Background: Na/K-ATPase decrease has been reported in patients with heart failure and is related to cardiac dysfunction.
Results: Reducing Na/K-ATPase activates caspase 9 and induces cardiac dilation when treated with marinobufagenin.
Conclusion: Reduction of Na/K-ATPase potentiates marinobufagenin-induced cardiac myocyte apoptosis.
Significance: Decreased Na/K-ATPase content together with increased cardiotonic steroids levels is a novel mechanism that may account for cardiac dysfunction.
Keywords: Apoptosis; Cardiomyopathy; Cardiovascular Disease; Na,K-ATPase; Signal Transduction; Cardiotonic Steroids
Abstract
Decreases in cardiac Na/K-ATPase have been documented in patients with heart failure. Reduction of Na/K-ATPase α1 also contributes to the deficiency in cardiac contractility in animal models. Our previous studies demonstrate that reduction of cellular Na/K-ATPase causes cell growth inhibition and cell death in renal proximal tubule cells. To test whether reduction of Na/K-ATPase in combination with increased cardiotonic steroids causes cardiac myocyte death and cardiac dysfunction, we examined heart function in Na/K-ATPase α1 heterozygote knock-out mice (α1+/−) in comparison to wild type (WT) littermates after infusion of marinobufagenin (MBG). Adult cardiac myocytes were also isolated from both WT and α1+/− mice for in vitro experiments. The results demonstrated that MBG infusion increased myocyte apoptosis and induced significant left ventricle dilation in α1+/− mice but not in their WT littermates. Mechanistically, it was found that in WT myocytes MBG activated the Src/Akt/mTOR signaling pathway, which further increased phosphorylation of ribosome S6 kinase (S6K) and BAD (Bcl-2-associated death promoter) and protected cells from apoptosis. In α1+/− myocytes, the basal level of phospho-BAD is higher compared with WT myocytes, but MBG failed to induce further activation of the mTOR pathway. Reduction of Na/K-ATPase also caused the activation of caspase 9 but not caspase 8 in these cells. Using cultures of neonatal cardiac myocytes, we demonstrated that inhibition of the mTOR pathway by rapamycin also enabled MBG to activate caspase 9 and induce myocyte apoptosis.
Introduction
Reduction of Na/K-ATPase has been documented in patients and experimental animals with congestive heart failure (1, 2), aging (3, 4), diabetes with hypertension (5–7), and neurological disorders (8, 9). In heart tissue biopsies from patients with dilated cardiomyopathy, the Na/K-ATPase amount is decreased by up to 40% (1). Reduction of cardiac Na/K-ATPase is also related with decreased cardiac contractile function in humans and in animals (1, 10, 11). Previous reports showed that exposure of cells to low concentrations of cardiotonic steroids (CTS)2 stimulates cell growth (12–14). Our recent work also shows that binding of ouabain to Na/K-ATPase in pig renal proximal tubule cells stimulates Src/Akt/mTOR signaling pathways and results in a stimulation of cell growth. Interestingly, the stimulatory effects of CTS on cell growth are abolished when cellular Na/K-ATPase is reduced by siRNA (15).
In addition to the reduction of Na/K-ATPase, studies have found that levels of endogenous CTS increase in patients and in experimental animals with volume expanded hypertension and chronic renal disease (16–18). It was also found that endogenous ouabain-like compounds increased in patients with left ventricular dysfunction due to dilated myocardium (19). Because myocyte death may play an important role in development of myocardium dilation and heart failure (20), the current study tested whether reduction of Na/K-ATPase potentiates CTS-induced myocyte apoptosis and cardiac dysfunction.
EXPERIMENTAL PROCEDURES
Animals
All animal experimentation described in this article was conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals Committee. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Toledo. The Na/K-ATPase heterozygous (α1+/−) mice and their wild type littermates were generated as described previously (11). Genomic DNA was obtained from tail biopsies and used for PCR-based genotyping. Adult mice at 2∼3 months were used for the study. All mice were kept in a 12-h dark/light cycle and fed standard chow ad libitum. Experimental mice were anesthetized and an osmotic mini-pump from Alzet (Cupertino, CA) was placed under the skin to constantly infuse MBG at the dose of 10 μg/kg/day. Mice infused with solvent only (50% dimethyl sulfoxide and 50% saline) were used as control. Doppler imaging study was performed at the end of the 4th week of infusion as described before (21). Mice were sacrificed at the end of 4th week after echocardiographic measurements. Blood was collected, and the heart was then removed. The whole heart and isolated left ventricle were weighed. Afterward, half of the left ventricle was snap frozen in liquid nitrogen, whereas the other half was fixed in 4% formalin solution for histology analysis. Tissue homogenates for biochemistry analysis were obtained by grinding tissues in liquid nitrogen and then homogenized in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.25% deoxycholic acid, 1% Nonidet P-40, 1 mm EDTA). RIPA buffer was supplemented with 1 mm PMSF, 1 mm sodium orthovanadate, and 1% protease inhibitor cocktails from Sigma before use.
Adult Cardiac Myocyte Preparation from Mice
Ca2+-tolerant adult mouse cardiac myocytes were prepared from isolated hearts as described previously by O'Connell et al. (22) with minor modifications. Briefly, mice were heparinized and anesthetized with Nembutal (100–140 mg/kg) via intraperitoneal injection. The isolated heart was cannulated via the aorta and perfused with calcium-free solution for 5 min followed by a solution containing 1 mg/ml type II collagenase. The yield of myocytes per heart was >1.0 million cells with 70–90% viable rod-shaped cells. Cell attachment rate was ∼80%. Myocytes were plated at the density of 25,000/ml on laminin (1.25 μg/cm2)-coated Petri dishes in modified Eagle's medium with Hanks' balanced salt solution supplemented with fetal bovine serum (10%), 2,3-butanedione monoxime (10 mmol/liter) and 100 units/ml penicillin in a 2% CO2 incubator for 1 h. Cells were then cultured in modified Eagle's medium with 10 mm butanedione monoxime and 0.1% bovine serum albumin (BSA) at 5% CO2 before any treatment.
