and amyloid-β peptide on structure and function of mixed lipid films and pulmonary surfactant BLES: an atomic force microscopy study. Nanomedicine 6, 808–814 [DOI] [PubMed] [Google Scholar]
- 36. Panchal M., Loeper J., Cossec J. C., Perruchini C., Lazar A., Pompon D., Duyckaerts C. (2010) Enrichment of cholesterol in microdissected Alzheimer's disease senile plaques as assessed by mass spectrometry. J. Lipid Res. 51, 598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rojo L., Sjöberg M. K., Hernández P., Zambrano C., Maccioni R. B. (2006) Roles of cholesterol and lipids in the etiopathogenesis of Alzheimer's disease. J. Biomed. Biotechnol. 2006, 73976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gervais F., Paquette J., Morissette C., Krzywkowski P., Yu M., Azzi M., Lacombe D., Kong X., Aman A., Laurin J., Szarek W. A., Tremblay P. (2007) Targeting soluble Aβ peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging 28, 537–547 [DOI] [PubMed] [Google Scholar]
- 39. Schaefer E. J., Bongard V., Beiser A. S., Lamon-Fava S., Robins S. J., Au R., Tucker K. L., Kyle D. J., Wilson P. W., Wolf P. A. (2006) Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch. Neurol. 63, 1545–1550 [DOI] [PubMed] [Google Scholar]
- 40. Liu Y., Yang L., Conde-Knape K., Beher D., Shearman M. S., Shachter N. S. (2004) Fatty acids increase presenilin-1 levels and γ-secretase activity in PSwt-1 cells. J. Lipid Res. 45, 2368–2376 [DOI] [PubMed] [Google Scholar]
- 41. Calon F., Cole G. (2007) Neuroprotective action of ω-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot. Essent. Fatty Acids 77, 287–293 [DOI] [PubMed] [Google Scholar]
- 42. Choi J. S., Braymer J. J., Nanga R. P., Ramamoorthy A., Lim M. H. (2010) Design of small molecules that target metal-Aβ species and regulate metal-induced Aβ aggregation and neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 107, 21990–21995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bazoti F. N., Bergquist J., Markides K., Tsarbopoulos A. (2008) Localization of the noncovalent binding site between amyloid-β peptide and oleuropein using electrospray ionization FT-ICR mass spectrometry. J. Am. Soc. Mass Spectrom. 19, 1078–1085 [DOI] [PubMed] [Google Scholar]
- 44. Galanakis P. A., Bazoti F. N., Bergquist J., Markides K., Spyroulias G. A., Tsarbopoulos A. (2011) Study of the interaction between the amyloid β peptide1–40 and antioxidant compounds by NMR spectroscopy. Biopolymers 96, 316–327 [DOI] [PubMed] [Google Scholar]
- 45. Nerelius C., Sandegren A., Sargsyan H., Raunak R., Leijonmarck H., Chatterjee U., Fisahn A., Imarisio S., Lomas D. A., Crowther D. C., Strömberg R., Johansson J. (2009) α-Helix targeting reduces amyloid-β peptide toxicity. Proc. Natl. Acad. Sci. U.S.A. 106, 9191–9196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kinghorn K. J., Crowther D. C., Sharp L. K., Nerelius C., Davis R. L., Chang H. T., Green C., Gubb D. C., Johansson J., Lomas D. A. (2006) Neuroserpin binds Aβ and is a neuroprotective component of amyloid plaques in Alzheimer disease. J. Biol. Chem. 281, 29268–29277 [DOI] [PubMed] [Google Scholar]
- 47. Narayanan S., Kamps B., Boelens W. C., Reif B. (2006) αB-crystallin competes with Alzheimer's disease β-amyloid peptide for peptide-peptide interactions and induces oxidation of Aβ-Met35. FEBS Lett. 580, 5941–5946 [DOI] [PubMed] [Google Scholar]
- 48. Mandal P. K., Williams J. P., Mandal R. (2007) Molecular understanding of Aβ peptide interaction with isoflurane, propofol, and thiopental: NMR spectroscopic study. Biochemistry 46, 762–771 [DOI] [PubMed] [Google Scholar]
- 49. Mandal P. K., Pettegrew J. W., McKeag D. W., Mandal R. (2006) Alzheimer's disease: halothane induces Aβ peptide to oligomeric form: solution NMR studies. Neurochem. Res. 31, 883–890 [DOI] [PubMed] [Google Scholar]
- 50. Mandal P. K., Pettegrew J. W. (2004) Alzheimer's disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with Aβ1–40 peptide in a membrane mimic environment. Neurochem. Res. 29, 447–453 [DOI] [PubMed] [Google Scholar]
- 51. Bieschke J., Herbst M., Wiglenda T., Friedrich R. P., Boeddrich A., Schiele F., Kleckers D., Lopez del Amo J. M., Grüning B. A., Wang Q., Schmidt M. R., Lurz R., Anwyl R., Schnoegl S., Fändrich M., Frank R. F., Reif B., Günther S., Walsh D. M., Wanker E. E. (2012) Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nat. Chem. Biol. 8, 93–101 [DOI] [PubMed] [Google Scholar]