Background: S6KII (RSK) kinase is important for neuronal plasticity and behavior.
Results: Using genetic approaches, we identified functional S6KII domains relevant for circadian behavior.
Conclusion: ERK binding to S6KII and activation of its C-terminal kinase modulate circadian period whereas the N-terminal kinase is dispensable for circadian function.
Significance: This is the first in vivo structure/function analysis of S6KII (RSK) in the context of behavior.
Keywords: Circadian, ERK, Rsk, S6 Kinase, Signal Transduction, p90 Ribosomal S6 Kinase
Abstract
A detailed structure/function analysis of Drosophila p90 ribosomal S6 kinase (S6KII) or its mammalian homolog RSK has not been performed in the context of neuronal plasticity or behavior. We previously reported that S6KII is required for normal circadian periodicity. Here we report a site-directed mutagenesis of S6KII and analysis of mutants, in vivo, that identifies functional domains and phosphorylation sites critical for the regulation of circadian period. We demonstrate, for the first time, a role for the S6KII C-terminal kinase that is independent of its known role in activation of the N-terminal kinase. Both S6KII C-terminal kinase activity and its ERK-binding domain are required for wild-type circadian period and normal phosphorylation status of the protein. In contrast, the N-terminal kinase of S6KII is dispensable for modulation of circadian period and normal phosphorylation of the protein. We also show that particular sites of S6KII phosphorylation, Ser-515 and Thr-732, are essential for normal circadian behavior. Surprisingly, the phosphorylation of S6KII residues, in vivo, does not follow a strict sequential pattern, as implied by certain cell-based studies of mammalian RSK protein.
Introduction
p90 ribosomal S6 kinase (RSK)2 is a well-conserved serine/threonine kinase and a target of the Ras-mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) signaling cascade (1). RSK was first identified as a kinase that phosphorylates the 40 S ribosomal subunit protein S6 in Xenopus larval extracts (2), although it has since been determined that S6 protein may not be a relevant substrate in vivo (3–5). As with other elements of the Ras-ERK pathway, RSK kinase activity has been linked to a wide range of critical cellular processes including transcriptional and translational regulation, cell proliferation, survival, growth, and cell motility (6), reviewed in Refs. 7, 8.
RSK is unique among serine/threonine kinases in that it contains two distinct and functional kinase domains joined by a linker region (9, 10). The kinases are thought to be sequentially activated by a series of phosphorylation events, beginning with phosphorylation by activated ERK. However, recent studies indicate that kinase activation may occur in the absence of this full sequential series of modifications (11, 12). As the majority of these studies were conducted in vitro, there is insufficient evidence as to whether sequential phosphorylation/kinase activation is required in vivo. The sole known purpose of the RSK carboxyl (C)-terminal kinase is to activate the amino (N)-terminal kinase through autophosphorylation. In contrast, the N-terminal kinase is responsible for phosphorylation of other protein substrates (9, 13, 14). N-terminal kinase activity was thought to be essential for RSK function until a recent study demonstrated an alternative role for RSK in fly eye development as a non-catalytic, scaffolding protein (15).
The mammalian genome encodes four isoforms of RSK (RSK 1–4) while only a single isoform has been described for Drosophila melanogaster (dRSK or S6KII) that has ∼60% amino acid identity with RSK1 (16). Whereas there have been extensive studies of RSK structure and function using mammalian cell-based assays, detailed studies of fly S6KII functional domains have not been reported even though the kinase is known to be important for memory functions and circadian behavior (17–19). The use of Drosophila for such an analysis will permit critical domains and phosphorylation sites of S6KII to be delineated, in vivo, in the context of behavior and plasticity, with obvious relevance for mammalian RSK function.
Even though S6KII is ubiquitously expressed throughout Drosophila development and in all embryonic tissues (15), S6KII null mutant flies are viable (15, 19). Interestingly, S6KII null (S6KIIignorant or S6KIIign) flies exhibit circadian molecular and behavioral phenotypes previously described by our laboratory (17). Specifically, the mutant exhibits a short-period phenotype that appears to result from alteration of the PERIOD (PER)-based circadian oscillator. Based on those and other studies, we have proposed a role for S6KII in modulating the Drosophila circadian molecular oscillator that involves interaction and cooperation with the known clock kinase casein kinase 2 (CK2) (17). Given that S6KII also interacts with numerous other partners in a variety of ERK pathway roles, it is possible that S6KII modulation of oscillator function is controlled by ERK signaling. In addition, it is not known whether S6KII serves as a kinase or alternatively as a scaffolding protein in the circadian system. Finally, we wondered whether the sequence of RSK phosphorylation and kinase activation observed in mammals is relevant, in vivo, for S6KII modulation of fly circadian behavior.
To further understand the role of S6KII in the circadian system, we set out to evaluate the importance of conserved protein domains and phosphorylation sites for kinase regulation of locomotor activity rhythms. We report that S6KII's circadian function does not require its N-terminal kinase activity, similar to findings reported for Drosophila eye development (15). In contrast, C-terminal kinase activity, previously thought to be responsible only for N-terminal kinase activation, is required for normal circadian behavior. Our studies also suggest that ERK binding to and phosphorylation of S6KII threonine 732 (T732) within clock neurons is essential for normal rhythmicity. Whereas S6KII was shown to negatively regulate ERK in the fly eye (15) and at the neuromuscular junction (20), our work indicates that activation of S6KII by ERK is required for modulation of the circadian clock. Further, we show that both ERK binding and C-terminal kinase activity are important for autophosphorylation of S6KII serine 515 (S515) and T732 phosphorylation, whereas phosphorylation at S357, which activates the N-terminal kinase, is not dependent on these activities. Phosphorylation of S6KII S515 or T732 is not required for normal phosphorylation of the protein, but it is required for wild-type circadian behavior. These studies provide novel insights about the function of S6KII, in vivo, and support a model in which ERK regulates S6KII in clock cells, thereby, modulating circadian behavior.
EXPERIMENTAL PROCEDURES
Stock Maintenance and Generation of Fly Strains
Drosophila cultures were reared at 25 °C and 60% relative humidity in an LD 12:12 cycle on a modified standard medium containing wheat germ. For genetic crosses and behavioral experiments, flies were collected using C02 anesthesia.