Rat Neonatal Cardiac Myocyte Preparation
Primary cultures of rat neonatal cardiac myocytes were prepared as described previously with minor modifications (23). Myocytes were dispersed from ventricles of 1- to 2-day-old Sprague-Dawley rats using 0.04% collagenase II and 0.05% pancreatin at 37 °C. Non-cardiomyocytes were eliminated by pre-plating for 1.5 h at 37 °C. Myocytes were cultured in cell culture medium containing Dulbecco's modified Eagle's medium and M199 (4:1 v/v) with 10% fetal bovine serum. The neonatal cardiac myocytes were serum-starved for 48 h before experimentation. For experiments containing 10% fetal bovine serum, cells were cultured 48 h after plating with one time media change before treatment.
Lactate Dehydrogenase Measurement, Annexin V Staining, and TUNEL Assay for Cell Apoptosis
For lactate dehydrogenase measurement, rat neonatal myocytes were treated with MBG or the combination of MBG and rapamycin. The culture medium of treated cells was collected and centrifuged at 16,000 × g for 5 min to remove dead cells. The supernatant was transferred to a new tube for lactate dehydrogenase measurement using a commercial kit from Roche Applied Science. Rat neonatal myocytes grown on glass coverslips were used for annexin V staining and TUNEL assay. The annexin V staining kits was from Invitrogen. The manufacturer's instructions were followed for annexin V staining with minor modifications. Briefly, rat neonatal myocytes were stained with annexin V and PI in annexin V assay buffer for 15 min. The cells were washed twice with PBS and slightly fixed with 2% paraformaldehyde for 15 min. These cells were then examined using a Leica confocal microscope (Buffalo Grove, IL) on the same day. TUNEL assay was performed on rat neonatal myocytes using a commercial kit from Millipore (Billerica, MA) following the manufacturer's instructions. Treated cells were first fixed and permeabilized with cold methanol. TUNEL-positive cells were stained with streptavidin-FITC after terminal deoxynucleotidyl transferase incubation and examined using a Leica confocal microscope. PI is used for counterstaining the nuclei.
Statistics
Continuous data are presented as the mean ± S.E. Statistical analysis was performed using the Student's t test, and significance was accepted at p < 0.05.
RESULTS
MBG Infusion Induces Myocyte Apoptosis, Cardiac Dysfunction, Ventricular Dilation, and Reduced Wall Thickness in α1+/− Mice
Decreased Na/K-ATPase and increased endogenous CTS have been found in patients with congestive heart failure and chronic kidney diseases (16–18). To test whether Na/K-ATPase reduction potentiates CTS-induced cardiac dysfunction, Na/K-ATPase α1+/− mice with one allele of the Na/K-ATPase α1 gene deleted, and their wild type littermates were used in the MBG infusion studies. Both WT and α1+/− mice were infused with MBG through an osmotic mini pump at a dose of 10 μg/kg/day. This concentration of MBG has been used in our previous studies without observable toxicity (24, 25). Mice infused with solvent only were used as control. Echocardiograph measurement was performed at the end of 4th week post implantation of the mini pump. As shown in Table 1, MBG infusion significantly increased the systolic and diastolic dimension of the left ventricle in α1+/− mice when compared with the wild type controls. Consistently, the septal wall thickness reduced over time after MBG infusion in α1+/− mice from 1.31 ± 0.38 mm at base line to 0.78 ± 0.09 mm at the end of 4th week, whereas in the WT mice, there was no significant change observed. Functionally, MBG infusion also caused >45% reduction in contractile function of the left ventricle in α1+/− mice but had less of an effect in WT mice.
TABLE 1.
Doppler image study in WT and Na/K-ATPase knockdown (α1+/−) mice with MBG infusion
EDD, end diastolic dimension; ESD, end systolic dimension; BW, body weight; HW, heart weight; LVW, left ventricle weight; SWT, anterior wall thickness; PWT, posterior wall thickness; shortening (%) = (EDD − ESD)/EDD × 100). Data are presented as mean ± S.E. (*, p < 0.05, base line versus 4 weeks).
