Background: Binding of LPSs and lipopeptides to Toll-like receptors depends on their lipid moieties.
Results: Synthetic triacylated LPS-like molecules bind to TLR2 (weak agonists) and TLR4 (strong antagonists) and block phagocytosis of Gram-negative bacteria.
Conclusion: Triacylated LPS-like molecules are potent TLR4 antagonists.
Significance: These compounds may represent valuable LPS antagonists and immunomodulators.
Keywords: Lipopolysaccharide (LPS), Lipoprotein, Phagocytosis, Sepsis, Toll-like Receptors (TLR)
Abstract
Recognition of microbial molecules by mammalian host receptors is essential to mount an immune response. Hexaacylated LPS is the prototypic example of a bacterial molecule recognized by the receptor complex TLR4/MD-2 with its lipid A moiety, whereas bacterial lipopeptides are recognized by TLR2. Here we show that a series of synthetic triacylated lipid A-like molecules are weak Toll-like receptor (TLR) agonists (mainly TLR2 agonists) but very potent TLR4/MD-2 antagonists (submicromolar range). Not only do they block human cell responses to LPS but also to whole Gram-negative bacteria, and they inhibit the phagocytosis of Gram-negative bacteria. These compounds may represent promising immunomodulatory agents.
Introduction
The recognition of bacteria by mammalian cells is key to mount an innate response necessary to control infection. Several bacterial antigens known as pathogen-associated molecular patterns (PAMPs) are sensed by host cells using Toll-like receptors (TLRs)3. LPS is the prominent ligand among Gram-negative bacteria and signals via a receptor complex comprising TLR4, MD-2, and CD14 (1–3) Recent structural studies have allowed us to better understand the intimate molecular relationship between bacterial ligands and their receptors. This is particularly true for TLR2 and TLR4, which have been crystallized with their respective ligands (2, 4). Individual LPS molecules may act as agonists in some mammalian species and as antagonists in others (5–8). As an example, monomerized LPS binds CD14 and is then transferred onto MD-2 and TLR4. Five of six acyl chains of LPS insert into a hydrophobic pocket of the MD-2 bound to TLR4 (2). The sixth acyl chain binds TLR4, inducing ligand-dependent receptor cross linking and signaling via the intracellular portion of TLR4 (2). Bacterial lipopeptides, through their acyl chains, directly cross-link TLR2 with TLR6 or TLR1, depending on whether they are made of two or three acyl chains, respectively (4).
LPS lipid A structures containing less than six acyl chains are poor TLR4 agonists or even antagonists, such as Rhodobacter sphaeroides LPS, lipid IVa, or its synthetic molecular mimics Eritoran (E5564), containing four acyl chains. Eritoran blocks LPS activation of cells by inserting itself into the MD-2 hydrophobic pocket (9) TLR2 and TLR4 antagonists may prove to be important immunomodulators in the future because these two receptors account for nearly all the cell activation that is observed in Gram-negative and Gram-positive bacterial infections (10).
We aimed to test a series of newly developed synthetic triacylated lipid A-like molecules for their agonistic versus antagonistic effects on cells bearing TLRs and associated receptor molecules. We found that most of the triacylated Lipid A-like molecules activated cells via TLR2 (acting as lipopeptides), except for one that was a TLR4 agonist. Some of them blocked TLR4 signaling, activation of cells induced by whole Gram-negative bacteria, and also phagocytosis of Gram-negative bacteria and are promising immunomodulatory agents.
EXPERIMENTAL PROCEDURES
Reagents
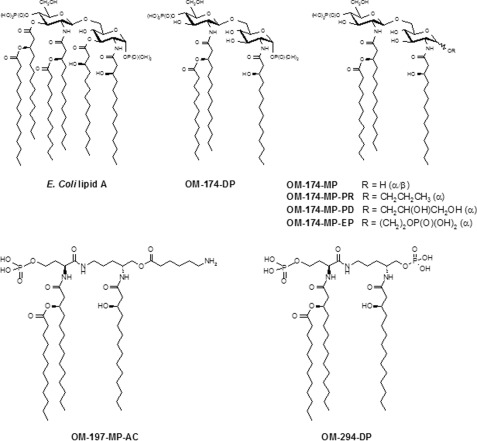
Molecular structures of triacylated lipid A-like molecules are shown in Fig. 1. These molecules were produced by OM Pharma®, and methods for the production and/or synthesis of these compounds has been published elsewhere (11). OM-174-DP was originally derived from Escherichia coli LPS. Both the core and the O-specific polysaccharidic antigens were chemically removed from the E. coli LPS, as well as three fatty acid chains in lipid A (11). There only remained the diglucosamine backbone of native E. coli lipid A and three fatty acid acyl chains. OM-174-DP was subsequently purified by RP-HPLC (> 96% purity), and the sodium salt was shown to be water-soluble (> 100 mg/ml). OM-174-DP is a fully synthetic diphosphate (DP) triacylated lipid A-like molecule obtained by chemical synthesis. Using the chemical route developed for the synthesis of OM-174-DP, several synthetic triacylated derivatives of OM-174-DP were produced where the anomeric phosphate group was replaced by the following residues: hydrogen (MP); 2,3 dihydroxypropyl (PD); 2-(phosphonooxy)ethyl (EP), or propyl (PR). The compounds of acyclic lipid A mimics series, namely OM-197-MP-AC and OM-294-DP, are fully chemically synthesized triacylated Lipid A-like molecules where the diglucosamine from lipid A was substituted by a pseudodipeptide backbone (OM-Triacyl®, Fig. 1). OM-294-DP bears two phosphate groups, and OM-197-MP-AC has an aminocaproyl (AC) in place of the second phosphate. OM-174, OM-197-MP-AC, and OM-294 derivatives had very low endotoxic activity in the limulus assay and very low pyrogenicity (in vivo rabbit assay) (11–13). E. coli K12LCD25 LPS and PAM3CSK4 were purchased from InvivoGen (San Diego, CA). The E. coli and Staphylococcus aureus bacterial strains were obtained from P. Rohner (clinical isolates, Clinical Microbiology Laboratory, University Hospitals of Geneva, Switzerland). Heat-killed bacteria were produced as described previously (10).
FIGURE 1.
Molecular structure of triacylated lipid A analogs. E. coli lipid A characterized by a diglucosamine backbone, six acyl chains, and two phosphate groups. The triacylated compound OM-174-DP is a fully synthetic molecule that was originally produced by deacylation of three of the six acyl chains of E. coli lipid A, maintaining the diglucosamine backbone of lipid A. OM-174-MP, OM-174-MP-PD, OM-174-MP-EP, and OM-174-MP-PR were derivatives of OM-174-DP obtained by chemical synthesis where the anomeric phosphate group was substituted by the following residues (R): hydrogen (MP), PD, EP, or PR. OM-294-DP is a completely synthetic triacylated molecule where the diglucosamine backbone has been replaced by a pseudodipeptide moiety plus the two phosphate groups (11). OM-197-MP-AC is also a triacylated synthetic molecule with a pseudodipeptide backbone identical to OM-294-DP with the substitution of the second phosphate group by an aminocaproyl (AC) residue.
