Background: Fut8−/− mice show emphysematous lesions, the major risk factor for which is exposure to cigarette smoke (CS).
Results: Fut8+/− mice developed CS-induced emphysematous lesions, which are associated with an aberrant Smad7-Smad2-matrix metalloproteinase signaling pathway.
Conclusion: Genetic ablation of Fut8 increases sensitivity to CS-induced emphysema.
Significance: Core fucosylation appears to be involved in the development of chronic obstructive pulmonary disease.
Keywords: Animal Models; Chronic Obstructive Pulmonary Disease (COPD); Cigarette Smoke; Glycosyltransferases; Matrix Metalloproteinase (MMP); α1,6-Fucosyltransferase; Smad7; Emphysema; Heterozygous Knock-out Mice
Abstract
We previously demonstrated that a deficiency in core fucosylation caused by the genetic disruption of α1,6-fucosyltransferase (Fut8) leads to lethal abnormalities and the development of emphysematous lesions in the lung by attenuation of TGF-β1 receptor signaling. Herein, we investigated the physiological relevance of core fucosylation in the pathogenesis of emphysema using viable heterozygous knock-out mice (Fut8+/−) that were exposed to cigarette smoke (CS). The Fut8+/− mice exhibited a marked decrease in FUT8 activity, and matrix metalloproteinase (MMP)-9 activities were elevated in the lung at an early stage of exposure. Emphysema developed after a 3-month CS exposure, accompanied by the recruitment of large numbers of macrophages to the lung. CS exposure substantially and persistently elevated the expression level of Smad7, resulting in a significant reduction of Smad2 phosphorylation (which controls MMP-9 expression) in Fut8+/− mice and Fut8-deficient embryonic fibroblast cells. These in vivo and in vitro studies show that impaired core fucosylation enhances the susceptibility to CS and constitutes at least part of the disease process of emphysema, in which TGF-β-Smad signaling is impaired and the MMP-mediated destruction of lung parenchyma is up-regulated.
Introduction
Chronic obstructive pulmonary disease (COPD)4 is a severe, slowly progressive, and disabling disease associated with an accelerated decline in lung function and is currently the fourth leading cause of death worldwide. Emphysema and chronic bronchitis are important phenotypes of COPD (1, 2). Oxidative stress (3) and an imbalance in protease-antiprotease (4) in the lungs are thought to be key components of the pathogenesis of COPD.
It is now clear that exposure to cigarette smoke (CS) is a major risk factor for the development of COPD. Smoking-initiated inflammation leads to changes in both the airways and lung parenchyma. The main known contribution of CS is the activation and recruitment of inflammatory cells to the lungs (5–7). CS induces the release of proinflammatory cytokines from cultured macrophages, epithelial cells, and fibroblasts and also increases the expression of genes that encode chemoattractants and proinflammatory mediators, including TNF-α and IL-1β (8, 9), in whole lungs and bronchoalveolar lavage (BAL) fluid.
Matrix metalloproteinase (MMP) appears to play a role as a mediator of emphysema and is associated with both the destruction of elastin and the aberrant remodeling of damaged alveoli. Exposure to CS results in an increase in the levels of MMP-2, -9, -12, -13, and -14 in mice (10–12). Mice that are lacking MMP-12 have been shown to be completely protected against emphysema (13). On the other hand, it has been well established that MMP-9 is a major mediator of emphysema in humans (14).
We recently reported that the growth of α1,6-fucosyltransferase knock-out (Fut8−/−) mice is severely retarded and that the mortality rate was ∼70% during the first 3 postnatal days. Interestingly, the lungs of the surviving adult mice showed emphysematous changes, which can be partly attributed to a lack of α1,6-fucosylation of the TGF-β1 receptor, resulting in the dysregulation of TGF-β1 receptor activation and signaling. As a consequence of the impaired TGF-β1 signaling at steady state, the Fut8−/− mice also showed evidence of the overexpression of MMP-9, -12, and -13 in the lungs (15). The Fut8 gene encodes the α1,6-fucosyltransferase (FUT8) that catalyzes the transfer of a fucose residue from GDP-fucose to position 6 of the innermost GlcNAc residue of hybrid and complex types of N-linked oligosaccharides on glycoproteins, and the product is referred to as a core fucose (16, 17). The loss of core fucosylation also results in the down-regulation of the EGF receptor-mediated cellular signaling pathway and proteinase-activated receptor and integrin activities, which may contribute to the growth retardation observed in Fut8−/− mice (18, 19). Core fucosylation not only affects ligand-receptor binding and receptor-mediated signaling but has also been reported to affect the expression level of VEGF receptor-2, which is involved in alveolar cell apoptosis (20).
On the basis of the fact that gene-targeted Fut8−/− mice show airspace enlargement due to developmental abnormal lung morphogenesis, we hypothesized that a lowered FUT8 enzyme activity might be associated with susceptibility to emphysema in adults. In this study, Fut8+/− mice were exposed to CS, and the degree of alveolar destructions, inflammatory cell accumulation, and changes in MMP levels in the lung were evaluated.
EXPERIMENTAL PROCEDURES
Mice
Fut8+/− mice were generated on a pure C57BL/6J background (>10 backcrosses). Age- and sex-matched WT C57BL/6J mice were used as controls. Their genotypes were determined by PCRs. We used 10–12-week-old female mice for CS exposure. All experimental protocols and procedures were approved by the Ethical Committees on Animal Research of RIKEN and the Gunma University Graduate School of Medicine.
CS Exposure
Groups of Fut8± and WT female mice, all 10–12 weeks of age, were subjected to smoke from four unfiltered Kentucky cigarettes per day, 6 days per week, for 2 weeks to 3 months, using a previously described smoking apparatus (13). Mice tolerated CS exposure without any obvious evidence of toxicity (carboxyhemoglobin levels of 10% and no weight loss).