| Variables | WT |
α1+/− |
||||||
|---|---|---|---|---|---|---|---|---|
| Control |
MBG |
Control |
MBG |
|||||
| Base line | 4 wk | Base line | 4 wk | Base line | 4 wk | Base line | 4 wk | |
| HR (beat/min) | 439 ± 11 | 473 ± 40 | 453 ± 31 | 451 ± 26 | 475 ± 29 | 492 ± 20 | 422 ± 29 | 447 ± 11 |
| EDD (mm) | 3.86 ± 0.27 | 3.75 ± 0.14 | 3.15 ± 0.27 | 3.37 ± 0.19 | 3.77 ± 0.21 | 3.65 ± 0.16 | 3.43 ± 0.23 | 4.29 ± 0.14* |
| ESD (mm) | 2.25 ± 0.18 | 2.12 ± 0.19 | 1.61 ± 0.25 | 2.10 ± 0.14 | 2.27 ± 0.15 | 2.23 ± 0.10 | 1.81 ± 0.26 | 3.12 ± 0.13* |
| PWT (mm) | 1.05 ± 0.10 | 1.27 ± 0.16 | 1.06 ± 0.05 | 0.99 ± 0.07 | 0.90 ± 0.07 | 1.03 ± 0.14 | 0.92 ± 0.04 | 0.71 ± 0.04* |
| SWT (mm) | 1.07 ± 0.01 | 0.94 ± 0.05 | 1.42 ± 0.14 | 1.32 ± 0.10 | 1.03 ± 0.07 | 0.92 ± 0.04 | 1.31 ± 0.38 | 0.78 ± 0.09* |
| Shortening (%) | 41.8 ± 1.7 | 43.2 ± 4.8 | 50.4 ± 4.6 | 39.3 ± 2.5 | 40.0 ± 1.3 | 36.5 ± 2.0 | 47.8 ± 4.2 | 24.3 ± 4.5* |
| HW/BW (mg/g) | 3.9 ± 0.2 | 4.0 ± 0.2 | 3.8 ± 0.1 | 4.1 ± 0.1 | ||||
| LVW/BW (mg/g) | 2.9 ± 0.3 | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.2 ± 0.1 | ||||
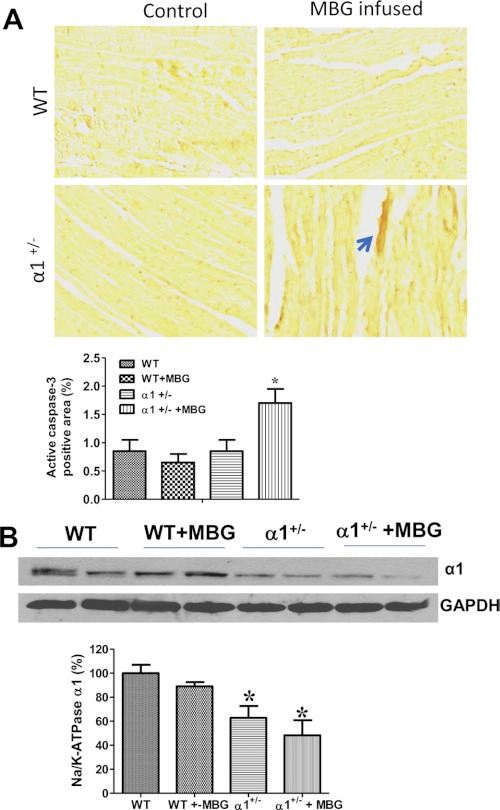
Cardiac myocyte apoptosis has been found to cause decreased wall thickness and increased chamber dilation (26, 27), whereas we have shown that reduction of Na/K-ATPase potentiates cell death in cultured epithelial cells (15). To test whether cardiac myocyte death plays a role in left ventricle dilation and cardiac dysfunction in the Na/K-ATPase α1+/− mice, we first examined the left ventricle slides stained with hematoxylin and eosin (H&E) and found no significant tissue damage or vacuoles in any of the experimental groups. We then further examined whether cardiac myocytes undergo apoptosis in these tissue slides. Although TUNEL assay is a commonly used method for detection of cell apoptosis, it is a demonstration of a late event in the process of cell death and is detectable only for a few hours (28). Thus, we used immunohistochemistry against active caspase 3 as an indicator of cells undergoing apoptosis. As shown in Fig. 1A, WT and α1+/− control mice as well as MBG-infused WT mice exhibit very low positive staining of active caspase 3 (<1% of the total area) in heart tissue, but the positive staining of active caspase 3 more than doubled in MBG-infused α1+/− mice, indicating that Na/K-ATPase reduction potentiates MBG-induced cell apoptosis. Fig. 1B demonstrates that expression of α1 Na/K-ATPase in cardiac tissue homogenates from α1+/− mice was ∼38% lower than that observed in WT control mice, which is consistent with previous observations (11). Infusion of MBG tended to further decrease the expression of Na/K-ATPase α1 but in a manner that was not statistically significant in heart tissues from α1+/− mice (Fig. 1B).
FIGURE 1.
MBG infusion induces caspase 3 activation in α1+/− mice. A, immunohistochemistry of active caspase 3 on left ventricle tissue from control and MBG-infused WT and α1+/− mice. The tissue slides were incubated with anti-active caspase 3 antibody after performing antigen retrieval. They were then incubated with a secondary antibody conjugated with HRP, and the brown color was developed by adding its substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB). The upper panel is a representative image, and the lower panel shows the quantitative data of positive staining using ImageJ analysis. B, Na/K-ATPase α1 expression in left ventricle homogenates from control or MBG-infused WT and α1+/− mice. GAPDH was used as loading control. *, p < 0.05 versus WT control. *, p < 0.05, WT versus α1+/− (n = 6 for each group).