Mouse anti-huCD14 28C5 mAb (mouse IgG1) was a gift from P. S. Tobias (Scripps, La Jolla, CA). Mouse anti-huTLR2 T2.5 mAb (mouse IgG1) was purchased from eBioscience (San Diego, CA). Mouse anti-human TLR1 and TLR6 (mouse IgG1) mAb were purchased from InvivoGen. Mouse anti huTLR4 15C1 mAb (mouse IgG1) and mouse anti-huMD-2 18H10 mAb (mouse IgG2b) were obtained from NovImmune SA, Geneva, Switzerland, and described in Refs. 10, 14–16. IgG isotype controls were purchased from BD Biosciences. IL-6 and IL-8 ELISA were performed using couples of MAbs (Endogen, Woburn, MA) as described elsewhere (15). Human IP-10 was measured by ELISA (Hycult Biotechnology b.v., The Netherlands) as described elsewhere (10). In some experiments, levels of multiple inflammatory cytokines were measured in conditioned supernatants using the BD cytometric bead array (BD Biosciences).
Cells
HEK 293 cells were obtained from the ATCC. HEK-293 cells stably expressing huTLR4/MD-2 or TLR2 were produced as described elsewhere (10). TLR2(+) and TLR4(+)HEK293Bluetm cells were obtained from InvivoGen.
Cell Stimulation Assays
Transfected HEK293 cells were seeded into a 96-well plate at the density of 6 × 104 cells/well. Triacylated LPS-like molecules were added to cells at the concentrations of 10, 3.3, 1.1, 0.37, 0.12, 0.04, 0.013, and 0 μg/ml diluted in culture media containing 5% FCS. E. coli K12CD25 LPS was used as a positive control for TLR4 stimulation and PAM3CSK4 as a TLR2 agonist. Markers of cell activation were IL-8 in supernatant for HEK293 transfectants and alkaline phosphatase for HEK293Bluetm cells, with levels measured according to the protocol of the manufacturer. Cell stimulation experiments were performed in triplicates, and each experiment was repeated at least three times.
Activation of Whole Blood by Triacylated Lipid A-like Molecules
Fresh heparinized blood from healthy volunteers was obtained by venipuncture. Blood was diluted 1:1 in RPMI 1640 medium, and 60 μl were distributed in each well of a 96-well plate. In some experiments, 10 μg/ml anti-TLRs, anti-MD-2, or anti-CD14 mAbs or their isotype controls were added to blood 60 min prior to stimulation with agonists. Triacylated lipid A-like molecules, E. coli K12 LPS, or PAM3CSK4 was added to blood and incubated for 6 or 24 h at the concentration specified above. The final dilution of blood was 1:4 in RPMI. IL-6 levels were measured in conditioned supernatants by ELISA.
Antagonist Effect of Triacylated Lipid A-like Molecules
Triacylated lipid A-like molecules were added to the cells at the concentration of 10, 3.3, 1.1, 0.37, 0.12, 0.04, 0.013, and 0 μg/ml 30 min prior to cell stimulation. E. coli K12 LPS, heat-killed S. aureus (HKSA), heat-killed Gram-negative bacteria (E. coli, Klebsiella pneumoniae) was then added to diluted whole blood or TLR4(+)HEK293Bluetm cells for 6 or 24 h. Markers of cell activation were alkaline phosphatase activity (TLR4(+)HEK293Bluetm cells), inflammatory cytokines (whole blood, human inflammation kit BD cytometric bead array), as well as human IP-10. In other experiments, we incubated TLR2(+)HEK293 Bluetm cells with a fixed concentration of PAM3CSK4 in the presence of increasing concentrations of OM compounds.
Phagocytosis Assay
Leukocytes from human heparinized blood obtained by venipuncture in healthy subjects were isolated using a gelatin gradient (Physiogel®, Braun Medical, Emmenbrücke, Switzerland). Red blood cells were lysed with a 0.15 m ammonium chloride shock. To opsonize bacteria, fluorescent E. coli (BODIPY FL bioparticles, Invitrogen) was incubated with 20 μg/ml recombinant human soluble MD-2 (rhusMD-2, produced as described previously (15) or alternatively with 2% normal human decomplemented plasma for 1 h at 37 °C. Opsonized bacteria were then centrifuged at 1,000 g for 15 min at 4 °C and washed once in PBS. To test phagocytosis, 3 × 106 fluorescent E. coli was added to 106 leukocytes, corresponding to a bacteria-to-cell ratio of 3:1. OM-174-DP compound was added on the cells for 30 min prior to fluorescent bacteria at different concentrations (0, 1, 5, and 10 μg/ml). Phagocytosis was performed at 37 °C for 10 and 20 min in the same buffer as used during opsonization. Cells were centrifuged at 300 g for 4 min at 4 °C and further washed with ice-cold PBS. To only quantify internalized E. coli, extracellular fluorescence was quenched with 0.05% trypan blue (Invitrogen), and leukocytes were analyzed by flow cytometry (FACScalibur). The neutrophil population was gated according to its forward and side-scatter characteristics. The negative control consisted of neutrophils pretreated 30 min with 100 μm cytochalasin D (Sigma). Phagocytosis was quantified using a “phagocytic index” as defined by the percentage of cells becoming green (positive) multiplied by their geometric mean of fluorescence, as described previously (17). In some experiments, 10 μg/ml anti-TLR4 mAb was added to the leukocytes before initiating the phagocytosis assay.
