Background: Verotoxin internalization and retrograde transport to the Golgi/ER is mediated by Gb3 glycolipid.
Results: Amphipathic Gb3 mimics can alter binding, trafficking, and cytotoxicity of verotoxins.
Conclusion: The lipid moiety of Gb3 analogues determines the trafficking of verotoxins.
Significance: Synthetic glycolipid analogues can function as membrane receptors to internalize bound ligand and subvert endogenous GSL traffic.
Keywords: Bacterial Toxins, Glycobiology, Receptor Endocytosis, Receptor Modification, Trafficking, Glycosphingolipid, Verotoxin, Glycosphingolipid Mimics, Intracellular Traffic, Cell Cytotoxicity
Abstract
The verotoxin (VT) (Shiga toxin) receptor globotriaosyl ceramide (Gb3), mediates VT1/VT2 retrograde transport to the endoplasmic reticulum (ER) for cytosolic A subunit access to inhibit protein synthesis. Adamantyl Gb3 is an amphipathic competitive inhibitor of VT1/VT2 Gb3 binding. However, Gb3-negative VT-resistant CHO/Jurkat cells incorporate adaGb3 to become VT1/VT2-sensitive. CarboxyadaGb3, urea-adaGb3, and hydroxyethyl adaGb3, preferentially bound by VT2, also mediate VT1/VT2 cytotoxicity. VT1/VT2 internalize to early endosomes but not to Golgi/ER. AdabisGb3 (two deacyl Gb3s linked to adamantane) protects against VT1/VT2 more effectively than adaGb3 without incorporating into Gb3-negative cells. AdaGb3 (but not hydroxyethyl adaGb3) incorporation into Gb3-positive Vero cells rendered punctate cell surface VT1/VT2 binding uniform and subverted subsequent Gb3-dependent retrograde transport to Golgi/ER to render cytotoxicity (reduced for VT1 but not VT2) brefeldin A-resistant. VT2-induced vacuolation was maintained in adaGb3-treated Vero cells, but vacuolar membrane VT2 was lost. AdaGb3 destabilized membrane cholesterol and reduced Gb3 cholesterol stabilization in phospholipid liposomes. Cholera toxin GM1-mediated Golgi/ER targeting was unaffected by adaGb3. We demonstrate the novel, lipid-dependent, pseudoreceptor function of Gb3 mimics and their structure-dependent modulation of endogenous intracellular Gb3 vesicular traffic.
Introduction
Verotoxin (VT)3 comprises a family of Escherichia coli-derived AB5 subunit toxins (also termed Shiga toxins). Verotoxin cytopathology is targeted via the B subunit pentamer of the holotoxin binding to its receptor glycosphingolipid (GSL), globotriaosyl ceramide (Gb3; also known as the pk blood group antigen (1) and CD77, a human B cell marker (2)). VT1 and VT2 (60% identical at the nucleotide level (3)) are the primary verotoxins associated with clinical disease (4). Gastrointestinal infection with verotoxin-producing E. coli can result in the pathology of hemorrhagic colitis, which may precede the more severe hemolytic uremic syndrome (HUS), a renal pathology characterized by a triad of symptoms, thrombocytopenia, anemia, and renal glomerular microangiopathy (5). Hemorrhagic colitis is mediated via VT targeting Gb3 within the submucosal microvasculature of the GI tract. Subsequent systemic verotoxemia results in toxin access to renal glomerular endothelial cells, which also express Gb3 (6) to mediate endothelial cell damage, blood vessel occlusion, glomerular infarct, and subsequent hemolysis. HUS, primarily a disease of the very young and elderly (7), currently retains an approximately 5% mortality, and estimates of morbidity range as high as 30%. The recent German outbreak of enteroaggregative, VT2-expressing E. coli infections (8, 9) with an HUS incidence reaching 25% and a preponderance of female adult cases indicates major, unsuspected knowledge gaps in VT-induced pathology.
For reasons as yet unclear, VT2 is more frequently associated with clinical disease than VT1 (10, 11), despite the fact that VT1 is a more potent cytotoxin in vitro (12) and both toxins bind to the same receptor (13). Despite a common receptor Gb3, VT1 and VT2 preferentially bind different and shared epitopes within the Gb3 carbohydrate (14, 15), which may be differentially available within different lipid contexts (12). Such differential receptor binding results in coincident but also discreet VT1 and VT2 binding sites on the surface of sensitive cells (12, 16) and within human renal tissue (15, 17). Cholesterol within human renal glomeruli can mask Gb3 to prevent VT1 and VT2 binding (15, 17). Unlike VT1, VT2 can induce the formation of intracellular vacuoles in a subfraction of susceptible renal epithelial cells (12).
Cell membrane GSL carbohydrate presentation for ligand binding is complex, being a function of both the highly heterogeneous composition of the membrane-embedded ceramide and a lateral association with other membrane lipids, most notably cholesterol (18), to form domains of differential membrane order (19). Molecular simulation shows that the cholesterol-GSL interaction can alter the GSL carbohydrate conformation (18, 20) from a membrane-perpendicular to -parallel format. Cholesterol can mask GSLs to prevent appropriate ligand binding in tissues (15, 17, 20) and in model and cell membranes (20, 21). Nevertheless, to mediate cell cytotoxicity in vitro, Gb3 must be expressed in what are termed cell surface lipid microdomains or rafts (22, 23), in which the concentration of GSLs and cholesterol and membrane order are significantly increased (24). The renal glomerulus is the target of the VT-induced pathology of HUS, and glomerular Gb3 is within such domains (17). We propose that adaGSLs, unlike the parent GSL, will not interact with cholesterol; indeed, the adamantane frame may partially substitute for cholesterol to provide a mimic of the GSL-cholesterol complex (25).
Membrane Gb3 within such domains mediates both clathrin-dependent (26) and -independent (27) VT internalization and subsequent “retrograde transport,” from the cell surface through endosomes, trans-Golgi network (TGN), and Golgi to the ER (28), where the A subunit is translocated to the cytosol for inhibition of protein synthesis (29). When VT binds to non-raft Gb3, internalization of the toxin receptor complex mediates the transport of the toxin to the lysosome for degradation, without the induction of cytotoxicity (22, 30). This may be similar to the abnormal transport of accumulated GSLs and cholesterol to lysosomes in GSL storage diseases (31). Cholesterol depletion (27) or modulation (32) can also prevent the GSL endosome-TGN transition. A balance of GSL-cholesterol interaction may be required for retrograde transport.
Verotoxin binding to the Gb3 oligosaccharide is modulated by the lipid moiety of Gb3 (33, 34) and the membrane environment in which Gb3 is presented (35). This has been termed “aglycone modulation of GSL receptor function” (36). The requirement for retrograde transport of the toxin receptor complex to the endoplasmic reticulum is shared by the cholera toxin/GM1 receptor interaction (37, 38). Exogenous GSL analogues in which the native fatty acid is replaced by a fluorescent group (e.g. BODIPY or NBD) also demonstrate retrograde transport from the cell surface to the Golgi (39).
Several groups have developed receptor analogues based on the Gb3 carbohydrate sequence coupled to polymeric or pentameric scaffolds (40–42) to develop specific receptor-based means to prevent the in vivo cytotoxicity, which may follow verotoxin-producing E. coli infection. The binding affinity of the VT B subunit pentamer for the lipid-free oligosaccharide is much reduced compared with native Gb3 glycolipid (43), but this can be largely countered by multivalency (41), particularly when tailored to accommodate the pentameric geometry of the receptor B subunits displayed within the VT holotoxin (40, 42).