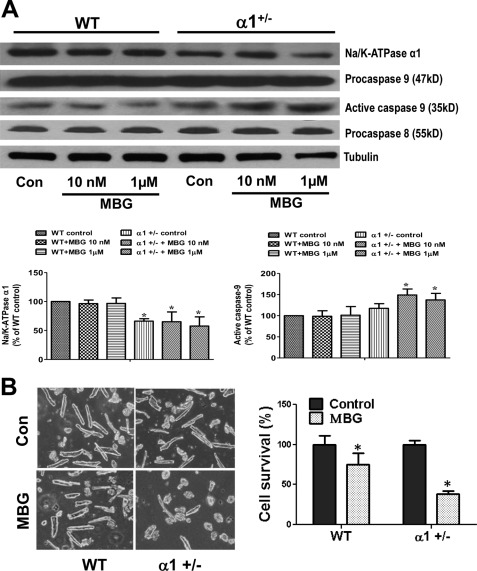
Caspase 3 is one of the executive caspases that can be activated by caspase 8 through the death receptor-initiated apoptotic pathway or by caspase 9 of the mitochondria-involved apoptotic pathway. To delineate the specific pathway in MBG-induced myocyte apoptosis, we isolated adult cardiac myocytes from both WT and α1+/− mice as described under “Experimental Procedures” and treated the cells with MBG for 24 h. After the treatment period, cells were lysed in RIPA buffer, and cell lysates were used to probe for the presence of caspase 8 and caspase 9. The anti-caspase 8 and anti-caspase 9 antibodies recognize both the pro- and active forms of the respective caspases. As shown in Fig. 2A, MBG treatment increased active caspase 9 levels (the 35-kDa band resulted from the cleavage of a 47-kDa procaspase 9) in cells isolated from α1+/− mice but not in cells from WT mice. However, no cleavage of caspase 8 was detected in any of the control or treated groups. To examine whether cardiac myocytes from hearts of α1+/− mice are more sensitive to MBG-induced cell death, we measured cell survival rate of adult cardiac myocytes isolated from wild type and α1+/− mice after treatment with MBG. Live (rod-shaped) and dead (round-shaped) cells were counted under a 20× light microscope. As shown in Fig. 2B, MBG treatment for 24 h resulted in much more cell death in α1+/− myocytes compared with that the level of cell death observed in WT myocytes. Definitive assertions are difficult to assign to the morphological results obtained in these studies. Although observing changes in cell morphology (i.e. round up) in adult myocytes is a sensitive indicator of cell damage, however, it is difficult to identify the specific mechanism of cell death based only on cell counting. When the results are combined with the data detailing the activation of apoptotic pathway components, these results become clearer and suggest that reduction of Na/K-ATPase α1 may potentiate MBG-induced cardiac myocyte apoptosis through activation of caspase 9, although it is hard to exclude the involvement of necrosis or other cell death mechanisms.
FIGURE 2.
Na/K-ATPase reduction potentiates MBG-induced activation of caspase 9 and cardiac cell death. A, cardiac myocytes were isolated from both WT and α1+/− mice and were treated with different concentration of MBG for 24 h. The cell lysates were collected in RIPA buffer to probe for Na/K-ATPase α1, caspase 9, and caspase 8 using Western blot. Tubulin was used for loading control (Con). The 35-kDa band of caspase 9 indicates the activation of procaspase 9 (a 47-kDa protein). B, isolated adult cardiac myocytes were treated with 1 μm MBG for 24 h. Live cells (rod-shaped) and dead cells (round-shaped) were counted under a microscope (20×) for each group. The left panel shows the representative images of adult myocytes of control and MBG-treated cells. The right panel is the quantification data from three different preparations. *, p < 0.05, control versus MBG treatment.
Na/K-ATPase Reduction Attenuates Survival Signaling Pathways in Cardiac Myocytes
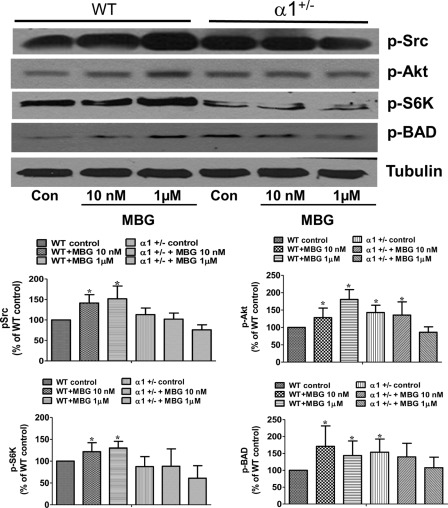
It is reported that activation of the AKT/mTOR signaling pathway protects cells from death by regulating the phosphorylation of BAD (Bcl-2-associated death promoter) and downstream caspase 9 activity (29). To further understand how reduction of Na/K-ATPase potentiates MBG-induced cardiac cell death, we analyzed the activation of the AKT/mTOR pathway in cardiac myocytes isolated from both α1+/− mice and their WT littermates. The isolated adult cardiac myocytes were treated with different concentrations of MBG for 15 min and then assayed for activation of the Src and mTOR signaling pathways using Western blots. As shown in Fig. 3, MBG induces phosphorylation of Src, AKT, and S6K in cardiac myocytes from wild type mice. The activation of S6K also results in an increased phosphorylation of BAD at Ser136, which has been reported to block the activation of the caspase 9 pathway (29). However, in myocytes isolated from α1+/− mice, MBG treatment cannot further phosphorylate S6K and BAD, despite the slightly higher basal phosphorylation levels of these proteins compared with the cells from WT mice, indicating that Na/K-ATPase reduction causes deficiency of cell survival signaling.
FIGURE 3.
Na/K-ATPase reduction attenuates MBG-induced survival signaling in cardiac myocytes. Adult cardiac myocytes were isolated from WT and α1+/− mice and treated with different concentrations of MBG for 15 min. The phosphorylation of Src (p-Src), AKT (p-AKT), p70s6K (p-S6K), and BAD (p-BAD) were probed using Western blot. Tubulin was used as loading control. The upper panel shows the representative Western blots. The lower panel shows the quantification data for p-Src, p-Akt, p-S6K and p-BAD from three different experiments. *, p < 0.05, control (Con) versus MBG treatment.
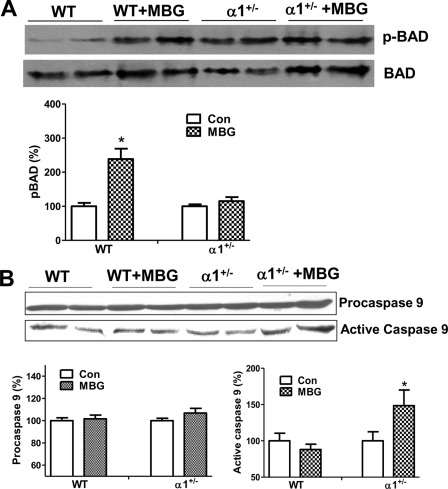
To examine the deficiency of the survival signaling pathways in vivo, we carried out Western blots on left ventricle homogenates to probe for BAD phosphorylation and activation of caspase 9. As shown in Fig. 4A, MBG infusion in WT mice induced phosphorylation of BAD at Ser136 but fails to do so in the α1+/− mice. Meanwhile, MBG-infusion induces the activation of caspase 9 in heart tissue of α1+/− mice but not in that of WT mice (Fig. 4B).