RESULTS
Most Triacylated Lipid A-like Molecules Are TLR2 Agonists
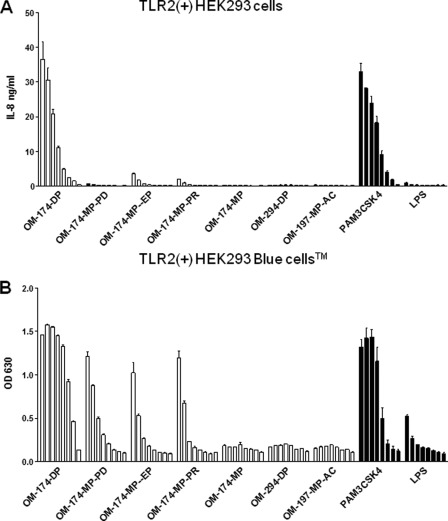
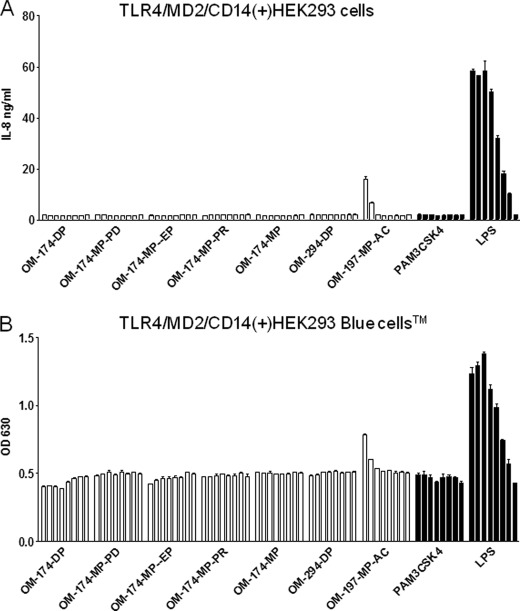
Several E. coli LPS-derived and synthetic triacylated lipid A-like molecules stimulated HEK293 cells via TLR2 (Fig. 2, A and B). The level of activation was strongest for OM-174-DP in this cell system, with levels of cell activation comparable with that of the classical TLR2 agonist PAM3CSK4 lipopeptide. Interestingly, the OM-174-MP (monophosphate) and the OM-294-DP (synthetic, pseudodipeptide backbone) molecules did not activate TLR2-expressing HEK293 cells. We observed a difference of the sensitivity between the two cells lines to TLR2 agonists (Fig. 2, A and B) because of the presence of CD14 in HEK-Bluetm-hTLR2 and a more sensitive detection system (alkaline phosphatase) enhancing TLR2 responses. One lipid A-like molecule (OM-197-MP-AC) did not activate cells via TLR2, but was a TLR4 agonist, the only one in our panel of triacylated molecules (Fig. 3, activation of TLR4(+)HEK293 cells). Notably, the level of activation with this compound was much lower than that obtained with the classical TLR4 agonist, E. coli LPS (Fig. 3). None of the other OM molecules tested herein activated cells via a TLR4-dependent pathway (Fig. 3).
FIGURE 2.
TLR2 activation by triacylated compounds using target TLR2-tranfected HEK293 cells. A, activation of TLR2(+)HEK293 cells by a series of triacylated compounds. Each triacylated compound was tested with a dose response ranging from 10 μg/ml up to 1.37 ng/ml, diluted 1:3 in RPMI. PAM3CSK4 (starting concentration 1 μg/ml, diluted 1:3) served as a positive control (TLR2 agonist), and LPS (TLR4 agonist) was used as a negative control in this assay. IL-8 production was measured in conditioned supernatants after 24 h of incubation time. Data are expressed as mean ± S.D. from triplicates for each point. This is a representative experiment of three experiments with similar results. B, same experiment, but with TLR2/CD14(+)HEK293 Bluetm cells expressing alkaline phosphatase under the control of TLR2 activation, a cell-based assay slightly more sensitive than that of classical TLR2(+)HEK293 cells. Alkaline phosphatase was measured in conditioned supernatants after 24 h incubation time using an alkaline substrate in a colorimetric assay. This is a representative experiment of three experiments with similar results.
FIGURE 3.
TLR4 activation by triacylated compounds using target TLR4-tranfected HEK293 cells. A, activation of TLR4/MD-2/CD14(+)HEK293 cells by a series of triacylated compounds. Each triacylated compound was tested with a dose response ranging from 10 μg/ml up to 1.37 ng/ml, diluted 1:3 in RPMI. LPS (starting concentration 1 μg/ml, diluted 1:3) served as a positive control (TLR4 agonist), and PAM3CSK4 (TLR2 agonist) was used as a negative control in this assay. IL-8 production was measured in conditioned supernatants after 24 h of incubation time. Only OM-197-MP-AC was a (weak) TLR4 agonist in this assay. Data are expressed as mean ± S.D. from triplicates for each point. This is a representative experiment of three experiments with similar results. B, same experiment but with TLR4/MD-2/CD14(+)HEK293 Bluetm cells expressing alkaline phosphatase under the control of TLR4 activation. Again, only OM-197-MP-AC was a (weak) TLR4 agonist in this assay. Alkaline phosphatase was measured in conditioned supernatants after 24 h of incubation time using an alkaline substrate in a colorimetric assay. This is a representative experiment of three experiments with similar results.
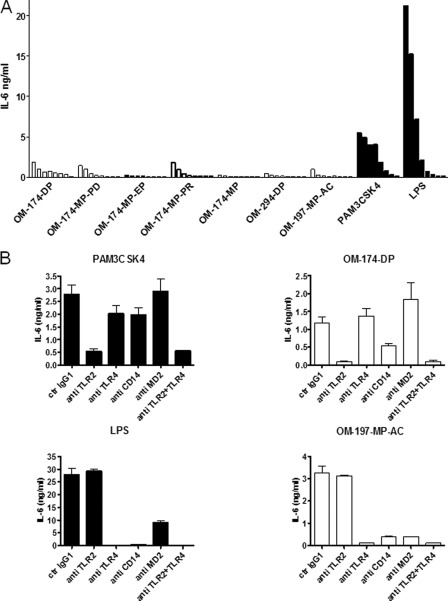
Similar results were obtained when leukocytes were incubated with triacylated lipid A-like molecules (whole blood stimulation assay). Monocytes and neutrophils (phagocytes) present in blood are known to carry both TLR2 and TLR4 receptors and to be responsive to their respective agonists. OM compounds activated phagocytes in whole blood to secrete IL-6 (Fig. 4A), except for the OM-174-MP, which was not stimulatory. Triacylated compounds found to be TLR2 agonists in a HEK293 cell assay (Fig. 2) were also found to activate human phagocytes in whole blood via TLR2, as demonstrated with specific anti-TLRs mAbs. In Fig. 4B, results of antibody blockade experiments are shown as an example for the OM-174-DP compound. Other TLR2 agonists had the same TLR2-CD14-dependent profile (results not shown here). Similar to experiments performed with HEK293 transfectants, OM-197-MP-AC-activated phagocytes in whole blood in a TLR4-dependent manner (Fig. 4B). Interestingly, an anti-CD14 mAb also inhibited activation of phagocytes in whole blood induced by triacylated lipid A-like molecules, both for compounds depending on TLR2 and TLR4 (Fig. 4B). All triacylated TLR2 agonists tested showed this CD14 dependence for cell activation. Blocking anti-MD-2 antibody only inhibited cell activation by the TLR4 agonist OM-197-MP-AC. These results indicate that CD14 is an accessory molecule for cell activation induced by triacylated compounds, irrespective of their TLR receptor. These results also show that MD-2 is an accessory molecule important for TLR4 activation by OM-197-MP-AC, similar to the classical TLR4 agonist LPS. Notably, levels of activation of phagocytes in whole blood were markedly less important with OM compounds as compared with classical TLR2 and TLR4 agonists (PAM3CSK4 and LPS, respectively).
FIGURE 4.
