Background: Synaptotagmin-1, a Ca2+ sensor of neuronal exocytosis, interacts with the anionic phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2).
Results: Microscale thermophoresis shows that PIP2 binding to the polybasic patch of synaptotagmin-1 increases the Ca2+ affinity by >40-fold.
Conclusion: PIP2 and Ca2+ binding to synaptotagmin-1 is strongly cooperative.
Significance: Understanding the interplay between Ca2+, synaptotagmin-1, and PIP2 is crucial for our understanding of neurotransmitter release.
Keywords: Calcium-binding Proteins, Lipid-binding Protein, Neurobiology, Phosphatidylinositol, Synaptotagmin, Thermodynamics, Thermophoresis
Abstract
Synaptotagmin-1 is the main Ca2+ sensor of neuronal exocytosis. It binds to both Ca2+ and the anionic phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), but the precise cooperativity of this binding is still poorly understood. Here, we used microscale thermophoresis to quantify the cooperative binding of PIP2 and Ca2+ to synaptotagmin-1. We found that PIP2 bound to the well conserved polybasic patch of the C2B domain with an apparent dissociation constant of ∼20 μm. PIP2 binding reduced the apparent dissociation constant for Ca2+ from ∼250 to <5 μm. Thus, our data show that PIP2 makes synaptotagmin-1 >40-fold more sensitive to Ca2+. This interplay between Ca2+, synaptotagmin-1, and PIP2 is crucial for neurotransmitter release.
Introduction
In the synaptic terminal, neurotransmitter release is mediated by fusion of synaptic vesicles with the plasma membrane. Fusion is triggered by a sudden increase in the cytoplasmic Ca2+ concentration in response to membrane depolarization. The protein synaptotagmin-1 (together with synaptotagmin-2 and synaptotagmin-9) is the main Ca2+ sensor of the fast phase of neuronal exocytosis (reviewed in Ref. 1). Synaptotagmin-1 contains a single transmembrane domain close to the N terminus, which anchors the protein to synaptic vesicles. The transmembrane domain is connected by a 61-residue unstructured linker to two C2 domains, C2A and C2B. The mechanism by which synaptotagmin-1 triggers membrane fusion is still debated, but structural rearrangements of the plasma membrane and/or interactions with SNARE proteins have been implicated (1).
Ca2+ binding to synaptotagmin-1, originally demonstrated by equilibrium dialysis using native protein (2), has been characterized by isothermal titration calorimetry (3) and NMR (4–6) using a soluble fragment containing both C2 domains (C2AB fragment, residues 97–421). The C2A domain binds to three Ca2+ ions with affinities ranging from 50 μm to 10 mm. The C2B domain binds two Ca2+ ions, both with ∼200 μm affinity.
In the presence of Ca2+, the C2 domains of synaptotagmin-1 also bind to membranes containing anionic phospholipids, with little specificity for the phospholipid species (3, 6–14). Interestingly, binding already occurs at Ca2+ concentrations well below the Ca2+ affinity of free synaptotagmin-1. Here, anionic phospholipid headgroups complement the Ca2+-binding sites, increasing the affinity of C2AB for Ca2+ to ∼5–100 μm (3, 6–8, 11, 13). In the absence of Ca2+, a conserved polybasic lysine patch located on the C2B domain can also bind to anionic lipids, and this binding is strongly preferential for the polyanionic phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2)4 (3, 9–14). Binding of PIP2 to the polybasic patch might increase the Ca2+ affinity (12), although this is still controversial (3) and has hitherto not been characterized in detail.
