Background: The KSHV-encoded vIRF-3 is a multifunctional protein expressed in latently infected primary effusion lymphoma.
Results: vIRF-3 associates with Skp2 ubiquitin ligase and stimulates the ubiquitylation and transcription activity of c-Myc.
Conclusion: vIRF-3 activates the c-Myc-regulated pathway that may lead to oncogenic transformation.
Significance: The study of the mechanism by which vIRF-3 manipulates c-Myc function contributes to the understanding of KSHV-associated lymphomagenesis.
Keywords: Herpesvirus, Myc, Proteasome, Transcription Factors, Ubiquitylation, KSHV, Skp2, c-Myc Transcription Activity, vIRF-3
Abstract
The Kaposi sarcoma-associated herpesvirus (KSHV) has been linked to Kaposi sarcoma, body cavity-based lymphoma, and Castleman disease. vIRF-3 is a KSHV latent gene that is critical for proliferation of KSHV-positive lymphoid cells. Furthermore, vIRF-3 contributes to KSHV-associated pathogenesis by stimulating c-Myc transcription activity. Here we show that vIRF-3 can associate with Skp2, a key component of the SCFskp2 ubiquitin ligase complex. Skp2 is a transcriptional co-factor for c-Myc that was shown to regulate the stability of c-Myc protein as well as c-Myc-dependent transcription. In this study, we show that vIRF-3 binds to the F-box of Skp2 and recruits it to c-Myc-regulated promoters to activate c-Myc-dependent transcription. Additionally, cells overexpressing vIRF-3 exhibit higher levels of c-Myc ubiquitylation, suggesting that ubiquitylation is necessary for c-Myc-mediated transcription. Moreover, vIRF-3 can stabilize the c-Myc protein by increasing its half-life. Collectively, these results indicate that vIRF-3 can effectively manipulate c-Myc stability and function and thus contribute to c-Myc-induced KSHV-associated lymphomagenesis.
Introduction
The genome of Kaposi sarcoma-associated herpesvirus (KSHV),2 which is a member of the γ herpesvirus family (1), contains a cluster of four genes with homology to cellular interferon regulatory factors (IRFs) (reviewed in Ref. 2). The viral interferon regulatory factor-3 (vIRF-3, also referred to as latency-associated nuclear antigen 2 (LANA2)) (3, 4) is a multifunctional nuclear protein that is constitutively expressed in KSHV-positive primary effusion lymphoma (PEL) and Castleman disease tumors (5–8). vIRF-3 exhibits pleiotropic effects on different cellular targets that are involved in apoptosis, cell cycle regulation, and oncogenic transformation as well as antiviral immunity (2). vIRF-3 was shown to inhibit p53-induced apoptosis (4) and down-modulate NFκB-dependent transcription (9) and is required for the continuous proliferation of PEL cells (10). Furthermore, interaction of vIRF-3 with the c-Myc oncogene results in the stimulation of c-Myc-dependent transcription (11).
c-myc, a well characterized proto-oncogene, has been implicated in controlling cellular growth and cell survival (12). Constitutive expression of c-myc reduces the growth factor requirement, prevents growth arrest, and blocks cellular differentiation (13, 14). The importance of rapid c-Myc turnover to normal growth control is suggested by the extremely short half-life of c-Myc protein (∼30 min (15)). More recent studies have identified the ubiquitin (Ub) ligase, Skp2, to be an important factor in the regulation of c-Myc protein stability (16–19). skp2 encodes the F-box protein of the ubiquitin ligase SCFskp2 complex consisting of Skp2, Cul1, and Skp1, which participates in the ubiquitylation and proteasomal degradation of c-Myc (20). Surprisingly, the Skp2 ubiquitin ligase is also required for induction of c-Myc-responsive genes, suggesting that ubiquitylation not only promotes c-Myc turnover but also stimulates its transcription activity (16–19). Moreover, Skp2 has been suggested to be an oncogene because it is overexpressed in transformed cells (21, 22). Here we further explored the possible regulation of c-Myc, an important cell cycle regulator, in KSHV-infected cells. We show that the KSHV latent oncoprotein, vIRF-3, can stabilize c-Myc protein and that vIRF-3-mediated recruitment of c-Myc and its co-factor, Skp2, to c-Myc-regulated promoters can efficiently enhance c-Myc-dependent transcription. Interestingly, cells overexpressing vIRF-3 exhibit higher levels of c-Myc ubiquitylation, suggesting that ubiquitylation is necessary for c-Myc-mediated transcription. These results further demonstrate the importance of vIRF-3 in the activation of the c-Myc-regulated pathway that may lead to uncontrolled proliferation and oncogenic transformation.
EXPERIMENTAL PROCEDURES
Cell Lines and Culture Conditions
BCBL-1, BC-3, and BJAB cells were grown in RPMI 1640 supplemented with 10% (20% for BC-3 cells) fetal bovine serum (FBS). HeLa and HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS. BJAB/vIRF-3 and BJAB/pcDNA3.1 cell lines were cultured as described previously (23).
Plasmids and Antibodies
Full-length vIRF-3 (vIRF-3-FL; amino acids 1–566), vIRF-3-N′ (amino acids 1–254), vIRF-3-C′ (amino acids 254–566), and vIRF-3-GST were described previously (3). The expression plasmid c-myc-HA and cdk4 reporter construct, WT MBS1–4, were kindly provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD). The skp2-Myc and skp2-GST plasmids were kindly provided by Dr. Yue Xiong (Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, NC). c-myc and skp2 various truncations were PCR-amplified using primers containing HA and Myc tag sequences, respectively. PCR products were gel-purified and cloned between EcoRI/BamHI and EcoRI/HindIII sites of pcDNA3.1 vector (Invitrogen), respectively. vIRF-3 deletion constructs were cloned by PCR amplification of vIRF-3-FL plasmid DNA. The 5′ primers carried a Myc tag sequence that was in-frame with the vIRF-3 open reading frame. The PCR products were subcloned into pcDNA3.1 vector (Invitrogen). c-myc-GST was cloned by PCR amplification of c-myc cDNA and was inserted into pGEX4T vector (Amersham Biosciences). The fidelity of all constructs was verified on the ABI PRISMTM 377 automated DNA sequencer (Applied Biosystems, Foster City, CA). The His-Myc-Ub plasmid was kindly provided by Dr. Michele Pagano (New York University Cancer Institute, New York, NY). The following antibodies were used: antibodies against c-Myc (N-262) and β-actin, HA, Skp2, Ub, IRF-3, IRF-4, and Myc (9E10) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Production and purification of polyclonal antibodies against vIRF-3 was described previously (23).
Transfections
All transfections were carried out using the SuperFect transfection reagent (Qiagen Inc., Valencia, CA). For c-Myc half-life, ubiquitylation, and DNA pulldown studies, HEK293 or HeLa cells were transfected in two rounds. First, to ensure the equal transfection efficiency of exogenous c-myc, the cells were transfected with c-myc-HA expression plasmid. Sixteen hours later, the cells were trypsinized and co-transfected with vIRF-3, skp2-Myc expression plasmids, or an empty vector (pcDNA3.1). At 24 h (or 48 h) after transfection, cells were treated as indicated (25 μg/ml cycloheximide or 50 μm MG115 (Sigma)), and cell lysates were analyzed by immunoprecipitation or Western blot.