FIGURE 4.
Na/K-ATPase reduction causes MBG-induced caspase activation and attenuates MBG-induced BAD phosphorylation. A, left ventricles from WT and α1+/− mice were homogenized and probed for BAD phosphorylation at Ser136 using Western blot. B, left ventricle homogenates from WT and α1+/− mice were probed for procaspase 9 and active caspase 9. *, p < 0.05, MBG treatment versus control (Con).
Inhibition of mTOR Activation Makes Cardiac Myocytes Vulnerable to MBG Treatment
The above data indicate that the deficiency of the Akt/mTOR signaling pathway in cardiac myocytes of α1+/− mice may potentiate MBG-induced caspase 9 activation. To further assess the role of mTOR activation in MBG-induced apoptosis of cardiac myocytes, we measured the effect of an mTOR inhibitor, rapamycin, on MBG-induced cell death. Neonatal myocytes were employed for this set of experiments because a large number of adult myocytes in culture were annexin V-positive even under control experimental conditions after being cultured for long periods of time.
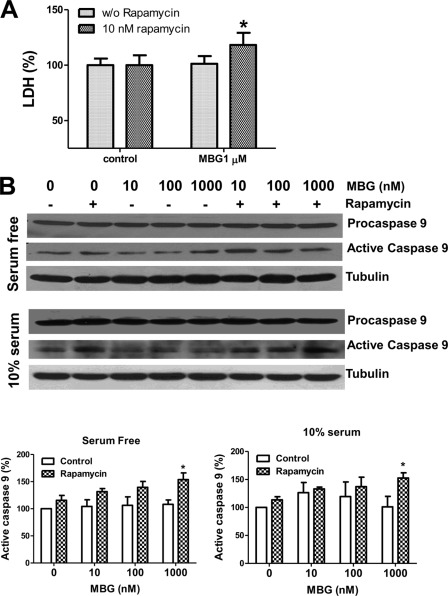
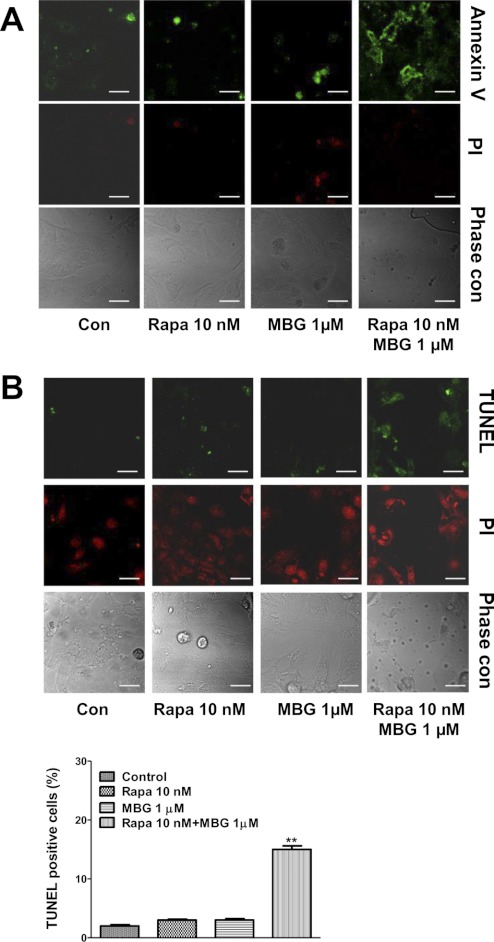
As depicted in Fig. 5A, rat neonatal myocytes were pretreated with rapamycin for 1 h, and then both control and rapamycin-pretreated cells were exposed to MBG for 72 h in serum-free medium. Although MBG alone at 1 μmol/liter did not induce significant cell death, indicated by a lower activity of lactate dehydrogenase in the culture medium, the combination of MBG and rapamycin significantly increased lactate dehydrogenase release (Fig. 5A). To test whether MBG induces cardiac myocyte death via caspase 9-involved apoptosis, rat neonatal cardiac myocytes were treated with MBG alone or in combination with rapamycin for 72 h with or without 10% fatal bovine serum, and cell lysates were collected in RIPA buffer to probe for caspase 9 activation using Western blot. As shown in Fig. 5B, pretreatment with rapamycin potentiated MBG-induced caspase 9 activation. We also cultured neonatal myocytes on coverslips and treated them with MBG alone for 72h or in combination with 10 nm rapamycin as described above. The cells were then subject to annexin V staining or TUNEL assay to detect apoptosis. For the annexin V assays, staining of phosphatidylserine with green fluorescence indicates the integrity of the cell membrane has been compromised, a feature found in apoptosis. The red fluorescence of PI indicates the necrotic or late apoptotic cells. As shown in Fig. 6A, rapamycin treatment potentiated MBG-induced apoptosis but did not induce significant necrosis. Through the use of TUNEL assays, we further confirmed that mTOR pathway inhibition by rapamycin increased the ratio of apoptotic cells as indicated by the green fluorescence staining in the cell nuclei (Fig. 6B). PI was used for counterstaining to calculate the TUNEL-positive cell ratio in the TUNEL assay.
FIGURE 5.