Experimentally, measuring synaptotagmin-1 binding to PIP2 and/or Ca2+ is not trivial. Isothermal titration calorimetry and NMR require high (100 μm to 1 mm) concentrations of protein (3–5). Therefore, high affinities well below these concentrations cannot be accurately determined with these approaches. Binding of synaptotagmin to PIP2 is often inferred from binding of the C2 domains to artificial membranes containing a defined fraction of PIP2 (e.g. by FRET (3), pulldown assays (11, 13), or density flotations (3, 12)). However, it is difficult to quantitatively distinguish Ca2+ from PIP2 binding with these approaches. We have recently shown (10) that Ca2+ binding to synaptotagmin-1 can be directly measured with a new technique called microscale thermophoresis (MST) (15, 16). MST is based on the principle that molecules move along a temperature gradient in a capillary (the Soret effect). Upon binding to Ca2+ or PIP2, the surface properties of synaptotagmin-1 change, resulting in an altered thermophoretic behavior. In this study, we applied MST to study PIP2 and Ca2+ cooperative binding to synaptotagmin-1.
EXPERIMENTAL PROCEDURES
The C2AB fragment of synaptotagmin-1 (rat sequence, residues 97–421) was expressed in Escherichia coli and purified as described (3, 10). The single cysteine mutant (C278S/S342C) was labeled with Alexa Fluor 488-maleimide (Invitrogen) as described (3, 10). Liposomes were prepared by extrusion of rehydrated lipid films through 100-nm pores (polycarbonate membranes, Avestin) (17). All lipids were from Avanti Polar Lipids. MST was measured with ∼50 nm fluorescently labeled C2AB in 20 mm HEPES, 150 mm KCl, and 2.5 mg/ml BSA at pH 7.4. The samples were added to hydrophobic capillaries (NanoTemper Technologies), and MST was measured with a NanoTemper Monolith NT.015 system (25% light-emitting diode, 40% IR laser power). The label-free (tryptophan) experiments were performed with 1 μm wild-type C2AB, no BSA, and the NanoTemper Monolith NT.LabelFree instrument (80% UV light-emitting diode, 40% IR laser power). The MST curves were fitted with simple Michaelis-Menten kinetics to obtain the apparent dissociation constant for Ca2+ (KCa) or PIP2 (KPIP2). For Ca2+ binding, T = A − B/(KCa + [Ca2+]), where T is the percentage of fluorescence after heating, [Ca2+] is the total calcium concentration in the capillary, and A and B are conversion factors for the thermophoresis.
RESULTS
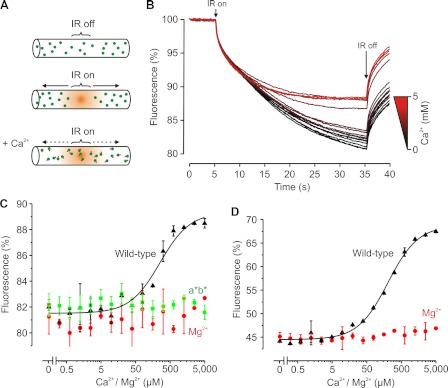
We performed MST measurements on the Alexa Fluor 488-labeled C2AB fragment of synaptotagmin-1 (residues 97–421). With this technique, a glass capillary is filled with a dilute protein solution (50 nm). Fluorescence is then measured at a spot in the capillary that is heated with a focused IR laser beam. Heating (by ∼5 °C) results in the generation of a temperature gradient along the axis of the capillary (Fig. 1, A and B). The C2AB fragment thermodiffuses out of this heated spot (measured by fluorescence recording), resulting in a protein gradient that is reversed when the IR laser is switched off. The amount of fluorescence decrease at the heated spot (the MST signal) was changed in the presence of Ca2+, thus providing a direct readout of Ca2+ binding to the C2AB fragment. Evidently, Ca2+ binding alters the thermophoretic (i.e. surface, charge) properties and thereby the thermodiffusion of synaptotagmin (10). Varying the calcium concentration in the capillary thus allowed us to obtain a binding curve (Fig. 1C).
FIGURE 1.