An additional approach is to try to utilize the inherent high affinity binding of the native Gb3 glycolipid. Substitution of the Gb3 fatty acid with an adamantane frame provided a water-soluble analog of Gb3 that retained high affinity VT1 binding in an aqueous environment (44, 45). Although this analog proved an effective competitor to prevent VT1 and VT2 cytotoxicity in vitro (12), in vivo, the analog was found to augment rather than reduce VT2 cytopathology (46).
Our present studies indicate that this is due to a previously unrecognized property of such GSL analogues. We now show that adaGb3 can partition into receptor-negative cells to render them VT1- and VT2-sensitive. This pseudoreceptor function is mediated via a novel intracellular routing pathway involving early endosomes. Moreover, we find that this adaGb3 pathway can also hijack the endogenous cellular Gb3-mediated retrograde transport of VT in sensitive cells to reroute traffic and induce resistance. This may relate to loss of interaction with cholesterol that we show for the adaGSL mimic. We have designed several new soluble adaGb3 analogues and show this dominant rerouting of native intracellular GSL trafficking is prevented by chemical substitution within the adamantane frame, suggesting the existence of intramembrane vesicular trafficking cues.
A dimeric Gb3 analog retains the VT1/VT2 inhibitory activity of adaGb3 in solution but is unable to insert into cell membranes to show VT1/VT2 pseudoreceptor function. These studies demonstrate the importance of the lipid chemistry of Gb3 in membrane incorporation and intracellular trafficking and illustrate a new approach against VT-induced cytopathology. Exogenous GSL mimics can be functionally trafficked in cells and, according to their lipid structure, subvert endogenous intracellular membrane GSL trafficking pathways.
EXPERIMENTAL PROCEDURES
Synthetic Compounds
Synthesis of the adamantyl analogues of Gb3 (Fig. 1) is described in the supplemental material. Analogues were prepared for cell insertion as follows. Compounds were dried from solution in CHCl3-CH3OH (2:1, v/v) under N2, resuspended in ethanol, sonicated briefly, dried under N2, and resuspended in water at a concentration of 100 μm. To allow the analogues to reach an equilibrium state in solution, solutions were vortexed and sonicated for 30 s and then incubated at 37 °C for 2 h. Aliquots were dispensed into glass tubes, rapidly frozen on dry ice, and lyophilized overnight. The compounds were redissolved in chilled serum-free culture medium immediately before the addition to cells.
FIGURE 1.
Scheme for adaGb3, carboxyadaGb3, urea-adaGb3, OHEtadaGb3, and adabisGb3.
Reagents and Antibodies
Verotoxin 1 (VT1) and VT2 and VT1 B-subunit were purified as described previously (34, 46). Antibodies used were as follows. Mouse anti-VT1 mAb PH-1, reactive against the VT1 B-subunit and polyclonal rabbit anti-VT1 B-subunit, were prepared in our laboratory. Rabbit anti-VT2 was a generous gift of Dr. Glen Armstrong (University of Calgary). Goat anti-EEA1 (Santa Cruz Biotechnology, Inc.), mouse anti-Lamp-2 (Developmental Studies Hybridoma Bank, clone H4B4), rabbit anti-Rab6 (Santa Cruz Biotechnology, Inc.), and rabbit anti-calnexin (Enzo Life Sciences) were obtained as indicated. Brefeldin A, cholera toxin B-subunit, sphingomyelin (SPM), methyl-β-cyclodextrin (MβCD), cholesterol, egg phosphatidylcholine (PC), and crystal violet were from Sigma. Fluorescence mounting medium was from Dako, and paraformaldehyde was from EM Sciences. [3H]cholesterol and Cy3 were from GE Healthcare. Alexa Fluor 488 pentafluorophenyl ester, DiI LDL, DAPI, and Texas Red sulfonyl chloride were from Molecular Probes. Proteins were labeled with fluorophores using standard conditions as recommended by the manufacturer and isolated by gel filtration using G-25.
Cell Culture
Vero cells were grown in Eagle's minimum essential medium, 5% FCS, CHO; HEK-293 cells were grown in DMEM, 10% FCS; and Jurkat cells were grown in RPMI 1640, 10% FCS at 37 °C, 5% CO2. All tissue culture media and buffers were obtained from Wisent Inc.
Insertion of GSL Analogues into Cells
Cells were washed twice with serum-free RPMI 1640 with 20 mm Hepes (H-RPMI) and chilled on ice. Cells were then incubated with freshly dissolved adaGb3-solution for 1 h at 4 °C. Serum-containing medium was added for 37 °C incubations exceeding 1 h.
Cell Cytotoxicity Assays
Vero and CHO cells seeded in 96-well cell culture plates (3 × 104 cells/well) were grown at 37 °C overnight. Jurkat cells were centrifuged, washed twice with chilled H-RPMI, distributed in 96-well plates (5 × 104 cells/well), and chilled on ice.
Dilutions of adamantyl analogues were added to the cells and incubated on ice for 1 h. 10-fold serial VT1/VT2 or ricin dilutions were then added to the cells and incubated at 37 °C. After 4 h, serum was added to a final concentration of 5%, and cells were incubated at 37 °C for another 68 h. In some experiments, 0.5 μg/ml brefeldin A (BFA) was added for 30 min at 37 °C before the addition of VT and maintained throughout. Due to the long term toxicity of BFA, cytotoxicity was measured after 18 h of toxin treatment.
At the end of the incubation period, live cells were fixed onto the wells using 2% formalin in PBS and stained with crystal violet as described (47). Dye was solubilized with 100 μl of 10% acetic acid, and optical density was read at 560 nm using an ELISA plate reader. Cell viability was expressed as a percentage of control cells, which were treated with neither VT nor adaGb3 analogues.
The ability of the adamantyl Gb3 analogues in solution to block VT cytotoxicity to Gb3+ve cells was assessed by prebinding the toxin and analogues prior to the addition to Vero cells. Dilutions (50 ng/ml to 0.03 pg/ml) of VT1 or VT2 in Eagle's minimum essential medium were prepared and mixed with an equal volume of adaGb3 or adabisGb3 (100, 50, 25, 12.5, 6.3, and 3.1 μm). After incubation at 37 °C for 60 min, 50 μl of the mixture was added to Vero cells in 96-well plates and incubated for 1 h at 37 °C. Cells were washed with Eagle's minimum essential medium and then incubated in complete medium for 72 h at 37 °C. Cell viability was measured as described above by crystal violet staining.
Confocal Fluorescence Microscopy
CHO and Jurkat cells were grown to 80% confluence on 12-mm gelatin-treated glass coverslips. Cells were washed twice with H-RPMI, chilled on ice, and then incubated with 50 μm adabisGb3 or 20 μm adaGb3 (CHO) or 10 μm adaGb3 (Jurkat) in H-RPMI for 1 h on ice. For labeling of the cells with VT, 4 μg/ml Alexa Fluor 488-VT1 or Texas Red-VT2 was bound on ice for 1 h. Cells were washed with cold PBS and fixed with 4% paraformaldehyde in PBS. To observe the intracellular trafficking of VT, bound VT was internalized at 37 °C for 10 min, 1 h, or 6 h. At the end of the incubation period, the cells were fixed with 4% paraformaldehyde in PBS, permeabilized for 15 min at ambient temperature with 0.2% Triton X-100, and then blocked with 2% BSA. Verotoxins were detected with VT1- or VT2-specific antibodies and the organelle-specific antibodies to EEA1 (early endosomes), Rab6 (Golgi), Lamp-2 (late endosome/lysosome), or calnexin (ER), followed after washing with Alexa Fluor 488 or 546 anti-goat or 594 anti-rabbit secondary antibody.