RNAi Assay
Synthetic siRNAs targeted at vIRF-3 were selected using the Sigma-Aldrich and Dharmacon siRNA design centers. The following siRNA sequences were used: si291, 5′-GGUCGUACAGGGAAUUAAU-3′; si999, 5′-GGAGAUAAGUGACGAAGAA-3′. The negative control, nonsense siRNA 5′-AAGACUACCGUUGUAUAGUAG-3′, which shows no homology to any known human or KSHV gene, was used previously (10). For small interfering RNA (siRNA) transfection, 3 days prior to transfection, PEL cells (BC-3) were maintained at a density of 4 × 105 cells/ml. A total of 2 × 106 PEL cells were then transfected with 6 μg of siRNA using the HiPerFect transfection reagent (Qiagen) according to the manufacturer's instructions.
Immunoprecipitation and Western Blot Analysis
Cells were lysed in co-immunoprecipitation buffer (20 mm HEPES (pH 7.9), 50 mm NaCl, 5 mm EDTA, 2 mm EGTA, 0.1% Nonidet P-40, 10% glycerol, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, and 0.2 mm protease inhibitor mixture (Sigma)). The protein extracts (400 μg) were incubated with the respective antibodies at 4 °C for 1 h. Then 30 μl of protein A/G-Sepharose beads (Santa Cruz Biotechnology) was added followed by overnight incubation at 4 °C. Immune complexes were extensively washed with co-immunoprecipitation buffer, and precipitated proteins were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot.
Luciferase Assays
For luciferase assays, HEK293 cells were seeded in 6-well tissue culture plates 12 h before transfection. Subconfluent cells were transfected with equal amounts (1 μg) of luciferase reporter and vIRF-3, c-myc-HA, or skp2-Myc expression plasmids together with control plasmid pRL-SV40 (0.1 μg; Promega Corp., Madison, WI) using the SuperFect transfection reagent (Qiagen). Forty-eight hours after transfection, the cells were lysed with the 1× reporter lysis buffer (Promega), and luciferase activity was measured in 20 μl of the lysate using the Dual-Luciferase reporter assay kit (Promega) as recommended by the manufacturer. Each experiment was repeated three times. Renilla luciferase activity levels were used to normalize the differences in the transfection efficiency.
GST Pulldown
In vitro translated proteins were synthesized using either the TnT T7 quick coupled transcription/translation system (Promega) or the Escherichia coli T7 S30 extract system for circular DNA (used only in Fig. 8C; Promega) according to the manufacturer's instructions. GST fusion proteins (0.5 μg) bound to glutathione-Sepharose beads (Amersham Biosciences) were incubated with 3–10 μl of the reaction mixture consisting of in vitro translated proteins in 500 μl of binding buffer (10 mm Tris (pH 7.6), 100 mm NaCl, 0.1 mm EDTA (pH 8.0), 1 mm dithiothreitol, 5 mm MgCl2, 0.05% Nonidet P-40, 8% glycerol, 0.2 mm protease inhibitor mixture (Sigma)) at 4 °C for 90 min. After five 10-min washes with binding buffer supplemented with 1% Nonidet P-40, the proteins that were bound to the beads were resolved by SDS-PAGE and detected by Western blotting with specific antibodies.
FIGURE 8.
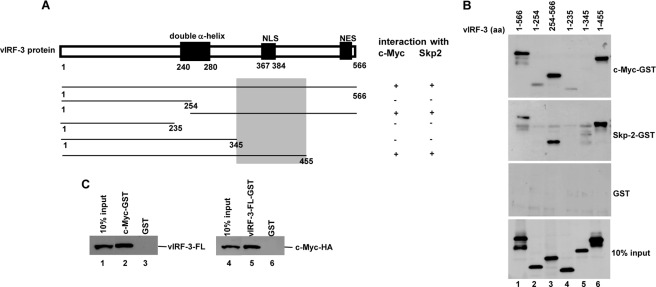
c-Myc and Skp2 interact with vIRF-3 (amino acids 346–455). A, schematic representation of vIRF-3 N- and C-terminal deletion mutants. The shaded box indicates the common domain for c-Myc and Skp2 binding. NLS, nuclear localization signal; NES, nuclear export signal. B, vIRF-3 deletion mutants were in vitro translated using TnT T7 quick coupled transcription/translation system and incubated with c-Myc or Skp2 fused to GST or GST alone immobilized on glutathione-Sepharose beads. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot with anti-vIRF-3 antibodies. 10% of vIRF-3 deletion mutant protein input is shown below (10% input). aa, amino acids. C, vIRF-3 (left panel) or c-Myc-HA (right panel) was in vitro translated using E. coli T7 S30 extract system and incubated with c-Myc-GST or vIRF-3-FL-GST, respectively. Incubation with GST alone served as a negative control. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot with either anti-vIRF-3 or anti-HA antibodies. 10% of in vitro translated vIRF-3 or c-Myc-HA is also shown (10% input).
Oligonucleotide Pulldown
The DNA pulldown assay was done as described previously (24). Briefly, double-stranded oligomers corresponding to the WT MBS4 in the human cdk4 promoter region (5′-CCCTCAGCGCATGGGTGGCGGTCACGTGCCCAGAACGTCCGG-3′; E-box sequence underlined) or mutMBS4 (5′-CCCTCAGCGCATGGGTGGCGGTCACCTGCCCAGAACGTCCGG-3′; mutated E-box sequence underlined) were synthesized and biotin-labeled at the 5′ end of the sense strand and coupled with streptavidin magnetic beads (Dynal, Invitrogen). Whole cell lysates (350 μg) were then incubated with the DNA bound to magnetic beads for 3 h at 4 °C. After extensive washing, the bound proteins were resolved by SDS-PAGE and analyzed by Western blot.
Reverse Transcription and Quantitative PCR
RNA was isolated using the TRIzol reagent (Invitrogen) according to the manufacturer's manual. Contaminating genomic DNA was degraded by treatment with TURBO DNase (Ambion, Austin, TX). RNA integrity was evaluated by electrophoresis, and 0.5 μg of RNA was reverse-transcribed with the RevertAid first-strand cDNA synthesis kit (Fermentas, Burlington, ON, Canada) according to the manufacturer's manual. Complementary DNA was 10-fold diluted, and 2 μl was used for quantitative RT-PCR performed in an ABI 7300 PCR system using the Universal PCR master mix (Applied Biosystems, Carlsbad, CA). The expression of glyceraldehyde-3-phosphate dehydrogenase (gapdh) and c-myc was analyzed using specific probes, Hs99999905_m1 and Hs00905030_m1, respectively (Applied Biosystems). Relative c-myc expression levels were calculated using the 2−ΔΔCT method (25) and normalized to the reference gapdh gene.