Ca2+ binding to C2AB measured by MST. A, principle of MST. A capillary containing 50 nm Alexa Fluor 488-labeled C2AB is locally heated by a focused IR laser (IR on). C2AB thermodiffuses away from the heated spot, causing a local depletion and a drop in fluorescence. Ca2+ binding changes the thermophoretic properties of C2AB, resulting in a decreased thermodiffusion. B, MST time traces of 16 different Ca2+ concentrations (ranging from 0 to 5 mm). Note that thermodiffusion is reduced at high Ca2+ concentrations. C, dependence of the MST signal on the Ca2+ concentration (measured 30 s after turning on heating; data from B). The solid line is a fit with Michaelis-Menten kinetics, yielding an apparent dissociation constant of KCa = 221 μm. No change in the MST signal was observed in the presence of Mg2+ or when a mutant impaired in Ca2+ binding was used (D178A/D230A/D232A/D309A/D363A/D365A, called C2a*b* (a*b*)). D, same as C but using unlabeled C2AB. MST was measured using intrinsic tryptophan fluorescence and fitted, yielding KCa = 206 μm. Error bars indicate the range of data points obtained from at least two measurements.
We fitted the binding curves with simple Michaelis-Menten kinetics assuming a single binding site (see “Experimental Procedures”). This model does not take into account binding of multiple Ca2+ ions (or PIP2 molecules; see below), and for some curves, this simplification may affect the quality of the fit. However, the overall quality of the data did not warrant fitting with a more sophisticated binding model. Thus, we could not differentiate between the different calcium-binding sites, and we report only the apparent dissociation constant (KCa).
C2AB bound to Ca2+ with KCa = 221 ± 23 μm (n = 3). Control experiments with Mg2+ or a mutant with disrupted Ca2+ binding (D178A/D230A/D232A/D309A/D363A/D365A, called C2a*b*) (3, 10) showed that the change in the MST signal was indeed due to binding of Ca2+ ions to the established binding sites in the C2 domains. Furthermore, the MST measurements were not affected by the presence of the dye because a similar binding constant of KCa = 206 ± 40 μm was obtained with the unlabeled C2AB fragment using the intrinsic tryptophan fluorescence as the readout (C2AB has three tryptophans) (Fig. 1D). We then set out to study the cooperativity of Ca2+ and PIP2 binding.
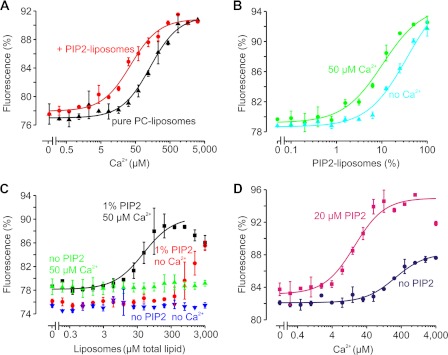
No apparent change in the Ca2+-dependent thermophoretic behavior of C2AB was observed in the presence of liposomes composed of pure 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (2.5 mm total lipid concentration; KCa = 226.7 ± 50.7 μm) (Fig. 2A). In contrast, the apparent affinity for Ca2+ increased by ∼5-fold when only 10% of these liposomes were replaced with a liposome population composed of 95% 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine and 5% PIP2 (KCa = 46.0 ± 5.9 μm). Accordingly, the addition of 50 μm Ca2+ (well below the KCa of C2AB) resulted in ∼4-fold stronger binding to PIP2-containing liposomes (from KPIP2 = 45.3 ± 9.25 μm to 13.3 ± 2.9 μm total PIP2 concentration) (Fig. 2B). 50 μm Ca2+ also increased C2AB binding to liposomes containing a more physiological lipid composition (phosphatidylcholine/phosphatidylethanolamine/phosphatidylserine/cholesterol at a molar ratio of 5:2:1:1) but only if 1 mol % PIP2 was present (Fig. 2C). Thus, synaptotagmin-1 binds to anionic membranes and Ca2+ in a cooperative manner, as reported previously (3, 6–13). We performed a set of experiments with water-solubilized PIP2 to further characterize this cooperativity.
FIGURE 2.