The UAS-S6KII+ transgenic line was obtained from the Bloomington Drosophila Stock Center. UAS-S6KIIK231R, UAS-S6KIIR902A, UAS-S6KIIK231R/R902A, and UAS-S6KIIK231M/K597M flies were generously donated by J. Chung (KAIST, Korea) and described in (15). Additional S6KII mutants were generated from a pUAST-myc-S6KII construct obtained from Marc Bourouis (University of Nice, France) that was used to create Bloomington's UAS-S6KII+ transgenic line. A portion of the S6KII C terminus containing the C-terminal kinase dead mutation K597M was excised from pUAST-myc-S6KIIK231M/K597M (from J. Chung) using the enzymes SrgAI and BsiWI and cloned into the pUAST-myc-S6KII+ to create pUAST-myc-S6KIIK597M. The QuikChange XL Site-Directed Mutagenesis kit (Stratagene) was used to make pUAST-myc-S6KIIT732A, pUAST-myc-S6KIIT732E, pUAST-myc-S6KIIS515A, and pUAST-myc-S6KIIS515D. Mutagenesis primers were: T732A forward 5′-CGGCCTCCTGATGGCGCCATGCTACAC-3′, T732A reverse 5′-GTGTAGCATGGCGCCATCAGGAGGCCG-3′, T732E forward 5′-CAACGGCCTCCTGATGGAGCCATGCTACACAGCC-3′, T732E reverse 5′-GGCTGTGTAGCATGGCTCCATCAGGAGGCCGTTG-3′, S515A forward 5′-GATCTTCCGCGGGTTCGCCTTTGTGGCTCCTGTC-3′, S515A reverse 5′-GACAGGAGCCACAAAGGCGAACCCGCGGAAGATC-3′, S515D forward 5′-GATCTTCCGCGGGTTCGACTTTGTGGCTCCTGTC-3′, S515D reverse 5′-GACAGGAGCCACAAAGTCGAACCCGCGGAAGATC-3′. Constructs were sequenced to confirm the presence of new mutations. For the T732 transgenes, an S6KII fragment containing the relevant mutation was excised and ligated appropriately into pUAST-myc-S6KII+ due to additional sequence alterations in another region of the mutagenesis construct. The S515 mutations were produced in a ∼3.2 kb S6KII+ fragment within pBluescript II, which was then subcloned into pUAST. Transgenic strains carrying the new mutant constructs were produced by Genetic Services, Inc. (Cambridge, MA).
Analysis of Behavioral Rhythmicity
Locomotor activity data from individual flies were collected using the Drosophila Activity Monitor (DAM) system (Trikinetics, Waltham, MA). Flies were entrained at 20 °C to an LD 12:12 cycle for 4 days and then transferred to constant darkness (DD) at the same temperature for approximately 2 weeks. Our previous work (17) shows that the S6KII mutant phenotype is most severe at 20 °C. To estimate period and visualize actograms, we used a MATLAB (MathWorks)-based signal processing toolbox (21). We employed a time series analysis called autocorrelation to look for periodicity in the activity data and generate a correlogram (with peaks representing harmonics in the data). In accordance with the standard in the field, period was estimated from the third peak of the correlogram. Differences in circadian period were assessed for statistical significance using a nonparametric ANOVA (Kruskal-Wallis Test) with Dunn's Multiple post-hoc comparisons (GraphPad InStat).
Western Analyses
Fly heads were collected and homogenized in 3 volumes of Head Extraction Buffer (50 mm KCl, 10 mm HEPES, 5 mm Tris-HCL, 10% glycerol, 2 mm EDTA, 1% Triton X-100) with 1 mm DTT, 0.4% Nonidet P-40, 0.5 mm PMSF, 10 mm pNPP, and a 1:100 dilution of Halt protease inhibitor mixture (Pierce). Extract buffer for pS6KII blots also contained a 1:10 dilution of PhosSTOP phosphatase inhibitor mixture (Roche). Standard immunoblotting techniques were used to examine protein abundance for S6KII and phosphorylated S6KII (pS6KII). Protein lysates to be examined with antibodies against phosphorylation sites were only heated to 55 °C for 10 min while other proteins were heated to 95 °C for 5 min. To generate gels for blotting, 50 μg or 75 μg of protein per time point were loaded for the detection of S6KII or pS6KII, respectively. The following antibodies were used: guinea pig anti-S6KII, 1:1000 (19); rabbit anti-pSer380-RSK, 1:1000 (Cell Signaling); rabbit anti-pSer221-RSK, 1:1000 (Abcam); rabbit anti-pThr573-RSK, 1:200 (Cell Signaling); rat anti-Drosophila (D) N-Cadherin, 1:500 (Developmental Studies Hybridoma Bank). Bands were visualized using the ECL or ECL Plus Western blotting detection systems (Amersham Biosciences). Band density and background were measured using NIH ImageJ. After background subtraction, total S6KII was normalized to N-Cadherin level to account for differences in loading. pS6KII signal was expressed as a fraction of mean total S6KII amount for each genotype. Quantitative differences in S6KII phosphorylation status and total abundance were evaluated using an ANOVA with Dunnett's post test.
RESULTS
Transgenic Expression of Mutant Proteins for the Assay of S6KII Functional Domains in Vivo
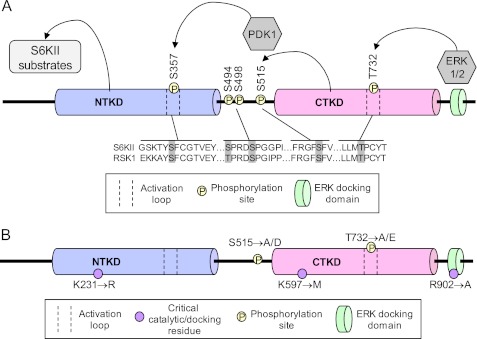
To discern the role S6KII plays in circadian rhythms, we examined the behavioral and molecular effects of mutations that either block the function of a particular conserved domain (kinase or ERK-binding domains) or alter phosphorylation status of specific residues of the protein. Fig. 1A presents a current model for the sequential activation of S6KII, based on studies of mammalian RSK. The indicated phosphorylation sites are conserved in all mammalian RSK isoforms, and in the Drosophila and Caenorhabditis elegans homologs (22). ERK is thought to initiate the sequential phosphorylation of multiple S6KII residues by binding to a specific docking domain at the terminus of the protein, resulting in phosphorylation of residue T732 (and perhaps also S498) (T573 and S363 in RSK, respectively) and subsequent activation of the C-terminal kinase (22–24). The C-terminal kinase autophosphorylates S515 (RSK S380), creating a binding site for PDK1, which then docks and phosphorylates S6KII S357 (RSK S221), activating the N-terminal kinase domain and enabling the phosphorylation of S6KII target substrates (14, 25–27).
FIGURE 1.
S6KII domains and phosphorylation sites that may play a role in its circadian function. A, composition of the fly S6KII protein. Two kinase domains, the N-terminal kinase domain (NTKD) and the C-terminal kinase domain (CTKD), are joined by a linker region. S6KII also contains an ERK1/2-binding domain at its C terminus. Schematic depicts the series of events thought to lead to S6KII kinase activation (right to left), beginning with ERK binding to the C terminus and phosphorylation of T732. Arrows indicate phosphorylation events. Homology between S6KII and human RSK1 phosphorylation sites is shown. B, point mutations generated to disrupt N-terminal kinase activity (lysine, K to arginine, R), C-terminal kinase activity (lysine, K to methionine, M) and ERK-binding (arginine, R to alanine, A) are shown below the S6KII protein schematic. Serine and threonine phosphorylation sites mutated to be pseudophosphorylated (aspartate, D or glutamate, E) or unphosphorylatable (alanine, A) are depicted above the schematic.
We utilized multiple site-directed mutants of S6KII, summarized in Fig. 1B, to determine which conserved domains and phosphorylation events are required for the protein modulation of circadian oscillator function. All mutant S6KII transgenes carried single amino acid changes that were produced in vitro (see “Experimental Procedures”).