Microscope Image Acquisition
Fluorescently stained cells were viewed with a Leica DMRE2 confocal microscope under oil at ×63 with a numerical aperture of 1.4 at ambient temperature. Fluorophores used were DAPI for nuclear staining, Alexa488 (for VT), Cy3 (for cholera toxin (CT)), Texas Red (for VT2), and Alexa 594 (secondary antibody). Images were captured with a Hamamatsu EM-CCD C9100 digital camera using Volocity 5.5.0. Confocal stacks were deconvolved with Volocity software by iterative restoration using calculated point spread functions. Composite images were assembled using Photoshop CS4 and Zeiss Image Examiner.
Isolation of Total Lipids from Cells and Detection by TLC
CHO cells (1 × 107 cells) and Jurkat cells (2.4 × 106 cells) were treated with 20 μm adaGb3 or 50 μm adabisGb3 for 1 h on ice and then washed with PBS. Cell suspensions in water were transferred into chloroform/methanol (2:1, v/v) and shaken vigorously overnight. After filtering the cell debris, the solvents were dried down, resuspended in methanolic NaOH for saponification, and then neutralized with NH4HOAc and HCl. The samples were desalted using Sep-Pak C18 cartridges (Waters, Milford, MA). The lipids were eluted with methanol and chloroform/methanol (2:1), dried down, and redissolved in 100 μl of chloroform/methanol (2:1). The samples (10 μl each) were separated on two identical TLC plates (chloroform/methanol/water; 65:25:4), one for detection with orcinol and one for VT binding.
VT1 and VT2 Binding to Gb3 and Analogues by TLC Overlay
After GSL separation, the TLC plates were dried and incubated with 1% fish gelatin in TBS for 3 h at room temperature and washed twice with TBS. The plates were incubated with VT1 B-subunit (0.35 μg/ml) or VT2 (2.5 μg/ml) overnight at 4 °C, with polyclonal rabbit anti-VT1 B-subunit (VT1B) or anti-VT2 for 3 h at room temperature, and with HRP-conjugated goat anti-rabbit IgG for 1 h at room temperature. Bound toxin was detected by development using 0.6 mg/ml 4-chloro-1-naphthol, 0.015% H2O2 in TBS.
Liposomal Assay of Glycosphingolipid-Cholesterol Interaction
The ability of Gb3 as compared with adaGb3 to interact with cholesterol in membranes was quantitated in liposomes by measuring the induced resistance to cholesterol extraction by MβCD, based on previous studies (48, 49). Multilamellar PC, [3H]cholesterol liposomes with or without Gb3, SPM, or adaGb3 were prepared. (a) 0.2 μmol of PC + 0.07 μmol of cholesterol (500,000 dpm), (b) 0.14 μmol of PC + 0.07 μmol of cholesterol + 0.07 μmol of Gb3, and (c) 0.07 μmol of PC + 0.07 μmol of cholesterol + 0.07 μmol of Gb3 or SPM + 0.07 μmol of adaGb3 were dried together from organic solvent under N2, freeze-dried, and vortexed in PBS for 30 min at room temperature to give a total lipid concentration of 500 μm in a total volume of 500 μl. Liposomes were briefly sonicated and incubated at 85 °C for 30 min with vortexing every 5 min, cooled to room temperature, centrifuged at 11,000 × g for 10 min, and washed in PBS. 50-μl aliquots were mixed with 50 μl of PBS with or without 0.5 mm MβCD at room temperature for 60 min with frequent vortexing. The suspension was centrifuged at 11,000 × g for 10 min, and tritium in the supernatant was counted in a scintillation counter.
RESULTS
Adamantyl Gb3 Analogues
Five soluble adamantyl Gb3 analogues were synthesized from deacyl(lyso)-Gb3 (see supplemental material). The structures of these species are shown in Fig. 1. Different substitutions at the 2-position of the adamantane frame generated an acidic carboxyadaGb3, a basic urea-adaGb3, and a neutral hydroxyethyl adaGb3 (OHEtadaGb3). Coupling a second lyso-Gb3 to carboxyadaGb3 generated the dimeric adamantylbisGb3 (adabisGb3).
VT1/VT2 Binding to Synthetic Gb3 Analogues
The VT binding activities of Gb3, adaGb3, OHEtadaGb3, carboxyadaGb3, urea-adaGb3, and adabisGb3 were compared by TLC overlay (Fig. 2). Gb3 and each adaGb3 analog were bound by VT1 and VT2. VT2/Gb3 binding was weaker than that of VT1, as shown previously (46). However, VT2 bound all adaGb3 species in preference to native Gb3. AdaGb3 and adabisGb3 bound strongly to both VT1 and VT2.
FIGURE 2.
VT1 and VT2 binding to Gb3 and adaGb3 analogues by TLC overlay. The glycolipids (1.3 μg each) were separated by TLC and detected by orcinol or by binding of either VT1 or VT2. The binding to adaGb3, OHEtadaGb3, carboxyadaGb3, urea-adaGb3, and adabisGb3 relative to Gb3 was calculated from the band intensities: VT1, 80, 20, 6, 35, and 68%; VT2, 400, 275, 140, 310, and 315%.
AdaGb3 Induction of VT1/VT2 Cytotoxicity to Gb3-negative Cells
To assess the potential pseudoreceptor function of these adaGb3 analogues, the Gb3-negative, VT-resistant Jurkat and CHO cell lines were selected as potential targets for receptor “reconstitution.”
Cells were incubated in the presence of adaGb3 and then treated with VT1 or VT2. The resulting cytotoxicity curves (Fig. 3A) show that untreated CHO cells were resistant to VT1 and VT2 below 10 μg/ml and that Jurkat cells were resistant at <1 μg/ml, whereas 10–20% cells were killed at 10 μg/ml. Cells treated with adaGb3, carboxyadaGb3, OHEtadaGb3, or urea-adaGb3 all showed increased susceptibility to VT1 and VT2 cytotoxicity. Significantly greater VT sensitivity was induced in Jurkat, as compared with CHO cells. Indeed, reconstituted CHO cells were only sensitive to the highest dose of VT1 and VT2 tested and essentially only at the highest concentration of adaGb3 analog added. The receptor-incorporated CHO cells were in general slightly more sensitive to VT1 than VT2. However, Jurkat cell VT1 and VT2 sensitivity was significantly increased following treatment with any of the adaGb3 analogues. Again susceptibility to VT2 was slightly less than that of VT1, and the different adaGb3 analogues showed varying degrees of induction of VT1 and VT2 sensitivity. Cells in which adaGb3 was incorporated showed the greatest response to VT1 and VT2, with a CD50 of between 0.1 and 10 ng/ml according to adaGb3 dosage. CarboxyadaGb3-treated cells and OHEtadaGb3-treated cells showed less VT1/VT2 sensitivity, with CD50 ranging from 1 to 100 ng/ml for VT1 and from 50 to 10,000 ng/ml for VT2 according to adaGb3 analog dosage. Cells treated with urea-adaGb3 were yet less sensitive to VT1 and VT2.