Whole blood leukocyte activation by triacylated lipid A analogs and effect of specific anti-TLR2, anti-TLR4, and anti-CD14 mAb on the simulation of blood leukocytes by OM-174-DP and OM-197-MP-AC. A, activation of leukocytes in whole heparinized blood from a healthy donor by a series of triacylated compounds. Each triacylated compound was tested with a dose response ranging from 10 μg/ml up to 1.37 ng/ml, diluted 1:3 in RPMI, final blood dilution 1:4. PAM3CSK4 (TLR2 agonist, starting concentration 500 ng/ml, diluted 1:3,) and LPS (TLR4 agonist, starting concentration 100 ng/ml, diluted 1:3) served as a positive controls in this assay. IL-6 production was measured in conditioned supernatants after 6 h of incubation time. This is a representative experiment of three experiments with different blood donors with similar results. B, activation of leukocytes in whole heparinized blood from a healthy donor by OM-174-DP and OM-197-MP-AC. Left panels show whole blood leukocyte response (IL-6 production) after 6 h of incubation with PAM3CSK4 (500 ng/ml, upper left panel) and LPS (1 ng/ml, lower left panel). Leukocyte activation by PAM3CSK4 was TLR2-dependent, whereas LPS activation was TLR4/MD-2/CD14-dependent using specific blocking MAbs, as expected. OM-174-DP was also TLR2-dependent, with an effect more pronounced with anti-CD14 that that of PAM3CSK4. Similar results were obtained with the other TLR2-dependent triacytaled lipid A-like molecules (data not shown). OM-197-MP-AC activation of whole blood leukocytes was TLR4/MD-2/CD14-dependent, similar to that of LPS. Data are expressed as mean ± S.D. from triplicates for each point. This is a representative experiment of four experiments with different blood donors with similar results.
The addition of anti-TLR4 plus anti-TLR2 MAbs did not modify the level of inhibition observed with only one antibody, indicating that these compounds are pure TLR2 or TLR4 (OM-197-MP-AC) agonists (Fig. 4B).
Antagonistic Activities of Triacylated Lipid A-like Compounds
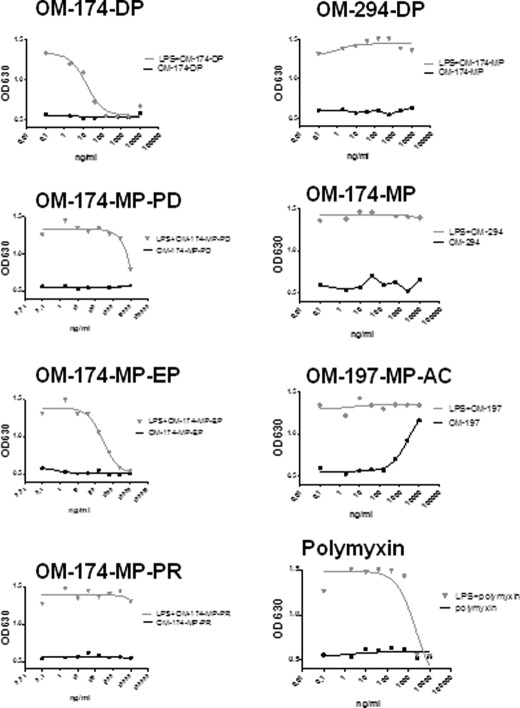
Because triacylated compounds have a molecular structure close to lipid A but lack a complete set of hexaacylated lipid A, we tested the hypothesis that they may act as inhibitors of cell activation by hexaacylated E. coli LPS. We first tested their inhibitory activity in LPS-treated HEK293 cells transfected with TLR4/MD-2/CD14. We observed that several triacylated compounds were very potent LPS antagonist (Fig. 5). The dose of LPS chosen to activate cells was 10 ng/ml, an intermediate concentration in the dose-response curve for those cells (Fig. 5). The 50% inhibitory concentration (IC50) of OM compounds were < 10 ng/ml for OM-174-DP, ∼ 100 ng/ml for OM-174-MP-EP, and ∼2,000 ng/ml for OM-174-MP-PD. We did not observe antagonistic activity with compounds OM-174-MP-PR, OM-174-MP, and OM-294-DP. Polymyxin B was used as a classical LPS antagonist control in this system. In data not shown here, we found that the two compounds that lacked TLR2 agonistic activities (OM-174-MP and OM-294-DP) also lacked antagonistic activity in TLR2(+)cells stimulated with PAM3CSK4.
FIGURE 5.
Triacylated lipid A-like molecules are LPS antagonists in TLR4/MD-2/CD14(+)HEK293 Blue cellstm. TLR4/MD-2/CD14(+)HEK293 Blue cellstm were incubated with a fixed amount of E. coli K12 LPS (10 ng/ml). Thirty minutes prior to LPS addition, triacylated compounds were added to cells at different concentrations (dose response ranging from 10 μg/ml up to 1.37 ng/ml, diluted 1:3 in culture media). As controls, OM compounds were added to cells without LPS. Polymyxin B (starting concentration of 10 μg/ml) was used as a classical LPS blocker in this system. Alkaline phosphatase was measured in conditioned supernatants after 24 h of incubation time using an alkaline substrate in a colorimetric assay. Only OM-174-DP, OM-174-MP-PD, and OM-174-MP-EP showed evidence of LPS antagonism in this assay. This is a representative experiment of three experiments with similar results.
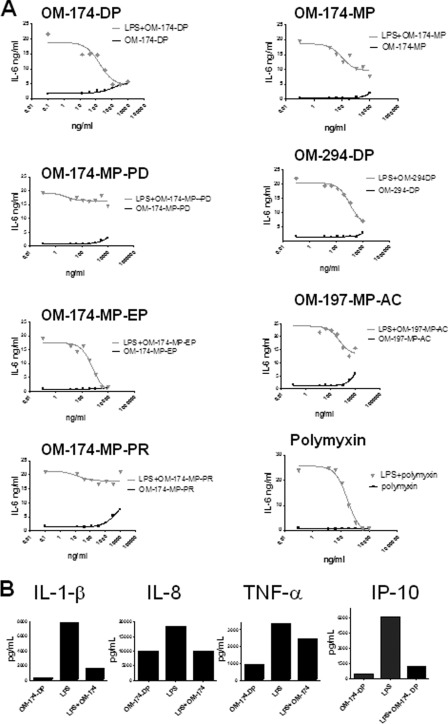
These antagonistic effects observed with transfected HEK293 cells were confirmed with leukocytes in whole human blood from volunteer as other and more relevant target cells. In whole blood stimulated with 1 ng/ml LPS (which corresponds to maximal whole blood stimulation), OM-174-DP and OM-174-MP-EP were also strong LPS antagonists (IC50 ∼100 ng/ml and ∼200 ng/ml, respectively, Fig. 6A). This was the case when markers of leukocyte activation were dependent of both the MyD88 and the TiR-domain-containing adapter-inducing interferon-β (TRiF) pathways (Fig. 6B). In this system, OM-174-MP-PD was also a weak antagonist. Interestingly, OM-174-MP and OM-294-DP, which were not antagonists in HEK293 cells, were weak antagonists in whole blood (Fig. 6A). Although at high doses of the OM-197-MP-AC compound behaved as a TLR4 agonist, at some concentrations (200 ng/ml) it showed an LPS antagonistic effect without agonistic activity, most likely by blocking LPS binding to TLR4/MD-2 (Fig. 6A).