An N-terminal kinase-dead mutant called S6KIIK231R carries a Lys-231 to Arg change while C-terminal kinase-dead S6KIIK597M contains a Lys-597 to Met alteration (Fig. 1B). Arg-902 was mutated to Ala to obtain an ERK binding-deficient allele (S6KIIR902A). It was previously demonstrated that S6KIIK231R and S6KIIK597M are kinase-dead alleles, based on labeled phosphate incorporation into the S6 ribosomal protein, a known substrate of S6KII, and that S6KIIR902A lacks ERK-binding activity (15). The S6KIIK231R/R902A line contains a combination of the K231R (N-terminal kinase-dead) and R902A (ERK binding-deficient) mutations in the same S6KII transgene. Pseudo-phosphorylated (S6KIIT732E) or unphosphorylatable (S6KIIT732A) mutants of S6KII were generated in our laboratory by Thr to Glu and Thr to Ala changes, respectively. Similarly, we generated pseudo-phosphorylated (S6KIIS515D) or unphosphorylatable (S6KIIS515A) S515 mutants by Thr to Asp and Thr to Ala changes, respectively (Fig. 1B). Mutant proteins were expressed specifically in clock cells, for immunochemical and behavioral studies, using the Gal4/UAS binary expression system (28). A summary of all S6KII mutants employed in this study with abbreviations is shown in supplemental Table S2.
Mutant Forms of S6KII Can Be Detected in Head Tissues and Clock Neurons of the Fly Brain
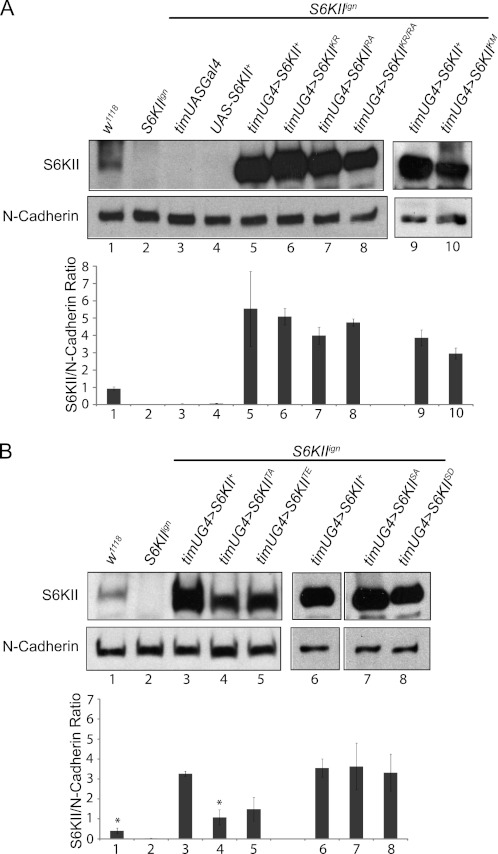
Prior to behavioral testing, we verified that mutant S6KII proteins could be detected in whole head or brain tissues. Detection of proteins was accomplished using an antibody specific for fly S6KII (19). To distinguish transgene-encoded S6KII from the endogenous protein, we performed immunoblotting experiments in an S6KII protein-null background using Df(1)ignΔ58/1, a deletion of the S6KII gene (hereafter referred to as S6KIIign) (19). As expected, S6KII protein was detected in head lysates from S6KII+ genetic background control flies (w1118), but not in lysates from S6KIIign mutants (Fig. 2A). Importantly, control lysates from flies, which carried only the Gal4 driver (S6KIIign;timUASGal4) or the UAS responder transgene (S6KIIign;UAS-S6KII+) did not exhibit detectable S6KII protein. However, transgene-encoded wild-type and domain mutant (K231R, R902A, or K231R/R902A) S6KII proteins, when expressed using timUASGal4, were easily detected in an S6KIIign background, at higher than endogenous levels in head lysates. S6KII S515 phosphorylation-mutant proteins (S515A, S515D) were also detected at comparable, high levels, and not significantly different from the transgene-encoded wild-type S6KII+ (Fig. 2B). Using two different antibodies, T732 phosphorylation-mutant proteins (T732A, T732E) were detected at levels higher than endogenous S6KII (see w1118 control), but less than that observed with timUASGal4-driven S6KII+ expression (Fig. 2B and data not shown). We note, however, that only T732A is statistically different from the S6KII+ control (Fig. 2B). Reduced abundance may be caused by genomic position effects on transgene expression or reduced stability of the mutant proteins. Nonetheless, S6KIIT732A and S6KIIT732E proteins are expressed at a level higher than endogenous S6KII, which we know is sufficient for normal circadian behavior (17).
FIGURE 2.
Transgenes carrying site-directed mutations express in vivo. A, S6KII proteins with mutations of specific domains can be detected when UAS transgenes are expressed within clock cells by timUAS-Gal4 in an S6KIIign-null background. S6KII is absent in S6KIIign and in the Gal4- or UAS- alone genetic controls. Expression of transgenes does not occur without the presence of a Gal4 driver (data not shown). Transgene abbreviations: K231R (KR), R902A (RA), K231R/R902A (KR/RA), K597M (Km). B, S6KII phosphorylation site mutant proteins can also be detected when expressed within clock cells using timUAS-Gal4. N-cadherin was employed as a loading control. Transgene abbreviations: S515A (SA), S515D (S.D.), T732A (TA), T732E (TE). All blots are representative of at least two independent experiments which are quantified in the histograms (mean ± range) at the bottom of each panel. Numbers below the histograms correspond to the numbered lanes on the blots. *, p = 0.005 compared with S6KII+.
The pdfGal4 driver expresses in 16 critical pacemaker neurons (a subset of the TIM-containing clock cells) and was utilized in certain behavioral experiments. Using immunoblotting techniques, we failed to detect S6KII protein in head extracts of pdfGal4>UAS-S6KII transgenic flies. Thus, immunostaining procedures were employed to detect the S6KIIK597M, S6KIIT732A and S6KIIT732E mutant proteins in these pacemaker neurons. Using whole mounts of the brain (supplemental Fig. S1), both wild-type and mutant S6KII proteins (K597M, T732A, T732E) were detected in the PDF neurons, although the level of expression varied slightly among strains. Nevertheless, studies described below (Figs. 3 and 5) demonstrated that certain mutant proteins can rescue behavior of S6KIIign flies even when expressed at levels lower than that of transgene-encoded wild-type protein. In addition, a very high level of wild-type protein did not cause behavioral alterations (Fig. 3; Ref. 17), indicating that S6KII abundance is not limiting for circadian behavior.
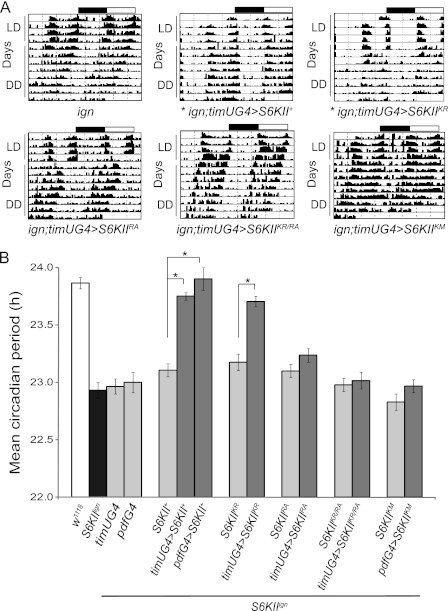
FIGURE 3.