Ca2+ dependence of MST signal of C2AB in presence of PIP2-containing liposomes. A, Ca2+ binding of the C2AB fragment in the presence of 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (PC)-containing liposomes (2.5 mm total lipid concentration) yielded an apparent dissociation constant of KCa = 226.7 ± 50.7 μm (black). However, when 10% of the 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine-containing liposomes contained 5 mol % PIP2, the affinity increased by ∼5-fold to KCa = 46.0 ± 5.9 μm (red). B, liposome binding as a function of the fraction of PIP2-containing liposomes. In all cases, the total lipid concentration was 2.5 mm, but the fraction of liposomes containing 5 mol % PIP2 varied. In the absence of Ca2+, C2AB bound to the PIP2 membranes with KPIP2 = 36.2 ± 7.4% (or 45.3 μm PIP2; cyan). In the presence of 50 μm Ca2+, the affinity increased by 4-fold to KPIP2 = 10.6 ± 2.3% (or 13.3 μm PIP2; green). C, binding of C2AB to liposomes composed of a 5:2:1:1 molar ratio of brain isolated phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and cholesterol. C2AB did not bind to liposomes lacking PIP2 regardless of the presence (green) or absence (blue) of 50 μm Ca2+. In contrast, C2AB bound to liposomes containing 1 mol % PIP2 already in the absence of Ca2+ (red). Similar to B, 50 μm Ca2+ increased the binding affinity (KPIP2 = 50.9 ± 20.0 μm total lipid concentration; black). D, Ca2+ binding curve of C2AB in the presence (KCa = 17.7 ± 0.7 μm; pink) or absence (KCa = 265.2 ± 27.4 μm; blue) of 20 μm PIP2 in solution. 1 mm Mg2+ was present to suppress potentially unspecific Ca2+-PIP2 interactions. Error bars indicate the range of data points obtained from at least two measurements.
One of the main advantages of MST compared with alternative techniques for measuring Ca2+ binding is the low concentration of protein that is required: measurements could be carried out with C2AB concentrations as low as 50 nm, which is 3–4 orders of magnitude below that reported for isothermal titration calorimetry (3) or NMR (4–6). This low concentration allowed us to measure PIP2 binding by adding PIP2 directly to the capillary (Fig. 2D). Even PIP2 isolated from porcine brain with long fatty acid acyl chains (dominant species C18:0 and C20:4) is water-soluble at concentrations up to ∼9 mm and does poorly form micelles because of its high anionic charge (18).
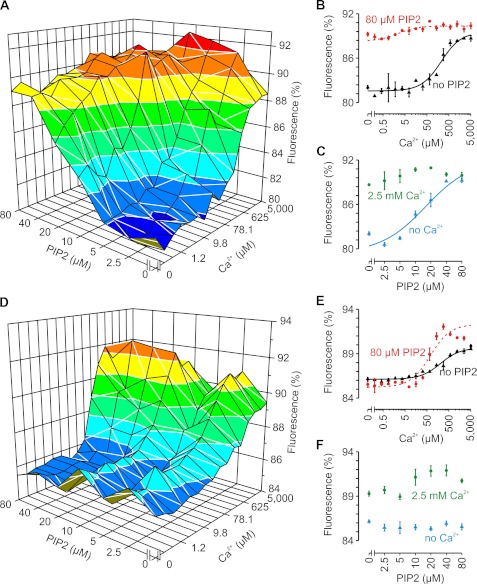
Strikingly, the affinity for Ca2+ binding increased by 15-fold in the presence of 20 μm PIP2 (from KCa = 265.2 ± 27.4 μm to 17.7 ± 0.7 μm) (Fig. 2D). In this experiment, an excess of 1 mm Mg2+ was present to suppress potential nonspecific interactions of Ca2+ with PIP2 or C2AB. At higher PIP2 concentrations, the Ca2+ affinity increased even further (to >40-fold; KCa = 3.3 ± 1.3 μm at 40–80 μm PIP2 compared with 221 ± 23 μm without PIP2) (Fig. 3, A–C). Accordingly, the addition of Ca2+ progressively increased the binding affinity of C2AB for PIP2 (from KPIP2 = 20 ± 5 μm without Ca2+ to <2 μm at >20 μm Ca2+). This cooperativity is not specific for PIP2 or the length of the acyl chains because another phosphoinositide (20 μm phosphatidylinositol 3,5-bisphosphate) or short-chain PIP2 (20 μm 1,2-dioctanoyl-sn-glycero-3-phosphatidylinositol 4′,5′-bisphosphate; C8:0) also increased the apparent Ca2+ affinity (KCa = 11 ± 5 and 8 ± 5 μm, respectively).
