Background: There is a lack of information about the roles of neurotrophin BDNF in adult taste buds.
Results: We generated taste cell-specific BDNF-overexpressing mice. Increased BDNF elevates TrkB activation, increases transcription of NCAM-TrkB, leads to larger taste buds, and increases taste cell number and taste-specific innervation.
Conclusion: BDNF has local and neuronal influences.
Significance: BDNF might be involved in the supertasting phenomenon.
Keywords: Brain-derived Neurotrophic Factor (BDNF), Development, Growth Factors, Neurotrophins, Transgenic Mice, Gustation, NCAM, Taste Buds, TrkB
Abstract
Brain-derived neurotrophic factor (BDNF) is the most potent neurotrophic factor in the peripheral taste system during embryonic development. It is also expressed in adult taste buds. There is a lack of understanding of the role of BDNF in the adult taste system. To address this, we generated novel transgenic mice in which transgene expression was driven by an α-gustducin promoter coupling BDNF expression to the postnatal expression of gustducin in taste cells. Immunohistochemistry revealed significantly stronger BDNF labeling in taste cells of high BDNF-expressing mouse lines compared with controls. We show that taste buds in these mice are significantly larger and have a larger number of taste cells compared with controls. To examine whether innervation was affected in Gust-BDNF mice, we used antibodies to neural cell adhesion molecule (NCAM) and ATP receptor P2X3. The total density of general innervation and specifically the gustatory innervation was markedly increased in high BDNF-expressing mice compared with controls. TrkB and NCAM gene expression in laser capture microdissected taste epithelia were significantly up-regulated in these mice. Up-regulation of TrkB transcripts in taste buds and elevated taste cell-specific TrkB phosphorylation in response to increased BDNF levels indicate that BDNF controls the expression and activation of its high affinity receptor in taste cells. This demonstrates a direct taste cell function for BDNF. BDNF also orchestrates and maintains taste bud innervation. We propose that the Gust-BDNF transgenic mouse models can be employed to further dissect the specific roles of BDNF in the adult taste system.
Introduction
More than half a century ago, pioneering work by Rita Levi Montalcini and colleagues (1) led to the discovery of growth factors. Nerve growth factor (NGF), the first growth factor discovered, is the prototype member of the neurotrophin family of neurotrophic factors. The neurotrophin family has four family members in mammals: NGF, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and neurotrophin 4 (NT-4) (2). BDNF has been shown to have a wide range of functions from trophic effect on neurons in the peripheral nervous system (3) to orchestrating the innervation of sensory organs (4–6). One such sensory organ is the chemical sensors (taste buds) in the oral cavity of mammals. Taste buds contain taste cells that are specialized epithelial cells (7) with neuronal properties; namely, releasing neurotransmitters and the ability to depolarize and form synapses (8). BDNF is expressed in taste progenitor cells prior to taste bud formation and innervation (9) and in developing taste buds, suggesting a role in gustatory innervation (6). BDNF expression continues in adult taste buds (9–11). However, its physiological role in the adult taste system is not clear. Mammalian taste cells turn over continuously throughout life (10, 11). The rather rapid turnover of taste receptor cells necessitates continuous formation of new connections between the nerve fibers and taste receptors cells. Taste bud development, innervation, and functional connectivity to the brain are important to understand from a developmental point of view.
Taste buds contain three distinct anatomical/histological types of elongated taste cells: type I, type II, and type III (12, 13). Type I cells are the most abundant cells in taste buds and have been suggested to have glial-like properties (8). Type II cells express T1R and T2R taste receptors and their downstream signaling components such as phospholipase Cβ2, IP3R3 (14, 15), and gustducin (16, 17). Type II cells respond to bitter, sweet taste, and umami stimuli. Type III cells respond to sour taste and carbonation (18, 19) and have synaptic contacts with the gustatory nerve fibers. They express neural cell adhesion molecule (NCAM)2 (20) as well as the synaptic membrane protein SNAP25 (21). Although both type II and III cells express BDNF, BDNF is expressed by a larger number of type III cells (22).
It has been shown that a 7.7-kb segment of DNA upstream to the protein coding portion of the mouse α-gustducin gene functions provides a fully functional promoter: it drove strong expression of β-galactosidase in a temporospatial pattern similar to that of endogenous α-gustducin (23). α-Gustducin is not expressed embryonically and is found in early postnatal ages in mouse taste buds (23). Thus, α-gustducin expression commences after the period of embryonic neurogenesis in gustatory and cranial ganglia has ended (24). The relationship between the gustatory neuron and the taste buds it innervates is a dynamic process, and one neuron sends branches that innervate multiple taste buds (25, 26). We and others have targeted BDNF expression to different lingual structures using nestin (27) and CK14 (28) promoters. These strategies led to ectopic embryonic expression of BDNF in lingual muscles and anterior lingual epithelial cells, respectively. With the embryonic expression, it is impossible to separate trophic effect from other functions that BDNF might have. Most importantly, BDNF was not targeted for expression in taste buds in either approach. To address these shortcomings, we utilized the α-gustducin promoter to express BDNF in postnatal taste buds to study the possible roles of BDNF in adult taste buds.
Using the α-gustducin 7.7-kb promoter we have generated transgenic mice that overexpress BDNF in taste buds of adult mice. Continued overexpression of BDNF in taste buds in postnatal mice leads to elevated levels of TrkB activation in taste cells in vivo and influences taste bud morphology and innervation, suggesting a role for BDNF in maintenance of gustatory innervation.
EXPERIMENTAL PROCEDURES
Production and Genotyping of Gust-BDNF Transgenic Mice
The 7.7-kb α-gustducin promoter (Gust) acts as a taste cell-specific promoter and to drive transgene expression in developing and adult taste buds (23). Following the promoter, three Kozak nucleotide bases, ACC (29), were added before the ATG start of the coding sequence for BDNF followed by bovine growth hormone polyadenylation site (BGH-PA). BGH-PA has been shown to successfully stabilize neurotrophin transcripts under the CK14 promoter (30). It is also commonly used in commercially available mammalian expression vectors (for instance see invitrogen.com or stratagene.com). The Kozak sequence was added to the PCR primers to amplify the full-length BDNF gene, and BGH-PA fragment was obtained from a commercially available expression vector (pCDNA 3.1; Invitrogen). The mature BDNF sequence in the transgene was sequenced several times after insertion into the construct to examine the integrity of the sequence and to prevent mutations. PCR primers were also used to sequence the transgene fragments over the ligation sites, and we have verified the exact identity of these fragments to eliminate ligation of cutoff DNA fragments. The transgene can be removed with NotI from the pBSKSII backbone (Stratagene).