N-terminal kinase-dead S6KII (K231R) rescues the S6KIIign short-period phenotype while C-terminal kinase-dead (K597M) and ERK binding-deficient S6KII (R902A) do not. A, mutant S6KII transgene expression was driven in clock cells. Panels show representative actograms from single flies of the indicated genotypes. Each genotype was examined in three independent experiments. The black and white rectangles above the actograms represent the light/dark cycles. Flies were entrained to 12 h light: 12 h dark (LD) for 4 days and then maintained in constant darkness (DD) for a period of 8–14 days (first 8 days are shown). timUG4 indicates the timUAS-Gal4 driver; pdfG4 indicates the pdf-Gal4 driver. Transgene abbreviations: K231R (KR), R902A (RA), K231R/R902A (KR/RA), K597M (Km). * signifies genotypes with significant rescue of the short-period phenotype. Results for other control genotypes are shown in supplemental Table S1. B, histograms show mean circadian period (h) ± S.E. for three independent experiments. *, p < 0.001 compared with control.
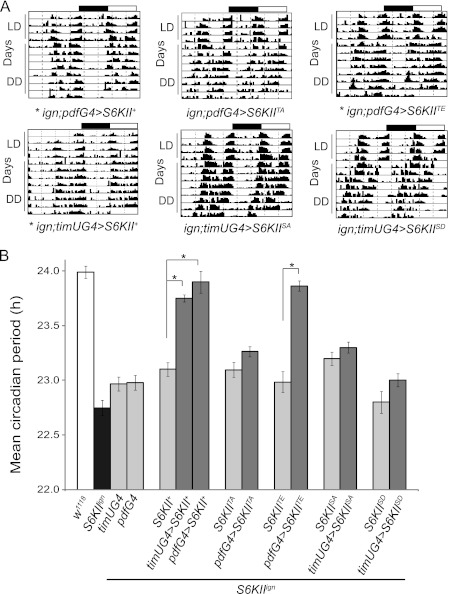
FIGURE 5.
Pseudo-phosphorylated S6KII (TE) rescues the S6KIIign short period phenotype while unphosphorylatable S6KII (TA), unphosphorylated S6KII (SA), and pseudophosphorylated S6KII (SD) do not. A, mutant S6KII transgene expression was driven in the clock (PDF or TIM-containing) cells. Panels show representative actograms from single flies of the indicated genotypes. Each genotype was examined in three independent experiments. Flies were entrained to 12 h light: 12 h dark (LD) for 4 days and then maintained in constant darkness (DD) for a period of 8–12 days (first 8 days are shown). timUG4 indicates the timUAS-Gal4 driver; pdfG4 indicates the pdf-Gal4 driver. Transgene abbreviations: S515A (SA), S515D (SD), T732A (TA), T732E (TE). A * signifies genotypes with significant rescue of the short-period phenotype. Results for other control genotypes are shown in supplemental Table S1. B, histogram shows mean circadian period (h) ± S.E. for three independent experiments. *, p < 0.001.
S6KII N-terminal Kinase Activity Is Dispensable for Circadian Behavior
Cell-based assays suggest that mammalian RSKs function primarily as kinases while in Drosophila S6KII enzymatic activity is thought to be dispensable for the protein function in eye and wing development (15). To establish whether S6KII kinase activities are relevant for the modulation of circadian period, we determined if expression of various mutant S6KII proteins in clock cells was sufficient to rescue the S6KIIign short-period locomotor-activity phenotype (Fig. 3, A and B; supplemental Table S1). The short period phenotype of S6KIIign was described in detail in a previous publication from our laboratory (17). Briefly, the wild-type period for the locomotor activity rhythm is ∼24.0 h whereas S6KIIign mutants have an average period of ∼22.8 h. As shown previously (17) and in Fig. 3, expression of UAS-S6KII+ in TIM-containing clock cells (timUASGal4) or PDF neurons (pdfGal4) is able to completely rescue the S6KIIign short-period phenotype (Fig. 3, A and B, supplemental Table S1). Similarly, timUASGal4-driven expression of S6KIIK231R, an N-terminal kinase-dead allele, restored normal circadian period to S6KIIign mutants, demonstrating that this kinase domain is not required for circadian behavior (Fig. 3, A and B, supplemental Table S1).
S6KII C-terminal Kinase Activity Is Required for Normal Circadian Period
We next asked whether C-terminal kinase activity is required for modulation of circadian behavior using expression of the S6KIIK597M mutant transgene. As shown in Fig. 3, A and B and supplemental Table S1, expression of the S6KIIK597M mutant in PDF neurons of S6KIIign flies did not significantly rescue circadian period, i.e. circadian periods were similar in S6KIIign;pdf-Gal4>UAS-S6KIIK597M flies and control mutants carrying only the UAS transgene. We note, however, that activity bouts are longer in flies expressing the K597M mutant, relative to S6KII+ expression, although we do not know the cause of this effect. Nonetheless, there is no difference in percent rhythmicity (not shown) or circadian period (supplemental Table S1) between the genotypes. Lack of rescue was also observed for a mutant in which both N-terminal and C-terminal kinase activities are eliminated (29), presumably due to lack of C-terminal kinase activity. These results demonstrate that C-terminal kinase activity is important for the circadian function of S6KII, and they represent the first link between the S6KII C-terminal kinase and behavior.
ERK Binding to S6KII Is Required for Normal Circadian Behavior
ERK binding to S6KII is essential for Drosophila eye and wing development (15). Thus we asked whether the same is true for circadian behavior. As shown in Fig. 3, A and B, and supplemental Table S1, expression of an ERK-binding mutant (S6KIIR902A) in TIM-containing clock cells did not rescue mutant behavior; i.e. circadian period of S6KIIign; timUASGal4>S6KIIR902A flies is similar to that of mutants carrying only the UAS transgene (the genetic background control). This implies that the ability of S6KII to bind to ERK is important for its circadian function. We have also shown that a double S6KII mutant (S6KIIK231R/R902A) does not rescue the short-period phenotype, attributable to its ERK-binding deficit, as N-terminal kinase activity is not required for normal circadian function.