Tissue Preparation and Histological Analysis
On completion of the smoking protocol, the mice were killed by CO2 inhalation. Lungs were inflated by instilling 10% formalin at a constant pressure of 25 cm of H2O (for 10 min), and the inflated lungs were fixed for 24 h. Serial midsagittal sections were obtained for morphological and histological analyses.
After fixation, the paraffin-embedded tissues were sectioned (4 μm) and stained with hematoxylin and eosin. Mean linear intercepts were calculated based on 20 randomly selected fields in each section at ×100 magnification with two crossed test lines.
BAL Fluid Analysis
BAL fluid was prepared via a 22-gauge intravenous catheter inserted into the tracheas of the mice. Total cell counts of the BAL fluid were determined with a hemocytometer after the lysis of red blood cells. Cell differentials in BAL fluid were examined by Cytospin preparation with Hema 3 (Biochemical Sciences) or Diff-Quik reagent (Sysmex International Reagents, Kobe, Japan) staining. Differential counts were obtained by examining >300 cells under a standard light microscope.
FUT8 Activity Assay
The lung tissues were homogenized by sonication and then assayed for FUT8 activity as described previously (21, 22).
Cell Cultures
Mouse embryonic fibroblasts (MEFs) derived from wild-type and Fut8-null mice were established and maintained as described before (15). MicroRNAs specific for mouse Fut8 for use in RNA interference were designed on the Invitrogen website, and the single-stranded RNA sequences were as follows: TGCTGATAACTGGATGTTTGAAGCCAGTTTTGGCCACTGACTGACTGGCTTCACATCCAGTTAT (top) and CCTGATAACTGGATGTGAAGCCAGTCAGTCAGTGGCCAAAACTGGCTTCAAACATCCAGTTATC (bottom). A stable Fut8 knockdown cell line (Fut8KD) was established using the above probes and exhibited ∼50% of the FUT8 activity of wild-type MEF cells as shown in supplemental Fig. 1. For Smad signaling pathway analysis, TGF-β1 and a CS extract (CSE) were used.
RNA Extraction and Quantification by Real-time PCR
Total RNA was extracted from tissues using TRIzol reagent (Invitrogen), and reversed-transcribed cDNA was subjected to PCR amplification using THUNDERBIRD SYBR qPCR mix (TOYOBO, Tsuruga, Japan). RT-qPCR was performed on the Mx3000P real-time qPCR system (Stratagene). The forward and reverse primers used were as follows: MMP-9, 5′-TGAACAAGGTGGACCATGAG-3′ (forward) and 5′-CGGTTGAAGCAAAGAAGGAG-3′ (reverse); MMP-12, 5′-GCTAGAAGCAACTGGGCAAC-3′ (forward) and 5′-ACCGCTTCATCCATCTTGAC-3′ (reverse); RPL4 (ribosomal protein L4), 5′-GTTCAAAGCTCCCATTCGAC-3′ (forward) and 5′-AATTCACTGACGGCATAGGG-3′ (reverse); mouse Smad2, 5′-GTCAACCAGGGTTTTGAAGC-3′ (forward) and 5′-CTGTCTGCCTCCGATATTCTG-3′ (reverse); and mouse Smad7, 5′-TTTACAACCGCAGCAGTTACC-3′ (forward) and 5′-AGCCTTGATGGAGAAACCAG-3′ (reverse).
Assay for MMP Activity
The levels of MMP-9 activity in tissue homogenates were assessed using a SensoLyteTM 520 MMP-9 assay kit (AnaSpec, Freemont, CA) following the recommended protocols. Before reaction, the homogenized tissues were desalted using centrifugal filter devices with regenerated cellulose (molecular weight cutoff of 30,000; Millipore). The reaction was terminated by adding 50 mm EDTA.
In the case of MMP-12, a two-step method was used. The levels of total MMP activities in tissue samples were first assessed directly by cleavage of the quenched fluorescent substrate 2,4-dinitrophenyl-Pro-b-cyclohexyl-Ala-Gly-Cys(Me)-His-Ala-Lys(N-Me-2-aminobenzoyl)-NH2 (Calbiochem) according to the recommendations of the manufacturer. Total MMP activity was then measured again in the presence of a peptide inhibitor of MMP-12. The difference between activity values was considered to denote the relative activity of endogenous MMP-12. The percentage of activity of MMP-12 to that of total MMP was compared among all of the samples. This peptide inhibitor of MMP-12 was a generous gift from Drs. Vincet Dive and Fabrice Beau (Commissariat à l'Energie Atomique, Institute de Biologie et des Technologies de Saclay, France) (23).
TGF-β1 Stimulation
Cells (5 × 105) were seeded on 60-mm culture dishes and subjected to serum starvation for 24 h. After washing with chilled PBS containing 0.1% BSA, the cells were incubated for 2 h at 4 °C with TGF-β1 at the indicated concentrations. After washing, the cells were lysed for Western blot analysis.
Preparation of Aqueous CSE
Research-grade cigarettes (2R4F) were obtained from Kentucky Tobacco Research (Lexington, KY). The CSE was prepared by bubbling smoke from one cigarette into 10 ml of culture medium, and this medium is referred as a 100% CSE solution (24). The CSE was sterile-filtered through a 0.45-μm filter. The CSE was freshly prepared for each experiment and diluted with culture medium immediately before use. The final concentration of the CSE was 10%. At this concentration, cell viability was consistently >95%, as evidenced by trypan blue exclusion experiments.