FIGURE 6.
Triacylated lipid A-like molecules are LPS antagonists in whole blood. A, whole heparinized human blood was incubated with a fixed amount of E. coli K12 LPS (1 ng/ml). Thirty minutes prior to LPS addition, triacylated compounds were added to blood at different concentrations (dose response ranging from 10 μg/ml up to 1.37 ng/ml, diluted 1:3 in culture media). As controls, OM compounds were added to blood without LPS. Polymyxin B (starting concentration of 10 μg/ml) was used as a classical LPS blocker in this system. IL-6 was measured in conditioned supernatants after 6 h of incubation time by ELISA. Only OM-174-DP, OM-174-MP-EP, OM-174-MP, OM-294-DP, and OM-197-MP-AC showed evidence of LPS antagonism in this assay. This is a representative experiment of three experiments with different blood donors with similar results. B, whole heparinized human blood was incubated with a fixed amount of E. coli K12 LPS (50 ng/ml). Thirty minutes prior to LPS addition, OM-174-DP was added at the concentration of 10 μg/ml. The cytokines IL-1β, IL-8, TNF-α, and IP-10 were measured in conditioned supernatants after 6 h of incubation time by cytometric bead array for IL-1β, IL-8, and TNF-α and by ELISA for IP-10. OM-174-DP also inhibited LPS activation of these cytokines. This is a representative experiment of three experiments with different blood donors with similar results.
Triacylated Lipid A Analogs Inhibit Human Leukocyte Activation by Gram-negative Bacteria and Bacterial Phagocytosis
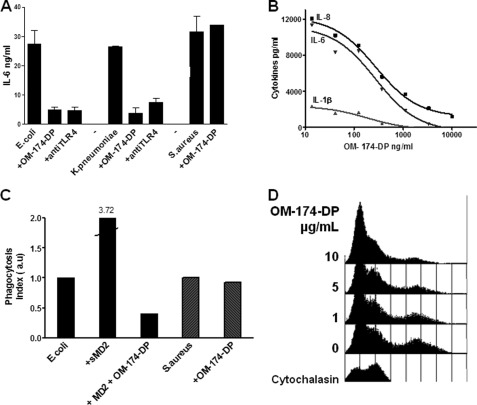
As a prototypic triacylated Lipid A-analog with high LPS antagonistic activity, we used OM-174-DP in the following experiments. OM-174-DP blocked the activation of whole blood leukocytes stimulated by either heat-killed E. coli or K. pneumoniae bacteria. OM-174-DP inhibited the secretion by blood leukocytes of TNF-α (not shown) IL-1β, IL-6, and IL-8 (and IP-10, not shown here) to levels similar to those obtained with a TLR4 mAb (Fig. 7, A and B). In contrast, it did not block the activation of whole blood leukocytes stimulated with the heat-killed Gram-positive S. aureus bacteria (Fig. 7A). With the concentration of Gram-negative bacteria used in this assay (100,000 bacteria/ml), the IC50 of OM-174-DP was ∼100 ng/ml in a 24-h whole blood stimulation assay (data not shown).
FIGURE 7.
Triacylated lipid A-like molecules block blood leukocyte activation by Gram-negative bacteria and phagocytosis of Gram-negative bacteria by human neutrophils. A, whole heparinized human blood was incubated with heat-killed E. coli (105 bacteria/ml for 6 h), heat-killed K. pneumoniae (105 bacteria/ml for 6 h), or heat-killed S. aureus (5 × 107 bacteria/ml for 24 h). OM-174-DP or anti-TLR4 blocking mAb were added to the blood at concentrations of 10 μg/ml. IL-6 was measured in conditioned supernatants by ELISA.OM-174-DP was as efficient an LPS antagonist as that measured with the anti-TLR4 mAb. This is a representative experiment of three experiments with different blood donors with similar results. B, whole heparinized human blood was incubated with heat-killed E. coli (105 bacteria/ml for 24 h). Thirty minutes prior to incubation with bacteria, serial dilutions of OM-174-DP were added to blood (dose response ranging from 10 μg/ml up to 1.37 ng/ml, diluted 1:3 in RPMI, final blood dilution 1:4). IL-1β, IL-6, and IL-8 production were measured in conditioned supernatants. OM-174-DP had a dose-dependent inhibitory effect on E. coli-induced leukocyte activation. This is a representative experiment of three experiments with different blood donors with similar results. C, freshly isolated normal human neutrophils were incubated with fluorescent E. coli or S. aureus particles for 10 min in 2% normal human plasma. Thirty minutes prior to incubation with bacteria, 10 μg/ml OM-174-DP and 20 μg/ml recombinant soluble MD-2 (as an opsonin) were added to neutrophils. Although soluble MD-2 (sMD-2) increased the phagocytosis of E. coli because of its opsonic effect (16), the pretreatment of neutrophils with OM-174-DP blocked E. coli phagocytosis. In contrast, OM-174-DP did not have an effect on the phagocytosis of Gram-positive S. aureus bacteria. Results expressed as phagocytic index (arbitrary units, a.u.). This is a representative experiment of three experiments with different neutrophil donors with similar results. D, flow cytometry analysis of fluorescent E. coli incorporation into freshly isolated neutrophils (10 min). The right shift of the histograms corresponds to fluorescent E. coli incorporation into neutrophils (phagocytosis). OM-174-DP inhibited E. coli phagocytosis with effects increasing with increasing concentrations of OM-174-DP. Cytochalasin D served as a control, completely inhibiting bacteria phagocytosis.
Interestingly, OM-174-DP also inhibited the phagocytosis of heat-killed E. coli by human neutrophils. We had shown previously that soluble MD-2 was an opsonin for Gram-negative bacteria and enhanced phagocytosis of these bacteria by neutrophils (16). We had also shown that phagocytosis of Gram-negative bacteria was partially TLR4/MD-2-dependent, where 50% of E. coli phagocytosis could be inhibited by either anti-TLR4 or anti-MD-2 mAbs (16). We isolated human blood leukocytes by gelatin gradient and incubated leukocytes with fluorescent bacteria opsonized with soluble MD-2. The addition of OM-174-DP markedly decreased E. coli phagocytosis by human neutrophils (Fig. 7C). The highest dose of OM-174-DP almost completely blocked E. coli phagocytosis, to a level comparable with those measured with the phagocytosis inhibitor cytochalasin D (Fig. 7D). In contrast, OM-174-DP did not influence phagocytosis of Gram-positive S. aureus by neutrophils (Fig. 7C). OM-174-MP-PD and OM-174-MP-EP were strong LPS antagonist but weak inhibitors of phagocytosis (not shown). Interestingly, OM-174-MP-PR that did not antagonize LPS activation of cells showed an inhibitory effect on phagocytosis of E. coli by neutrophils (IC50 of 10 μg/ml in our system, Fig. 7E). OM-174-MP, which did not show an LPS antagonist effect, had a small effect on E. coli phagocytosis only at the highest dose of 10 μg/ml (not shown here). OM-294-DP and OM-197 MP-AC (lacking the diglucosamine backbone) did not influence E. coli phagocytosis at any dose tested.