RESULTS
vIRF-3 Regulates c-Myc Stability
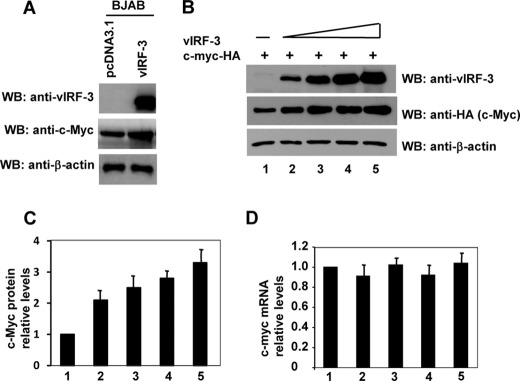
vIRF-3 associates with c-Myc and activates its transcription activity (11); however, the precise mechanism of this phenomenon has not yet been determined. We found that the vIRF-3-expressing stable line, BJAB/vIRF-3, shows reproducibly higher expression of endogenous c-Myc protein when compared with the control BJAB/vector line (Fig. 1A). To confirm this initial observation, we repeated the experiment with different cell lines. Because a majority of cancer cells carry mutations in c-myc that render it more stable than the wild-type form, we assayed the protein stability of transfected exogenous wild-type c-Myc-HA in HeLa cells. To ensure an equal transfection efficiency in all tested samples, first, HeLa cells were transfected with c-myc-HA expression construct. After this first round of transfection, cells were trypsinized and transfected with increasing amounts of vIRF-3 expression plasmid. As shown in Fig. 1B, although the levels of the endogenous β-actin remained unchanged, the levels of c-Myc-HA were found to be increased by the introduction of vIRF-3 in a dose-dependent manner. Fig. 1C shows the quantification of multiple independent experiments with similar results. Interestingly, transfection of vIRF-3 expression construct did not affect the relative levels of c-myc mRNA (Fig. 1D), an indication that vIRF-3 enhances the stability of the c-Myc protein.
FIGURE 1.
c-Myc stability is enhanced in presence of vIRF-3. A, BJAB stable cell lines expressing vIRF-3 (BJAB/vIRF-3) show higher levels of c-Myc protein when compared with control cells (BJAB/pcDNA3.1). WB, Western blot. B, HeLa cells were co-transfected with constant amounts of c-myc-HA and increasing amounts of vIRF-3 expression constructs (0, 0.5, 1.5, 3.0, and 6.0 μg). Forty-eight hours after transfection, cell extracts were prepared. The proteins in the extracts were separated on a 10% polyacrylamide gel and blotted with anti-HA (c-Myc), anti-vIRF-3, and anti-β-actin antibodies. C, quantitation of the immunoblot signals from panel B. c-Myc was normalized to the β-actin signal and then plotted against the signal obtained at 0 μg of vIRF-3 transfection. D, total RNA was isolated from cells used in panel B and analyzed by real-time RT-PCR using c-myc- and gapdh-specific probes. Error bars represent standard errors for four independent experiments. Each experiment was performed in duplicates.
Knockdown of vIRF-3 Expression by siRNAs Is Associated with Increased c-Myc Protein Stability
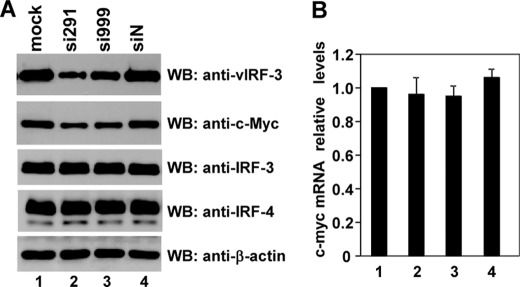
To ensure that the observed phenomenon also operates in a native environment, we employed the method of RNA interference (RNAi) to knock down vIRF-3 expression in KSHV-positive PEL cell line, BC-3. The target sequences of two vIRF-3-specific siRNAs, si291 and si999, started at positions 291 and 999 of vIRF-3 cDNA, respectively. BC-3 cells were either mock-treated or transfected with nonsense siRNA or vIRF-3-specific siRNAs (si291 or si999). At 2 days after transfection, the PEL cells were lysed, and the proteins were analyzed by Western blot and quantified by densitometry (Fig. 2A). The amount of vIRF-3 protein was reduced by 63 and 41% 2 days after transfection of BC-3 cells with si291 and si999, respectively (Fig. 2A). Actin levels remained constant in all tested samples. Additionally, there was no effect of nonsense siRNA on vIRF-3 expression. Interestingly, knockdown of vIRF-3 was associated with significant reduction of c-Myc protein (Fig. 2A, lanes 2 and 3) by 58 and 35% in cells transfected with si291 and si999, respectively. The observed reduction of c-Myc protein detected by Western blot was not reflected on the RNA level. As estimated by real-time RT-PCR, the relative levels of c-myc mRNA remained constant in all tested samples (Fig. 2B). To exclude any off-target effects of siRNAs used, the expression analyses of the closest vIRF-3 homologues, cellular IRF-3 and IRF-4, were included as negative controls. As shown in Fig. 2A, neither si291 nor si999 affected cellular IRF-3 or IRF-4 expression. Therefore we conclude that vIRF-3 expression leads to increased stability and accumulation of c-Myc protein in KSHV-positive PEL cells.
FIGURE 2.
Reduction of c-Myc protein in PEL cells after transient knockdown of vIRF-3 expression. A, BC-3 cells were either mock-treated or transfected with nonsense siRNA (siN) or vIRF-3-specific siRNAs (si291 and si999). At 2 days after transfection, half of the cells were harvested, and 8 μg of protein was analyzed by Western blot (WB) using anti-vIRF-3, anti-c-Myc, anti-β-actin, anti-IRF-3, and anti-IRF-4 antibodies. B, the other half of the cells used in panel A was harvested for total RNA isolation and analyzed by real-time RT-PCR using c-myc- and gapdh-specific probes. Error bars represent standard errors for four independent experiments. Each experiment was performed in duplicates.
vIRF-3 Increases c-Myc Protein Half-life
To confirm the effect of vIRF-3 on the enhanced stability of c-Myc, c-Myc half-life in HeLa cells was examined using cycloheximide treatment (Fig. 3A). Western blot of extracts from HeLa cells transfected with wild-type c-myc-HA alone (Fig. 3A, left panel) or together with vIRF-3 (Fig. 3A, right panel) and treated with cycloheximide showed that transfected c-Myc was significantly stabilized in the presence of vIRF-3. In agreement with previous studies, the half-life of wild-type c-Myc was ∼40 min. However, in the presence of vIRF-3, the half-life of c-Myc was considerably extended to ∼150 min. Fig. 3B shows the quantification of multiple independent experiments with similar results.
FIGURE 3.
Expression of vIRF-3 extends half-life of c-Myc. A, HeLa cells were co-transfected with constant amounts of c-myc-HA, vIRF-3, or pcDNA3.1 expression constructs. Forty-eight hours after transfection, cells were treated with 25 μg/ml cycloheximide (CHX) for the indicated times. Cell lysates were analyzed by Western blotting using anti-HA (c-Myc), anti-vIRF-3, and anti-β-actin antibodies. B, quantitation of the immunoblot signals from panel A. c-Myc was normalized to the β-actin signal and then plotted against the signal obtained at 0 h of cycloheximide treatment. Error bars represent standard errors for four independent experiments. Each experiment was performed in duplicates.