Western Blot Analysis
Anti-Smad2, anti-phospho-Smad2, and anti-Smad7 antibodies were obtained from Cell Signaling Technology. Anti-MMP-9 antibody was from Santa Cruz Biotechnology, and anti-β-actin antibody was from Sigma. TGF-β receptor II was immunoprecipitated with an anti-TGF-β receptor II antibody (Millipore) and was detected using another anti-TGF-β receptor II antibody (Cell Signaling Technology). Biotinylated Aspergillus oryzae lectin, a kind gift from Dr. K. Matsumura (Gekkeikan, Kyoto, Japan) (25), was used for lectin blotting of TGF-β receptor II. At the end of the TGF-β1 or CSE treatment, MEF cells were collected, and the cell lysates were analyzed by 10% SDS-PAGE. Gels were blotted onto PVDF membranes. Membranes were incubated with the primary antibody overnight at 4 °C, followed by washing and exposure to horseradish peroxidase-labeled secondary antibodies for 30 min at room temperature. The immunocomplexes were visualized using an enhanced chemiluminescence detection system and quantified by densitometric scanning. β-Actin was included for normalization in this quantification.
Data Analysis
Data are expressed as the mean ± S.E. Differences between groups were assessed by analysis of variance. Statistical significance was set at p < 0.05.
RESULTS
Exposure to CS Results in Decrease in FUT8 Enzyme Activity
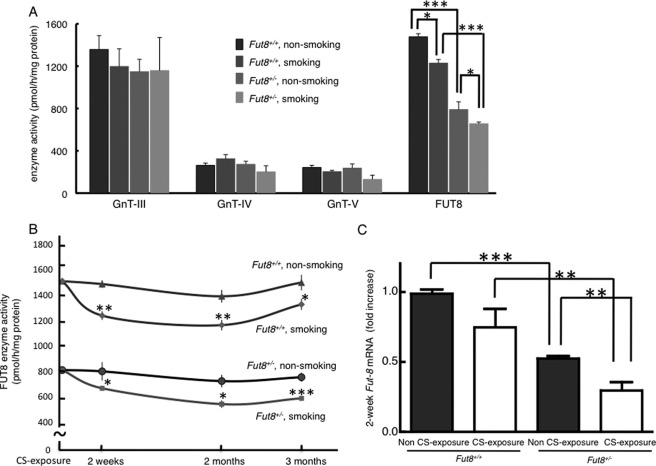
To evaluate the physiological relevance of FUT8 enzyme activity and CS exposure, we analyzed the activities of four glycosyltransferases related to the synthesis of the N-glycan core, β1,4-N-acetylglucosaminyltransferases III and IV, β1,6-N-acetylglucosaminyltransferase V, and FUT8 (supplemental Fig. 2), in the lung. Only the activity of FUT8 was decreased in all of the CS-exposed cohorts (Fig. 1A). The decrease in enzyme level was detectable after a 2-week exposure to CS, and the lowered enzyme activity level persisted for the entire period of the CS exposure. The difference in FUT8 enzyme activity reached statistical significance at all of the time points between the CS-exposed and non-CS-exposed groups (Fig. 1B). Furthermore, we could detect an emphasized reduction of Fut8 mRNA levels in Fut8+/− mouse lung samples at 2 weeks of CS exposure (Fig. 1C).
FIGURE 1.
CS exposure results in decreased FUT8 enzyme activity. A, CS specifically decreased FUT8 enzyme activity. In addition to FUT8, other N-glycosyltransferases (β1,4-N-acetylglucosaminyltransferases III (GnT-III) and IV (GnT-IV) and β1,6-N-acetylglucosaminyltransferase V (GnT-V)) were compared in all 2-week CS-exposed mice and their controls. B, the decline in FUT8 enzyme activity in the lung tissues persisted for the entire CS exposure period. FUT8 enzyme activities in all CS-exposed mice at each time point (2 weeks, 2 months, and 3 months) are shown. C, the reduction of gene expression levels of Fut8 caused by a 2-week CS exposure was much more significant in Fut8+/− mice. Data are the mean ± S.E. (n = 10). *, p < 0.05; **, p < 0.02; ***, p < 0.01 versus the matched groups connected with bars.
Lungs of Fut8+/− Mice Have Increased MMP Expression Levels and Activities after Short-term Exposure to CS
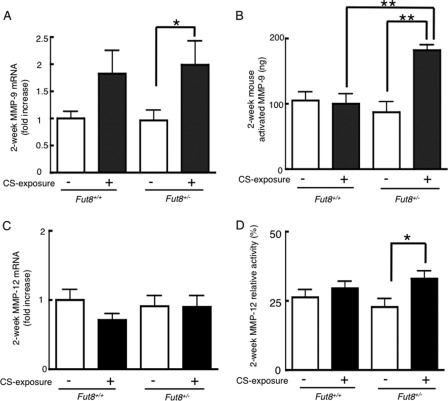
At the 2-week point, the CS-exposed Fut8+/− mice showed an ∼2-fold increase in MMP-9 gene expression levels (p < 0.05 versus non-CS-exposed mice) (Fig. 2A). The difference in MMP-12 mRNA expression among all of the groups was subtle (Fig. 2C).
FIGURE 2.
CS exposure enhances expression of MMP-9 and MMP-12 at early stage in Fut8+/− mice. Total RNAs from 2-week CS-exposed lungs were used for real-time PCR analysis of MMP-9 (A) and MMP-12 (C). Data are the mean ± S.E. (n = 10). Values were normalized to RPL4 levels and are expressed as the -fold increases against the value for wild-type (Fut8+/+) non-CS-exposed mice. *, p < 0.05; p < 0.01 versus the matched groups connected with bars. The protein expression and/or enzyme activity of MMP-9 (B) and MMP-12 (D) was measured as described under “Experimental Procedures.” Values for MMP-9 are corrected with the activity of commercial recombinant protein and are expressed as the amount of activated MMP-9.
The increased MMP gene expression in the early stage of CS exposure corresponded to increased enzyme activity. At the 2-week point, the CS-exposed Fut8+/− mice showed a marked increase in the amount of activated MMP-9 compared with all of the other groups (p < 0.01) (Fig. 2B). Meanwhile, up-regulated MMP-12 activity was observed in the CS-exposed Fut8+/− mice (p < 0.05 versus non-CS-exposed Fut8+/− mice) at the 2-week point (Fig. 2D).