DISCUSSION
Here we show that relatively simple triacylated lipid A-derived synthetic molecules can have profound effects on the activation of cells, either by directly activating them through TLRs or by blocking the activation of cells by natural bacterial ligands or by whole bacteria. Interestingly, some of these compounds also inhibited phagocytosis of Gram-negative bacteria.
The triacylated molecules used in this study have been designed starting from the LPS lipid A structure, removing three of six acyl chains originally present in the lipid A moiety. Some of the molecules also had removal of one of the two phosphate groups or had addition of residues at the level of the missing phosphate group. Two compounds had synthetic substitutes for the diglucosamine backbone with a pseudodipeptide, one with addition of a phosphate group and another with the addition of two phosphate groups simulating the original lipid A structure. The biochemical synthesis of the principal of these compounds has been published previously (11, 12).
A majority of the triacylated synthetic molecules tested in this study had TLR2 agonistic effects. The maximal TLR2 agonist effect was observed with OM-174-DP with the level of activation of TLR2-expressing cells comparable with that observed with the classical TLR2 ligand PAM3CSK4. Substituting one phosphate group with various residues decreased the TLR2 agonistic activity by a factor of ∼100× (OM-174-MP-PD, MP-EP, and MP-PR), whereas completely removing one phosphate without substitution abolished the TLR2 agonistic effect (OM-174-MP). The triacylated lipid A-like molecules for which a natural diglucosamine backbone was substituted for a synthetic pseudodipeptide (OM-294-DP and OM-197-MP-AC) did not activate TLR2-expressing cells, irrespective of the presence of one or two phosphate groups.
Interestingly, one of the synthetic triacylated monophosphated compound, OM-197-MP-AC, had a small TLR4 agonistic effect, with levels of cell activation > 1000× less than that observed with E. coli LPS in our cell systems. Savoy et al. (13) showed that this compound contained a very small amount of endotoxic activity, i.e. < 0.125 endotoxin unit/mg of OM-197-MP-AC in the limulus lysate assay. Furthermore, these authors showed that this compound was not pyrogenic in rabbits up to 0.9 mg/kg. OM-197-MP-AC also weakly stimulated circulating leukocytes from normal whole human blood. This compound was, however, shown to be a potent adjuvant for a vaccine, most probably through its TLR4/MD-2 agonistic activity, and represents a promising adjuvant because of its low pyrogenic effect and its strong activation of human dendritic cells, promoting a TH1-type immune response (13, 18, 19). OM-197-MP-AC also showed a very potent inhibitory activity in TLR4-expressing cells stimulated with LPS. These results are in accordance with studies showing that different lipid A-derived structures can be TLR4/MD-2 agonists or antagonists and others TLR2 agonists. The critical factors for their agonistic versus antagonistic effects and their promiscuity for TLR2 versus TLR4 are the number of acyl chains, the length of the carbon chains, the number and the distribution of negative charges, the asymmetry of the acyl chains, and the species of TLR4 (5–8). It has been shown that lipid IVa, for example, can be an agonist in mouse and horse TLR4/MD-2 but is clearly an LPS antagonist in human TLR4/MD-2 (8, 20). It is now believed that molecules capable of inserting into the hydrophobic grove of MD-2, preventing LPS to insert, have antagonist activity. This has been well described for lipid IVa (21), Eritoran, a lipid IVa analog also containing four acyl chains (22), as well as curcuma (23, 24). Other triacylated compounds have TLR4/MD-2 antagonistic activities, most likely by inserting into the hydrophobic pocket of MD-2, such as CRX-527, an aminoalkyl glucosaminide 4-phosphate (25). This latter compound can be a TLR4/MD-2 agonist or LPS antagonist, depending on the length of the acyl chain (25). Interestingly, CRX-527 increased survival in mice infected with Yersinia pestis (26) or Francisella tularensis (27) in pneumonia models. Another lipid A mimetic of the aminoalkyl glucosaminide 4-phosphate type (triacylated monosaccharide or monoglucosamine lipid A, GLA) had TLR4/MD-2 agonistic activity when the acyl chains took a conical shape and an antagonistic activity for cylindrical shaped lipid chains (28, 29). Importantly, the agonistic versus antagonistic effects of both TLR2 and TLR4 ligands depend on their conical versus cylindrical shape, respectively (30). The agonistic effect of triacylated lipid A-like compounds was also noted for OM compounds such as those studied in this work (12). In the latter work, it was shown that the angle between the glucosamine backbone and the acyl chains was also critical for the antagonistic effect (12, 31). Other molecules were shown to be LPS antagonists, most likely through the interference with LPS binding to TLR4/MD-2, such as some omega-3 fatty acids (32) and synthetic rhamnolipids (33).
Most of the OM compounds tested in this study showed a TLR2-agonistic effect. This was particularly true for triacylated lipid A-like molecules diphosphated (the most potent) or with the addition of various types of chains at the site of the second phosphate group (OM-174 compounds). They also stimulated leukocytes from whole blood in a TLR2-dependent manner, as shown by the inhibition of specific anti-TLR2 mAb. The anti-TLR4 mAb had no effect, in contrast with anti-CD14 mAb, which partially inhibited leukocyte stimulation by OM-174 compounds, suggesting a participation of CD14 in the recognition of these molecules and cell activation (34, 35). It is our hypothesis that, given their triacylated structure, these compounds will cross-link TLR2 and TLR1, with two acyl chains binding to TLR2 and one acyl chain binding to TLR1, similar to that shown by Jin et al. (4) with triacylated lipopeptides (Pam3CSK4), and thereby induce TLR2-dependent cell signaling. In experiments not shown here, only anti-TLR1 and anti-TLR2, but not anti-TLR6, blocked the agonistic activity of triacylated molecules. Interestingly, despite their TLR2 agonistic activity, OM-174 triacylated compounds potently blocked LPS-dependent activation of cells (TRL4(+)HEK293 transfectants and whole blood), most probably through their insertion into the hydrophobic groove of MD-2. This is in accordance with the lipid A-like conical molecular structure determined by Brandenburg et al. (12) under physiological conditions. It was also demonstrated that OM-174-DP existed in solution mainly as monomers (active forms), and a concentration of more than 1000× was necessary to create micelles when compared with lipid A from LPS (12, 31). This certainly explains that these compounds function as LPS antagonists in the absence of monomerizing proteins such as LBP and CD14. The fact that monophosphated OM-174-MP compounds were less potent as LPS antagonists is certainly because of the loss of the second phosphate group replaced by substitutes groups, at a site where the phosphate group is important for ligand binding and stabilization in the pocket of MD-2 pocket (2). OM 294-DP was a strong antagonist in whole blood, whereas this effect could not be shown in TLR4(+)HEK293 transfectants. This may be due to the lack of a cofactor in the transfectant system, a cofactor present in whole blood (36). Taken together, these results show that, in the absence of LPS, OM-174-derived compounds are weak TLR2 agonists but show strong LPS antagonistic activities at concentrations where their TLR2 agonistic activity is not or minimally present, particularly in whole blood. These results suggest that these triacylated lipids A molecules could be used as LPS antagonists without fearing to get an intrinsic inflammation that is TLR2-dependent.