FIGURE 3.
Ca2+ and PIP2 binding to C2AB measured by MST. A, MST as a function of both Ca2+ and PIP2. Each x and y curve (thus with the same Ca2+ or PIP2 concentrations) was fitted with Michaelis-Menten kinetics to obtain the apparent dissociation constants (KCa and KPIP2; see Fig. 4). B, two Ca2+ binding curves from A and their corresponding fits in the absence (KCa = 221 μm; black) or presence (KCa = 4.6 μm; red) of 80 μm PIP2. C, two PIP2 binding curves from A in the absence (KPIP2 = 20 μm; blue) or presence (KPIP2 < 5 μm; green) of 2.5 mm Ca2+. D–F, same as A–C but for the KAKA mutant (K326A/K327A) (12). Compared with the wild type, the amplitude of the fluorescence changes of the KAKA mutant was reduced due to the altered thermophoretic properties that resulted from the substitution of charged residues. In E, the solid (no PIP2) and dashed (80 μm PIP2) lines are fits with KCa = 195 and 61 μm, respectively. Note that for the KAKA mutant, PIP2 binding was dramatically reduced compared with the wild type. Each experiment was repeated at least twice; error bars show the range of data points.
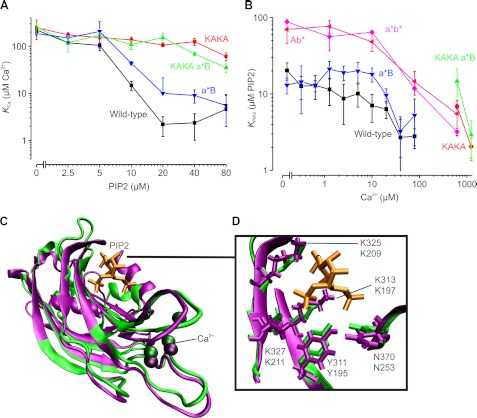
PIP2 binding required the well conserved polybasic patch that is located on the C2B domain because removal of two lysines from this patch (K326A/K327A, the so-called KAKA mutant (12)) (Fig. 3, D–F, and Fig. 4, A and B) almost completely abolished PIP2-dependent MST changes, even at very high Ca2+ concentrations. Accordingly, the apparent affinity for Ca2+ was increased by only ∼3-fold in the presence of 80 μm PIP2 (from KCa = 195 ± 35 μm to 61 ± 11 μm). Thus, we could detect only PIP2 binding to the polybasic patch and did not observe PIP2 binding via the Ca2+-binding sites on the C2A and C2B domains, in contrast to previous observations by us and others (3, 10–12, 14). It is likely that, for the interaction of the Ca2+-binding pockets with the membrane, hydrophobic residues surrounding these pockets must insert into the membrane (6–8, 11, 12, 14), although we cannot exclude that PIP2 binding to the Ca2+ sites is silent (i.e. does not change the MST signal). Nevertheless, the Ca2+-binding pocket of the C2B domain does affect PIP2 binding to the polybasic patch because disruption of Ca2+ binding to the C2B domain (D309A/D363A/D365A, called C2Ab*) reduced the affinity for PIP2 by ∼4-fold (from KPIP2 = 20.4 ± 5.2 μm to 70 ± 24 μm) (Fig. 4B).
FIGURE 4.