FIGURE 3.
A, induction of VT1/VT2 toxicity in adaGb3 analog-treated CHO and Jurkat cells. CHO or Jurkat cells were incubated with 0, 10, 20, or 50 μm adaGb3 at 4 °C for 1 h. Cells were treated with 10-fold serial diluted VT1 or VT2 and incubated at 37 °C. Cell viability was monitored after 72 h and expressed as a percentage of control cells, which were treated with neither VT nor adaGb3 analogues. B, effect of adaGb3 analogues on CHO/Jurkat cell viability. CHO or Jurkat cells were incubated with 0, 10, 20, or 50 μm adaGb3 (closed circles), OHEtadaGb3 (triangles), carboxyadaGb3 (squares), urea-adaGb3 (diamonds), or adabisGb3 (open circles) at 37 °C. Cell viability was monitored after 72 h and expressed as a percentage of non-treated control cells.
In contrast to the other adaGb3 derivatives, treatment of Jurkat or CHO cells with adabisGb3 had no subsequent effect on VT1 or VT2 sensitivity. AdabisGb3 treated cells remained resistant to VT1/VT2.
At high dosage, some adaGb3 analogues were toxic to Jurkat cells (Fig. 3B). A concentration of >20 μm adaGb3 or urea-adaGb3 was toxic, whereas OHEtadaGb3, carboxyadaGb3, and adabisGb3 were non-toxic at all doses. No analog showed cytotoxicity to CHO cells.
VT1/VT2 Binding to Gb3 Analog-treated CHO/Jurkat Cells
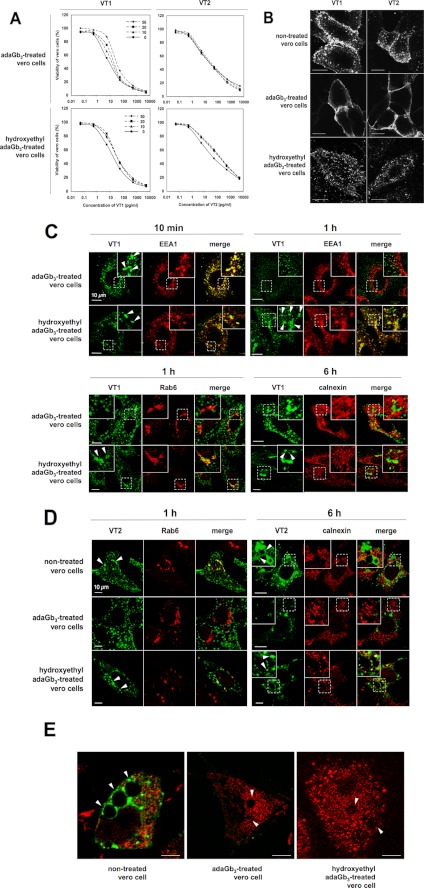
AdaGb3-treated CHO or Jurkat cells showed significant cell surface binding of fluorescent VT1 or VT2 at 4 °C (Fig. 4A). Cell membrane labeling was punctate, particularly for CHO cells. No VT binding to adabisGb3-treated CHO or Jurkat cells was detected.
FIGURE 4.
A, binding of VT1 or VT2 to adaGb3- or adabisGb3-treated Gb3-negative cells. CHO cells were treated with 20 μm adaGb3 (left) or 50 μm adabisGb3 (right), and Jurkat cells were treated with 10 μm adaGb3 (left) or 50 μm adabisGb3 (right). Then 4 μg/ml Alexa488-VT1B or Texas Red-VT2 was added at 4 °C. After 1 h, cells were washed and fixed. Stained cells were viewed by confocal microscopy. B and C, TLC of total lipids from adaGb3 or adabisGb3-treated cells. Glycolipids were isolated from CHO and Jurkat cells, separated by TLC, and visualized with orcinol (left). VT binding to the lipids was detected by TLC overlay with VT1 B-subunit (right). B, lane 1, adaGb3; lane 2, adabisGb3; lane 3, total lipids of untreated CHO cells; lane 4, total lipids of adaGb3-treated CHO cells; lane 5, total lipids of adabisGb3-treated CHO cells. C, lane 1, adaGb3; lane 2, adabisGb3; lane 3, total lipids of untreated Jurkat cells; lane 4, total lipids of adaGb3-treated Jurkat cells; lane 5, total lipids of adabisGb3-treated Jurkat cells. S, standard glycolipid mixture (from the top, glucosylceramide, galactosylceramide, lactosylceramide, Gb3, Gb4, and Gb5). D and E, trafficking of VT1 to the early endosome, Golgi, and ER in Vero cells compared with adaGb3-treated or OHEtadaGb3-treated CHO/Jurkat cells. VT1 was internalized at 37 °C for 10 min (D), 1 h (D and E), or 6 h (E) as indicated. After fixation and permeabilization, VT1 was detected with mAb PH-1/Alexa488 anti-mouse IgG (for EEA1 colocalization) or polyclonal anti-VT1B/Alexa488 anti-rabbit IgG (for RAb6 and calnexin colocalization). The early endosome marker EEA1 (D), Golgi marker Rab6 (E), or ER marker calnexin (E) was detected with Alexa546-labeled anti-goat or Alexa594 anti-rabbit antibodies (red). Fluorescently stained cells were viewed with a confocal microscope. Toxin colocalization (arrowheads) with organelle markers was quantitated (supplemental Table 1).
Cellular Uptake of AdaGb3 or AdabisGb3
To determine whether adabisGb3 is incorporated into the target cell membrane but becomes receptor-inactive, we extracted total lipids from adaGb3 or adabisGb3-treated CHO (Fig. 4B) and Jurkat cells (Fig. 4C) and detected the analogues by VT1 binding in a TLC overlay assay. AdaGb3 was incorporated into both Jurkat and CHO cells, whereas adabisGb3 was not detected in the extracts of adabisGb3-treated cells. These results show adaGb3 inserted into the cell membrane of Gb3-negative cells to provide a pseudoreceptor for VT1 and VT2. In contrast, adabisGb3 does not incorporate into the cell membrane of Gb3-negative cells.
Intracellular VT1 Trafficking in AdaGb3- or OHEtadaGb3-reconstituted Cells
Confocal microscopy was used to compare the intracellular trafficking of fluorescent VT1 bound to endogenous Gb3 in Vero cells and adaGb3 inserted into CHO or Jurkat cells. After 10 min at 37 °C, a large fraction of internalized VT1 colocalized with the early endosome marker EEA1 in both Vero cells and adaGb3-treated CHO/Jurkat cells (Fig. 4D). The VT1 also colocalized with transferrin in the “reconstituted” cells at this time (not shown). After 1 h at 37 °C in Vero cells, some of the internalized VT1 overlaps with the Golgi marker, Rab6 (Fig. 4E, arrowheads), and some remained colocalized with EEA1 (Fig. 4D). In adaGb3- or OHEtadaGb3-treated CHO/Jurkat cells, Rab6 coincidence with internalized VT1 was insignificant compared with Vero cells (Fig. 4E), although VT1 staining was much less overall. VT1 did not colocalize with the lysosomal marker, Lamp-2, at any time (supplemental Fig. 1). After 6 h, most VT1 colocalized with the ER marker calnexin in Vero cells (Fig. 4E). In contrast, most of VT1 was lost and rarely overlapped with calnexin in adaGb3- or OHEtadaGb33-treated CHO/Jurkat cells (Fig. 4E). Essentially the same results were found for OHEtadaGb3-treated CHO and Jurkat cells (Fig. 4, D and E) (and for cells treated with carboxyl or urea-adaGb3. Cells treated with adaSGC (sulfatide) did not bind VT (supplemental Fig. 2). Thus, these Gb3 analogues mediate VT1 internalization to early endosomes, but trafficking to Golgi/ER, as seen in Gb3-expressing, VT1/VT2-sensitive Vero cells, is not detectable. The differential targeting (quantitated in supplemental Table 1) provides an explanation for the reduced efficacy of adaGb3, compared with natural Gb3, to mediate VT cytotoxicity.