We had shown previously that the TLR4/MD-2 pathway was the one important in leukocyte activation by whole Gram-negative bacteria despite the presence of other potential TLR ligands at the surface of bacteria and their counter Toll-like receptors (10). We confirmed these findings by showing that leukocyte activation by Gram-negative bacteria (E. coli and K. pneumoniae) was blocked by OM compounds, particularly by OM-174-DP (submicromolar range), to levels similar to those obtained with our best inhibitory anti-TLR4 mAb. This inhibition was observed for TNF-α, IL-1β, IL-6, IL-8 (MyD88-dependent inflammatory markers), and IP-10 (TRIF-dependent chemokine) secretion by leukocytes. In contrast, OM compounds did not influence leukocyte activation induced by whole Gram-positive bacteria (S. aureus).
The TLR4/MD-2 pathway was shown to be important for the phagocytosis of Gram-negative bacteria (16, 37). Here we show that OM compounds were also efficient blockers of phagocytosis of Gram-negative bacteria by human neutrophils, although they did not influence phagocytosis of Gram-positive bacteria.
In conclusion, our results demonstrate that synthetic triacylated lipid A-like are in general weak TLR2 agonists, certainly by cross-linking TLR2 and TLR1. At submicromolar concentrations, they are very potent LPS antagonists, blocking leukocyte stimulation by LPS and whole Gram-negative bacteria, and also inhibiting phagocytosis of Gram-negative bacteria. Because kilograms of these synthetic compounds can easily be produced4) and show poor intrinsic proinflammatory effects, they are promising immunomodulatory agents in the context of sepsis, for example (38, 39). The TLR4 triacylated agonist (OM-197-MP-AC) is a promising adjuvant given its low endotoxic activity and its strong Th1-inducing stimulatory effect on dendritic cells (40).
Acknowledgments
We thank Carlo Chiavaroli for help and guidance and Greg Elson for the gift of anti-MD-2 and anti-TLR4 mAbs.
This work was supported by Swiss National Foundation for Scientific Research Grant 122034 (to J. P.).
S. Moutel and J. Bauer, personal communication.
- TLR
- Toll-like receptor
- DP
- diphosphate
- PD
- 2,3 dihydroxypropyl
- EP
- 2-(phosphonooxy)ethyl
- PR
- propyl.
REFERENCES
- 1. Kim J. I., Lee C. J., Jin M. S., Lee C. H., Paik S. G., Lee H., Lee J. O. (2005) Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J. Biol. Chem. 280, 11347–11351 [DOI] [PubMed] [Google Scholar]
- 2. Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 [DOI] [PubMed] [Google Scholar]
- 3. Shin H. J., Lee H., Park J. D., Hyun H. C., Sohn H. O., Lee D. W., Kim Y. S. (2007) Kinetics of binding of LPS to recombinant CD14, TLR4, and MD-2 proteins. Mol. Cells 24, 119–124 [PubMed] [Google Scholar]
- 4. Jin M. S., Kim S. E., Heo J. Y., Lee M. E., Kim H. M., Paik S. G., Lee H., Lee J. O. (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 [DOI] [PubMed] [Google Scholar]
- 5. Bryant C. E., Ouellette A., Lohmann K., Vandenplas M., Moore J. N., Maskell D. J., Farnfield B. A. (2007) The cellular Toll-like receptor 4 antagonist E5531 can act as an agonist in horse whole blood. Vet. Immunol. Immunopathol. 116, 182–189 [DOI] [PubMed] [Google Scholar]
- 6. Meng J., Drolet J. R., Monks B. G., Golenbock D. T. (2010) MD-2 residues tyrosine 42, arginine 69, aspartic acid 122, and leucine 125 provide species specificity for lipid IVA. J. Biol. Chem. 285, 27935–27943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meng J., Lien E., Golenbock D. T. (2010) MD-2-mediated ionic interactions between lipid A and TLR4 are essential for receptor activation. J. Biol. Chem. 285, 8695–8702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh C., Gangloff M., Monie T., Smyth T., Wei B., McKinley T. J., Maskell D., Gay N., Bryant C. (2008) Elucidation of the MD-2/TLR4 interface required for signaling by lipid IVa. J. Immunol. 181, 1245–1254 [DOI] [PubMed] [Google Scholar]
- 9. Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., Lee J. O. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130, 906–917 [DOI] [PubMed] [Google Scholar]
- 10. Elson G., Dunn-Siegrist I., Daubeuf B., Pugin J. (2007) Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 109, 1574–1583 [DOI] [PubMed] [Google Scholar]
- 11. Martin O. R., Zhou W., Wu X., Front-Deschamps S., Moutel S., Schindl K., Jeandet P., Zbaeren C., Bauer J. A. (2006) Synthesis and immunobiological activity of an original series of acyclic lipid a mimics based on a pseudodipeptide backbone. J. Med. Chem. 49, 6000–6014 [DOI] [PubMed] [Google Scholar]
- 12. Brandenburg K., Lindner B., Schromm A., Koch M. H., Bauer J., Merkli A., Zbaeren C., Davies J. G., Seydel U. (2000) Physicochemical characteristics of triacyl lipid A partial structure OM-174 in relation to biological activity. Eur. J. Biochem. 267, 3370–3377 [DOI] [PubMed] [Google Scholar]
- 13. Savoy F., Nicolle D. M., Rivier D., Chiavaroli C., Ryffel B., Quesniaux V. F. (2006) Synthetic triacylated lipid A derivative activates antigen presenting cells via the TLR4 pathway and promotes antigen-specific responses in vivo. Immunobiology 211, 767–777 [DOI] [PubMed] [Google Scholar]
- 14. Dunn-Siegrist I., Leger O., Daubeuf B., Poitevin Y., Dépis F., Herren S., Kosco-Vilbois M., Dean Y., Pugin J., Elson G. (2007) Pivotal involvement of Fcγ receptor IIA in the neutralization of lipopolysaccharide signaling via a potent novel anti-TLR4 monoclonal antibody 15C1. J. Biol. Chem. 282, 34817–34827 [DOI] [PubMed] [Google Scholar]
- 15. Pugin J., Stern-Voeffray S., Daubeuf B., Matthay M. A., Elson G., Dunn-Siegrist I. (2004) Soluble MD-2 activity in plasma from patients with severe sepsis and septic shock. Blood 104, 4071–4079 [DOI] [PubMed] [Google Scholar]
- 16. Tissières P., Dunn-Siegrist I., Schäppi M., Elson G., Comte R., Nobre V., Pugin J. (2008) Soluble MD-2 is an acute-phase protein and an opsonin for Gram-negative bacteria. Blood 111, 2122–2131 [DOI] [PubMed] [Google Scholar]