Cooperative Ca2+ and PIP2 binding to C2AB. The apparent dissociation constants for Ca2+ binding (KCa; A) and PIP2 binding (KPIP2; B) were determined by MST. Wild-type C2AB (see Fig. 3, A–C) and various mutants were tested: KAKA (K326A/K327A; see Fig. 3, D–F), C2a*B (a*B; D178A/D230A/D232A), C2Ab* (Ab*; D309A/D363A/D365A), C2a*b* (a*b*; D178A/D230A/D232A/D309A/D363A/D365A), and KAKA/C2a*B (KAKA a*B). The KAKA/C2Ab* and KAKA/C2a*b* mutants are not shown in the figure because PIP2 and Ca2+ binding could not be detected with MST (see Fig. 1C). Error bars show the range of data points obtained from at least two measurements. C, conservation of the PIP2-binding sites. The crystal structure of the C2B domain (purple; Protein Data Bank code 1TJX (26)) was overlapped with that of the PIP2-bound PKCα C2 domain (green; code 3GPE (25)). D, all residues that stabilize the PIP2 headgroup (orange) are conserved in the C2B domain (see also Ref. 25).
We then performed MST experiments with mutants disrupted in Ca2+ binding to the C2A domain (D178A/D230A/D232A, called C2a*B). Surprisingly, only a small and insignificant PIP2- or Ca2+-dependent change in the MST signal of C2a*B was observed compared with the wild type (Fig. 4, A and B). Accordingly, the combination of C2a*B with the KAKA mutation did not markedly differ from the KAKA mutant with all Ca2+-binding sites intact. Apparently, Ca2+ binding to the C2A domain does not result in a detectable change in the thermophoretic properties of the C2AB fragment. In contrast, Ca2+ binding could no longer be detected by MST upon disruption of the C2B domain. Thus, only Ca2+ binding to the C2B domain seems to change the thermophoretic properties of the C2AB fragment, indicating that the calcium-dependent changes reported above are exclusively mediated by the C2B domain. Perhaps this selectivity is related to the thermodynamically divergent modes of Ca2+ binding of synaptotagmin-1: Ca2+ binding to the C2A domain is endothermic, and that to the C2B domain is exothermic (3). Finally, Ca2+ concentrations above 100 μm increased the apparent PIP2 affinity of synaptotagmin-1 even when both Ca2+-binding sites were disrupted (double mutant C2a*b*) (Fig. 4B). This indicates that Ca2+ was still able to bind to the double mutant at very high Ca2+ concentrations in the presence of PIP2, perhaps by binding directly to PIP2 (19, 20).
DISCUSSION
In this work, we have shown that PIP2 binds to the polybasic patch of the C2B domain of synaptotagmin-1, in agreement with earlier studies (10–14, 21). PIP2 binding to the polybasic patch increases the apparent affinity of the C2B domain for Ca2+ by >40-fold. Conversely, Ca2+ binding to the C2B domain increases the affinity for PIP2 by >10-fold. Cooperative PIP2 and Ca2+ binding to synaptotagmin-1 has been observed previously (12). This cooperativity is probably not caused by complementation of the Ca2+-binding sites, as suggested earlier by us and others (3, 6–8), because the polybasic patch and the Ca2+-binding sites are located quite far apart (Fig. 4C). Instead, PIP2 may interact in a structurally less defined manner with the polybasic patch and other solvent-exposed basic residues (9, 12), and this may increase the Ca2+ affinity simply by charge screening. Alternatively, the polybasic patch may form a structurally defined complex with PIP2 similar to the C2 domains of rabphilin-3A and PKCα (22–25). In fact, cooperative PIP2 and Ca2+ binding has been observed for these C2 domains (22–24), very similar to our observations for the C2B domain. Moreover, the crystal structure of the C2B domain (26) can be superimposed with that of the PIP2-bound C2 domain of PKCα (25), rendering it likely that PIP2 binds to the C2AB fragment of synaptotagmin-1 in a similar manner (Fig. 4, C and D). Thus, it is conceivable that such PIP2 binding increases the Ca2+ affinity via a conformational change. However, how PIP2 and Ca2+ precisely bind in a cooperative manner to synaptotagmin-1 remains to be elucidated.