AdaGb3 Treatment of VT-sensitive Vero Cells Subverts Endogenous Gb3-mediated VT1 and VT2 Retrograde Transport
To assess any relationship between these “exogenous” versus “endogenous” trafficking pathways, Gb3-expressing Vero cells were also “reconstituted” with adaGb3 or OHEtadaGb3. Cells were treated with VT1 or VT2, and cell viability was monitored after 72 h (Fig. 5A). Vero cell VT1 sensitivity was reduced 10-fold after adaGb3 treatment, but little effect on VT2 sensitivity was seen. OHEtadaGb3 treatment of Vero cells had significantly less effect on VT1 sensitivity (Fig. 5A).
FIGURE 5.
A, toxicity of VT1/VT2 to adaGb3- or OHEtadaGb3-treated Vero cells. Vero cells were incubated with 0, 10, 20, or 50 μm adaGb3 or OHEtadaGb3 at 4 °C for 1 h. Cells were treated with 10-fold serially diluted VT1 or VT2 and incubated at 37 °C. Cell viability was monitored after 72 h and expressed as a percentage of control cells, which were treated with neither VT nor adaGb3 analogues. B, staining of non-treated, adaGb3-treated, or OHEtadaGb3-treated Vero cells with fluorescent VT1 or VT2. Vero cells were treated with or without adaGb3 or OHEtadaGb3. Then Alexa488-VT1B or Texas Red-VT2 was bound on ice for 1 h. Cells were washed, fixed, and viewed with a confocal microscope. Bar, 10 μm. C, trafficking of VT1 in adaGb3 or OHEtadaGb3-treated Vero cells. VT1 was internalized at 37 °C for 10 min, 1 h, or 6 h in adaGb3- or hydroxyethyl adaGb3-treated Vero cells. VT1, EEA1, Rab6, and calnexin were localized as described in the legend to Fig. 4. D, trafficking of VT2 to the Golgi and ER in non-treated, adaGb3-treated, or hydroxyethyl adaGb3-treated Vero cells. Bound VT2 was internalized at 37 °C for 1 or 6 h. Cells were fixed, permeabilized, and labeled with anti-Rab6 (Golgi) or anti-calnexin (ER). Fluorescently stained cells were viewed with a confocal microscope. (Texas Red-VT2 is pseudocolored green, and organelle markers detected with anti-rabbit-Alexa488 colored red, for ease of comparison). E, VT2-induced vacuolation in non-treated, adaGb3-treated, or OHEtadaGb3-treated Vero cells. VT2 was bound and internalized at 37 °C for 6 h. VT2 (green) and calnexin (red) were detected as described for D. Fluorescently stained cells were viewed with a confocal microscope. Arrowhead, vacuoles; bar, 10 μm. Toxin colocalization with organelle markers was quantitated (supplemental Table 2).
AdaGb3 treatment of Vero cells altered the VT1 and VT2 cell surface staining pattern (Fig. 5B). Gb3-expressing VT-sensitive Vero cells showed punctate cell surface binding, as observed previously (12). However, Vero cells treated with adaGb3 showed a more uniform cell surface VT1/VT2 labeling pattern, as if adaGb3 had served to fuse previously separate cell surface Gb3 domains. In contrast, OHEtadaGb3-treated Vero cells retained the punctate cell surface binding of non-treated Vero cells (Fig. 5B). Quantitation of cell surface binding showed that adaGb3 treatment reduced the amount of Alexa488-VT1B bound by 20–30% (supplemental Fig. 3), consistent with loss of a GSL clustering component in membrane binding affinity (50).
The intracellular trafficking of VT1 in non-treated (Fig. 4, D and E) and in adaGb3- or OHEtadaGb3-treated Vero cells (Fig. 5C) was then compared. After 10 min at 37 °C, a large portion of internalized VT1 colocalized with early endosomal EEA1 in all cases. In non-treated Vero cells after 1 h at 37 °C, some internalized VT1 colocalized with the Golgi marker, Rab6 (Fig. 4E, arrowheads), and some remained with EEA1 (Fig. 4D). However, in adaGb3-treated Vero cells after 1 h at 37 °C, little VT1 colocalized with EEA1, and VT1 coincidence with Rab6 (Fig. 5C) was far less than in non-treated Vero cells. In contrast, in OHEtadaGb3-treated Vero cells, a large portion of internalized VT1 remained colocalized with EEA1 at 1 h (Fig. 5C) as in non-treated Vero cells. After 6 h, most VT1 colocalized with the ER marker calnexin in non-treated Vero cells (Fig. 4E). However, VT1 rarely overlapped with calnexin in adaGb3-treated cells. In contrast, VT1/calnexin overlap at 6 h was retained in OHEtadaGb3-treated Vero cells (Fig. 5C) although reduced compared with in non-treated Vero cells.
Because adaGb3 was less effective to reduce VT2, as compared with VT1, Vero cell sensitivity (Fig. 5A), the intracellular trafficking of VT2 was compared in Vero and adaGb3-treated Vero cells (Fig. 5D). After 1 h at 37 °C, some internalized VT2 was overlapping with the Golgi marker Rab6, in non-treated Vero cells (Fig. 5C, arrowheads). In adaGb3-treated Vero cells, Rab6 coincidence with VT2 was barely detectable at 1 h, but in OHEtadaGb3-treated Vero cells, internalized VT2 colocalized with Rab6 as for non-treated Vero cells. After 6 h of culture, significant VT2 colocalization with the ER marker calnexin in non-treated Vero cells was found. In contrast, VT2 showed no overlap with calnexin in adaGb3-treated Vero cells at this time. However, for OHEtadaGb3-treated cells, VT2/calnexin overlap (Fig. 5C) was similar to that in non-treated Vero cells after 6 h.
Thus, adaGb3 changed the intracellular VT1 and VT2 trafficking in Gb3-expressing cells. Initial endosomal entry was retained, but subsequent Golgi/ER traffic was compromised. OHEtadaGb3 treatment had little obvious effect.
As previously reported (12), intracellular perinuclear vacuoles were detected in some VT2-treated Vero cells (Fig. 5E, arrowheads). VT2 vacuolation was retained for adaGb3- or OHEtadaGb3-treated Vero cells, but VT2 staining of these vesicles was lost (Fig. 5, D and E).