Mice were generated by pronuclear microinjection of the transgene construct (α-gustducin promoter-Kozak sequence-BDNF-BGH-PA) into fertilized eggs collected from female C57BL/6J mice at the University of Michigan transgenic core facility. Procedures were approved by the Institutional Animal User Committee at the University of Michigan. The microinjected fertilized eggs were reimplanted in pseudopregnant mice. Four founder mice were generated, but three lines survived (denoted as Gust-BDNF 739, 755, and 759). Transgene expression was verified by several construct-specific PCR primers spanning the 3′ end of the promoter to 5′ regions of the mature BDNF and 3′ region of BDNF and 5′ region of the BGH-PA. No PCR product could be generated on wild-type genomic DNA. All experiments were performed on 2–4-month-old mice unless otherwise mentioned.
Histology and Immunohistochemistry
Mice were euthanized using CO2 and perfused with 2% or 4% paraformaldehyde in phosphate-buffered saline (PBS) through the ascending aorta. Tongues were dissected, postfixed for 1 h, rinsed, and stored at 4 ºC in 10% sucrose until use. These procedures were approved by the Institutional Animal Care and User Committee at the University of Tennessee Health Science Center.
To measure taste bud size, immunohistochemistry was performed on 14-μm sections using the Troma-1 (rat, Hybridoma bank, 1:80) antibody. Troma-1 is a monoclonal antibody against intermediate filaments, which identifies taste buds by its reaction with cytokeratin 8 found within the taste buds (31, 32). The slides were incubated with Troma-1 overnight at 4 °C, rinsed in PBS, and incubated with cyanine-2-coupled antibody (Cy-2, 1:200; Jackson ImmunoResearch Laboratories) or with Alexa Fluor-conjugated anti-rat IgG (1:400; Molecular Probes) for 60 min at room temperature. The slides were rinsed in PBS and cover-slipped by using glycerol/PBS (1:2) mounting medium. Images were collected with a Nikon microscope (Nikon 80i, Tokyo, Japan). Taste buds were measured using ImageJ software.
BDNF and TrkB Immunohistochemistry
Gust-BDNF 739 and 759 and wild-type circumvallate papillae and brain tissues containing the hippocampal formation and cortex were sectioned at 14 μm and incubated in blocking solution with 5% normal goat serum (NGS), 1% BSA, and 1% H2O2 in 0.3% Triton-X for 40 min at room temperature. The slides were incubated for 36 h with antibodies against BDNF (1:50–1:500, rabbit; Santa Cruz Biotechnology) or phosphorylated TrkB (pTrkB, 1:100–1:500, rabbit; Abcam). Sections were rinsed in PBS and incubated with anti-rabbit Alexa Fluor 488 (1:200) diluted in 1% NGS in 0.3% Triton-X for 90 min. For diaminobenzidine staining, sections were incubated for 45 min with anti-rabbit biotinylated secondary antibody and incubated with Vectastain ABC reagent (Vector Laboratories) for 45 min. Sections were thereafter incubated in diaminobenzidine substrate kit for peroxidase until the desired staining was obtained and rinsed in tap water. Slides were then dehydrated and mounted with Permount. To analyze intensity, we measured optical density of BDNF and pTrkB labeling in grayscale photomicrographs using ImageJ (National Institutes of Health) software. Black and white were set at 0 and 100%. An equal area was outlined in ImageJ with the strongest labeling in Gust-BDNF 739 and 759 and wild-type circumvallate papillae (n = 5), and optical density was measured and expressed as percentage.
P2X3 Receptor/Troma-1
Gust-BDNF 739, 755, and 759 and wild-type circumvallate papillae were sectioned at 40 μm. The mounted tongue sections were hydrated with three 10-min washes in PBS. The slides were blocked with 5% NGS and 1% BSA for 45 min and incubated overnight with antibodies to P2X3 (1:5000, guinea pig; Chemicon) and Troma-1. These antibodies identify the purinergic P2X3 receptor and its nerve fibers and taste receptor cells, respectively. Sections were rinsed followed by incubation with anti-guinea pig Alexa Fluor 594 (1:400) and anti-rat Cy-2 (1:200) for 60–90 min.
NCAM
Gust-BDNF 739, 755, and 759 and wild-type circumvallate papillae were sectioned at 40 μm and incubated in blocking solution with 5% NGS and 1% BSA, followed by incubation with NCAM (1:400, rabbit; Chemicon). Sections were incubated with secondary antibodies for 1 h with anti-rabbit Alexa Fluor 594. NCAM is expressed on the surface of neurons and nerve fibers and type III cells in taste buds.
Confocal Microscopy
Digital images of taste buds were captured with a Zeiss laser scanning confocal microscope (LSM700) using a 20× lens. The system laser was used to excite Troma at 488 nm and NCAM at 594 nm. A pinhole size of 1.0 airy unit was used. The scan rate was 1 frame/s, and a total of 2 frames were averaged. Scan speed was selected at 5. Optical sections (0.7-μm spacing) were collected for the z-stack images.
Size of Fungiform and Circumvallate Taste Buds and Statistical Analysis
To examine the possible effect of BDNF overexpression on the size of circumvallate and fungiform taste buds, we compared the size of taste buds in circumvallate papillae and fungiform papillae on the anterior 140 μm of the tongue in Gust-BDNF lines with wild-type mice. Tissue sections that had been incubated with Troma-1, followed by secondary antibody (Cy-2) were processed and photographed. Fungiform and circumvallate taste buds were outlined in ImageJ, and their area, maximum widths and heights for each taste bud were measured. A detailed description of the measured parameters has been reported previously (33). Statistical analysis was performed by using Student-Newman-Keuls multiple comparisons test (InStat, Graphpad) to test whether there were any significant differences in taste bud area, width/height between Gust-BDNF lines and wild-type mice. One-way analysis of variance (ANOVA) was used for statistical analysis, and a p value of ≤0.05 was considered significant.
For circumvallate taste bud measurements, the five largest taste buds from three mice were measured on a tissue section. We also counted the number of taste buds in the sections that had the highest number of taste buds. We measured the area of the inner core part of the papillae by choosing the biggest area of the section in circumvallate papillae. We also measured the width of the inner part of the circumvallate papilla and the width of the whole papillae including the trench epithelium. For fungiform taste bud measurements, the papillae at the tip of the tongue were selected (anterior 140 μm of the tongue). The 10 largest taste buds were chosen in Gust-BDNF 739, 759, and wild-type mice (n = 30).
Propidium Iodide Staining and Taste Cell Number
Following incubation with Troma-1 and Cy-2, the slides were washed three times with PBS and propidium iodide (Sigma) was then added at a final concentration of 250 μg/ml (in PBS) as described previously (34). The slides were incubated for 15 min at room temperature in dark and washed three times with PBS and coverslipped with glycerol/PBS. All taste cell nuclei were counted in five taste buds on five different slides in Gust-BDNF 739, 755, and 759 and in wild-type mice (n = 3). The calculated number of taste cells in wild type was set to 100%.