- 17. Rämet M., Manfruelli P., Pearson A., Mathey-Prevot B., Ezekowitz R. A. (2002) Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416, 644–648 [DOI] [PubMed] [Google Scholar]
- 18. Byl B., Libin M., Bauer J., Martin O. R., De Wit D., Davies G., Goldman M., Willems F. (2003) OM197-MP-AC induces the maturation of human dendritic cells and promotes a primary T cell response. Int. Immunopharmacol. 3, 417–425 [DOI] [PubMed] [Google Scholar]
- 19. Véran J., Mohty M., Gaugler B., Chiavaroli C., Olive D. (2004) OM-197-MP-AC adjuvant properties. The in vitro maturation of normal and leukemic dendritic cells in a serum-free culture model. Immunobiology 209, 67–77 [DOI] [PubMed] [Google Scholar]
- 20. Muroi M., Ohnishi T., Tanamoto K. (2002) MD-2, a novel accessory molecule, is involved in species-specific actions of Salmonella lipid A. Infect. Immun. 70, 3546–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. (1990) Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J. Exp. Med. 172, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahn J. H., Shin M. S., Jun M. A., Jung S. H., Kang S. K., Kim K. R., Rhee S. D., Kang N. S., Kim S. Y., Sohn S. K., Kim S. G., Jin M. S., Lee J. O., Cheon H. G., Kim S. S. (2007) Synthesis, biological evaluation and structural determination of β-aminoacyl-containing cyclic hydrazine derivatives as dipeptidyl peptidase IV (DPP-IV) inhibitors. Bioorg. Med. Chem. Lett. 17, 2622–2628 [DOI] [PubMed] [Google Scholar]
- 23. Wong S. W., Kwon M. J., Choi A. M., Kim H. P., Nakahira K., Hwang D. H. (2009) Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 284, 27384–27392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gradisar H., Keber M. M., Pristovsek P., Jerala R. (2007) MD-2 as the target of curcumin in the inhibition of response to LPS. J. Leukocyte Biol. 82, 968–974 [DOI] [PubMed] [Google Scholar]
- 25. Stöver A. G., Da Silva Correia J., Evans J. T., Cluff C. W., Elliott M. W., Jeffery E. W., Johnson D. A., Lacy M. J., Baldridge J. R., Probst P., Ulevitch R. J., Persing D. H., Hershberg R. M. (2004) Structure-activity relationship of synthetic toll-like receptor 4 agonists. J. Biol. Chem. 279, 4440–4449 [DOI] [PubMed] [Google Scholar]
- 26. Airhart C. L., Rohde H. N., Bohach G. A., Hovde C. J., Deobald C. F., Lee S. S., Minnich S. A. (2008) Induction of innate immunity by lipid A mimetics increases survival from pneumonic plague. Microbiology 154, 2131–2138 [DOI] [PubMed] [Google Scholar]
- 27. Lembo A., Pelletier M., Iyer R., Timko M., Dudda J. C., West T. E., Wilson C. B., Hajjar A. M., Skerrett S. J. (2008) Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia. J. Immunol. 180, 7574–7581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brandenburg K., Matsuura M., Heine H., Müller M., Kiso M., Ishida H., Koch M. H., Seydel U. (2002) Biophysical characterization of triacyl monosaccharide lipid a partial structures in relation to bioactivity. Biophys. J. 83, 322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Netea M. G., van Deuren M., Kullberg B. J., Cavaillon J. M., Van der Meer J. W. (2002) Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 23, 135–139 [DOI] [PubMed] [Google Scholar]
- 30. Schromm A. B., Howe J., Ulmer A. J., Wiesmüller K. H., Seyberth T., Jung G., Rössle M., Koch M. H., Gutsmann T., Brandenburg K. (2007) Physicochemical and biological analysis of synthetic bacterial lipopeptides. Validity of the concept of endotoxic conformation. J. Biol. Chem. 282, 11030–11037 [DOI] [PubMed] [Google Scholar]
- 31. Brandenburg K., Andrä J., Müller M., Koch M. H., Garidel P. (2003) Physicochemical properties of bacterial glycopolymers in relation to bioactivity. Carbohydr. Res. 338, 2477–2489 [DOI] [PubMed] [Google Scholar]
- 32. Singer P., Shapiro H., Theilla M., Anbar R., Singer J., Cohen J. (2008) Anti-inflammatory properties of omega-3 fatty acids in critical illness. Novel mechanisms and an integrative perspective. Intensive Care Med. 34, 1580–1592 [DOI] [PubMed] [Google Scholar]
- 33. Howe J., Bauer J., Andrä J., Schromm A. B., Ernst M., Rössle M., Zähringer U., Rademann J., Brandenburg K. (2006) Biophysical characterization of synthetic rhamnolipids. FEBS J 273, 5101–5112 [DOI] [PubMed] [Google Scholar]
- 34. Nakata T., Yasuda M., Fujita M., Kataoka H., Kiura K., Sano H., Shibata K. (2006) CD14 directly binds to triacylated lipopeptides and facilitates recognition of the lipopeptides by the receptor complex of Toll-like receptors 2 and 1 without binding to the complex. Cell. Microbiol. 8, 1899–1909 [DOI] [PubMed] [Google Scholar]
- 35. Bas S., Neff L., Vuillet M., Spenato U., Seya T., Matsumoto M., Gabay C. (2008) The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J. Immunol. 180, 1158–1168 [DOI] [PubMed] [Google Scholar]
- 36. Triantafilou M., Brandenburg K., Kusumoto S., Fukase K., Mackie A., Seydel U., Triantafilou K. (2004) Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem. J. 381, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jain V., Halle A., Halmen K. A., Lien E., Charrel-Dennis M., Ram S., Golenbock D. T., Visintin A. (2008) Phagocytosis and intracellular killing of MD-2 opsonized gram-negative bacteria depend on TLR4 signaling. Blood 111, 4637–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cristofaro P., Opal S. M. (2006) Role of Toll-like receptors in infection and immunity: clinical implications. Drugs 66, 15–29 [DOI] [PubMed] [Google Scholar]
- 39. Opal S. M., Huber C. E. (2002) Bench-to-bedside review. Toll-like receptors and their role in septic shock. Crit. Care. 6, 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mascarell L., Van Overtvelt L., Lombardi V., Razafindratsita A., Moussu H., Horiot S., Chabre H., Limal D., Moutel S., Bauer J., Chiavaroli C., Moingeon P. (2007) A synthetic triacylated pseudo-dipeptide molecule promotes Th1/TReg immune responses and enhances tolerance induction via the sublingual route. Vaccine 26, 108–118 [DOI] [PubMed] [Google Scholar]