BFA, which prevents Golgi-dependent retrograde traffic, protects cells from VT1 (51). To confirm that intracellular VT trafficking in adaGb3-treated cells mediates toxicity without Golgi access, the effects of BFA on VT-induced cytotoxicity in adaGb3-treated CHO/Jurkat cells (Fig. 6A) and Vero cells (Fig. 6B) were compared. BFA was virtually ineffective to prevent VT1/VT2 killing of adaGb3-treated CHO cells and provided minimal protection to adaGb3-treated Jurkat cells (Fig. 6A). In contrast, BFA completely protected Vero cells against VT1/VT2 (Fig. 6B). However, for adaGb3-treated Vero cells, VT1/VT2 cytotoxicity became BFA-resistant. These data indicate that intracellular VT traffic and toxicity in adaGb3-treated CHO, Jurkat, and Vero cells is Golgi-independent.
FIGURE 6.
Effect of BFA on VT1- and VT2-induced cell killing. Cells were incubated ± 0.5 μg/ml BFA for 30 min prior to the VT addition. VT1 was added at 10 μg/ml for adaGb3-treated CHO/Jurkat cells (A), 0.1 ng/ml for non-treated Vero cells (B, top), and 100 ng/ml for adaGb3-treated Vero cells (B, bottom). VT2 was added at 10 μg/ml for adaGb3-treated CHO/Jurkat cells, 1 ng/ml for non-treated Vero cells, and 100 ng/ml for adaGb3-treated Vero cells. Cell viability was monitored after 21.5 h and expressed as a percentage of control cells in the absence of VT.
AdaGb3 Analog Inhibition of VT Cytotoxicity
Previously, we reported that adaGb3 competed with Gb3 for VT1 binding in receptor ELISA (44) and was effective to prevent the Vero cell binding of both VT1 and VT2 (12, 46). We therefore compared the efficacy of other adaGb3 analogues for protection of Vero cells from VT binding.
Increasing concentrations of adaGb3, hydroxylethyladaGb3, carboxyadaGb3, or adabisGb3 were preincubated with VT1/VT2 and tested for reduction of Vero cell cytotoxicity (Fig. 7). Of the “monomer” species, only adaGb3 showed significant protection against VT1 and, more effectively, VT2. CarboxyadaGb3 and hydroxylethyladaGb3 showed no protection. AdabisGb3 inhibited VT1/VT2 Vero cell cytotoxicity to a greater extent than adaGb3. Cytotoxicity of VT1/VT2 preincubated with 50 μm adalbisGb3 was reduced 150–250-fold. AdaGb3 had no effect on Vero cell susceptibility to ricin, which also undergoes Golgi/ER retrograde transport (52); thus, overall transport to the Golgi and ER was not blocked (supplemental Fig. 4).
FIGURE 7.
Inhibition of verotoxin cytotoxicity. Increasing concentrations of adaGb3, carboxyadaGb3, OHEtadaGb3, or adabisGb3 were premixed with VT1 or VT2 dilutions at 37 °C for 1 h and then added to Vero cells for 1 h. Cells were then washed and incubated at 37 °C for 72 h. Live cells were stained with crystal violet, and viability was plotted as a percentage of untreated Vero cells. From this, the CD50 values of VT1 (left)/VT2 (right) preincubated with adaGb3 analogues were determined and plotted as a function of analog concentration.
Unlike Sphingolipids, AdaGb3 Does Not Stabilize Membrane Cholesterol in Phospholipid Liposomes
Because cholesterol is central to intracellular membrane GSL traffic, the ability of Gb3 and adaGb3 to interact with cholesterol was compared by their ability to induce resistance to MβCD cholesterol extraction from a model phospholipid membrane (48, 53). Approximately 40% of the [3H]cholesterol in PC liposomes was extracted by 0.25 mm MβCD (Fig. 8) from the liposomal pellet. Inclusion of SPM or Gb3 within the liposomes significantly reduced the extracted cholesterol to 10 and 15%, respectively, indicating stabilization of the cholesterol within the membrane. In contrast, inclusion of adaGb3 consistently increased cholesterol susceptibility to MβCD extraction to 50%. Inclusion of adaGb3 together with SPM had no effect on SPM-cholesterol stabilization, but adaGb3 reduced the stabilizing effect of Gb3 on cholesterol by ∼30%, indicating that membrane adaGb3 interfered with the interaction between Gb3 and cholesterol. Thus, in contrast to Gb3 and SPM, adaGb3 destabilizes rather than stabilizes membrane cholesterol and partially reverses Gb3-cholesterol stabilization. This effect could explain the lack of adaGb3 Golgi/ER trafficking and the adaGb3 modulation of VT1-bound Gb3 intracellular trafficking observed.
FIGURE 8.
GSLs/adaGSL stabilize/destabilize cholesterol within PC liposomes. The effect of inclusion of SPM, Gb3, or adaGb3 (alone and in combination) on [3H]cholesterol availability to MβCD extraction from cholesterol/PC liposomes was determined. The percentage of cholesterol extracted by PBS (open bars) or 0.25 mm MβCD (gray bars) after 1 h at room temperature is shown. As expected, inclusion of the sphingolipid SPM or Gb3 increased resistance to cholesterol extraction by MβCD, but adaGb3 showed a reverse effect. Moreover the inclusion of adaGb3 together with Gb3, but not SPM, partially reversed the stabilizing effect on liposomal cholesterol. Error bars, S.D.
AdaGb3 Does Not Alter Internalization and Retrograde Traffic of CT
To address whether the effect of adaGb3 on native Gb3-mediated VT intracellular traffic might be in any way selective, we examined the intracellular retrograde traffic of GM1-bound Cy3-CTB in adaGb3-treated HEK-293 cells. VT1 and CT preferentially bind different Vero cell subsets during the cell cycle (54), making comparison of differential trafficking in a single cell difficult. CHO cells do not express GM1 (55) (or Gb3), and cell suspension cultures (Jurkat) are inconvenient to study intracellular traffic. We therefore treated HEK-293 cells (Gb3−ve, GM1+ve) with adaGb3 and monitored the cell binding and internalization of VT1B and CTB (Fig. 9). The cell surface binding of CTB to HEK-293 cells at 4 °C was largely unaffected by adaGb3 treatment (Fig. 9). VTB bound the HEK-293 cell surface only after adaGb3 treatment and colocalized extensively with CTB at 4 °C (Fig. 9). Warming to 37 °C induced plasma membrane-bound CTB internalization to the same juxtanuclear Golgi structures in both control and adaGb3-treated cells (Fig. 9). VTB, however, was internalized into punctate intracellular vesicles, for the most part, distinct from CTB-labeled Golgi (Fig. 9). VTB containing vesicles were in the Golgi area (as defined by CTB) but remained separate from CTB. Thus, cell surface-colocalized GM1-bound CTB and adaGb3-bound VTB are differentially trafficked to separate structures within the cell, such that GM1-CTB Golgi retrograde traffic is retained, whereas adaGb3-VT1 is trafficked to an alternative destination within the same cells.
FIGURE 9.
AdaGb3 insertion and VT trafficking do not perturb the intracellular traffic of cholera toxin. AdaGb3 was inserted into HEK-293 cells, and the simultaneous binding and internalization of Alexa488-VTB and Cy3-CTB were assessed. Top panels, binding at 4 °C; bottom panels, detection after 1 h of internalization at 37 °C. DAPI nuclear staining is shown for VT1 B-treated cells without adaGb3. Bar, 10 μm. Only plasma membrane staining is seen at 4 °C. AdaGb3-bound VTB and GM1-bound CTB show significant cell surface overlap. At 37 °C, Cy3-CTB is internalized to juxtanuclear Golgi in both control and adaGb3-treated cells. In contrast, Alexa488-VTB is internalized to punctate vesicles distinct from CTB-labeled Golgi in adaGb3-treated cells.