Laser Capture Microdissection (LCM), RNA Preparation, and Reverse Transcription-PCR (RT-PCR)
Adult (6 weeks and older) transgenic Gust-BDNF and wild-type mice were euthanized using CO2 asphyxiation. The circumvallate papillae were dissected, rapidly frozen on dry ice, and sectioned on a cryostat. Horizontal sections (10 μm) were collected on frosted slides and stored at −80 °C. A HistoGene LCM Frozen Section Staining kit (Arcturus) was used for dehydration and staining of the slides. HistoGene Staining Solution (Arcturus) was applied for 10 s to the tissue sections, followed by rinses in water, ethanol, and xylene. Slides were dried for 5 min, placed in a slide box containing fresh desiccant, and LCM was performed.
Arcturus Pixcell II was used for the LCM. The laser was fired on the epithelium of the circumvallate papillae containing taste buds. The tissue was collected on a cap, which was put on a 0.5-ml Eppendorf tube containing buffer (PicoPure RNA Isolation kit). Total RNA was isolated and purified from the tissue obtained by LCM using the PicoPure RNA Isolation kit (Arcturus). cDNA was synthesized (using 1 μg of RNA) by reverse transcription using the High Capacity cDNA Archive kit (Applied Biosystems) primed with random primers, allowing us to compare the levels of gene expression with the housekeeping gene cyclophilin-D. Reverse transcription was performed for 10 min at 25 °C and 120 min at 37 °C according to the manufacturer (Applied Biosystems).
Quantitative Real-time PCR
The primers and probes for the target genes were determined with the assistance of the “Universal Probe Library” (Roche Applied Science). Real-time PCR was performed using the LightCycler (Roche Applied Science) in the presence of TaqMan Probe (Universal Probe 67) according to the manufacturer's instructions to determine BDNF, Ntrk2, and Ncam1 transcripts in the gustatory epithelium of circumvallate papillae. The primers used were bdnf forward, gcctttggagcctcctctac and reverse, gcggcatccaggtaatttt; Ntrk2 forward, tgccgagtgctacaacctct and reverse, gcgtccttcagcgtcttc; Ncam1 forward, agggcaaggctgctttct and reverse, ccccatcatggtttggagt. Total volume reaction is 10 μl using 5 μl of Taqman Master Mix reagent (Roche), 2 μl of cDNA (1:10), 0.2 μl of 10 μm primer pairs (forward, 5′-3′ and reverse, 3′-5′, Invitrogen), 0.1 μl of Universal probe, and 2.7 μl of RNase free water.
Thermal cycling conditions included 2 min at 50 ºC, 10 min at 95 ºC, and 45 cycles for 15 s at 95 ºC, and 1 min at 60 ºC. To normalize the amount of total BDNF, Ntrk2, and Ncam1 transcripts in each reaction, levels of cyclophilin-D were monitored in parallel samples. The assay was performed in triplicate for each sample with Gust-BDNF 739, 755, 759, and wild-type (n = 5) and was repeated three times (biological triplicates). Results are expressed as relative levels of BDNF, Ntrk2, and Ncam1 transcripts, referred to as wild-type control samples. The amount of targets, normalized to an endogenous reference was defined by the Ct (threshold cycle) methods (Livak and Schmittgen, 2001).
Genomic DNA of Gust-BDNF and wild-type tail biopsies was purified using DNA easy-Qiagen kit. 10 μg of DNA was used for purification. 15 ng of genomic DNA from Gust-BDNF 739, 755, and 759 was used for real-time PCR to determine whether the transgenic transcript got incorporated into the genome. BDNF-primers, cyclophilin-D, and Universal probe 67 was used.
Statistical Analysis
The difference in the transcript expression levels of the target genes between the experimental groups were evaluated by using ANOVA.
RESULTS
Generation of Transgenic Mouse Lines
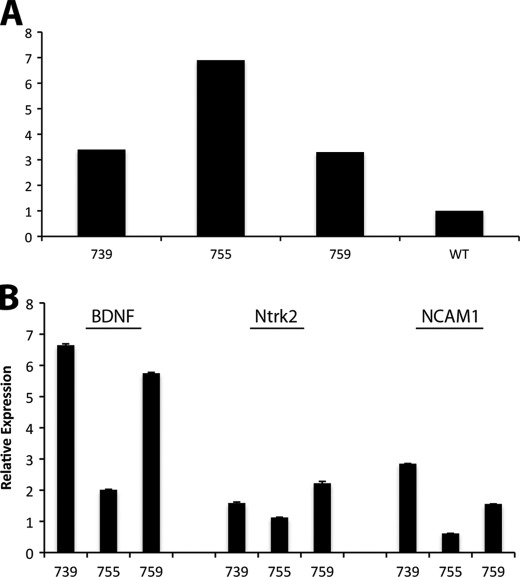
We overexpressed BDNF in postnatal taste buds to study its roles in the adult taste system, using a taste cell-specific promoter for α-gustducin. Three independent mouse lines were generated, Gust-BDNF 739, 755, and 759. The mice have been inbred for more than 10 generations, and we have achieved homozygosity in each of the transgenic mouse lines. Using mature BDNF-specific primers, we first examined the relative frequency of transgene incorporation in the genomic DNA. Normalized against wild-type mouse genomic DNA, the relative incorporation of BDNF in genomic DNA of Gust-BDNF was highest in the Gust-BDNF 755 line (Fig. 1A). To determine whether transgene copy number was a good indicator for transgene expression, we used laser capture microdissection to isolate circumvallate taste buds and gustatory epithelium from the lines, then generated cDNA. Using real-time PCR, we analyzed mature BDNF mRNA expression in circumvallate taste buds. Compared with wild-type littermates the assays revealed 6.6-fold higher mature BDNF expression in Gust-BDNF 739, 2.0-fold higher expression in Gust-BDNF 755, and 5.7-fold higher expression in Gust-BDNF 759 (Fig. 1B). Assays were done in triplicate in three independent experiments. Our results showed that although Gust-BDNF 755 had the highest number of transgenes incorporated into its genomic DNA, the taste bud-specific mature BDNF mRNA expression was highest in Gust-BDNF 759 followed by Gust-BDNF 739. TrkB (Ntrk2) and Ncam1 transcripts were also up-regulated in the high BDNF-expressing lines (Fig. 1B, see below). These results clearly demonstrate that we have generated transgenic mice with taste bud-specific overexpression of BDNF. Due to the modest BDNF overexpression in Gust-BDNF 755 line, it was not included in subsequent analyses.
FIGURE 1.
Quantitative real-time PCR was performed using LightCycler (Roche Applied Science) in the presence of TaqMan probes on mouse genomic DNA (A) and laser capture microdissected taste buds and gustatory epithelium (B). A, Gust-BDNF 755 transgenic mouse line has the highest number of transgene copies inserted into genomic DNA. BDNF in wild-type mice was used as reference. B, BDNF expression is highest in Gust-BDNF 739 followed by Gust-BDNF 759. High BDNF-expressing lines (739 and 759) have higher expression level of TrkB (Ntrk2) and NCAM1. Expression values were normalized against that of cyclophylin-D.