Enhanced Inflammation in Response to Long-term CS Exposure in Fut8+/− Mice
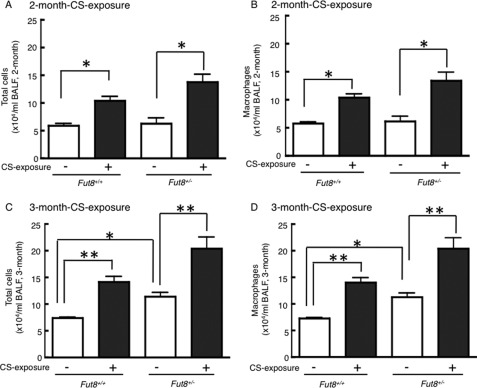
Because the development of emphysema is closely associated with inflammation, we evaluated the inflammatory cells that accumulated in response to CS exposure. The population of inflammatory cells in BAL fluid obtained from the mice was measured. A significant increase in the total cell number and macrophages was detected in 2-month (Fig. 3, A and B) and 3-month (Fig. 3, C and D) CS-exposed mice (p < 0.05), whereas the most prominent changes were found in 3-month CS-exposed Fut8+/− mice.
FIGURE 3.
Inflammatory cell content in BAL fluid in response to long-term CS exposure. The numbers of total cells (A and C) and macrophages (B and D) in the BAL fluid of WT (Fut8+/+) and heterozygous knock-out (Fut8+/−) mice are shown. Black bars, CS-exposed; white bars, non-CS-exposed. Cell counts were tabulated using a hemocytometer and Cytospin preparation with Hema 3 staining (n = 10 mice/group). *, p < 0.05; **, p < 0.02 versus the matched groups connected with bars.
Dramatic Airspace Enlargement in Fut8+/− Mice in Response to Long-term Exposure to CS
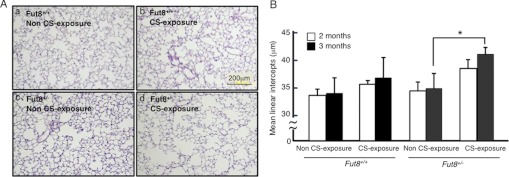
To estimate the morphological change in response to long-term CS exposure, we measured the mean linear intercept, which indicates the average distance between alveolar walls and is proportional to the degree of emphysema. Histological sections indicated that a 3-month CS exposure induced clear emphysematous changes in Fut8+/− mice compared with non-CS-exposed Fut8+/− mice and Fut8+/+ mice (Fig. 4A). In the Fut8+/− mice, the mean linear intercept was significantly increased by 18.6% in response to a 3-month CS exposure (34 ± 2.8 to 41 ± 1.3, p < 0.05 versus non-CS-exposed mice) (Fig. 4B). In contrast, the mean linear intercept was unchanged in response to a 2- or 3-month CS exposure in Fut8+/+ mice (33 ± 2.9 to 37 ± 3.8) (Fig. 4B). These results suggest that CS-induced emphysema is facilitated in Fut8+/− mice.
FIGURE 4.
Fut8+/− mice show enlarged airspace in response to 3-month exposure to CS. A, hematoxylin/eosin-stained sections were used for morphological analyses. Photographs of lungs from non-CS-exposed (panel a) or 3-month CS-exposed (panel b) Fut8+/+ mice and non-CS-exposed (panel c) or 3-month CS-exposed (panel d) Fut8+/− mice are shown. B, mean linear intercept, which is proportional to the extent of emphysema, was quantified. Data are the mean ± S.E. (n = 10). The mean linear intercept was significantly increased in Fut8+/− mice in response to a 3-month CS exposure. *, p < 0.05 versus the matched groups connected with bars.
Lungs of Fut8+/− Mice Have Impaired Smad Pathway Due to Exposure to CS
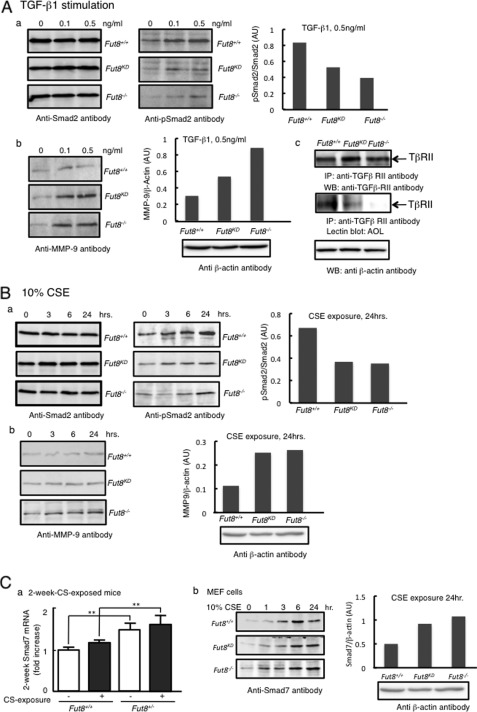
To clarify the molecular mechanism underlying CS-induced emphysema, we initially analyzed TGF-β1 signaling related to Smad activation in Fut8-deficient MEF cells (Fig. 5A) by immunoblot analyses using anti-Smad2, anti-phospho-Smad2, and anti-MMP-9 antibodies. Although the expression level of Smad2 was not affected, the phosphorylation of Smad2 in response to TGF-β1 was decreased in both Fut8KD and Fut8−/− cells. MMP-9 proteins that were secreted into the medium were essentially undetectable in wild-type embryonic fibroblasts, whereas they were clearly detected in Fut8-deficient cells. These data are consistent with previous findings obtained with Fut8−/− mice, where a lack of core fucose was found to lead to a marked down-regulation in the TGF-β1 signaling pathway, which leads to the dysregulation of expression and activation of MMP-9 (15).