DISCUSSION
The binding of the VT family of AB5 subunit toxins to their receptor GSL, globotriaosyl ceramide, is of interest for many reasons. First, the VT B subunit pentamer binding to Gb3 provides the basis for renal glomerular endothelial cell targeting following systemic verotoxemia and therefore plays a central role in the pathology of HUS (17, 56), which remains a life-threatening complication of gastrointestinal verotoxin-producing E. coli infection, an ever increasing threat in the developed world (9). Second, verotoxin binding to cell surface Gb3 provides an index of the complex manner in which cell surface GSLs can be presented within a bilayer for ligand recognition and is thus a probe for GSL membrane organization (57). Third, Gb3 and verotoxin internalization and intracellular traffic provide a probe of the molecular basis of retrograde transport to the ER (58). Fourth, Gb3 is up-regulated in many human tumor cells, and thus verotoxin itself (59, 60, 61) or the B subunit pentamer coupled to cytotoxic drugs (62, 63) offers new antineoplastic approaches (64). In this area also, as with HUS, endothelial cells within the neovasculature express Gb3 and are VT-sensitive (65, 66). Last, Gb3 expression is a key risk factor for HIV susceptibility (67), and aglycone modulation of gp120-Gb3 binding is similar to that of VT1 (16).
Membrane GSL organization and its role in intracellular vesicular traffic are poorly understood but are of high potential significance (68–70). The amphipathic GSL analogues we have made, which in part retain the receptor function of membrane GSLs (71), provide new insight into these processes and the means to alter cellular GSL metabolism selectively (72). We now show that adaGSLs have an immediate effect on plasma membrane GSL receptor function and intracellular traffic. Our results, summarized in Scheme 1, include several novel observations as described below.
SCHEME 1.
1–3, endogenous Gb3 within cell surface lipid rafts mediates VT internalization (1), endosomal transport to Golgi-associated vesicle (2), and retrograde transport to Golgi and thence ER (3). 4, plasma membrane adaGb3 can mediate VT internalization to early endosomes without further retrograde transport. 5, A-subunit may be released into cytosol. 6, toxin-adaGb3 complex may be recycled and lost from the cell surface. Some VT may undergo lysosomal degradation. 7, mixing of endogenous and adaGb3 alters Gb3 organization to disburse Gb3 from raft restriction. In combination, Gb3 and adaGb3 mediate VT internalization to endosomes (5) or Golgi-associated vesicles (3), but retrograde transport (8) to Golgi and ER does not occur.
Induction of VT1/VT2 Cell Sensitivity
We synthesized a series of modified adaGb3 species and found preferential VT2 (cf. VT1) binding. These Gb3 mimics incorporated into the plasma membrane of receptor negative cells to induce cell VT1/VT2 cytotoxicity. This is the first report in which a Gb3 derivative has been shown capable of this function and opens the potential to make any cell sensitive to VT cytopathology. Significantly, in such “pseudoreceptor”-reconstituted cells, the toxin-receptor complex was internalized to endosomes but did not mediate Golgi/ER retrograde transport, as for endogenous Gb3-mediated VT traffic (Scheme 1). Despite the different functional groups present, this was seen for all adaGb3 species. Prolonged association with EEA1 vesicles was seen for OHEtadaGb3 but not other adaGb3-treated cells. Lack of Golgi/ER targeting suggests that A-subunit cytosolic translocation from endosomes mediates the induced toxicity (51, 73). The internalized toxin was less long-lived compared with that within Vero cells. (70–100% versus 30–50% loss in 1–6 h; see supplemental Table 2). This may indicate proteolysis or, more likely, loss due to recycling from endosomes to the cell surface (Scheme 1). This VT loss from endosomes may contribute to the lack of Golgi/ER VT detection. We have not observed any adaGSL breakdown within the time frame of the present experiments.
The intracellular transport of adaGb3 is distinct from exogenous BODIPY and NBD-GSL analogues, which readily traffic from the cell surface to the Golgi (31). The more planar structure of these fluorescent substituents may permit a cholesterol interaction. This clearly shows that the lipid structure of membrane GSLs can provide differential intracellular membrane addresses for exogenous (and, by inference, endogenous) GSL species. AdaGSLs may be defective in lateral membrane “connectivity” (74).
AdaGb3-reconstituted CHO cells were significantly less sensitive to VT1/VT2 than similarly reconstituted Jurkat cells. This indicates that properties in addition to receptor status regulate cytotoxicity.
AdabisGb3 Does Not Induce VT1/VT2 Cell Sensitivity
Our second novel observation is that adabisGb3, in which two lyso-Gb3s are coupled to a single adamantane frame, does not incorporate into the cell membrane. This lack of membrane partitioning of adabisGb3 is of significance and must be a structure-related property. Although the hydrophobicity of adabisGb3 is significantly reduced compared with adaGb3, gangliosides of greater polarity are readily taken up into the membranes of cultured cells (75). It is possible that the 1–3 coupling to the adamantane frame positions the sphingosine tails in a skewed orientation relative to one another, and as such, the non-parallel alkyl chains may be unable to insert and stack in a lamellar bilayer to prevent plasma membrane incorporation. Nevertheless, in solution, adabisGb3 can bind to VT1/VT2 tightly to function as an extracellular inhibitor of VT1/VT2 cell binding. Furthermore, adabisGb3 itself is not toxic to Jurkat cells, whereas high concentrations of adaGb3 can be toxic. This is probably a function of the lack of cell membrane insertion of adabisGb3. Thus, adabisGb3 provides a potential basis for protection against verotoxemia.
AdaGb3 Compromises Endogenous Gb3-mediated VT1 Retrograde Transport
Our third observation is that adaGb3 can subvert the natural retrograde transport of VT1/VT2 bound to endogenous cellular Gb3. This property is dependent on the lipid structure of the Gb3 mimic; OHEtadaGb3 did not have this effect. In addition, the effect was selective, in that GM1-CTB intracellular traffic was virtually unaffected. AdaGb3 plasma membrane incorporation altered the surface distribution of VT1 and VT2 overall, generating a more uniform cell surface receptor (Gb3 + adaGb3) distribution. This implies cooperation between the membrane-incorporated adaGb3 and non-uniformly distributed Gb3 (Scheme 1). The subsequent retrograde transport of VT1/VT2 from endosomes to Golgi and hence to ER was largely circumvented in adaGb3-treated Vero cells. Consistent with the lack of retrograde Golgi/ER transport, VT1/VT2 cytotoxicity for adaGb3-treated cells became insensitive to BFA protection. VT1 (but not VT2) cytotoxicity was significantly reduced for adaGb3-treated Vero cells, suggesting more effective cytosolic VT2 A-subunit translocation from endosomes.
Lipid Structural Dependence
The early intracellular transport of VT-bound adaGb3 in Gb3-negative cells is similar to endogenous Gb3-bound VT, in that the toxin is rapidly targeted to early endosomes. We did not observe any VT/adaGb3 colocalization with Lamp-2, indicating a fate other than lysosomal degradation. For Gb3-positive plasma membranes into which adaGb3 is incorporated, VT1 and VT2 must simultaneously bind both endogenous Gb3 and incorporated adaGb3. The VTB subunit pentamer will probably bind five Gb3 molecules (76) to induce membrane curvature by compaction (77). Membrane adaGb3 has a larger molecular area and is more resistant to compression than Gb3 (25), and inclusion within this toxin-GSL membrane complex could compromise compaction/membrane curvature. The non-uniform Vero cell distribution of Gb3, as detected by VT1 or VT2 binding, was rendered more uniform after adaGb3 incorporation, consistent with a lack of clustering (77). This redistribution was not seen after OHEtadaGb3 incorporation and correlates with protection against VT cytotoxicity by adaGb3 but not OHEtadaGb3.