F-box Domain of Skp2 Is Sufficient for Interaction with vIRF-3
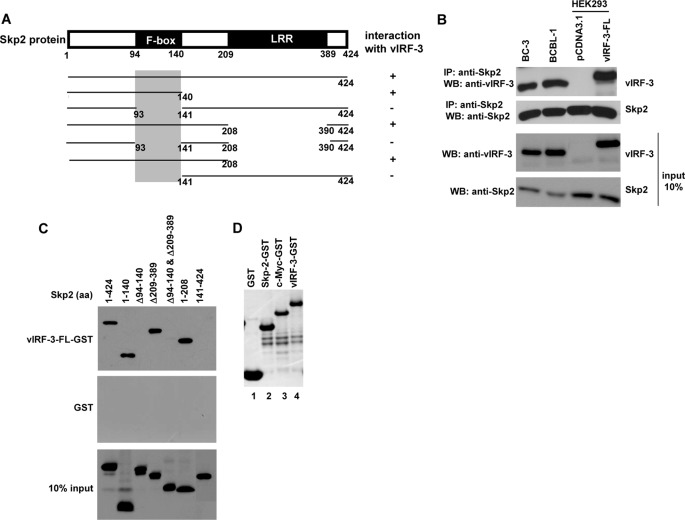
c-Myc is a short-lived transcription factor, and its rapid turnover is mediated by the ubiquitin/proteasome pathway, involving Skp2 as a major c-Myc-specific E3 ubiquitin ligase (16, 26). Based on our initial observation, we wanted to determine whether vIRF-3 could recruit components of the SCFskp2 ubiquitin ligase complex and regulate c-Myc stability. Co-immunoprecipitation experiments showed that endogenous vIRF-3 associated with Skp2 in both BC-3 and BCBL-1 PEL cells (Fig. 4B). These results were further supported by co-immunoprecipitation of transfected vIRF-3 and endogenous Skp2 in HEK293 cells. To identify the region of Skp2 that interacted with vIRF-3, we generated various skp2 deletion mutants (Fig. 4A) and analyzed the vIRF-3/Skp2 interaction by GST pulldown assay with GST-tagged full-length vIRF-3 (the quality of purified recombinant GST and vIRF-3-GST proteins is shown in Fig. 4D, lanes 1 and 4, respectively). The analysis revealed that the F-box of Skp2 (residues 94–140) was important for the interaction with vIRF-3 (Fig. 4C). All fragments of Skp2 failed to interact with the GST control, suggesting that interaction was specific for vIRF-3. The ability of vIRF-3 to interact with the F-box of Skp2 is interesting because the F-box domain was reported to carry ubiquitylation activity and has been implicated in the regulation of c-Myc stability (16).
FIGURE 4.
vIRF-3 interacts with F-box of Skp2. A, schematic representation of Skp2 deletion mutants tagged with Myc tag. The shaded box indicates the vIRF-3-binding domain. LRR, leucine-rich region. B, co-immunoprecipitation of Skp2 and vIRF-3 in PEL cells, BC-3 and BCBL-1, and transfected HEK293 cells. Protein lysates (400 μg) were immunoprecipitated (IP) with anti-Skp2 antibodies, and the immunoprecipitated complexes were analyzed by Western blot (WB) with anti-vIRF-3 antibodies. The relative levels of Skp2 and vIRF-3 in 40 μg of protein lysates are shown for comparison (input 10 %). The higher molecular weight of transfected vIRF-3-FL is due to Myc-His tag at the carboxyl terminus. C, Myc-tagged skp2 deletion mutants were in vitro translated using the TnT T7 quick coupled transcription/translation system and incubated with vIRF-3-FL fused to GST or GST alone immobilized on glutathione-Sepharose beads. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot with anti-Myc (9E10) antibodies. 10% of Skp2 deletion mutant protein input is shown below (10% input). aa, amino acids. D, Coomassie Blue staining of purified GST, Skp2-GST, c-Myc-GST, and vIRF-3-GST used in GST pulldown experiments.
Residues 129–142 and 379–418 of c-Myc Are Important for Interaction with vIRF-3
The c-Myc protein contains several regions that are highly conserved across the species (Fig. 5A). The amino terminus of the protein has two domains that are important for interaction with SCF. The first region (residues 45–63) interacts with the SCFfbw7 ubiquitin ligase complex, whereas the more distal region (residues 129–147) interacts with the SCFskp2 ubiquitin ligase complex. The carboxyl terminus of c-Myc contains the binding/dimerization domain (BR-HLH-LZ). The function of this domain is to specify homo- or heterodimerization through the helix-loop-helix-leucine zipper (HLH-LZ) region and interaction with DNA through the basic region (BR) (27–29). Within this domain, there is a region (residues 379–418) that also binds the Skp2 ubiquitin ligase (16). We have shown previously the interaction between vIRF-3 and c-Myc (11). Here we mapped precisely the regions of c-Myc that interact with vIRF-3. A series of c-myc deletion mutants (Fig. 5A) was translated in vitro and used for GST pulldown assay with vIRF-3-GST recombinant protein (Fig. 5B). Of all c-Myc deletion mutants tested, only the c-Myc (Δ129–142 and Δ379–418) failed to interact with full-length vIRF-3-GST. These results indicate that there are at least two regions within the c-Myc protein that are important for association with vIRF-3. Thus, in the carboxyl-terminal half of the c-Myc protein, a vIRF-3-binding site is localized between residues 379 and 418. Within the amino-terminal part of the c-Myc protein, the binding domain (residues 129–142) coincides with a highly conserved element, Myc box II (MBII). This region of c-Myc was shown to be involved not only in c-Myc proteolysis but also in the activation of c-Myc-mediated transcription and the ability to induce oncogenic transformation (12).
FIGURE 5.
Analysis of c-Myc interaction with vIRF-3. A, schematic representation of c-myc deletion constructs. The shaded boxes indicate vIRF-3-binding domains. MBI and MBII, Myc conserved domains I and II; TAD, transcription activation domain; NLS, nuclear localization signal; BR, basic region; HLH, helix-loop-helix; LZ, leucine zipper. B, HA-tagged c-Myc deletion mutants were in vitro translated using the TnT T7 quick coupled transcription/translation system and incubated with vIRF-3-FL fused to GST or GST alone immobilized on glutathione-Sepharose beads. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot with anti-HA antibodies. 10% of c-Myc deletion mutant protein input is shown below (10% input). aa, amino acids; N.S., nonspecific.