S515 Phosphorylation Correlates with S6KII Rescue of Short Period
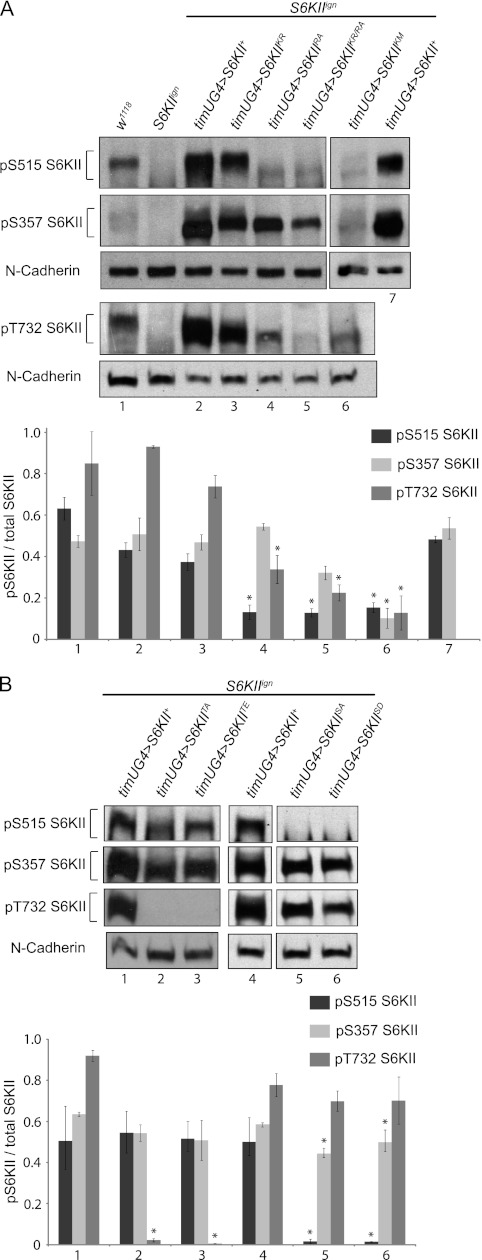
In mammals, RSK T732, S515, and S357 are sequentially phosphorylated in a cascade of phosphorylation events that involve ERK and the C-terminal kinase domain (see Fig. 1A) (9, 13, 14). To determine which domains might regulate S6KII phosphorylation, we employed anti-phospho-RSK (pRSK) antibodies to monitor phosphorylation at several different residues. As these commercially available pRSK antibodies were generated against the mammalian protein, we validated the specificity of the serine antibodies for fly phospho-S6KII (pS6KII) (supplemental Fig. S2). Using these anti-phospho-RSK antibodies, S6KII pT732, pS515, and pS357 were examined in S6KIIign flies that expressed wild-type or mutant isoforms of the protein. pS6KII signal was normalized to total S6KII levels for wild type and the mutants to account for possible differences in S6KII abundance. Although ERK is also known to phosphorylate T359/S363 of RSK (22), there are no anti-phospho antibodies that recognize the homologous residues of fly S6KII (S494/S498).
As shown in Fig. 4A, there was robust phosphorylation of residue S515 on the S6KII+ and S6KIIK231R proteins. For mutant proteins that did not rescue circadian period (S6KIIR902A, S6KIIK231R/R902A, and S6KIIK597M) we observed significantly decreased levels of pS515 immunoreactivity. Decreased pS515 signal in the S6KIIK597M mutant strongly suggests that the C-terminal kinase autophosphorylates at this site, as in mammals. A similar result for S6KIIR902A and S6KIIK231R/R902A mutants indicates that ERK binding to S6KII is an important regulator of autophosphorylation. Interestingly, however, the pS515 proteins in these mutants exhibited an apparent mobility shift (migrating faster), and this might be due to reduced overall phosphorylation of the S6KII protein. Analogous to the model for RSK, ERK binding may initiate a series of events that leads to autophosphorylation at S515.
FIGURE 4.
S6KII kinase activity and ERK-binding are required for phosphorylation of the protein. A, blot containing S6KII K231R, R902A, and K231R/R902A protein, probed with antibodies against mammalian pS380 RSK (fly pS515 S6KII), mammalian pS221 RSK (fly pS357 S6KII) and N-cadherin as a loading control. A separate blot with S6KII K597M protein was similarly probed. A third blot with proteins from all domain mutants was probed with an antibody against mammalian pT732 RSK (fly pT573 S6KII). Variations in the amount of total phosphorylation on the S6KII protein cause the mutants to have different electrophoretic mobilities. Transgene abbreviations: K231R (KR), R902A (RA), K231R/R902A (KR/RA), K597M (Km). B, blots containing the phosphorylation-site mutant proteins were probed sequentially with all three phospho-specific antibodies and anti-N-cadherin. The first three lanes (+, TA, TE) are imaged from one blot while the last three lanes (+, SA, SD) are all from a second blot. Transgene abbreviations: S515A (SA), S515D (SD), T732A (TA), T732E (TE). timUG4 indicates the timUAS-Gal4 driver. Histograms in A and B represent phospho signal normalized to protein abundance for the S6KII isoform (mean ± range). Numbers below the histograms in panels A and B correspond to the numbered lanes on the blots above. A star (*) represents a significant difference versus S6KII+. RA, KRRA (pS515), p = 0.02; KM (pS515), p = 0.008; SA, S.D. (pS515), p = 0.001; KM (pS357), p = 0.03); SA, S.D. (pS357), p = 0.01; RA, KRRA, KM, TA, TE (pT732), p = 0.001. All blots are representative of at least two independent experiments that yielded similar results.
T732 Phosphorylation Also Correlates with Ability to Rescue Circadian Period
S6KII T732 is homologous to the site within the C-terminal region of mammalian RSK that is phosphorylated by ERK (23, 24). Both S6KII+ and S6KIIK231R rescued circadian period and also displayed high levels of pT732 immunoreactivity (Fig. 4A). On the contrary, very low levels of pT732 immunoreactivity were observed for mutant proteins that did not rescue behavior (S6KIIR902A, S6KIIK231R/R902A, and S6KIIK597M). Lower pT732 signal in the S6KIIR902A and S6KIIK231R/R902A mutant lines suggests that ERK binding is required for wild-type levels of phosphorylation at this site. While T732 phosphorylation is thought to be “upstream” of C-terminal kinase activation (see model in Fig. 1A), low levels of pT732 on S6KIIK597M suggests that phosphorylation of this residue is dependent, at least in part, on C-terminal kinase activation.
Phosphorylation of S357 Is Not Altered by S6KII Mutations Affecting ERK Binding or N-terminal Kinase Activity, but Is Dependent upon C-terminal Kinase Activity
Based on the RSK activation model, phosphorylation of S6KII S515 modulates “downstream” phosphorylation events including pS357, a postulated target of the PDK1 kinase (26, 27). PDK1 phosphorylation is thought to be the last step in the activation of the N-terminal kinase. To determine if phosphorylation of S357 is altered by the S6KIIR902A, S6KIIK231R/R902A, and S6KIIK597M mutations, we stripped and reprobed the pS515 blots shown in Fig. 4, A and B with anti-pS357 antibody. Wild-type and mutant S6KII proteins all had substantial (and not significantly different) immunoreactive signals, relative to total S6KII, indicative of phosphorylation at S357, with the exception of S6KIIK597M (Fig. 4A). Thus, a deficiency in ERK binding does not affect phosphorylation of S357. However, S357 phosphorylation is dependent upon upstream C-terminal kinase activity, as evidenced by the low pS357 immunoreactivity in S6KIIK597M. Given that pS357 levels do not correlate with ability to rescue, we suggest that S357 phosphorylation and the subsequent activation of S6KII N-terminal kinase is not critical for the modulation of circadian period.