BDNF Protein Expression and TrkB Activation Assay
To determine whether the gustducin promoter-driven transgene affected the translational activity in taste cells and led to increased amounts of BDNF protein, we used immunohistochemistry (Fig. 2, A–F). The main selection criteria for the specific antibody was proper positive staining in cortical neurons and in the hippocampal formation and being distinct from NGF- and NT-3-like immunoreactivity in these areas (35). We screened multiple commercially available antibodies, and only one gave appropriate and reproducible results, enabling us to quantify the relative amounts of BDNF protein in wild-type and transgenic taste buds. Weak BDNF labeling was seen in taste cells of wild-type mice (Fig. 2, A and D). However, BDNF labeling was much stronger in the taste cells of Gust-BDNF mice (Fig. 2, B, C, E, and F), indicating a higher level of BDNF production in these mice than in wild-type mice. The average intensity of BDNF labeling in circumvallate taste buds in Gust-BDNF 739 (34.9%) and Gust-BDNF 759 (42.6%) was significantly higher (p < 0.0001) than in wild-type mice (19.4%) (ANOVA, post test Tukey-Kramer).
FIGURE 2.
BDNF and pTrkB immunoreactivity in the circumvallate taste buds of wild-type and Gust-BDNF mice. A, weak BDNF labeling is seen in the circumvallate taste buds in wild-type mice. B and C, strong BDNF labeling is observed in Gust-BDNF circumvallate papillae, indicating that BDNF levels are significantly elevated in these mice compared with wild-type controls. D–F, higher magnification of circumvallate taste buds in wild-type and Gust-BDNF mice. Arrowheads in D point to weak BDNF labeling in taste cells. A larger number of taste cells are strongly labeled in Gust-BDNF mice (E and F). G, pTrkB immunoreactivity is present in wild-type taste buds, and the labeling becomes stronger and is seen in a larger number of taste cells of Gust-BDNF transgenic mice (H and I). J–L, higher magnification of circumvallate taste buds in wild-type and Gust-BDNF mice labeled for pTrkB. A stronger pTrkB labeling is seen in a larger number of taste cells in Gust-BDNF mice (K and L) compared with wild-type controls (J). Brightness was adjusted identically in images in each row to visualize the difference in staining intensity accurately. Scale bar in A represents 200 μm in A–C and G–I. Scale bar in D represents 20 μm in D–F and J–L.
Although BDNF has been shown to orchestrate gustatory innervation, its possible roles in taste cells per se have not been studied. To examine BDNF function in taste cells, we studied TrkB activation and phophorylation in taste cells. We used a well characterized antibody to phosphorylated TrkB (pTrkB) that detects both the presence and activation of this receptor (36, 37). The specificity was also verified in our experiments by evaluating pTrkB labeling in brain tissue sections. We detected phosphorylated TrkB in taste cells (Fig. 2, G and J), and brain neurons, of wild-type mice. Weak labeling was also detected in some subepithelial nerve fibers. This shows not only that taste cells express TrkB, it also indicates a local functional activation of TrkB in taste cells in response to BDNF in taste buds. Labeling in the peripheral terminals of either gustatory or somatosensory neurons lends itself to the dual action of BDNF in taste buds and taste innervation. As mentioned previously, BDNF is overexpressed and overproduced in taste buds of Gust-BDNF mice, and thus, it would be valuable to use Gust-BDNF mice as a model to examine receptor activation and downstream signaling of BDNF in taste cells. Using the same antibody, we detected strong labeling for pTrkB in taste cells and in a larger number of taste cells in the high BDNF-expressing Gust-BDNF mice (Fig. 2, H–K). We also detected labeling in subepithelial nerve fibers. Interestingly and parallel to the increased levels of BDNF in taste buds of Gust-BDNF mice, there was a significant increase in the levels of TrkB signaling in taste cells of Gust-BDNF mice. The average intensity of pTrkB immunoreactivity in Gust-BDNF 739 (43.9%) and Gust-BDNF 759 (40.3%) was significantly higher (p < 0.0001) than in wild-type mice (22.2%) (ANOVA, post test Tukey-Kramer). This shows that increased levels of BDNF protein in taste buds correspond to an increase in TrkB activation and downstream signaling in taste cells.
Increased Size of Taste Buds
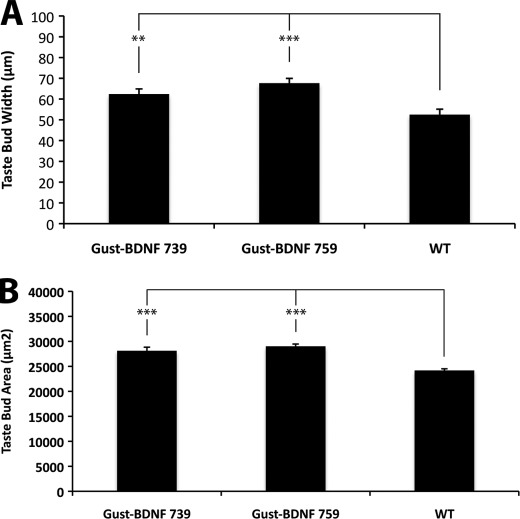
To determine whether taste buds in high BDNF-expressing transgenic mice differed in size compared with wild-type mice, we utilized the Troma-I antibody. Gustducin BDNF 739 and 759 had larger fungiform and circumvallate taste buds than wild-type control mice (Fig. 3A). The average maximum width of taste buds in circumvallate papillae of Gust-BDNF 739 (62.4 μm ± 2.5 μm; p < 0.01; taste buds = 15, n = 3) and Gust-BDNF 759 (67.7 μm ± 2.3 μm; p < 0.001; taste buds = 15, n = 3) mice are significantly wider than those in wild-type mice (52.3 μm ± 2.6; taste buds = 15, n = 3) (Fig. 3A). We also found that the average maximum surface area of fungiform taste buds (Fig. 3B) in Gust-BDNF 739 mice (28110 ± 729 μm2; p < 0.001; taste buds = 30, n = 3) and Gust-BDNF 759 (29,003 ± 458 μm2; p < 0.001; taste buds = 30, n = 3) are significantly larger than in wild-type mice (24,170 ± 349 μm2; taste buds = 30, n = 3) (ANOVA, post test Tukey-Kramer).
FIGURE 3.
Quantitative analysis of circumvallate and fungiform taste bud size in Gust-BDNF and wild type mice. A, taste buds in circumvallate papillae of Gust-BDNF 739 and 759 are significantly wider (p < 0.01and p < 0.001, respectively) than in wild-type mice. B, taste buds in fungiform papillae of Gust-BDNF 739 and 759 are significantly larger (p < 0.001 and p < 0.001, respectively) than in wild-type mice.