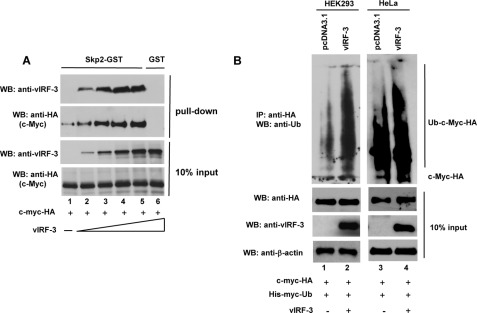
vIRF-3 neither Inhibits Skp2/c-Myc Interaction nor Blocks c-Myc Ubiquitylation
Because both vIRF-3 and Skp2 can bind to the same domains on c-Myc polypeptide, we examined whether vIRF-3 can interfere with the c-Myc/Skp2 interaction. To address this, constant amounts of in vitro translated c-myc-HA were preincubated with increasing amounts of in vitro translated vIRF-3 protein, and the binding of both vIRF-3 and c-Myc to Skp2-GST protein was examined by the GST pulldown assay (the quality of purified recombinant Skp2-GST protein is shown in Fig. 4D, lane 2). As shown in Fig. 6A, both c-Myc and vIRF-3 interacted with Skp2-GST. Interestingly, in the presence of vIRF-3, the binding of c-Myc to Skp2 was significantly increased in a dose-dependent manner. These data suggest that c-Myc/vIRF-3 heterodimer has higher binding affinity for Skp2 protein than c-Myc alone.
FIGURE 6.
vIRF-3 neither inhibits c-Myc/Skp2 interaction nor blocks c-Myc ubiquitylation. A, increased binding of c-Myc to Skp2 in the presence of vIRF-3. Constant amounts of in vitro translated c-Myc-HA protein were incubated with Skp2-GST immobilized to glutathione-Sepharose beads in the presence of increasing amounts of in vitro translated vIRF-3. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot (WB) with anti-HA (c-Myc) and anti-vIRF-3 antibodies. The binding to GST beads represents negative control (lane 6). 10% of c-Myc and vIRF-3 protein input is shown below (10% input). B, increased ubiquitylation of c-Myc in vIRF-3-expressing cells. HEK293 (lanes 1 and 2) and HeLa (lanes 3 and 4) cells were co-transfected with equal amounts of c-Myc-HA and His-Myc-Ub followed by transfection with either an empty vector (pcDNA3.1) or the vIRF-3-expressing construct. Prior to lysis, the cells were pretreated with proteasome inhibitor, 50 μm MG115, for 3 h. The cell lysates (400 μg) were analyzed by immunoprecipitation (IP) with anti-HA-specific antibodies followed by Western blotting with anti-Ub antibodies. The relative levels of transfected c-Myc-HA and vIRF-3 in 40 μg of cell lysates were estimated by Western blots (10% input).
Because the presence of vIRF-3 increases the ability of c-Myc to interact with Skp2, we examined the ubiquitylation status of c-Myc in vIRF-3-expressing cells. First, HEK293 and HeLa cells were transfected with constant amounts of His-Myc-tagged ubiquitin (His-Myc-Ub) and c-myc-HA expression constructs. After the first round of transfection, cells were trypsinized and transfected either with a vIRF-3 expression construct or with an empty vector (pcDNA3.1). c-Myc was immunoprecipitated from the cell lysates, and the level of ubiquitylation was assessed by Western blot using anti-Ub antibodies (Fig. 6B). Although c-Myc was already ubiquitylated in cells transfected with an empty vector (Fig. 6B, lanes 1 and 3), co-transfection with vIRF-3 expression construct resulted in significantly increased c-Myc ubiquitylation in both cell lines (Fig. 6B, lanes 2 and 4).
vIRF-3 Cooperates with Skp2 in Activation of c-Myc Target Genes
Skp2 was shown to be linked not only to the degradation of c-Myc, but it was also shown to be a transcriptional co-activator of c-Myc protein (16–19). Thus, Skp2 was shown to enhance the c-Myc-mediated transcription of a number of target promoters (16). We have previously shown that vIRF-3 can also stimulate c-Myc-regulated transcription (11). This stimulation was due to the association of vIRF-3 with c-Myc followed by binding of the vIRF-3·c-Myc complex to the E-box sequences in the promoter of WT cdk4 gene. The cdk4 promoter contains four putative c-Myc-binding sites (MBS1–4). Mutation analysis of individual MBS elements suggested that MBS3 and MBS4 were particularly important in the transactivation of the cdk4 promoter by c-Myc (30). Because vIRF-3 stimulates the association between c-Myc and Skp2, we tested whether vIRF-3 would further stimulate the activation of the WT cdk4 promoter in cells expressing both c-myc and skp2 genes. To address this, we transfected HEK293 cells with c-myc, vIRF-3, and skp2 expression plasmids together with a reporter construct containing the luciferase gene linked to the wild-type cdk4 promoter. Although c-Myc alone activated the cdk4 promoter ∼3-fold, co-transfection of skp2 or vIRF-3 resulted in further stimulation of cdk4 promoter activity to ∼5.2-and 4.9-fold, respectively (Fig. 7A). Importantly, co-transfection of all three expression plasmids, c-myc-HA, skp2, and vIRF-3, resulted in even higher up-regulation (7-fold) of transcription driven by the cdk4 promoter. These results support our previous observations and suggest that vIRF-3 cooperates with Skp2 in activation of c-Myc-regulated transcription.
FIGURE 7.
vIRF-3 cooperates with Skp-2 in activation of c-Myc-mediated transcription. A, effect of vIRF-3 on activation of cdk4 promoter. Human wild-type cdk4 reporter (WT MBS1–4) and pRL-SV40 plasmids were co-transfected into HEK293 cells with either an empty vector (pcDNA3.1) or c-myc-HA-, vIRF-3-, and skp2-Myc-expressing plasmids. Luciferase activity was analyzed 48 h after transfection as -fold activation relative to the basal level of reporter gene in the presence of control vector, pcDNA3.1. Results were normalized to Renilla luciferase activity. Error bars represent standard errors for three independent experiments. The bottom panel shows the expression levels of transfected proteins. RLU, relative luciferase units. B, association of c-Myc, vIRF-3, and Skp2 with the WT cdk4 promoter was analyzed by DNA pulldown assay. HEK293 cells were transfected with c-myc-HA-, vIRF-3-, and skp2-Myc-expressing plasmids as indicated. Twenty-four hours after transfection, cells were untreated or treated with 50 μm MG115 for 3 h followed by cell lysis. Lysates (350 μg) were incubated with WT cdk4 promoter (WT MBS4) oligodeoxynucleotides coupled to magnetic beads. The c-Myc-HA, vIRF-3, or Skp2-Myc proteins pulled down by DNA were identified by Western blot with anti-HA, anti-vIRF-3, and anti-Myc (9E10)-specific antibodies, respectively (upper panel). Binding to beads only (lanes 3 and 6) represents the negative control. Binding to mutated cdk4 promoter (mutMBS4) served as a negative control (middle panel). The relative levels of transfected vIRF-3, c-Myc-HA, and Skp-2-Myc in 35 μg of cell extracts were estimated by Western blot (10% input, bottom panel).