Blocking of the mTOR pathway by rapamycin potentiates MBG-induced caspase 9 activation in rat neonatal cardiac myocytes. Rat neonatal myocytes were serum-starved for 48 h and treated with different concentrations of MBG or MBG combined with 10 nm rapamycin for 72 h. A, lactate dehydrogenase (LDH) measurement using the culture media from control or MBG-treated cells. B, rat neonatal myocytes were treated with MBG alone or in combination with rapamycin in serum-free or 10% fatal bovine serum media. Cell lysates were collected in RIPA buffer for probing of caspase 9. The upper panel shows the representative Western blot of procaspase 9 and active caspase 9. The lower panel shows the quantitative data of active caspase 9 from three different preparations of neonatal cardiac myocytes. *, p < 0.05, MBG-treated cells in the presence of 10 nmol/liter rapamycin versus those in the absence of rapamycin.
FIGURE 6.
MBG induces cardiac myocyte apoptosis in the presence of rapamycin. Rat neonatal cardiac myocytes were serum-starved for 48 h and then treated with MBG 1 μmol/liter alone or in combination with 10 nmol/liter rapamycin (Rapa) for 72 h. Cell apoptosis was assayed by annexin V staining and TUNEL assay. A, a representative image of cell apoptosis measured by annexin V staining. The green fluorescence of annexin V staining on cell membrane indicates early stage apoptotic cells. The red fluorescence of PI indicates necrosis or later stages of apoptosis. B, TUNEL assay. Apoptotic cells are indicated by green fluorescence-stained nuclei. PI was used for counterstaining. Quantification data were determined by the percentage of TUNEL-positive nuclei. **, p < 0.01 versus control. The scale bar represents 20 μm. Con, control.
DISCUSSION
CTS compounds such as digoxin have been used in clinical treatment of heart failure despite the fact that their toxicity is well documented. The Digitalis Investigation Group Trial (30) found that there was a strong correlation between high serum digoxin concentration and increased mortality in congestive heart failure patients. Our current study suggests that the effect of CTS may also depend on changes in the amount of heart tissue Na/K-ATPase, the membrane receptor for CTS. Reduction of Na/K-ATPase in heart tissue potentiates MBG-induced myocyte apoptosis and cardiac dysfunction. Clinically, these potential pathological effects are highly relevant to the therapeutic use of digoxin. Decreases of Na/K-ATPase, which accompany some disease states, may reduce the tolerance of digoxin treatment. The combination of increased CTS levels and decreased Na/K-ATPase content may contribute to the worsened cardiac function observed with these diseases.
Previous studies have documented that CTS can regulate cell growth and cell survival in different cell types (12). Our previous publication (15) and the current study have demonstrated that Na/K-ATPase content is an important regulator of CTS-induced toxicity. Reducing Na/K-ATPase content by itself may not immediately cause cell death, but it potentiates MBG-induced myocyte apoptosis by activation of the caspase 9-regulated proapoptotic pathway. Unfortunately, it is not clear what upstream factors causes activation of caspase 9 in this process. However, one possibility is that long term exposure to MBG may cause an accumulation of ion concentration such as that expected with calcium overload, which can induce the activation of apoptotic pathways (31, 32). However, this may not fully explain the differences in cell apoptosis levels observed in myocytes from WT and Na/K-ATPase α1+/− mice because our previous studies have shown that reduction of Na/K-ATPase content by 60% results in only minimal differences in the 86Rb+ uptake in proximal tubule cells, i.e. Na/K-ATPase ion homeostatic activity (33). On the other hand, our results demonstrate that CTS compounds stimulate the Src/Akt/mTOR pathway, which is known to down-regulate caspase 9 activation and in doing so prevent cell death. We have shown in previous publications (33, 34) that reducing Na/K-ATPase using siRNA or blocking the signaling function of Na/K-ATPase in renal proximal tubule cells potentiates CTS-induced cell death. The current study further supports these findings suggesting that reduction of Na/K-ATPase affects MBG-induced further activation of this survival signaling pathway in cardiac myocytes. Thus, we reasoned that CTS compounds may have a potential to induce cell death pathways, but in normal cells, the death pathway is balanced by the activation of a survival signaling pathway and are therefore more resistant to CTS-induced cell death. Once Na/K-ATPase is reduced, the cell survival and proapoptotic events will become unbalanced and subsequently results in caspase 9 activation and cell apoptosis. The usage of rapamycin further demonstrated that inhibition of mTOR survival signaling pathway is sufficient to potentiate the MBG-induced cardiac myocyte apoptosis.
Despite the finding that signaling events stimulated by CTS play a role in protecting normal cells from apoptosis, we also noticed that reduction of Na/K-ATPase increases the basal level of Src, Akt, and BAD phosphorylation in the myocytes but has no protective effect on cell death or on caspase 9 activation when treated with MBG (Figs. 3 and 4). In in vitro studies, CTS compounds usually induce a transient activation of Src and its downstream signaling components at a time peak of 15–30 min (34, 35), but long term effects in in vivo experiments have not been well studied. Our results indicate that the transient response to MBG treatment seems to be correlated with myocyte survival while the constant increased phosphorylation of these kinases did not protect cells from MBG-induced cell apoptosis. Interestingly, transient activation of ERK promotes cell growth, whereas constant activation of ERK induces cell death (36). However, it is not clear whether a similar mechanism may apply to the kinases studied in our experimental conditions. We also acknowledge that our experiments did not exclude the involvement of other cell death mechanisms such as necrosis or oncosis after MBG treatment. A major problem in identifying these mechanisms is the lack of specific markers for these pathways, and levels of activation are often judged by morphological changes of the cells (37). Because most of our in vitro experiments were conducted in serum-free medium to avoid the confounding effect of other growth factors in serum, they may not fully represent the physiological conditions. However, as shown in Fig. 5B, MBG induces activation of caspase 9 at both serum-free and serum-containing conditions in the presence of rapamycin. Taken together with our in vivo study, we believe that the studies presented here support the relationship between Na/K-ATPase reduction, signaling activation, and cardiac myocyte apoptosis.