FIGURE 5.
Smad pathway is impaired by exposure to CS in Fut8 mutant mice. A, the alteration of Smad signaling pathway by TGF-β1 stimulation was examined. Serum-starved MEF cells were treated with or without TGF-β1 at the indicated concentrations as described under “Experimental Procedures.” Panel a, the cell lysates were detected by immunoblotting with anti-Smad2 antibody (left) and anti-phospho-Smad2 antibody (middle), and the ratio of phospho-Smad2 (pSmad2) to Smad2 for samples stimulated with 0.5 ng/ml TGF-β1 is shown (right). Panel b, the production of MMP-9 was checked using anti-MMP-9 antibody (left), and the quantity of MMP-9 (arbitrary units (AU)) is shown for samples stimulated with 0.5 ng/ml TGF-β1 (right). Panel c, the fucosylation levels on TGF-β receptor II were analyzed. TGF-β receptor II (TβRII) was immunoprecipitated (IP) from whole cell lysates and then subjected to 10% SDS-PAGE. After electroblotting, blots were probed with anti-TGF-β receptor II antibody (upper) and A. oryzae lectin (AOL) (middle). The protein concentration of all of the lysates used for immunoprecipitation was normalized as shown by immunoblotting of β-actin (lower). WB, Western blot. B, the time course of the CSE effect on the Smad pathway was then examined. Cells were treated with 10% CSE for the indicated periods of time leading up to simultaneous harvest and analyzed by immunoblotting. Panel a, the blots were developed with anti-Smad2 antibody (left) and anti-phospho-Smad2 antibody (middle), and the ratio of phospho-Smad2 to Smad2 for samples stimulated with 10% CSE for 24 h is shown (right). Panel b, the production on MMP-9 related to the CS exposure was checked by immunoblotting using anti-MMP-9 antibody (left). As for samples stimulated with 10% CSE for 24 h, the quantity of MMP-9 (arbitrary units) is shown (right). C, the effect of CSE on the expression of the Smad7 protein. Panel a, real-time PCR analysis of Smad7 was performed for 2-week CSE-exposed mouse samples. Total RNAs from 2-week CSE-exposed Fut8+/+ and Fut8+/− mouse lungs were used as templates. Values were normalized to RPL4 levels and are expressed as -fold increase against the value for wild-type (Fut8+/+) non-CS-exposed mice. **, p < 0.02 versus the matched groups connected with bars. Panel b, Fut8-deficient MEF cells treated with 10% CSE for the indicated periods of time were harvested and analyzed with anti-Smad7 antibody (left). The band density of the samples stimulated by 10% CSE for 24 h is shown (right). For all of the experiments, β-actin from the cell lysate was included for normalization.
We next examined the expression and phosphorylation of Smad2 in response to CSE exposure (Fig. 5B). Smad2 protein expression showed no differential expression among all cell types, whereas the phosphorylation levels of Smad2 were increased in a time-dependent manner by CSE exposure. Compared with wild-type MEF cells, the phosphorylation of Smad2 in Fut8-deficient cells was down-regulated, which coincided with elevated MMP-9 expression. This is consistent with previous findings (41) that the phosphorylation of Smad2 controls the production of MMP.
Furthermore, we investigated the mechanism of the down-regulation of Smad2 phosphorylation by CS stimulation (Fig. 5C). The gene expression profile of the Smad family obtained by real-time PCR analyses revealed that the gene expression of Smad7 was increased to a greater extent in 2-week CS-exposed Fut8+/− mice than in wild-type mice, as well as in CSE-treated Fut8-deficient MEF cells. Fut8-deficient cells exhibited higher induction levels of Smad7 proteins compared with wild-type cells, especially at 24 h.
The above findings indicate that low expression of Fut8 resulted in high sensitivity to the CSE. Actually, the level of core fucosylation on TGF-β receptor II, detected by A. oryzae lectin, was decreased or disappeared in Fut8-deficient cells (Fig. 5A, panel c). CSE stimulation appeared to up-regulate the production of Smad7 and subsequently attenuated TGF-β1-Smad2 signaling, which was more obvious in Fut8-deficient cells and could be a key link between protein core fucosylation and CS-induced emphysema.
DISCUSSION
In this study, Fut8+/− mice developed emphysematous changes in the lungs after a 3-month period of CS exposure, which is the half the time required for wild-type mice to develop the same symptoms (26). The onset of emphysema in Fut8+/− mice is associated with the accumulation of macrophages, induction of MMP gene expression, activation of MMP enzyme, and an aberrant Smad signaling pathway.
Because the Smad pathway coordinates a delicate balance between fibrosis and excess extracellular matrix destruction, alterations in this pathway can contribute to the development of emphysema. We first confirmed that the TGF-β1-Smad2-MMP-9 pathway in Fut8-deficient MEF cells is defective compared with that in wild-type cells, which is consistent with our current in vivo knowledge of Fut8−/− mice. We then addressed the issue of whether the differences in the rate of onset of emphysema as observed in Fut8+/− mice could be attributed to an aberrant Smad pathway caused by CS (Fig. 6). According to our data, CS exposure had no effect on the expression level of Smad2, whereas the phosphorylation of Smad2 was down-regulated, which resulted in a much higher production of MMP-9 in Fut8-deficient cells. More importantly, the gene expression level of Smad7 was increased in both Fut8-deficient cells and Fut8+/− mice due to CS exposure. A previous study indicated that Smad7 can affect the above signaling pathway by inhibiting the phosphorylation of Smad2 and Smad3 and inducing the ubiquitination of their receptors (27). Smad7 regulates TGF-β1 signaling and mediates cross-talk between TGF-β and other signaling pathways (28). As CS presumably contains >5000 compounds, it is nearly impossible to identify the specific compound responsible for stimulating the expression of Smad7 in the case of CS-exposed Fut8+/− mice. Smad7 has been reported to be regulated by different stimuli, including TGF-β, TNF-α, and IFN-γ (29, 30). Knowledge of how a deficiency in core fucose could affect the function of Smad7 would provide some clues to help explain the pathology of CS-exposed Fut8+/− mice.