The circumvallate papillae core appeared to have similar width in all mice. The height of taste buds (apical-basal axis length) in the circumvallate papillae of transgenic and wild-type mice did not show a significant difference. This indicates that the gustatory epithelium thickness is similar in all mouse lines. Neither was there a statistically significant difference in the number of taste buds in circumvallate papillae (data not shown).
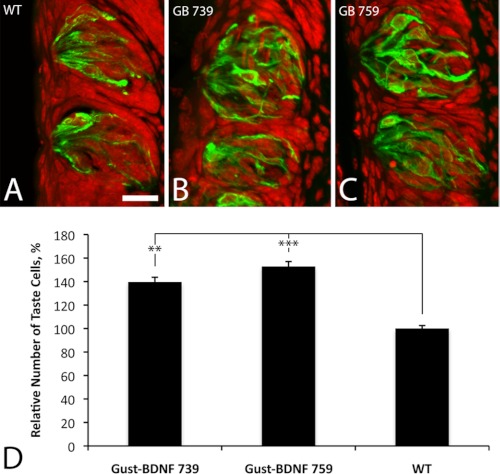
To examine whether the taste bud size increase in high BDNF-expressing transgenic mice was due to larger taste cells or a larger number of taste cells, we counted the number of taste cell nuclei in taste buds on tissue sections that had been stained with Troma-1 (Fig. 4, A–C). Counterstaining the cell nuclei with propidium iodide revealed 140 and 153% increased number of taste cells in Gust-BDNF 739 (p < 0.001) and 759 (p < 0.001), respectively (ANOVA and Tukey) compared with wild-type circumvallate taste buds (Fig. 4D). Whereas the height of taste buds is similar in the transgenic and wild-type mice, the taste buds in high BDNF-expressing transgenic lines (739 and 759) are significantly wider and thus a larger volume than in wild-type mice. The larger width and volume appear to correspond well to the increased number of cells in these taste buds.
FIGURE 4.
Quantitative analysis of relative number of taste cells in the circumvallate papillae of Gust-BDNF and wild-type mice. Taste bud boundary was marked by Troma-1 staining (green). Propidium iodide counterstaining (red) labels cell nuclei. Cell nuclei in taste buds with distinct boundary were counted. A–C, Gust-BDNF 739 (B) and 759 (C) showed an increased number of taste cells compared with wild-type mice (A). Scale bar in A represents 20 μm. D, Gust-BDNF 739 had 140% (p < 0.001) and GB 759 153% (p < 0.001) larger number of taste cells compared with wild-type mice. Data are expressed as mean ± S.E. (error bars), one-way analysis of variance (ANOVA) was used for statistical analysis.
NCAM, Phospholipase Cβ2, and Troma-1 Immunostaining
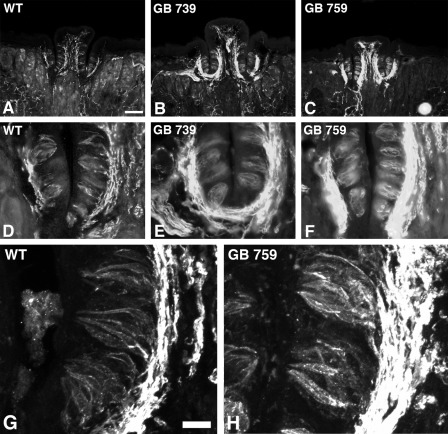
We showed that the Gust-BDNF taste buds are wider in the high BDNF-expressing transgenic mice and that they contain an increased number of taste cells compared with wild type. To examine whether BDNF overexpression had influenced cell identity as well as the previously established cell number increase, we used antibodies to NCAM, a nerve fiber, and type III cell marker and phospholipase Cβ2, a type II cell marker. We observed many more NCAM-positive cells and richer innervation as indicated by a much larger subepithelial plexus in Gust-BDNF 739 and 759 compared with wild-type littermates (Fig. 5). There was no apparent difference in phospholipase Cβ2 immunoreactivity between Gust-BDNF and wild-type mice (data not shown). Thus, there is an increase in the number of NCAM-positive type III cells in high BDNF-expressing Gust-BDNF mice (Fig. 5).
FIGURE 5.
NCAM immunoreactivity in the circumvallate papillae and taste buds of Gust-BDNF and wild-type mice. D–F are higher magnification views of the trench and associated inner and outer wall on the left side of each papilla represented in A–C. NCAM immunostaining shows a much stronger innervation pattern in the subepithelial plexus in Gust-BDNF 739 (C and D) and Gust-BDNF 759 (G and H) compared with wild-type mice (A and B). There is also a stronger intragemmal NCAM labeling in Gust-BDNF 759 and 739 than in wild-type mice. The intragemmal labeling is presumably the sum of labeling in intragemmal nerve fibers and taste cells. Scale bar represents 200 μm in A–C and 100 μm in D–F. G and H, confocal photomicrographs of representative taste buds in wild-type mice (G) and a high BDNF-expressing transgenic line (H, Gust-BDNF 759). There is stronger NCAM immunoreactivity in Gust-BDNF 759 taste buds than in wild-type taste buds. Brightness and contrast were adjusted identically for both images. Scale bar in G represents 20 μm and applies to G and H.
P2X3 Receptor and Troma-1 Immunostaining
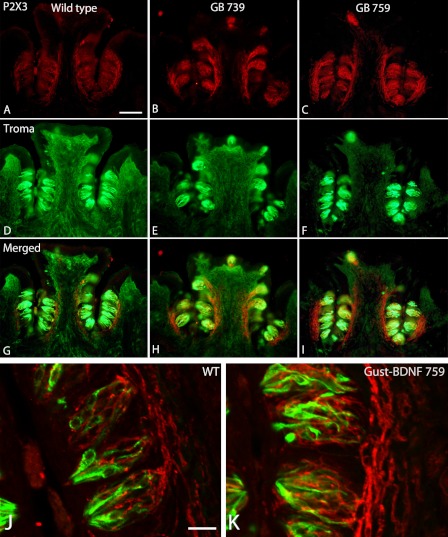
We next investigated whether there was a difference in the expression of the purinergic receptor P2X3 between the transgenic and wild-type mice. P2X3 has been proposed to function as a specific marker for gustatory nerve fibers (38). Nerve fibers innervating the circumvallate taste buds stained intensely with P2X3 receptor antibodies in both wild-type and high BDNF-expressing Gust-BDNF mice (Fig. 6). There was much stronger labeling in Gust-BDNF 759 followed by Gust-BDNF 739 than in wild-type mice.
FIGURE 6.