Phosphorylation of S6KII T732 Is Essential for Rescue of Circadian Behavior
Given that ERK binding is important for S6KII circadian function, and pT732 levels in the domain mutants correlate with circadian period rescue, we wondered whether S6KII phosphorylation by ERK directly affects circadian behavior. We mutated the ERK phosphorylation site (T732) on S6KII to create a pseudo-phosphorylated mutant (S6KIIT732E) and an unphosphorylatable mutant (S6KIIT732A). UAS transgenes encoding these proteins were expressed in the PDF clock cells of the S6KIIign mutant.
We observed that S6KIIT732E fully rescued the S6KIIign short period phenotype while S6KIIT732A did not (Fig. 5, A and B, supplemental Table S1; compare both to their respective UAS transgene controls). Thus, phosphorylation at T732 appears to be required for rescue of the S6KIIign circadian phenotype. We examined pT732 immunoreactivity on the S6KIIT732A and S6KIIT732E proteins with the intent of verifying the presence or absence of phospho-signal in flies expressing the mutant proteins. However, pT732 signal could not be detected on either mutant protein (Fig. 4B), suggesting that the amino acid change rendered the epitope unrecognizable to the phosphospecific antibody. Nevertheless, opposite effects of the two mutations on circadian behavior suggest that S6KIIT732E likely functions as a pseudo-phosphorylated protein. We had previously wondered whether ERK binding and phosphorylation of S6KII was under circadian control, yet rescue by the constitutively pseudo-phosphorylated S6KIIT732E suggests that phosphorylation at this site need not be rhythmic for the modulation of circadian behavior. These experiments further implicate ERK binding and phosphorylation at S6KII residue T732 in clock function.
Rescue of Circadian Behavior Requires Phosphorylation of S6KII S515
The requirement of a functional S6KII C-terminal kinase for rescue of circadian period motivated us to manipulate phosphorylation at the C-terminal autophosphorylation site. Thus, we created pseudo-phosphorylated (S6KIIS515D) and unphosphorylatable (S6KIIS515A) mutants of this residue. When expressed under control of timUASGal4, a UAS transgene encoding S6KIIS515A (S6KIIign; timUASGal4>S6KIIS515A) and control (S6KIIign; S6KIIS515A) flies had similar average periods (Fig. 5, A and B, supplemental Table S1), although period was slightly longer than flies carrying only S6KIIign (we attribute this period lengthening to an insertional effect of the UAS transgene). Thus, we conclude that clock cell expression of S6KIIS515A does not rescue mutant behavior. Indeed, neither did expression of S6KIIS515D, which we thought might behave as a pseudo-phosphorylated protein. Most likely, the mutant aspartate on S6KIIS515D does not mimic a phosphorylated residue as intended (Unlike replacement of T732 with glutamate which seemed to better mimic a pseudo-phosphorylated state). An antibody against pS515 did not recognize either S6KIIS515A or S6KIIS515D, similar to our attempt to detect pT732 in mutant T732 flies (Fig. 4B). Nonetheless, the inability of S6KIIS515A to rescue circadian period, suggests that phosphorylation of S6KII S515 is required for normal circadian behavior, and this result supports a role for autophosphorylation of this site by the C-terminal kinase.
Neither S515 nor S357 Phosphorylation Is Greatly Altered by Upstream Phosphorylation Events while pT732 Is Unaffected by Downstream S515 Phosphorylation
To ask about the interdependence of S6KII phosphorylation events, we examined the phosphorylation status of T732A, T732E, S515A, and S515D mutant proteins in vivo. T732 mutant (T732A or T732E) and S515 mutant (S515A or S515D) proteins all exhibited high levels of pS357 when signals were normalized to S6KII abundance (Figs. 4B and 2B). However, the S515A and S515D mutants had slightly but significantly decreased signal at this residue relative to S6KII+ (Fig. 4B). The presence of high levels of pS357 in the T732 and S515 mutants argues that phosphorylation of the latter residues does not contribute significantly to modification of S357. pT732 levels are similar to S6KII+ in both the unphosphorylatable S515A and pseudo-phosphorylated S515D mutants, indicating that T732 phosphorylation is independent of or upstream in the cascade leading to S515 phosphorylation (Fig. 4B). The unphosphorylatable T732A and pseudo-phosphorylated T732E proteins both exhibit levels of pS515 that are comparable to S6KII+ (Figs. 4B). Thus, while phosphorylation of T732 and S515 are both required for normal circadian behavior (Fig. 5, A and B), modification of S515 alone is not sufficient for rescue (i.e. in the absence of T732 phosphorylation).
Altogether, these immunoblotting experiments indicate that ERK binding is required for normal phosphorylation of S515, although phosphorylation of T732 (presumably by ERK) is not essential for downstream events of the cascade. Similarly, C-terminal kinase activity is necessary for robust phosphorylation of S357 while phosphorylation at S515 (regulated by the C-terminal kinase as demonstrated in Fig. 4A) is not required. Hence, the C-terminal catalytic and ERK-binding domains play a major role in regulating S6KII protein phosphorylation while the influence of the individual phosphorylation sites on each other's status is negligible (see summary in supplemental Table S2).
DISCUSSION
This study utilized wild-type and mutant forms of S6KII in genetic rescue experiments to identify domains that are critical for the protein's function in circadian behavior. To our knowledge, it represents the first study to identify domains of S6KII (RSK) that are required, in vivo, for a behavioral function. Although in many cases S6KII isoforms were expressed at higher than normal levels in transgenic flies, we do not think our results can be attributed to overexpression of the protein. Expression of wild-type S6KII at high levels has no discernable effects on circadian behavior or the phosphorylation pattern of S6KII. For example, as discussed below, high level expression of a C-terminal kinase-dead mutant (S6KIIKm) does not rescue behavior nor phosphorylation defects observed at several sites including S357, a postulated PDK1 docking site within the N kinase domain. We recognize, however, that there may be effects of S6KII overexpression that are not discernable in our molecular and behavioral assays.
The C-terminal Kinase of S6KII Is Required for Normal Circadian Behavior while N-terminal Kinase Activity Is Dispensable
In agreement with a previous study of fly development (15), we show that the S6KII N-terminal kinase is dispensable for its circadian function. This result contrasts with previous studies showing that RSK functions in the Ras/MAPK pathway as a kinase (1); it suggests that phosphorylation of downstream targets by the N-terminal kinase is not essential for modulation of the circadian clock. In support of a non-critical role for the N-terminal kinase, certain mutants that fail to rescue behavior nonetheless exhibit phosphorylation of S357, an event thought to activate RSK kinase activity. In addition, an S6KII mutant (S6KIIignΔ24−3) missing a large portion of the N-terminal region, including the N-terminal kinase, was shown to partially rescue an S6KII-null mutant in a previous study (30).
In contrast, our studies emphasize the importance of S6KII C-terminal kinase activity for modulation of the Drosophila circadian clock. This is the first evidence, in either vertebrate or invertebrate systems, of a function for the S6KII C-terminal kinase that is independent of activation of the N-terminal kinase. It is also the first direct link between the C-terminal kinase and behavior. Heretofore, the only known function of the RSK C-terminal kinase was autophosphorylation, which leads to activation of the N-terminal kinase (9, 13, 14). Our results suggest that either autophosphorylation serves an independent purpose (such as altering protein-protein interactions) or that the C-terminal kinase phosphorylates other proteins.