The current study also indicates that MBG-induced myocyte apoptosis may contribute to reduced left ventricle wall thickness and chamber dilation in α1+/− mice. Apoptosis may cause myocyte number to decrease, induce side to side slippage, as well as increase lengthening of myocytes within the ventricle wall, resulting in increased ventricle volume (20, 27). However, the decreased wall thickness did not seem to decrease the heart organ weight or its ratio to bodyweight in these mice (Table 1). It is not clear whether the lengthening of individual myocytes compensates for the overall mass loss or if the observed increase in myocyte death is not sufficient to elicit a significant organ weight loss in the time period of our experiment. Further studies are merited to elucidate the phenomena.
In summary, our experimental results suggest that Na/K-ATPase and its specific ligands are involved in the etiology of cardiac dysfunction. The reduction of Na/K-ATPase may serve as a novel mechanism that potentiates CTS-induced cardiac cell apoptosis and dilated cardiomyopathy.
Acknowledgments
We thank Dr. Christopher Drummond for excellent editorial assistance. We also thank Drs. Alexi Bagrov and Olga Fedorova at the National Institute on Aging for providing purified MBG.
This work was supported, in whole or in part, by National Institutes of Health, NHLBI Grants HL-105649, HL-109015, and HL-36573. This work was also supported by American Heart Association Grant 0980027N.
- CTS
- cardiotonic steroids
- MBG
- marinobufagenin
- mTOR
- the mammalian target of rapamycin
- RIPA
- radioimmune precipitation assay
- PI
- propidium iodide
- BAD
- Bcl-2-associated death promoter.
REFERENCES
- 1. Nørgaard A., Bagger J. P., Bjerregaard P., Baandrup U., Kjeldsen K., Thomsen P. E. (1988) Relation of left ventricular function and Na,K-pump concentration in suspected idiopathic dilated cardiomyopathy. Am. J. Cardiol. 61, 1312–1315 [DOI] [PubMed] [Google Scholar]
- 2. Semb S. O., Lunde P. K., Holt E., Tønnessen T., Christensen G., Sejersted O. M. (1998) Reduced myocardial Na+,K+-pump capacity in congestive heart failure following myocardial infarction in rats. J. Mol. Cell Cardiol. 30, 1311–1328 [DOI] [PubMed] [Google Scholar]
- 3. Fraser C. L., Arieff A. I. (2001) Na-K-ATPase activity decreases with aging in female rat brain synaptosomes. Am. J. Physiol. Renal Physiol. 281, F674–678 [DOI] [PubMed] [Google Scholar]
- 4. Kawamoto E. M., Munhoz C. D., Lepsch L. B., de Sá Lima L., Glezer I., Markus R. P., de Silva C. L., Camarini R., Marcourakis T., Scavone C. (2008) Age-related changes in cerebellar phosphatase-1 reduce Na,K-ATPase activity. Neurobiol. Aging 29, 1712–1720 [DOI] [PubMed] [Google Scholar]
- 5. Tirupattur P. R., Ram J. L., Standley P. R., Sowers J. R. (1993) Regulation of Na+,K+-ATPase gene expression by insulin in vascular smooth muscle cells. Am. J. Hypertens. 6, 626–629 [DOI] [PubMed] [Google Scholar]
- 6. Chen S., Yuan C., Clough D., Schooley J., Haddy F. J., Pamnani M. B. (1993) Role of digitalis-like substance in the hypertension of streptozotocin-induced diabetes in reduced renal mass rats. Am. J. Hypertens. 6, 397–406 [DOI] [PubMed] [Google Scholar]
- 7. Clerico A., Giampietro O. (1990) Is the endogenous digitalis-like factor the link between hypertension and metabolic disorders as diabetes mellitus, obesity, and acromegaly? Clin. Physiol. Biochem. 8, 153–168 [PubMed] [Google Scholar]
- 8. Harik S. I., Mitchell M. J., Kalaria R. N. (1989) Ouabain binding in the human brain. Effects of Alzheimer disease and aging. Arch. Neurol. 46, 951–954 [DOI] [PubMed] [Google Scholar]
- 9. Liguri G., Taddei N., Nassi P., Latorraca S., Nediani C., Sorbi S. (1990) Changes in Na+,K+-ATPase, Ca2+-ATPase and some soluble enzymes related to energy metabolism in brains of patients with Alzheimer disease. Neurosci. Lett. 112, 338–342 [DOI] [PubMed] [Google Scholar]
- 10. James P. F., Grupp I. L., Grupp G., Woo A. L., Askew G. R., Croyle M. L., Walsh R. A., Lingrel J. B. (1999) Identification of a specific role for the Na,K-ATPase α2 isoform as a regulator of calcium in the heart. Mol. Cell 3, 555–563 [DOI] [PubMed] [Google Scholar]
- 11. Moseley A. E., Cougnon M. H., Grupp I. L., El Schultz J., Lingrel J. B. (2004) Attenuation of cardiac contractility in Na,K-ATPase α1 isoform-deficient hearts under reduced calcium conditions. J. Mol. Cell Cardiol. 37, 913–919 [DOI] [PubMed] [Google Scholar]
- 12. Schoner W., Scheiner-Bobis G. (2007) Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 293, C509-C536 [DOI] [PubMed] [Google Scholar]
- 13. Aydemir-Koksoy A., Abramowitz J., Allen J. C. (2001) Ouabain-induced signaling and vascular smooth muscle cell proliferation. J. Biol. Chem. 276, 46605–46611 [DOI] [PubMed] [Google Scholar]
- 14. Liu L., Abramowitz J., Askari A., Allen J. C. (2004) Role of caveolae in ouabain-induced proliferation of cultured vascular smooth muscle cells of the synthetic phenotype. Am. J. Physiol. Heart Circ. Physiol. 287, H2173–2182 [DOI] [PubMed] [Google Scholar]