Double-label immunohistochemistry show expression of the purinergic receptor P2X3 (red) and Troma (green). A–C, Gust-BDNF 739 (A) and Gust-BDNF 759 (B) have a richer and denser innervation than wild type (C). D–F, Troma-I-reactive cells in the same sections of circumvallate papillae. G–I, merged images reveal P2X3 receptor expression in Troma-I-positive cells. Scale bar in A represents 100 μm and applies to A–I. J and K, high magnification confocal images of representative taste buds from wild-type (J) and Gust-BDNF 759 (K). Taste buds are larger and have significantly more P2X3-immunoreactive nerve fibers in Gust-BDNF 759 than in wild-type mice. Scale bar in J represents 20 μm and applies to J and K.
Gene Expression in Circumvallate Taste Buds
Because many intraepithelial NCAM-positive nerve fibers are present in the gustatory epithelia of circumvallate papillae, it makes it difficult to distinguish between immunostaining for NCAM-positive nerve fibers and NCAM-producing taste cells. In addition, to examine whether BDNF overexpression influences the morphological changes in taste buds, there is a requirement for BDNF downstream signaling. The transgene contains only the mature BDNF sequence, suggesting that only the mature protein would be overexpressed and overproduced. Thus, we also examined the taste epithelia expression of the high affinity receptor TrkB (Ntrk2) in circumvallate taste buds. Taste buds and gustatory epithelia were isolated using LCM on circumvallate papillae of 6-week-old Gust-BDNF mouse lines and their wild-type littermates. Real-time PCR confirmed an increase of transcript levels of BDNF, Ntrk2, and NCAM1 in Gust-BDNF 739 and 759 compared with wild-type littermates (Fig. 1B).
DISCUSSION
In the present study, we used a well characterized α-gustducin promoter (23) to drive site-specific overexpression of BDNF in taste buds. Three lines were established, two of which were high BDNF-overexpressing. We verified the increased transcriptional and translational activity of BDNF by PCR on LCM taste tissues and by immunohistochemistry on taste buds. We examined TrkB phosphorylation in response to BDNF overexpression and detected increased levels of TrkB activation in Gust-BDNF transgenic mice. High BDNF-overexpressing mice have larger taste buds and a larger number of taste cells. The larger size and cell number correspond to the increased density of the innervation in taste buds. High BDNF-overexpressing mice have hyperinnervated taste buds compared with wild-type mice. Interestingly, the increase in the gustatory innervation constitutes a major part of the general innervation density, as evaluated by immunohistochemistry to P2X3 ATP receptors. P2X3 antibodies have been suggested to identify gustatory nerve fibers in taste buds as well as some taste cells (38). In addition, there are a significantly lager number of NCAM- and pTrkB-positive cells in taste buds. NCAM is normally a marker for type III cells. This suggests that BDNF overexpression leads to an increased number of type III cells. BDNF overexpression did not cause an increased number of type II cells because phospholipase Cβ2 expression was similar to that found in wild-type mice.
NCAM-positive cells normally constitute only ∼10% of the cells in mature taste buds (20). There was a clear increase in the number of NCAM-positive cells in the taste buds of the transgenic mice, as also indicated by the significant increase in the NCAM gene expression in circumvallate taste epithelia. This shows not only that NCAM protein expression is highly up-regulated, but there is also a clear taste bud-specific increase in local transcription of NCAM. Thus, we suggest that BDNF directly or indirectly increases the transcription of neuronal genes in taste buds.
A previous study showed TrkB immunoreactivity in taste buds and its co-localization with BDNF (39). In the present study, we show for the first time TrkB activation in taste cells in response to BDNF. Increased levels of BDNF in taste buds directly correlated with the elevated activation levels of TrkB. Thus, not only does BDNF positively influence transcription of its high affinity receptor as shown in the gene expression section of this study, but also there is a higher level of TrkB phosphorylation in the taste cells of Gust-BDNF transgenic mice. We propose that Gust-BDNF mice would offer an excellent animal model to study the functional roles of BDNF and TrkB in a sensory organ that depends highly on BDNF for formation and function. In addition, there was a larger number of taste cells that contained labeling for pTrkB in Gust-BDNF taste buds, and there was an elevated level of pTrkB in the nerve fibers innervating the circumvallate papillae in these mice compared with wild-type controls. These findings suggest that BDNF is indeed involved in local taste cell-related events and in taste cell interaction with gustatory neurons. Interestingly, whereas pTrkB labeling in taste cells was altered in response to increased levels of BDNF, it was unchanged in the hippocampus and cortex, indicating taste cell specificity for α-gustducin promoter and BDNF overexpression in taste cells.
There is ample evidence that unprocessed secreted proneurotrophins function as ligands and bind to p75NTR with high affinity whereas mature neurotrophin proteins bind Trk receptors with high affinity (40). There is indeed p75NTR expression in taste buds and taste nerve fibers (41, 42). We show clearly that TrkB is active under such conditions, but its levels are elevated in Gust-BDNF mice. The simultaneous increased taste bud TrkB expression in response to increased levels of BDNF suggests that BDNF might control the expression of its own high affinity receptor in taste buds.
The relationship between BDNF and NCAM has been explored in previous studies. It was shown that NCAM phosphorylation in response to BDNF is involved in cortical neuron differentiation and survival (43). NCAM would bind BDNF to increase its concentration and participate in presentation of the ligand to its receptor TrkB. TrkB activation by BDNF would regulate many of its biological functions (44–48). Many receptor components are indeed involved in ligand concentration and presentation in the nervous system. However, it was later shown that there was a direct interaction between NCAM and TrkB molecules through their intracellular domains. TrkB activation enhanced by the presence of BDNF would lead to NCAM tyrosine phosphorylation, a process that is proposed to be involved in NCAM-mediated neurite outgrowth (49). NCAM-induced hippocampal neurite outgrowth is dependent on TrkB because reduction of TrkB expression inhibits this neurite outgrowth (50). In line with these reports, our findings postulate that overexpression of BDNF might directly promote TrkB binding to NCAM through its intracellular domain and lead to tyrosine phosphorylation of NCAM and thereby modulating NCAM-mediated functions. Increased BDNF expression, as seen in high BDNF-expressing transgenic mice, promotes both TrkB and NCAM expression, suggesting that BDNF activation of TrkB promotes its expression and thus modulates the functions of a neural cell adhesion molecule possibly by direct interaction.
Another plausible mechanism for BDNF function in the peripheral taste system might be related to its function in the pain transduction and nociceptive system (51). It was shown that calcitonin gene-related peptide (CGRP) promoted P2X3 transcription in trigeminal sensory neurons. BDNF appeared also to enhance P2X3 transcription. There is a significant increase in the density of P2X3-positive nerve fibers and to some extent taste cells in high BDNF-expressing transgenic mice. Overexpressed BDNF would lead either directly to increased transcription and synthesis of P2X3 or indirectly by promoting CGRP effect in taste buds. Perigemmal sensory nerve fibers are indeed CGRP-positive (52, 53).