To determine the molecular mechanism underlying this phenomenon, we analyzed the protein complex assembled on the WT cdk4 promoter using the DNA pulldown assay. In this assay, the oligodeoxynucleotides corresponding to MBS4 of the human wild-type cdk4 promoter were biotinylated, coupled to streptavidin-coated magnetic beads, and incubated with protein extracts isolated from HEK293 cells transfected with c-myc-HA, skp2, and vIRF-3 expression plasmids. The vIRF-3, c-Myc-HA and Skp2 proteins specifically bound to the WT MBS4 site oligodeoxynucleotides were detected by Western blot (Fig. 7B, upper panel). In agreement with our previous observations (11), c-Myc association with the WT cdk4-MBS4 site was increased in the presence of vIRF-3 (Fig. 7B, lanes 2 and 5). Interestingly, Skp2 was also found to be bound to the MBS4 oligodeoxynucleotides, and its association with this site was increased in cells expressing vIRF-3. A longer exposure of the Western blot detected the association of high molecular mass c-Myc (75–200 kDa) with the WT cdk4 promoter. Furthermore, the treatment with the proteasome inhibitor, MG115 (Fig. 7B, lanes 4 and 5), increased the levels of high molecular mass c-Myc present at the promoter, suggesting that this form of c-Myc corresponded to the ubiquitylated c-Myc protein. Notably, in the presence of vIRF-3, the association of Ub-c-Myc with the cdk4 promoter was significantly increased (Fig. 7B, lanes 2 and 5). To test the specificity of c-Myc·Skp2·vIRF-3 complex binding to the WT MBS4 site, we performed a DNA pulldown analysis with a mutated MBS4 oligodeoxynucleotide, mutMBS4, carrying a single nucleotide substitution within an E-box element (CACGTG → CACCTG). Hermeking et al. (30) previously reported that this particular mutation of the MBS4 site resulted in markedly diminished activation of the cdk4 luciferase reporter. As shown in Fig. 7B (middle panel), neither c-Myc nor Skp2 or vIRF-3 was able to associate with a mutated MBS4 oligonucleotide, confirming the specificity of observed complexes at the WT MBS4 element. Altogether, these data suggest that c-Myc, its ubiquitylated form, Skp2, and vIRF-3 form a multicomponent transcription complex that assembles on the cdk4 promoter and activates its transcription.
c-Myc and Skp2 Interact with vIRF-3 (Residues 346–455)
As a basis for future studies on the functional importance of the vIRF-3/c-Myc/Skp2 interaction, we identified the part of vIRF-3 required for binding to c-Myc and Skp2. As recently reported, the putative double α-helix motif of vIRF-3 (amino acids 240–280) (Fig. 8A) was sufficient to bind to both IRF-7 and IRF-5 proteins (31, 32). In vitro translated vIRF-3 deletion mutants were used for GST pulldown experiments with GST-tagged c-Myc and Skp2 recombinant proteins (Fig. 8B; the quality of purified recombinant Skp2-GST and c-Myc-GST is shown in Fig. 4D, lanes 2 and 3, respectively). This analysis revealed that the domain within the carboxyl-terminal half of the vIRF-3 protein (residues 346–455) was important for interaction with both c-Myc and Skp2. The specificity of this interaction was confirmed by the fact that GST alone did not bind to any of the vIRF-3 deletion mutants. The ability of c-Myc and Skp2 to interact with the same domain of vIRF-3 is interesting and supports our finding that both Skp2 and vIRF-3 are components of a co-activator complex involved in activation of c-Myc-mediated transcription.
The results suggesting that both c-Myc and Skp2 interact with the same domain of vIRF-3 as well as the finding that vIRF-3 binds to two domains of c-Myc protein shown to facilitate the c-Myc/Skp2 interaction were intriguing. There could be a possibility that in vitro translated proteins employed in GST pulldown assays may be contaminated with Skp2 present in a crude reticulocyte lysate. Under these conditions, Skp2 could serve as a bridge and recruit vIRF-3 to c-Myc to form a ternary complex. Therefore we decided to test and validate the c-Myc/vIRF-3 interaction in a system devoid of Skp2 or any other eukaryotic protein. Using a prokaryotic cell-free translation system (E. coli T7 S30 extract system for circular DNA), we in vitro translated vIRF-3 (Fig. 8C, left panel, lane 1) or c-Myc-HA (Fig. 8C, right panel, lane 4) and incubated with c-Myc-GST or vIRF-3-GST, respectively. As shown in Fig. 8C, the interaction between vIRF-3 and c-Myc also occurred in the absence of Skp2, suggesting that the vIRF-3·c-Myc complex formation is a result of a direct interaction. The specificity of the interaction was confirmed by the absence of GST binding to either in vitro translated vIRF-3 or in vitro translated c-Myc-HA.
DISCUSSION
c-myc is a proto-oncogene that controls proliferation, cell cycle, and cell survival (12). c-myc also plays an important role in the development of B-cell lymphomas (33, 34). Overexpression of c-myc as a consequence of reciprocal translocation involving the immunoglobulin loci can be seen in Epstein-Barr virus-positive B-cell lymphomas (35). Importantly, in most Burkitt lymphoma cell lines, c-myc amplification is often accompanied by its stabilization due to mutations at position Thr-48 (36). Although currently there are no reports of any c-myc mutations or locus rearrangements in KSHV-associated PEL cells (37–39), there is a growing evidence that c-Myc is an important cellular factor in the development of KSHV-associated neoplasia. Studies of Ahmad et al. (40) suggested that KSHV-encoded viral FLICE inhibitory protein (vFLIP) activates the NFκB pathway and cooperates with c-Myc to promote lymphoma in mice. Recently, two groups reported that another KSHV latent gene, latency-associated nuclear antigen (LANA), can stabilize and activate c-Myc by controlling its phosphorylation at threonine 58 and serine 62 (41, 42). In addition, the importance of c-Myc is well documented by the work of Li et al. (43), who show that c-Myc is required for maintenance of KSHV latency. Taken together, it appears that there are multiple mechanisms by which KSHV latent genes target and manipulate the function of c-Myc. In this study, we extended our observations regarding the vIRF-3-mediated stimulation of c-Myc transcription activity (11). We have shown that vIRF-3 can stabilize the c-Myc protein and extend its half-life by ∼4-fold. Because F-box protein Skp2 was reported to be involved in c-Myc protein turnover, we tested whether vIRF-3 can interact with Skp2. Although vIRF-3 was found to be able to associate with Skp2, it did not prevent formation of the c-Myc/Skp2 heterodimer. Consequently, the ubiquitylation of c-Myc appeared to be stimulated in the presence of vIRF-3.