- 15. Tian J., Li X., Liang M., Liu L., Xie J. X., Ye Q., Kometiani P., Tillekeratne M., Jin R., Xie Z. (2009) Changes in sodium pump expression dictate the effects of ouabain on cell growth. J. Biol. Chem. 284, 14921–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komiyama Y., Dong X. H., Nishimura N., Masaki H., Yoshika M., Masuda M., Takahashi H. (2005) A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 38, 36–45 [DOI] [PubMed] [Google Scholar]
- 17. Kashkin V. A., Bagrov A. Y., Fedorova O. V., Bagrov Y. Y., Agalakova N. I., Patkina N. A., Zvartau E. E. (2002) Marinobufagenin (MBG) suppression of ethanol-seeking behavior is associated with inhibition of brain cortex Na/K-ATPase in mice. Eur. Neuropsychopharmacol. 12, 217–223 [DOI] [PubMed] [Google Scholar]
- 18. Hamlyn J. M., Manunta P. (1992) Ouabain, digitalis-like factors and hypertension. J. Hypertens. Suppl. 10, S99–111 [PubMed] [Google Scholar]
- 19. Balzan S., Neglia D., Ghione S., D'Urso G., Baldacchino M. C., Montali U., L'Abbate A. (2001) Increased circulating levels of ouabain-like factor in patients with asymptomatic left ventricular dysfunction. Eur. J. Heart Fail 3, 165–171 [DOI] [PubMed] [Google Scholar]
- 20. Nadal-Ginard B., Kajstura J., Leri A., Anversa P. (2003) Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ. Res. 92, 139–150 [DOI] [PubMed] [Google Scholar]
- 21. Kennedy D. J., Elkareh J., Shidyak A., Shapiro A. P., Smaili S., Mutgi K., Gupta S., Tian J., Morgan E., Khouri S., Cooper C. J., Periyasamy S. M., Xie Z., Malhotra D., Fedorova O. V., Bagrov A. Y., Shapiro J. I. (2008) Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am. J. Physiol. Renal Physiol. 294, F450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Connell T. D., Rodrigo M. C., Simpson P. C. (2007) Isolation and culture of adult mouse cardiac myocytes. Methods Mol. Biol. 357, 271–296 [DOI] [PubMed] [Google Scholar]
- 23. Peng M., Huang L., Xie Z., Huang W. H., Askari A. (1996) Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J. Biol. Chem. 271, 10372–10378 [DOI] [PubMed] [Google Scholar]
- 24. Elkareh J., Kennedy D. J., Yashaswi B., Vetteth S., Shidyak A., Kim E. G. R., Smaili S., Periyasamy S. M., Hariri I. M., Fedorova L., Liu J., Wu L., Kahaleh M. B., Xie Z., Malhotra D., Fedorova O. V., Kashkin V. A., Bagrov A. Y., Shapiro J. I. (2007) Hypertension %R 10.1161/01.HYP. 0000252409.36927.05 49, 215–224 [DOI] [PubMed] [Google Scholar]
- 25. Tian J., Shidyak A., Periyasamy S. M., Haller S., Taleb M., El-Okdi N., Elkareh J., Gupta S., Gohara S., Fedorova O. V., Cooper C. J., Xie Z., Malhotra D., Bagrov A. Y., Shapiro J. I. (2009) Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension 54, 1313–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wencker D., Chandra M., Nguyen K., Miao W., Garantziotis S., Factor S. M., Shirani J., Armstrong R. C., Kitsis R. N. (2003) A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Invest. 111, 1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Q., Li B., Wang X., Leri A., Jana K. P., Liu Y., Kajstura J., Baserga R., Anversa P. (1997) Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. The J. Clin. Invest. 100, 1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haunstetter A., Izumo S. (1998) Apoptosis: Basic mechanisms and implications for cardiovascular disease. Circulation research 82, 1111–1129 [DOI] [PubMed] [Google Scholar]
- 29. Harada H., Andersen J. S., Mann M., Terada N., Korsmeyer S. J. (2001) p70S6 kinase signals cell survival as well as growth, inactivating the proapoptotic molecule BAD. Proc. Natl. Acad. Sci. U.S.A. 98, 9666–9670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rathore S. S., Curtis J. P., Wang Y., Bristow M. R., Krumholz H. M. (2003) Association of serum digoxin concentration and outcomes in patients with heart failure. Jama 289, 871–878 [DOI] [PubMed] [Google Scholar]
- 31. Basset O., Boittin F. X., Cognard C., Constantin B., Ruegg U. T. (2006) Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem. J. 395, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho S. Y., Lee J. H., Bae H. D., Jeong E. M., Jang G. Y., Kim C. W., Shin D. M., Jeon J. H., Kim I. G. (2010) Transglutaminase 2 inhibits apoptosis induced by calcium- overload through down-regulation of Bax. Exp. Mol. Med. 42, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang M., Tian J., Liu L., Pierre S., Liu J., Shapiro J., Xie Z. J. (2007) Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 282, 10585–10593 [DOI] [PubMed] [Google Scholar]
- 34. Liang M., Cai T., Tian J., Qu W., Xie Z. J. (2006) Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J. Biol. Chem. 281, 19709–19719 [DOI] [PubMed] [Google Scholar]
- 35. Haas M., Askari A., Xie Z. (2000) Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 275, 27832–27837 [DOI] [PubMed] [Google Scholar]
- 36. Cheung E. C., Slack R. S. (2004) Emerging role for ERK as a key regulator of neuronal apoptosis. Sci. STKE 2004, PE45. [DOI] [PubMed] [Google Scholar]
- 37. Fink S. L., Cookson B. T. (2005) Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 73, 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]