It has been reported that the density of fungiform papillae and fungiform taste buds correlate well with the perception of the bitter tasting compound 6-n-propylthiouracil (PROP). Perception of the bitter taste of PROP and the intensity with which human subjects rate its bitterness have led to identification of a subset of human subjects that have been called supertasters (54). Supertasters perceive an intense bitterness when exposed to PROP whereas other subjects might rate it tasteless. Not only do supertasters taste PROP, they also perceive stronger tastes from a variety of bitter and sweet compounds as well as oral irritants such as alcohol and capsaicin (54). The supertasting phenomenon in humans correlates directly with the number of taste receptors in the anterior portion of the tongue. Whereas the genetic background in rodents influences the ability of the animals to taste different compounds or require different concentrations of a compound to taste them (55, 56), there are no animal models in rodents that correspond to the phenomenon of supertasting in humans. Based on the anatomical evidence provided in the present study and our preliminary behavioral data, we propose that high BDNF-overexpressing mice may provide an animal model for supertasting. In high BDNF-overexpressing mice (i) taste buds are larger and have a larger number of taste cells and (ii) there is an increase in the density of gustatory innervation. Further studies are required to examine whether high BDNF-expressing mice might be a model for human supertasting. In addition, preliminary microarray data (data not shown) identified significant up-regulation of multiple genes involved in cell processes such as cell-cell signaling, neurogenesis, and development in high BDNF-expressing transgenic mice compared with low BDNF-expressing line and wild-type mice.
Acknowledgments
We thank Michelle Sims for mouse colony management, Carlos Ibanez, Sami Damak and Akira Ito for discussions.
This work was supported by National Institutes of Health Grant R01-RDC007628 through the NIDCD.
- NCAM
- neural cell adhesion molecule
- BGH-PA
- bovine growth hormone polyadenylation site
- CGRP
- calcitonin gene-related peptide
- Gust
- 7.7-kb α-gustducin promoter
- LCM
- laser capture microdissection
- NGS
- normal goat serum
- PROP
- 6-n-propylthiouracil.
REFERENCES
- 1. Cohen S., Levi-Montalcini R., Hamburger V. (1954) A nerve growth-stimulating factor isolated from sarcomas 37 and 180. Proc. Natl. Acad. Sci. U.S.A. 40, 1014–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewin G. R., Barde Y. A. (1996) Physiology of the neurotrophins. Annu. Rev. Neurosci. 19, 289–317 [DOI] [PubMed] [Google Scholar]
- 3. Ernfors P., Lee K. F., Jaenisch R. (1994) Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 368, 147–150 [DOI] [PubMed] [Google Scholar]
- 4. Fritzsch B., Silos-Santiago I., Bianchi L. M., Fariñas I. (1997) The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 20, 159–164 [DOI] [PubMed] [Google Scholar]
- 5. Ernfors P., Van De Water T., Loring J., Jaenisch R. (1995) Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 14, 1153–1164 [DOI] [PubMed] [Google Scholar]
- 6. Nosrat C. A., Blomlöf J., ElShamy W. M., Ernfors P., Olson L. (1997) Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development 124, 1333–1342 [DOI] [PubMed] [Google Scholar]
- 7. Barlow L. A., Northcutt R. G. (1995) Embryonic origin of amphibian taste buds. Dev. Biol. 169, 273–285 [DOI] [PubMed] [Google Scholar]
- 8. Chaudhari N., Roper S. D. (2010) The cell biology of taste. J. Cell Biol. 190, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nosrat I. V., Lindskog S., Seiger A., Nosrat C. A. (2000) Lingual BDNF and NT-3 mRNA expression patterns and their relation to innervation in the human tongue: similarities and differences compared with rodents. J. Comp. Neurol. 417, 133–152 [PubMed] [Google Scholar]
- 10. Beidler L. M., Smallman R. L. (1965) Renewal of cells within taste buds. J. Cell Biol. 27, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farbman A. I. (1980) Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 13, 349–357 [DOI] [PubMed] [Google Scholar]
- 12. Lindemann B. (1996) Chemoreception: tasting the sweet and the bitter. Curr. Biol. 6, 1234–1237 [DOI] [PubMed] [Google Scholar]
- 13. Murray R. G., Murray A. (1967) Fine structure of taste buds of rabbit foliate papillae. J. Ultrastruct. Res. 19, 327–353 [DOI] [PubMed] [Google Scholar]
- 14. Clapp T. R., Stone L. M., Margolskee R. F., Kinnamon S. C. (2001) Immunocytochemical evidence for co-expression of type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rebello M. R., Aktas A., Medler K. F. (2011) Expression of calcium binding proteins in mouse type II taste cells. J. Histochem. Cytochem. 59, 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boughter J. D., Jr., Pumplin D. W., Yu C., Christy R. C., Smith D. V. (1997) Differential expression of α-gustducin in taste bud populations of the rat and hamster. J. Neurosci. 17, 2852–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLaughlin S. K., McKinnon P. J., Margolskee R. F. (1992) Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357, 563–569 [DOI] [PubMed] [Google Scholar]
- 18. Chandrashekar J., Yarmolinsky D., von Buchholtz L., Oka Y., Sly W., Ryba N. J., Zuker C. S. (2009) The taste of carbonation. Science 326, 443–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang A. L., Chen X., Hoon M. A., Chandrashekar J., Guo W., Tränkner D., Ryba N. J., Zuker C. S. (2006) The cells and logic for mammalian sour taste detection. Nature 442, 934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson G. M., Finger T. E. (1993) Immunolocalization of different forms of neural cell adhesion molecule (NCAM) in rat taste buds. J. Comp. Neurol. 336, 507–516 [DOI] [PubMed] [Google Scholar]
- 21. Yang R., Crowley H. H., Rock M. E., Kinnamon J. C. (2000) Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J. Comp. Neurol. 424, 205–215 [DOI] [PubMed] [Google Scholar]
- 22. Yee C. L., Jones K. R., Finger T. E. (2003) Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J. Comp. Neurol. 459, 15–24 [DOI] [PubMed] [Google Scholar]
- 23. Wong G. T., Ruiz-Avila L., Margolskee R. F. (1999) Directing gene expression to gustducin-positive taste receptor cells. J. Neurosci. 19, 5802–5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carr V. M., Sollars S. I., Farbman A. I. (2005) Neuronal cell death and population dynamics in the developing rat geniculate ganglion. Neuroscience 134, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 25. Krimm R. F., Hill D. L. (1998) Innervation of single fungiform taste buds during development in rat. J. Comp. Neurol. 398, 13–24 [PubMed] [Google Scholar]