We show that the C-terminal kinase promotes phosphorylation of S515, a presumed autophosphorylation site and a residue within the hydrophobic motif site of AGC-type kinases. This region is important for stabilization of the catalytic domain of such kinases, including RSK, cAMP-dependent kinase and protein kinase C. We note that there is residual pS515 signal in a S6KII C-terminal kinase-dead mutant (S6KIIK597M) which may indicate that other kinases phosphorylate the site or that the mutant retains an undetectable amount of activity.
Our work also suggests that S6KII S357 and T732 phosphorylation events are modulated by the C-terminal kinase. The N-terminal kinase is dispensable for circadian regulation, but nevertheless our data suggests that it is activated by the C-terminal kinase via phosphorylation of S357; in agreement with cell-based studies of RSK. The C-terminal kinase may promote S357 phosphorylation through recruitment of PDK1 or another factor. While the mechanism for C-terminal kinase modulation of T732 phosphorylation is unknown, it is possible that kinase activity that is stimulated by ERK binding feeds back to activate ERK phosphorylation of T732. This and other alterations may also involve the actions of phosphatases as there is undoubtedly a dynamic interplay between the two types of modifying enzymes. Of interest, pS515 levels are not altered in the T732A/T732E mutants, indicating that T732 phosphorylation is not a prerequisite for S515 phosphorylation.
There is a positive correlation between S6KII variants that rescue S6KIIign mutant behavior and robust phosphorylation of S515; this suggests that phosphorylation of this residue is essential for normal circadian behavior. We note, however, that while S515 phosphorylation is correlated with rhythmicity, it is not sufficient for normal circadian behavior. Therefore, pS515 may simply be indicative of a functional C-terminal kinase whose kinase activity is necessary for modulating circadian behavior via phosphorylation of other unknown targets. In addition, phosphorylation of this residue does not affect the phosphorylation of other S6KII domains; instead C-terminal kinase activity and modification of S515 may serve to alter S6KII protein conformation and relevant protein-protein interactions. While we show that C-terminal kinase activity is important for rhythmicity, our experiments do not exclude the idea that S6KII functions as a scaffold in the circadian system, analogous to its role in Drosophila eye and wing development (15).
ERK Binding to and Phosphorylation of S6KII Modulates Circadian Function
We have shown that ERK binding to S6KII is required for transgenic rescue of circadian behavior, as it is for rescue of Drosophila eye development phenotypes (15). Consistent with a role for ERK in this pathway, we show that phosphorylation of S6KII at T732 (a known ERK phosphorylation site on RSK) is required for rescue of behavioral rhythms. ERK phosphorylation of T732, previously demonstrated for RSK in mammalian cell-based assays, was verified in the fly by the observation that pT732 is reduced in ERK binding-deficient mutants. ERK binding may promote S6KII function by facilitating activation of the C-terminal kinase, as evidenced by the decreased autophosphorylation of ERK-binding mutants. Alternatively, ERK binding may alter S6KII localization and/or binding to clock-related proteins such as CK2, similar to S6KII's regulation of ERK in fly eye development. Whatever the precise mechanism, phosphorylation of T732 and S515 are likely to be important for ERK's interaction with S6KII and the regulation of circadian period.
It was demonstrated in fly photoreceptor cells that S6KII negatively regulates ERK by retaining it in the cytoplasm. Using immunostaining procedures, however, we have shown that the localization pattern of ERK and diphosphorylated (activated) ERK are the same within PDF clock neurons (primarily cytoplasmic) in wild-type flies (w1118), S6KIIign-null mutants, and ERK-binding mutants (S6KIIign;timUG4>S6KIIR902A) (29). Hence, ERK may bind to and activate S6KII in clock cells, but there is no evidence that S6KII regulates ERK localization in this cell type.
Elements of Sequential Phosphorylation/Activation Occur in Vivo, but May Not Be Essential
RSK protein is thought to be activated by a sequence of protein binding and phosphorylation events, based on cell-based investigations of the protein. More recent cell-based assays question the validity of this model (12, 31, 32) and give added relevance to our studies as the first to examine this model in vivo.
We provide the first evidence that phosphorylation/activation of Drosophila S6KII can occur in the absence of a strict sequence of binding and phosphorylation events, but we note that there is some dependence of certain events on others. Although there is extremely low pS515 immunoreactivity in C-terminal kinase and ERK-binding mutants, indicating that S515 phosphorylation is a downstream event, there is residual phospho-signal on this residue in such flies. Thus, ERK binding and C-terminal kinase activation may not be the only events contributing to S515 phosphorylation. Consistent with this idea, in vitro analysis of mammalian RSK has demonstrated that S380 phosphorylation (S515 in S6KII) and C-terminal kinase activation can occur in the absence of RSK-ERK interactions (12). Similarly, ERK binding and C-terminal kinase activity are not the only contributors to S6KII T732 phosphorylation because residual pT732 signal exists in mutants lacking these functions. Our results also indicate that neither ERK binding nor phosphorylation at S515 or T732 is essential for phosphorylation S357 although C-terminal kinase activity influences this event. This result is in agreement with mammalian cell-based studies demonstrating that N-terminal kinase activation is not fully dependent upon C-terminal kinase activity (31, 32). Altogether, ERK-binding and C-terminal catalytic activity appear to play an important role in regulating phosphorylation of the S6KII protein, but the phosphorylation of individual sites is not absolutely required for the downstream phosphorylation of others.
Model for Circadian Clock Modulation
Our previous work indicated that S6KII modulates circadian function by negatively regulating the activity of the clock kinase CK2, via physical interaction with the CK2β subunit (17). Thus, it is possible that a prerequisite for the S6KII-CK2 interaction is activation of the S6KII C-terminal kinase or a conformational change in the protein resulting from ERK binding. CK2β may be a phosphorylation target of the S6KII C-terminal kinase (although there is no evidence of this), and this would provide a mechanism by which S6KII could regulate CK2 activity. Alternatively, a change in S6KII conformation might regulate interaction with CK2, thus modulating the previously documented effects of the kinases on the PER-based clock (17, 33). Finally, we cannot discount the possibility that S6KII regulates circadian clock function through a CK2-independent pathway. Further analysis of the S6KII binding partners and substrates may yield insights about the precise role of the C-terminal kinase and ERK-binding domains in circadian regulation.
Supplementary Material
Acknowledgments
We thank J. Chung of the Korean Advanced Institute of Science & Technology (KAIST) and Bloomington Stock Center (Bloomington, IN) for Drosophila strains; Marc Bourouis (University of Nice, France) for plasmids; J. Chung (KAIST) and the Developmental Studies Hybridoma Bank (Iowa City, IA) for antibodies, Genetic Services Inc. (Cambridge, MA) for transgenic services, Dan Cox for help with statistics and the Center for Neuroscience Research at Tufts University School of Medicine (Boston, MA) for confocal microscopy facilities.
This work was supported, in whole or in part, by National Science Foundation Fellowship DGE 0238731 (to M. M. T.), National Institutes of Health Grant T32 HD049341 (to F. S. N.), National Institutes of Health Grant R01 NS45817 (to F. R. J.), National Institutes of Health Grant R01 HL59873 (to F. R. J.), National Institutes of Health Grant R01 NS065900 (to F. R. J.), and National Institutes of Health Grant P30 NS047243 (to F. R. J.).