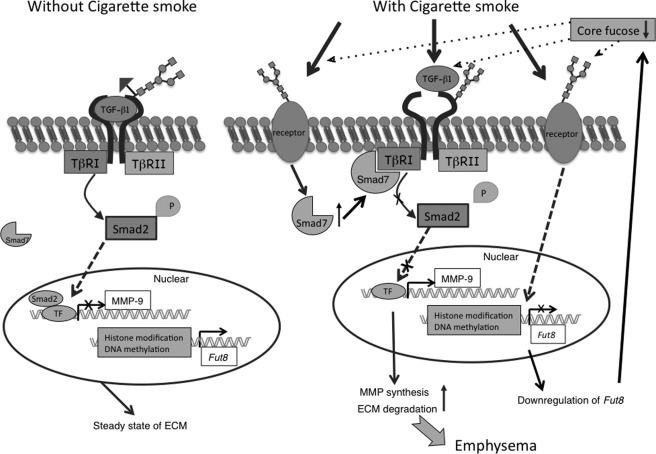
FIGURE 6.
Working model for onset of emphysema in Fut8+/− mice upon exposure to CS. It was reported previously (15) that Fut8−/− mice show emphysematous changes in the lung due to a lack of core fucosylation of the TGF-β1 receptor. In this study, Fut8 expression was down-regulated by exposure to CS, resulting a much lower level of core fucosylation. This change in N-glycosylation led to more severe impairment of the TGF-β1 signaling pathway. Moreover, CS evoked an up-regulation of Smad7, a key inhibitory factor associated with Smad2 phosphorylation. Comprehensively, a lower degree of core fucosylation appears to increase the susceptibility to CS-induced emphysemas. TβRI and TβRII, TGF-β receptors I and II, respectively; TF, transcription factor; ECM, extracellular matrix.
Down-regulation of FUT8 enzyme activity during exposure to CS was observed in all of the CS-exposed mice, but the mechanism for this is not clear. It has been reported that CS induces modifications in chromatin and epigenetic changes by causing the post-translational modification of histone acetyltransferases and histone deacetylases, leading to abnormal gene transcription (31). A recent study reported that the mechanism for the expression of glycosyltransferase was highly associated with epigenetic histone modifications (32). Taking these studies into account, it is entirely possible that the gene expression of Fut8 might also be regulated in an epigenetic manner (Fig. 6).
In addition to the accumulation of macrophages, the findings herein show that CS exposure also induces an increase in gene expression and activities of MMP-9 and MMP-12 in lung tissue of Fut8+/− mice. These two MMPs have been implicated in the genesis of pulmonary emphysema and have been reported to be increased in the lungs of patients with COPD (33, 34). The increased MMP production in Fut8+/− mice appears to occur very early in the CS exposure period. Our findings are similar to reports dealing with pulmonary hypertension and vascular remodeling (10). One possibility for the effect of the increased MMP induction in Fut8+/− mice is the early degradation of elastin, which sets the stage for the eventual functional abnormalities in the lung because it is known that elastin fragments produced as the result of cleavage by MMPs are major chemokines that recruit macrophages in lungs in response to exposure to CS in vivo (35).
Cigarette smoking is by far the most important risk factor for COPD. However, only a susceptible minority (∼15–20%) of tobacco smokers develop clinically significant COPD, suggesting that genetic factors must be involved in each individual's risk (35). Although several gene knock-out mice, e.g. the klotho gene (36), and tetraspanin CD9/CD81 double knock-out mice (37) showed emphysematous changes in the lung, the host factors that are involved in the pathogenesis of CS-induced COPD have not yet been identified, except for the rare hereditary deficiency of α1-antitrypsin (38). Our study of gene-environment interactions between Fut8 and CS is therefore of critical importance in terms of elucidating the effect of host factors on the development of COPD. The Fut8+/− mice developed emphysematous lesions in the alveolar wall after only a 3-month CS exposure (Fig. 1), whereas 6 months were generally required for wild-type mice. Our data suggest that mice with low expression levels of Fut8 are at a high risk of developing emphysema. Meanwhile, core fucosylation has been reported to be decreased in smokers by an analysis of N-glycans in the plasma of 1914 individuals (39), which confirmed our observations obtained with CS-exposed mice. Moreover, a recent clinical study that included 182 outpatients with COPD showed a Fut8 gene polymorphism (T267K) associated with human pulmonary emphysema (40). Further exploring the relationships between the enzyme activity of FUT8 and the onset of COPD in human samples would clearly be a worthwhile endeavor. Our unpublished data5 also suggest that a reduction in FUT8 activity is significantly associated with faster decline of FEV1, an important index for respiratory function in patients with prominent emphysema.
In conclusion, we have demonstrated that a lower degree of core fucosylation appears to increase the susceptibility to CS-induced emphysema. Our findings may have prognostic implications related to the incidence pattern, severity, and extent of emphysema for cigarette smokers.
Supplementary Material
Acknowledgments
We thank Dr. Rina Takamiya for valuable discussions and help with CS extraction and Miyuki Nomura and Tomoko Hasegawa for excellent technical assistance.
This work was supported in part by the Program for Promotion of Fundamental Studies in Health Science of the National Institute of Biomedical Innovation (NIBIO), the Global Center of Excellent (GCOE) Program, and Grant-in-aid for Scientific Research (A) 20249018 from the Ministry of Education, Science, Sports and Culture, Japan.

This article contains supplemental Figs. 1 and 2.
K. Kamio, T. Ishii, C. Gao, K. Korekane, F. Ota, T. Motegi, A. Azuma, A. Gemma, N. Taniguchi, and K. Kida, unpublished data.