- 26. Zaidi F. N., Krimm R. F., Whitehead M. C. (2007) Exuberant neuronal convergence onto reduced taste bud targets with preservation of neural specificity in mice overexpressing neurotrophin in the tongue epithelium. J. Neurosci. 27, 13875–13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ringstedt T., Ibáñez C. F., Nosrat C. A. (1999) Role of brain-derived neurotrophic factor in target invasion in the gustatory system. J. Neurosci. 19, 3507–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krimm R. F., Miller K. K., Kitzman P. H., Davis B. M., Albers K. M. (2001) Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev. Biol. 232, 508–521 [DOI] [PubMed] [Google Scholar]
- 29. Kozak M. (1987) An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15, 8125–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albers K. M., Wright D. E., Davis B. M. (1994) Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J. Neurosci. 14, 1422–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knapp L., Lawton A., Oakley B., Wong L., Zhang C. (1995) Keratins as markers of differentiated taste cells of the rat. Differentiation 58, 341–349 [DOI] [PubMed] [Google Scholar]
- 32. Zhang C., Cotter M., Lawton A., Oakley B., Wong L., Zeng Q. (1995) Keratin 18 is associated with a subset of older taste cells in the rat. Differentiation 59, 155–162 [DOI] [PubMed] [Google Scholar]
- 33. Nosrat I. V., Agerman K., Marinescu A., Ernfors P., Nosrat C. A. (2004) Lingual deficits in neurotrophin double knockout mice. J. Neurocytol. 33, 607–615 [DOI] [PubMed] [Google Scholar]
- 34. Gulbransen B. D., Finger T. E. (2005) Solitary chemoreceptor cell proliferation in adult nasal epithelium. J. Neurocytol. 34, 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friedman W. J., Black I. B., Kaplan D. R. (1998) Distribution of the neurotrophins brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the postnatal rat brain: an immunocytochemical study. Neuroscience 84, 101–114 [DOI] [PubMed] [Google Scholar]
- 36. Chen L. Y., Rex C. S., Pham D. T., Lynch G., Gall C. M. (2010) BDNF signaling during learning is regionally differentiated within hippocampus. J. Neurosci. 30, 15097–15101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshii A., Murata Y., Kim J., Zhang C., Shokat K. M., Constantine-Paton M. (2011) TrkB and protein kinase Mζ regulate synaptic localization of PSD-95 in developing cortex. J. Neurosci. 31, 11894–11904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ishida Y., Ugawa S., Ueda T., Yamada T., Shibata Y., Hondoh A., Inoue K., Yu Y., Shimada S. (2009) P2X(2)- and P2X(3)-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. J. Comp. Neurol. 514, 131–144 [DOI] [PubMed] [Google Scholar]
- 39. Yee C., Bartel D. L., Finger T. E. (2005) Effects of glossopharyngeal nerve section on the expression of neurotrophins and their receptors in lingual taste buds of adult mice. J. Comp. Neurol. 490, 371–390 [DOI] [PubMed] [Google Scholar]
- 40. Lee R., Kermani P., Teng K. K., Hempstead B. L. (2001) Regulation of cell survival by secreted proneurotrophins. Science 294, 1945–1948 [DOI] [PubMed] [Google Scholar]
- 41. Krimm R. F. (2006) Mice lacking the p75 receptor fail to acquire a normal complement of taste buds and geniculate ganglion neurons by adulthood. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288, 1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fan L., Girnius S., Oakley B. (2004) Support of trigeminal sensory neurons by nonneuronal p75 neurotrophin receptors. Brain Res. Dev. Brain Res. 150, 23–39 [DOI] [PubMed] [Google Scholar]
- 43. Vutskits L., Djebbara-Hannas Z., Zhang H., Paccaud J. P., Durbec P., Rougon G., Muller D., Kiss J. Z. (2001) PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur. J. Neurosci. 13, 1391–1402 [DOI] [PubMed] [Google Scholar]
- 44. Berkemeier L. R., Winslow J. W., Kaplan D. R., Nikolics K., Goeddel D. V., Rosenthal A. (1991) Neurotrophin-5: a novel neurotrophic factor that activates Trk and TrkB. Neuron 7, 857–866 [DOI] [PubMed] [Google Scholar]
- 45. Glass D. J., Nye S. H., Hantzopoulos P., Macchi M. J., Squinto S. P., Goldfarb M., Yancopoulos G. D. (1991) TrkB mediates BDNF/NT-3-dependent survival and proliferation in fibroblasts lacking the low affinity NGF receptor. Cell 66, 405–413 [DOI] [PubMed] [Google Scholar]
- 46. Klein R., Nanduri V., Jing S. A., Lamballe F., Tapley P., Bryant S., Cordon-Cardo C., Jones K. R., Reichardt L. F., Barbacid M. (1991) The TrkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 66, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Middlemas D. S., Lindberg R. A., Hunter T. (1991) TrkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol. Cell. Biol. 11, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Squinto S. P., Stitt T. N., Aldrich T. H., Davis S., Bianco S. M., Radziejewski C., Glass D. J., Masiakowski P., Furth M. E., Valenzuela D. M. (1991) TrkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell 65, 885–893 [DOI] [PubMed] [Google Scholar]
- 49. Cassens C., Kleene R., Xiao M. F., Friedrich C., Dityateva G., Schafer-Nielsen C., Schachner M. (2010) Binding of the receptor tyrosine kinase TrkB to the neural cell adhesion molecule (NCAM) regulates phosphorylation of NCAM and NCAM-dependent neurite outgrowth. J. Biol. Chem. 285, 28959–28967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doherty P., Fruns M., Seaton P., Dickson G., Barton C. H., Sears T. A., Walsh F. S. (1990) A threshold effect of the major isoforms of NCAM on neurite outgrowth. Nature 343, 464–466 [DOI] [PubMed] [Google Scholar]
- 51. Simonetti M., Giniatullin R., Fabbretti E. (2008) Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J. Biol. Chem. 283, 18743–18752 [DOI] [PubMed] [Google Scholar]
- 52. Montavon P., Lindstrand K. (1991) Immunohistochemical localization of neuron-specific enolase and calcitonin gene-related peptide in pig taste papillae. Regulatory Peptides 36, 235–248 [DOI] [PubMed] [Google Scholar]
- 53. Kinnman E., Aldskogius H. (1991) The role of substance P and calcitonin gene-related peptide containing nerve fibers in maintaining fungiform taste buds in the rat after a chronic chorda tympani nerve injury. Exp. Neurol. 113, 85–91 [DOI] [PubMed] [Google Scholar]
- 54. Bartoshuk L. M., Duffy V. B., Miller I. J. (1994) PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol. Behav. 56, 1165–1171 [DOI] [PubMed] [Google Scholar]
- 55. Bachmanov A. A., Li X., Reed D. R., Ohmen J. D., Li S., Chen Z., Tordoff M. G., de Jong P. J., Wu C., West D. B., Chatterjee A., Ross D. A., Beauchamp G. K. (2001) Positional cloning of the mouse saccharin preference (Sac) locus. Chem. Senses 26, 925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boughter J. D., Jr., Whitney G. (1997) Behavioral specificity of the bitter taste gene Soa. Physiol. Behav. 63, 101–108 [DOI] [PubMed] [Google Scholar]
- 57. Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]