Together, we conclude that PIP2 binding to the polybasic patch of synaptotagmin-1 dramatically increases the Ca2+ sensitivity. As discussed previously (12), this explains the reduced release probability of the KAKA mutant in hippocampal neurons (12, 27) and in Drosophila (28). It also explains why in vivo already 10 μm Ca2+ is sufficient for physiological release of neurotransmitters in the calyx of Held (29). PIP2 modulation of synaptotagmin-1 may well be of major physiological relevance when considering that PIP2 is the predominant phospholipid species at the sites of docked vesicles in PC12 cells (30).
Finally, our work demonstrates the value of MST for measuring molecular interactions. Although we were unable to detect Ca2+ binding to the C2A domain under our conditions, MST can be extremely sensitive and allows for monitoring medium and high affinity interactions with only picomoles of material. MST has the potential to complement the limited set of techniques available to measure Ca2+ and PIP2 binding to proteins under equilibrium conditions such as isothermal titration calorimetry and NMR.
Acknowledgment
We thank Stefan Duhr (NanoTemper Technologies GmbH) for advice and the label-free measurements.
This work was supported, in whole or in part, by National Institutes of Health Grant P01 GM072694 (to R. J.) This work was also supported by Deutsche Forschungsgemeinschaft Grant SFB803.
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- MST
- microscale thermophoresis.
REFERENCES
- 1. Chapman E. R. (2008) How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77, 615–641 [DOI] [PubMed] [Google Scholar]
- 2. Brose N., Petrenko A. G., Südhof T. C., Jahn R. (1992) Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256, 1021–1025 [DOI] [PubMed] [Google Scholar]
- 3. Radhakrishnan A., Stein A., Jahn R., Fasshauer D. (2009) The Ca2+ affinity of synaptotagmin-1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 284, 25749–25760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ubach J., Zhang X., Shao X., Südhof T. C., Rizo J. (1998) Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2 domain? EMBO J. 17, 3921–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez I., Araç D., Ubach J., Gerber S. H., Shin O., Gao Y., Anderson R. G., Südhof T. C., Rizo J. (2001) Three-dimensional structure of the synaptotagmin-1 C2B domain: synaptotagmin-1 as a phospholipid-binding machine. Neuron 32, 1057–1069 [DOI] [PubMed] [Google Scholar]
- 6. Fernández-Chacón R., Königstorfer A., Gerber S. H., García J., Matos M. F., Stevens C. F., Brose N., Rizo J., Rosenmund C., Südhof T. C. (2001) Synaptotagmin-1 functions as a calcium regulator of release probability. Nature 410, 41–49 [DOI] [PubMed] [Google Scholar]
- 7. Davletov B. A., Südhof T. C. (1993) A single C2 domain from synaptotagmin-1 is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 268, 26386–26390 [PubMed] [Google Scholar]
- 8. Zhang X., Rizo J., Südhof T. C. (1998) Mechanism of phospholipid binding by the C2A domain of synaptotagmin-1. Biochemistry 37, 12395–12403 [DOI] [PubMed] [Google Scholar]
- 9. Araç D., Chen X., Khant H. A., Ubach J., Ludtke S. J., Kikkawa M., Johnson A. E., Chiu W., Südhof T. C., Rizo J. (2006) Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13, 209–217 [DOI] [PubMed] [Google Scholar]
- 10. van den Bogaart G., Thutupalli S., Risselada J. H., Meyenberg K., Holt M., Riedel D., Diederichsen U., Herminghaus S., Grubmüller H., Jahn R. (2011) Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat. Struct. Mol. Biol. 18, 805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bai J., Tucker W. C., Chapman E. R. (2004) PIP2 increases the speed of response of synaptotagmin and steers its membrane penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 [DOI] [PubMed] [Google Scholar]
- 12. Li L., Shin O. H., Rhee J. S., Araç D., Rah J. C., Rizo J., Südhof T., Rosenmund C. (2006) Phosphatidylinositol phosphates as coactivators of Ca2+ binding to C2 domains of synaptotagmin-1. J. Biol. Chem. 281, 15845–15852 [DOI] [PubMed] [Google Scholar]