This article contains supplemental Tables S1 and S2 and Figs. S1 and S2.
- RSK
- p90 ribosomal S6 kinase (mammalian)
- S6KII
- p90 ribosomal S6 kinase (Drosophila)
- PER
- PERIOD
- CK2
- casein kinase 2
- pigment-dispersing factor
- p
- phosphorylated.
REFERENCES
- 1. Frödin M., Gammeltoft S. (1999) Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell Endocrinol. 151, 65–77 [DOI] [PubMed] [Google Scholar]
- 2. Erikson E., Maller J. L. (1985) A protein kinase from Xenopus eggs specific for ribosomal protein S6. Proc. Natl. Acad. Sci. U.S.A. 82, 742–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung J., Kuo C. J., Crabtree G. R., Blenis J. (1992) Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kDa S6 protein kinases. Cell 69, 1227–1236 [DOI] [PubMed] [Google Scholar]
- 4. Koh H., Jee K., Lee B., Kim J., Kim D., Yun Y. H., Kim J. W., Choi H. S., Chung J. (1999) Cloning and characterization of a nuclear S6 kinase, S6 kinase-related kinase (SRK); a novel nuclear target of Akt. Oncogene 18, 5115–5119 [DOI] [PubMed] [Google Scholar]
- 5. Blenis J., Chung J., Erikson E., Alcorta D. A., Erikson R. L. (1991) Distinct mechanisms for the activation of the RSK kinases/MAP2 kinase/pp90rsk and pp70-S6 kinase signaling systems are indicated by inhibition of protein synthesis. Cell Growth Differ. 2, 279–285 [PubMed] [Google Scholar]
- 6. Lewis T. S., Shapiro P. S., Ahn N. G. (1998) Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74, 49–139 [DOI] [PubMed] [Google Scholar]
- 7. Carriere A., Ray H., Blenis J., Roux P. P. (2008) The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci. 13, 4258–4275 [DOI] [PubMed] [Google Scholar]
- 8. Cargnello M., Roux P. P. (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75, 50–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher T. L., Blenis J. (1996) Evidence for two catalytically active kinase domains in pp90rsk. Mol. Cell Biol. 16, 1212–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones S. W., Erikson E., Blenis J., Maller J. L., Erikson R. L. (1988) A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc. Natl. Acad. Sci. U.S.A. 85, 3377–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen M. S., Hadjivassiliou H., Taunton J. (2007) A clickable inhibitor reveals context-dependent autoactivation of p90 RSK. Nat. Chem. Biol. 3, 156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richards S. A., Dreisbach V. C., Murphy L. O., Blenis J. (2001) Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1. Mol. Cell Biol. 21, 7470–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjørbaek C., Zhao Y., Moller D. E. (1995) Divergent functional roles for p90rsk kinase domains. J. Biol. Chem. 270, 18848–18852 [DOI] [PubMed] [Google Scholar]
- 14. Vik T. A., Ryder J. W. (1997) Identification of serine 380 as the major site of autophosphorylation of Xenopus pp90rsk. Biochem. Biophys. Res. Commun. 235, 398–402 [DOI] [PubMed] [Google Scholar]
- 15. Kim M., Lee J. H., Koh H., Lee S. Y., Jang C., Chung C. J., Sung J. H., Blenis J., Chung J. (2006) Inhibition of ERK-MAP kinase signaling by RSK during Drosophila development. EMBO J. 25, 3056–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wassarman D. A., Solomon N. M., Rubin G. M. (1994) The Drosophila melanogaster ribosomal S6 kinase II-encoding sequence. Gene 144, 309–310 [DOI] [PubMed] [Google Scholar]
- 17. Akten B., Tangredi M. M., Jauch E., Roberts M. A., Ng F., Raabe T., Jackson F. R. (2009) Ribosomal s6 kinase cooperates with casein kinase 2 to modulate the Drosophila circadian molecular oscillator. J. Neurosci. 29, 466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neuser K., Triphan T., Mronz M., Poeck B., Strauss R. (2008) Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244–1247 [DOI] [PubMed] [Google Scholar]
- 19. Putz G., Bertolucci F., Raabe T., Zars T., Heisenberg M. (2004) The S6KII (rsk) gene of Drosophila melanogaster differentially affects an operant and a classical learning task. J. Neurosci. 24, 9745–9751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer M., Raabe T., Heisenberg M., Sendtner M. (2009) Drosophila RSK negatively regulates bouton number at the neuromuscular junction. Dev. Neurobiol. 69, 212–220 [DOI] [PubMed] [Google Scholar]
- 21. Levine J. D., Funes P., Dowse H. B., Hall J. C. (2002) Signal analysis of behavioral and molecular cycles. BMC Neurosci. 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dalby K. N., Morrice N., Caudwell F. B., Avruch J., Cohen P. (1998) Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J. Biol. Chem. 273, 1496–1505 [DOI] [PubMed] [Google Scholar]
- 23. Smith J. A., Poteet-Smith C. E., Malarkey K., Sturgill T. W. (1999) Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 274, 2893–2898 [DOI] [PubMed] [Google Scholar]
- 24. Sutherland C., Campbell D. G., Cohen P. (1993) Identification of insulin-stimulated protein kinase-1 as the rabbit equivalent of rskmo-2. Identification of two threonines phosphorylated during activation by mitogen-activated protein kinase. Eur. J. Biochem. 212, 581–588 [DOI] [PubMed] [Google Scholar]
- 25. Frödin M., Jensen C. J., Merienne K., Gammeltoft S. (2000) A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 19, 2924–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richards S. A., Fu J., Romanelli A., Shimamura A., Blenis J. (1999) Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Curr. Biol. 9, 810–820 [DOI] [PubMed] [Google Scholar]
- 27. Jensen C. J., Buch M. B., Krag T. O., Hemmings B. A., Gammeltoft S., Frödin M. (1999) 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 274, 27168–27176 [DOI] [PubMed] [Google Scholar]
- 28. Brand A. H., Perrimon N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- 29. Tangredi M. M. (2010) Characterizing a role for the kinase S6KII in the Drosophila circadian system, Ph.D Thesis Tufts University School of Medicine, Boston, MA [Google Scholar]
- 30. Akten B. (2005) The role of two kinases, CK2 and S6KII, in the Drosophila circadian system Ph.D. thesis Tufts University School of Medicine, Boston, MA [Google Scholar]
- 31. Chrestensen C. A, Sturgill T. W. (2002) Characterization of the p90 ribosomal S6 kinase 2 carboxyl-terminal domain as a protein kinase. J. Biol. Chem. 277, 27733–27741 [DOI] [PubMed] [Google Scholar]
- 32. Roux P. P., Richards S. A., Blenis J. (2003) Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol. Cell Biol. 23, 4796–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akten B., Jauch E., Genova G. K., Kim E. Y., Edery I., Raabe T., Jackson F. R. (2003) A role for CK2 in the Drosophilla circadian oscillator. Nat. Neurosci. 6, 251–257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.