- COPD
- chronic obstructive pulmonary disease
- CS
- cigarette smoke
- BAL
- bronchoalveolar lavage
- MMP
- matrix metalloproteinase
- MEF
- mouse embryonic fibroblast
- CSE
- CS extract.
REFERENCES
- 1. Rabe K. F., Hurd S., Anzueto A., Barnes P. J., Buist S. A., Calverley P., Fukuchi Y., Jenkins C., Rodriguez-Roisin R., van Weel C., Zielinski J. (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176, 532–555 [DOI] [PubMed] [Google Scholar]
- 2. Balzano G., Stefanelli F., Iorio C., De Felice A., Melillo E. M., Martucci M., Melillo G. (1999) Eosinophilic inflammation in stable chronic obstructive pulmonary disease. Relationship with neutrophils and airway function. Am. J. Respir. Crit Care Med. 160, 1486–1492 [DOI] [PubMed] [Google Scholar]
- 3. Loukides S., Bakakos P., Kostikas K. (2011) Oxidative stress in patients with COPD. Curr. Drug Targets 12, 469–477 [DOI] [PubMed] [Google Scholar]
- 4. Abboud R. T., Vimalanathan S. (2008) Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int. J. Tuberc. Lung Dis. 12, 361–367 [PubMed] [Google Scholar]
- 5. Burgel P. R., Bourdin A., Chanez P., Chabot F., Chaouat A., Chinet T., de Blic J., Devillier P., Deschildre A., Didier A., Garcia G., Jebrak G., Laurent F., Morel H., Perez T., Pilette C., Roche N., Tillie-Leblond I., Verbanck S., Dusser D. (2011) Update on the roles of distal airways in COPD. Eur. Respir. Rev. 20, 7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siddiqui S., Hollins F., Saha S., Brightling C. E. (2007) Inflammatory cell microlocalization and airway dysfunction: cause and effect? Eur. Respir. J. 30, 1043–1056 [DOI] [PubMed] [Google Scholar]
- 7. Górska K., Maskey-Warzechowska M., Krenke R. (2010) Airway inflammation in chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 16, 89–96 [DOI] [PubMed] [Google Scholar]
- 8. Domagala-Kulawik J. (2008) Effects of cigarette smoke on the lung and systemic immunity. J. Physiol. Pharmacol. 59, Suppl. 6, 19–34 [PubMed] [Google Scholar]
- 9. Murugan V., Peck M. J. (2009) Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Exp. Lung Res. 35, 439–485 [DOI] [PubMed] [Google Scholar]
- 10. Wright J. L., Tai H., Wang R., Wang X., Churg A. (2007) Cigarette smoke up-regulates pulmonary vascular matrix metalloproteinases via TNF-α signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L125–L133 [DOI] [PubMed] [Google Scholar]
- 11. Stevenson C. S., Docx C., Webster R., Battram C., Hynx D., Giddings J., Cooper P. R., Chakravarty P., Rahman I., Marwick J. A., Kirkham P. A., Charman C., Richardson D. L., Nirmala N. R., Whittaker P., Butler K. (2007) Comprehensive gene expression profiling of rat lung reveals distinct acute and chronic responses to cigarette smoke inhalation. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1183–L1193 [DOI] [PubMed] [Google Scholar]
- 12. Shaykhiev R., Krause A., Salit J., Strulovici-Barel Y., Harvey B. G., O'Connor T. P., Crystal R. G. (2009) Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 183, 2867–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. (1997) Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004 [DOI] [PubMed] [Google Scholar]
- 14. Wallace A. M., Sandford A. J., English J. C., Burkett K. M., Li H., Finley R. J., Müller N. L., Coxson H. O., Paré P. D., Abboud R. T. (2008) Matrix metalloproteinase expression by human alveolar macrophages in relation to emphysema. COPD 5, 13–23 [DOI] [PubMed] [Google Scholar]
- 15. Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., Uozumi N., Ihara S., Lee S. H., Ikeda Y., Yamaguchi Y., Aze Y., Tomiyama Y., Fujii J., Suzuki K., Kondo A., Shapiro S. D., Lopez-Otin C., Kuwaki T., Okabe M., Honke K., Taniguchi N. (2005) Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uozumi N., Yanagidani S., Miyoshi E., Ihara Y., Sakuma T., Gao C. X., Teshima T., Fujii S., Shiba T., Taniguchi N. (1996) Purification and cDNA cloning of porcine brain GDP-l-Fuc:N-acetyl-β-d-glucosaminide α1→6-fucosyltransferase. J. Biol. Chem. 271, 27810–27817 [DOI] [PubMed] [Google Scholar]
- 17. Yanagidani S., Uozumi N., Ihara Y., Miyoshi E., Yamaguchi N., Taniguchi N. (1997) Purification and cDNA cloning of GDP-l-Fuc:N-acetyl-β-d-glucosaminide: α1-6-fucosyltransferase (α1-6FucT) from human gastric cancer MKN45 cells. J Biochem. 121, 626–632 [DOI] [PubMed] [Google Scholar]
- 18. Wang X., Gu J., Ihara H., Miyoshi E., Honke K., Taniguchi N. (2006) Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J. Biol. Chem. 281, 2572–2577 [DOI] [PubMed] [Google Scholar]
- 19. Taniguchi N. (2007) A sugar-coated switch for cellular growth and arrest. Nat. Chem. Biol. 3, 307–309 [DOI] [PubMed] [Google Scholar]
- 20. Wang X., Fukuda T., Li W., Gao C. X., Kondo A., Matsumoto A., Miyoshi E., Taniguchi N., Gu J. (2009) Requirement of Fut8 for the expression of vascular endothelial growth factor receptor-2: a new mechanism for the emphysema-like changes observed in Fut8-deficient mice. J. Biochem. 145, 643–651 [DOI] [PubMed] [Google Scholar]
- 21. Uozumi N., Teshima T., Yamamoto T., Nishikawa A., Gao Y. E., Miyoshi E., Gao C. X., Noda K., Islam K. N., Ihara Y., Fujii S., Shiba T., Taniguchi N. (1996) A fluorescent assay method for GDP-l-Fuc:N-acetyl-β-d-glucosaminide α1-6-fucosyltransferase activity, involving high performance liquid chromatography. J. Biochem. 120, 385–392 [DOI] [PubMed] [Google Scholar]