What is the biological role of the vIRF-3/c-Myc/Skp2 interaction? The study of the domains involved in this multicomponent interaction revealed that vIRF-3 binds to the F-box region of Skp2, which is presumably required for regulation of c-Myc ubiquitylation and stability (16). Another intriguing aspect of the vIRF-3/c-Myc/Skp2 interaction is in the way c-Myc interacts with vIRF-3. We found that vIRF-3 binds to two regions within c-Myc: i) residues 128–143 in the amino terminus corresponding to the well conserved Myc box II (MBII) domain and ii) residues 379–418 in the carboxyl terminus. Interestingly, both regions were reported to be necessary for c-Myc/Skp2 interaction (16). Furthermore, our mutational studies suggested that the carboxyl-terminal domain of vIRF-3 (residues 346–455) is essential for binding to both c-Myc and Skp2. Altogether, these results indicate that vIRF-3 recruits both c-Myc and its transcriptional co-activator Skp2 to the near proximity of each other, facilitating the c-Myc-regulated transcription. Indeed, we found that vIRF-3 can stimulate the c-Myc-regulated transcription from the cdk4 promoter, and this stimulation is further enhanced in the presence of Skp2. The activity of the cdk4 promoter is reflected by the presence of transcription factors and co-factors at its promoter. Previously, employing the chromatin immunoprecipitation (ChIP) assay, we have demonstrated recruitment of the c-Myc·vIRF-3 complex to the endogenous cdk4 promoter followed by increased histone H3 acetylation (11). Here we showed that expression of vIRF-3 resulted in higher levels of Skp2 as well as polyubiquitylated c-Myc proteins associated with the wild-type cdk4 promoter. Thus, our data support the observations that c-Myc ubiquitylation is linked to its transcription activity (16, 17). Additionally, we showed that c-Myc ubiquitylation can be further enhanced by vIRF-3. Paradoxically, we also found that vIRF-3 enhances the stability of c-Myc. One possible explanation might be that vIRF-3 inhibits the proteasomal degradation of c-Myc further downstream of ubiquitylation and thus uncouples the ubiquitylation/degradation pathway. A similar mechanism was observed with cancer-associated mutants of c-Myc, which can be efficiently ubiquitylated by Skp2, but are resistant to proteasomal degradation (16, 26). Alternatively, the ubiquitylation, which is under the control of vIRF-3, may utilize a different set of lysine residues that is not linked to proteasomal degradation. The exact mechanism by which vIRF-3 enhances the activity and stability of c-Myc is currently under investigation in our laboratory.
There is a growing evidence that a number of viruses have evolved various strategies to exploit the ubiquitin-proteasome system of the host. The Epstein-Barr virus nuclear antigen 3C (EBNA3C) was recently shown to stabilize and activate c-Myc by recruitment of the c-Myc/Skp2 heterodimer to c-Myc-dependent promoters (44). Similarly, the X protein of hepatitis B virus can stimulate c-Myc function by binding to Skp2 and inhibiting the ubiquitylation and proteasomal degradation of c-Myc (45).
In summary, we have elucidated a new mechanism by which KSHV-encoded vIRF-3 enhances transcription activity of c-Myc. Thus, this study extends our initial report showing the modulation of c-Myc transcription activity by the KSHV-encoded vIRF-3 (11). The previously unappreciated role of vIRF-3 in the stimulation of c-Myc ubiquitylation and protein stability provides a link between vIRF-3 and c-Myc-regulated pathways and a possible mechanism by which vIRF-3 contributes to the KSHV-associated PEL lymphoma. The association between the enhanced expression of c-myc and B-cells lymphomagenesis has been well documented. The study of the molecular mechanism by which vIRF-3 manipulates c-Myc function contributes to the understanding of KSHV-associated lymphomagenesis and indicates its critical function in KSHV-associated pathogenesis. It may also provide a new platform to its pharmacological regulation.
Acknowledgments
We thank Drs. Bert Vogelstein (Johns Hopkins University, Baltimore, MD), Yue Xiong (Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill), and Michele Pagano (New York University Cancer Institute, New York, NY) for providing plasmids and reagents. We are grateful to Dr. Jasper E. Manning for critical comments on the manuscript.
This work was supported by grants from the Grant Agency of the Czech Republic (Project 204/09/0773 to B. L.), from the Charles University (Projects PRVOUK-P24/LF1/3 and SVV-2012-264506), and from the Ministry of Education of the Czech Republic (Project MSM0021620806).
- KSHV
- Kaposi sarcoma-associated herpesvirus
- PEL
- primary effusion lymphoma
- IRF
- interferon regulatory factor
- vIRF-3
- viral interferon regulatory factor-3
- FL
- full-length
- MBS
- c-Myc-binding site
- Ub
- ubiquitin
- FLICE
- Fas-associated death domain interleukin-1/3-converting enzyme.
REFERENCES
- 1. Moore P. S., Gao S. J., Dominguez G., Cesarman E., Lungu O., Knowles D. M., Garber R., Pellett P. E., McGeoch D. J., Chang Y. (1996) Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 70, 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee H. R., Kim M. H., Lee J. S., Liang C., Jung J. U. (2009) Viral interferon regulatory factors. J. Interferon Cytokine Res. 29, 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lubyova B., Pitha P. M. (2000) Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74, 8194–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rivas C., Thlick A. E., Parravicini C., Moore P. S., Chang Y. (2001) Kaposi sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cesarman E., Chang Y., Moore P. S., Said J. W., Knowles D. M. (1995) Kaposi sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332, 1186–1191 [DOI] [PubMed] [Google Scholar]
- 6. Dupin N., Diss T. L., Kellam P., Tulliez M., Du M. Q., Sicard D., Weiss R. A., Isaacson P. G., Boshoff C. (2000) HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95, 1406–1412 [PubMed] [Google Scholar]
- 7. Schulz T. F. (2006) The pleiotropic effects of Kaposi sarcoma herpesvirus. J. Pathol. 208, 187–198 [DOI] [PubMed] [Google Scholar]
- 8. Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d'Agay M. F., Clauvel J. P., Raphael M., Degos L. (1995) Kaposi sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman disease. Blood 86, 1276–1280 [PubMed] [Google Scholar]
- 9. Seo T., Park J., Lim C., Choe J. (2004) Inhibition of nuclear factor κB activity by viral interferon regulatory factor 3 of Kaposi sarcoma-associated herpesvirus. Oncogene 23, 6146–6155 [DOI] [PubMed] [Google Scholar]
- 10. Wies E., Mori Y., Hahn A., Kremmer E., Stürzl M., Fleckenstein B., Neipel F. (2008) The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood 111, 320–327 [DOI] [PubMed] [Google Scholar]
- 11. Lubyova B., Kellum M. J., Frisancho J. A., Pitha P. M. (2007) Stimulation of c-Myc transcriptional activity by vIRF-3 of Kaposi sarcoma-associated herpesvirus. J. Biol. Chem. 282, 31944–31953 [DOI] [PubMed] [Google Scholar]
- 12. Grandori C., Cowley S. M., James L. P., Eisenman R. N. (2000) The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16, 653–699 [DOI] [PubMed] [Google Scholar]