- 13. Schiavo G., Gu Q. M., Prestwich G. D., Söllner T. H., Rothman J. E. (1996) Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. U.S.A. 93, 13327–13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuo W., Herrick D. Z., Cafiso D. S. (2011) Phosphatidylinositol 4,5-bisphosphate alters synaptotagmin-1 membrane docking and drives opposing bilayers closer together. Biochemistry 50, 2633–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wienken C. J., Baaske P., Rothbauer U., Braun D., Duhr S. (2010) Protein binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1, 100. [DOI] [PubMed] [Google Scholar]
- 16. Duhr S., Braun D. (2006) Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. U.S.A. 103, 19678–19682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Bogaart G., Mika J. T., Krasnikov V., Poolman B. (2007) The lipid dependence of melittin action investigated by dual-color fluorescence burst analysis. Biophys. J. 93, 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu A., Stefani E. (1991) Phosphatidylinositol 4,5-bisphosphate-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum terminal cisternal membranes. Ca2+ flux and single channel studies. J. Biol. Chem. 266, 7699–7705 [PubMed] [Google Scholar]
- 19. Carvalho K., Ramos L., Roy C., Picart C. (2008) Giant unilamellar vesicles containing phosphatidylinositol 4,5-bisphosphate: characterization and functionality. Biophys. J. 95, 4348–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levental I., Christian D. A., Wang Y. H., Madara J. J., Discher D. E., Janmey P. A. (2009) Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry 48, 8241–8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukuda M., Kojima T., Aruga J., Niinobe M., Mikoshiba K. (1995) Functional diversity of C2 domains of synaptotagmin family. Mutational analysis of inositol high polyphosphate-binding domain. J. Biol. Chem. 270, 26523–26527 [DOI] [PubMed] [Google Scholar]
- 22. Montaville P., Coudevylle N., Radhakrishnan A., Leonov A., Zweckstetter M., Becker S. (2008) The PIP2 binding mode of the C2 domains of rabphilin-3A. Protein Sci. 17, 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torrecillas A., Laynez J., Menéndez M., Corbalán-García S., Gómez-Fernández J. C. (2004) Calorimetric study of the interaction of the C2 domains of classical protein kinase C isoenzymes with Ca2+ and phospholipids. Biochemistry 43, 11727–11739 [DOI] [PubMed] [Google Scholar]
- 24. Guerrero-Valero M., Marín-Vicente C., Gómez-Fernández J. C., Corbalán-García S. (2007) The C2 domains of classical PKCs are specific PtdIns(4,5)P2-sensing domains with different affinities for membrane binding. J. Mol. Biol. 371, 608–621 [DOI] [PubMed] [Google Scholar]
- 25. Guerrero-Valero M., Ferrer-Orta C., Querol-Audí J., Marin-Vicente C., Fita I., Gómez-Fernández J. C., Verdaguer N., Corbalán-García S. (2009) Structural and mechanistic insights into the association of PKCα C2 domain with PtdIns(4,5)P2. Proc. Natl. Acad. Sci. U.S.A. 106, 6603–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng Y., Sequeira S. M., Malinina L., Tereshko V., Söllner T. H., Patel D. J. (2004) Crystallographic identification of Ca2+ and Sr2+ coordination sites in synaptotagmin-1 C2B domain. Protein Sci. 13, 2665–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borden C. R., Stevens C. F., Sullivan J. M., Zhu Y. (2005) Synaptotagmin mutants Y311N and K326A/K327A alter the calcium dependence of neurotransmission. Mol. Cell. Neurosci. 29, 462–470 [DOI] [PubMed] [Google Scholar]
- 28. Mackler J. M., Reist N. E. (2001) Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J. Comp. Neurol. 436, 4–16 [PubMed] [Google Scholar]
- 29. Schneggenburger R., Neher E. (2005) Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol. 15, 266–274 [DOI] [PubMed] [Google Scholar]
- 30. van den Bogaart G., Meyenberg K., Risselada H. J., Amin H., Willig K. I., Hubrich B. E., Dier M., Hell S. W., Grubmüller H., Diederichsen U., Jahn R. (2011) Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]