- 22. Ihara H., Ikeda Y., Taniguchi N. (2006) Reaction mechanism and substrate specificity for nucleotide sugar of mammalian α1,6-fucosyltransferase–a large-scale preparation and characterization of recombinant human FUT8. Glycobiology 16, 333–342 [DOI] [PubMed] [Google Scholar]
- 23. Devel L., Rogakos V., David A., Makaritis A., Beau F., Cuniasse P., Yiotakis A., Dive V. (2006) Development of selective inhibitors and substrate of matrix metalloproteinase-12. J. Biol. Chem. 281, 11152–11160 [DOI] [PubMed] [Google Scholar]
- 24. Janoff A., Carp H., Lee D. K., Drew R. T. (1979) Cigarette smoke inhalation decreases α1-antitrypsin activity in rat lung. Science 206, 1313–1314 [DOI] [PubMed] [Google Scholar]
- 25. Matsumura K., Higashida K., Ishida H., Hata Y., Yamamoto K., Shigeta M., Mizuno-Horikawa Y., Wang X., Miyoshi E., Gu J., Taniguchi N. (2007) Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae: a novel probe for core fucose. J. Biol. Chem. 282, 15700–15708 [DOI] [PubMed] [Google Scholar]
- 26. Wright J. L., Cosio M., Churg A. (2008) Animal models of chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L1–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi W., Sun C., He B., Xiong W., Shi X., Yao D., Cao X. (2004) GADD34-PP1c recruited by Smad7 dephosphorylates TGFβ type I receptor. J. Cell Biol. 164, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan X., Chen Y. G. (2011) Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signaling. Biochem. J. 434, 1–10 [DOI] [PubMed] [Google Scholar]
- 29. Hong S., Lee C., Kim S. J. (2007) Smad7 sensitizes tumor necrosis factor-induced apoptosis through the inhibition of antiapoptotic gene expression by suppressing activation of the nuclear factor-κB pathway. Cancer Res. 67, 9577–9583 [DOI] [PubMed] [Google Scholar]
- 30. Zandvoort A., Postma D. S., Jonker M. R., Noordhoek J. A., Vos J. T., Timens W. (2008) Smad gene expression in pulmonary fibroblasts: indications for defective ECM repair in COPD. Respir. Res. 9, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rajendrasozhan S., Yao H., Rahman I. (2009) Current perspectives on role of chromatin modifications and deacetylases in lung inflammation in COPD. COPD 6, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kizuka Y., Kitazume S., Yoshida M., Taniguchi N. (2011) Brain-specific expression of N-acetylglucosaminyltransferase IX (GnT-IX) is regulated by epigenetic histone modifications. J. Biol. Chem. 286, 31875–31884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kunz L. I., Lapperre T. S., Snoeck-Stroband J. B., Budulac S. E., Timens W., van Wijngaarden S., Schrumpf J. A., Rabe K. F., Postma D. S., Sterk P. J., Hiemstra P. S. (2011) Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir. Res. 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Babusyte A., Stravinskaite K., Jeroch J., Lötvall J., Sakalauskas R., Sitkauskiene B. (2007) Patterns of airway inflammation and MMP-12 expression in smokers and ex-smokers with COPD. Respir. Res. 8, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Houghton A. M., Quintero P. A., Perkins D. L., Kobayashi D. K., Kelley D. G., Marconcini L. A., Mecham R. P., Senior R. M., Shapiro S. D. (2006) Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Invest. 116, 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suga T., Kurabayashi M., Sando Y., Ohyama Y., Maeno T., Maeno Y., Aizawa H., Matsumura Y., Kuwaki T., Kuro-O M., Nabeshima Y., Nagai R. (2000) Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am. J. Respir. Cell Mol. Biol. 22, 26–33 [DOI] [PubMed] [Google Scholar]
- 37. Takeda Y., He P., Tachibana I., Zhou B., Miyado K., Kaneko H., Suzuki M., Minami S., Iwasaki T., Goya S., Kijima T., Kumagai T., Yoshida M., Osaki T., Komori T., Mekada E., Kawase I. (2008) Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease-like phenotype in mice. J. Biol. Chem. 283, 26089–26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hersh C. P., DeMeo D. L., Silverman E. K. (2008) National Emphysema Treatment Trial state of the art: genetics of emphysema. Proc. Am. Thorac. Soc. 5, 486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knezevic A., Gornik O., Polasek O., Pucic M., Redzic I., Novokmet M., Rudd P. M., Wright A. F., Campbell H., Rudan I., Lauc G. (2010) Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology 20, 959–969 [DOI] [PubMed] [Google Scholar]
- 40. Yamada M., Ishii T., Ikeda S., Naka-Mieno M., Tanaka N., Arai T., Kumasaka T., Gemma A., Kida K., Muramatsu M., Sawabe M. (2011) Association of fucosyltransferase-8 (FUT8) polymorphism T267K with pulmonary emphysema. J. Hum. Genet. 56, 857–860 [DOI] [PubMed] [Google Scholar]
- 41. Okamoto T., Takahashi S., Nakamura E., Nagaya K., Hayashi T., Fujieda K. (2009) Transforming growth factor-β1 induces matrix metalloproteinase-9 expression in human meningeal cells via ERK and Smad pathways. Biochem. Biophys. Res. Commun. 383, 475–479 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.