- 13. Alexandrow M. G., Kawabata M., Aakre M., Moses H. L. (1995) Overexpression of the c-Myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of transforming growth factor β1. Proc. Natl. Acad. Sci. U.S.A. 92, 3239–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hermeking H., Funk J. O., Reichert M., Ellwart J. W., Eick D. (1995) Abrogation of p53-induced cell cycle arrest by c-Myc: evidence for an inhibitor of p21WAF1/CIP1/SDI1. Oncogene 11, 1409–1415 [PubMed] [Google Scholar]
- 15. Hann S. R., Eisenman R. N. (1984) Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol. Cell. Biol. 4, 2486–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S. Y., Herbst A., Tworkowski K. A., Salghetti S. E., Tansey W. P. (2003) Skp2 regulates Myc protein stability and activity. Mol. Cell 11, 1177–1188 [DOI] [PubMed] [Google Scholar]
- 17. von der Lehr N., Johansson S., Wu S., Bahram F., Castell A., Cetinkaya C., Hydbring P., Weidung I., Nakayama K., Nakayama K. I., Söderberg O., Kerppola T. K., Larsson L. G. (2003) The F-box protein Skp2 participates in c-Myc proteasomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11, 1189–1200 [DOI] [PubMed] [Google Scholar]
- 18. Jin J., Harper J. W. (2003) A license to kill: transcriptional activation and enhanced turnover of Myc by the SCFSkp2 ubiquitin ligase. Cancer Cell 3, 517–518 [DOI] [PubMed] [Google Scholar]
- 19. von der Lehr N., Johansson S., Larsson L. G. (2003) Implication of the ubiquitin/proteasome system in Myc-regulated transcription. Cell Cycle 2, 403–407 [PubMed] [Google Scholar]
- 20. Frescas D., Pagano M. (2008) Deregulated proteolysis by the F-box proteins SKP2 and β-TrCP: tipping the scales of cancer. Nat. Rev. Cancer 8, 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gstaiger M., Jordan R., Lim M., Catzavelos C., Mestan J., Slingerland J., Krek W. (2001) Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl. Acad. Sci. U.S.A. 98, 5043–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Latres E., Chiarle R., Schulman B. A., Pavletich N. P., Pellicer A., Inghirami G., Pagano M. (2001) Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl. Acad. Sci. U.S.A. 98, 2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lubyova B., Kellum M. J., Frisancho A. J., Pitha P. M. (2004) Kaposi sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7. J. Biol. Chem. 279, 7643–7654 [DOI] [PubMed] [Google Scholar]
- 24. Au W. C., Pitha P. M. (2001) Recruitment of multiple interferon regulatory factors and histone acetyltransferase to the transcriptionally active interferon a promoters. J. Biol. Chem. 276, 41629–41637 [DOI] [PubMed] [Google Scholar]
- 25. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 26. Salghetti S. E., Kim S. Y., Tansey W. P. (1999) Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 18, 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blackwood E. M., Eisenman R. N. (1991) Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251, 1211–1217 [DOI] [PubMed] [Google Scholar]
- 28. Prendergast G. C., Ziff E. B. (1991) Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science 251, 186–189 [DOI] [PubMed] [Google Scholar]
- 29. Lüscher B., Larsson L. G. (1999) The basic region/helix-loop-helix/leucine zipper domain of Myc proto-oncoproteins: function and regulation. Oncogene 18, 2955–2966 [DOI] [PubMed] [Google Scholar]
- 30. Hermeking H., Rago C., Schuhmacher M., Li Q., Barrett J. F., Obaya A. J., O'Connell B. C., Mateyak M. K., Tam W., Kohlhuber F., Dang C. V., Sedivy J. M., Eick D., Vogelstein B., Kinzler K. W. (2000) Identification of CDK4 as a target of c-MYC. Proc. Natl. Acad. Sci. U.S.A. 97, 2229–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joo C. H., Shin Y. C., Gack M., Wu L., Levy D., Jung J. U. (2007) Inhibition of interferon regulatory factor 7 (IRF7)-mediated interferon signal transduction by the Kaposi sarcoma-associated herpesvirus viral IRF homolog vIRF3. J. Virol. 81, 8282–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wies E., Hahn A. S., Schmidt K., Viebahn C., Rohland N., Lux A., Schellhorn T., Holzer A., Jung J. U., Neipel F. (2009) The Kaposi sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J. Biol. Chem. 284, 8525–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keller U. B., Old J. B., Dorsey F. C., Nilsson J. A., Nilsson L., MacLean K. H., Chung L., Yang C., Spruck C., Boyd K., Reed S. I., Cleveland J. L. (2007) Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation and lymphomagenesis. EMBO J. 26, 2562–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barrios C., Castresana J. S., Ruiz J., Kreicbergs A. (1994) Amplification of the c-myc proto-oncogene in soft tissue sarcomas. Oncology 51, 13–17 [DOI] [PubMed] [Google Scholar]
- 35. Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. (1983) The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell 34, 779–787 [DOI] [PubMed] [Google Scholar]
- 36. Gregory M. A., Hann S. R. (2000) c-Myc proteolysis by the ubiquitin-proteasome pathway: stabilization of c-Myc in Burkitt lymphoma cells. Mol. Cell. Biol. 20, 2423–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carbone A., Cilia A. M., Gloghini A., Capello D., Todesco M., Quattrone S., Volpe R., Gaidano G. (1998) Establishment and characterization of EBV-positive and EBV-negative primary effusion lymphoma cell lines harboring human herpesvirus type-8. Br. J. Haematol. 102, 1081–1089 [DOI] [PubMed] [Google Scholar]
- 38. Arvanitakis L., Mesri E. A., Nador R. G., Said J. W., Asch A. S., Knowles D. M., Cesarman E. (1996) Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88, 2648–2654 [PubMed] [Google Scholar]
- 39. Gaidano G., Pastore C., Gloghini A., Volpe G., Ghia P., Saglio G., Carbone A. (1996) AIDS-related non-Hodgkin lymphomas: molecular genetics, viral infection, and cytokine deregulation. Acta Haematol. 95, 193–198 [DOI] [PubMed] [Google Scholar]
- 40. Ahmad A., Groshong J. S., Matta H., Schamus S., Punj V., Robinson L. J., Gill P. S., Chaudhary P. M. (2010) Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 cooperates with Myc to promote lymphoma in mice. Cancer Biol. Ther. 10, 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bubman D., Guasparri I., Cesarman E. (2007) Deregulation of c-Myc in primary effusion lymphoma by Kaposi sarcoma herpesvirus latency-associated nuclear antigen. Oncogene 26, 4979–4986 [DOI] [PubMed] [Google Scholar]
- 42. Liu J., Martin H. J., Liao G., Hayward S. D. (2007) The Kaposi sarcoma-associated herpesvirus LANA protein stabilizes and activates c-Myc. J. Virol. 81, 10451–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X., Chen S., Feng J., Deng H., Sun R. (2010) Myc is required for the maintenance of Kaposi sarcoma-associated herpesvirus latency. J. Virol. 84, 8945–8948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bajaj B. G., Murakami M., Cai Q., Verma S. C., Lan K., Robertson E. S. (2008) Epstein-Barr virus nuclear antigen 3C interacts with and enhances the stability of the c-Myc oncoprotein. J. Virol. 82, 4082–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kalra N., Kumar V. (2006) The X protein of hepatitis B virus binds to the F box protein Skp2 and inhibits the ubiquitination and proteasomal degradation of c-Myc. FEBS Lett. 580, 431–436 [DOI] [PubMed] [Google Scholar]