Subsequent internalization was similar for adaGb3-treated CHO or Jurkat cells and, initially, for untreated Vero cells, in that early endosomes were targeted, but in adaGb3-treated cells, the later retrograde transport to Golgi/ER was compromised. Thus, the adaGb3 internalization and trafficking route dominated that of endogenous Gb3. This was not observed for OHEtadaGb3-treated cells.
Retrograde transport overall was not affected because Vero cell susceptibility to ricin and Golgi traffic of cholera toxin/GM1 in HEK-293 cells were unaffected by adaGb3. Our previous studies showed partial cell surface colocalization but the separate internalization of VT1 and CT (78). Internalization is mediated through both clathrin-dependent and clathrin-independent mechanisms, both of which access Golgi/ER retrograde transport (27). Although VT2 bound adaGb3 in preference to native Gb3 by TLC overlay, adaGb3 did not have a greater effect on VT2 compared with VT1 intracellular traffic, indicating that membrane organization rather than binding per se is the key factor.
AdaGb3 Does Not Interact with Cholesterol
The endosome to TGN transport of VT is compromised by clathrin blockade (79, 80). Cholesterol depletion can reduce both clathrin-dependent (81) and caveolin-dependent (82) internalization and can block actin-dependent endosome-TGN CT (83) and endosome-TGN ricin retrograde transit (84). GSL-cholesterol interaction is key to the formation of liquid-ordered domains in model membranes (85) and increased order in cell membranes (74). Aberrant cholesterol and GSL retrograde transport (and metabolism) are intimately connected in sphingolipid storage diseases (86).
AdaGb3 was unable to stabilize liposomal membrane cholesterol and partially reduced cholesterol stabilization by Gb3; these novel biophysical properties may explain the effect of adaGb3 on intracellular VT routing. If the transition of VT1/VT2-bound Gb3 between endosomes and TGN is cholesterol-dependent, adaGb3-bound VT should transit ineffectively. Similarly, if the punctate cell surface Gb3 distribution were cholesterol-dependent, a more uniform distribution for adaGb3 would be expected.
In adaGb3-treated cells, VT1/VT2 cell surface distribution was altered, but internalization was similar. Thus, the cholesterol-dependent “decision” to undergo Golgi/ER retrograde transport may be taken at the cell surface.
GSL-cholesterol interaction can promote (25) or prevent ligand-GSL binding (21). This may be a function of GSL fatty acid content (16, 20), GSL/cholesterol ratio (20), and membrane curvature (21). The carbohydrate conformation of GSLs is changed when in complex with cholesterol (20). Hydrogen bonding of the sterol OH and adjacent GSL anomeric oxygen “bends” the carbohydrate from a membrane perpendicular to parallel orientation (18, 20, 87), restricts exogenous ligand binding (21), and shields the sterol from water interaction by an “umbrella” effect (87). In model membranes, the GSL glycan thickness is an inverse function of the cholesterol concentration, from perpendicular (thickest) to parallel (thinnest) (20), suggesting a range of intermediate cholesterol-dependent carbohydrate conformations. Nine potential membrane GSL carbohydrate conformations have been modeled (88).
The lack of cholesterol interaction we show predicts adaGSLs to be resistant to this masking effect, and such analogues might therefore have a binding advantage over natural GSL species in cholesterol-containing membranes. This could explain the dominant trafficking effect of adaGb3 in Gb3-expressing cells and the ability of adamantyl monohexosyl ceramides to modulate GSL metabolism (72). The lack of OHEtadaGb3 efficacy could be consistent with this mechanism. The GSL conformational change induced by cholesterol is mimicked in GSLs containing 2-hydroxy fatty acids (18). Hydrogen bonding between the sugar and fatty acid OH can similarly bend the carbohydrate (89). The OH of OHEtadaGb3 might similarly mediate such a carbohydrate conformational change, and the potential ligand binding advantage would be lost.
OHEtadaGb3 induced VT susceptibility in Gb3-negative cells. In OHEtadaGb3-treated (or carboxyadaGb3- or urea-adaGb3-treated), Gb3-negative cells, intracellular VT trafficking is similar to that of adaGb3-treated cells. This suggested that the incorporated adaGb3 analogues define a shared intracellular retrograde routing, which is different from natural Gb3 traffic. The differential VT cell sensitivity may due to binding differences between adaGb3 analogues. AdaGb3 is strongly bound by VT1/VT2. VT1-adaGb3 binding was similar to VT1-Gb3 binding, and VT2-adaGb3 binding was ∼4-fold greater than VT2-Gb3 binding. On the other hand, VT1/OHEtadaGb3 binding was significantly less than VT1/adaGb3 or Gb3. VT2/OHEtadaGb3 binding was similar to VT2/Gb3 binding, significantly weaker than VT2/adaGb3 binding. These binding differences are consistent with an OHEtadaGb3 conformational restriction that could explain the VT sensitivity, cell surface staining, and intracellular trafficking observed in adaGb3-treated versus OHEtadaGb3-treated Vero cells. The punctate VT1/VT2 Vero cell surface staining of Gb3-containing plasma membrane domains that we observed at 4 °C, as previously (12), was replaced in adaGb3-treated Vero cells by a more uniform membrane staining pattern. Membrane-incorporated adaGb3 may intercalate to disperse such domains by reducing cholesterol interaction and “fill the gaps” between domains (Scheme 1). This may reroute intracellular traffic similar to non-raft, as compared with raft Gb3 (22).
We found that the VT2-induced vacuolation we previously reported for a subpopulation of Vero cells (12) was retained for adaGb3-treated or OHEtadaGb3-treated Vero cells. Thus, this vacuolation response is independent of VT2 retrograde traffic to the Golgi/ER, which may relate to the increased clinical severity of VT2. In Vero cells, VT2 was present in the limiting membrane of the VT2-induced vacuoles. However, for adaGb3-treated or OHEtadaGb3-treated Vero cells, VT2 was not detected in the vacuolar membrane, indicating that the vacuoles arise from a signaling mechanism rather than direct effect of toxin membrane Gb3 binding.
In conclusion, we show the novel, lipid-dependent, pseudoreceptor function of adaGb3 mimics in receptor negative cells and their structure-dependent domination over native intracellular Gb3-dependent but not GM1-dependent traffic. This may be mediated by the lack of cholesterol association of adaGb3 mimics and their ability to preferentially reduce membrane Gb3-cholesterol interaction.
Supplementary Material
This work was supported by Canadian Institutes of Health Research Grant MT 13747 and grants from Ontario HIV Treatment Network and Canfar.

This article contains supplemental Tables 1 and 2 and Figs. 1–4.
- VT
- verotoxin
- GSL
- glycosphingolipid
- Gb3
- globotriaosyl ceramide
- adaGSL
- adamantyl GSL
- adaGb3
- adamantyl Gb3, (2S,3R,4E)-2-(1-adamantane)-acetamido-3-hydroxyl-4-octadecenyl-(α-d-galactotopyranosyl)-(1–4)-(β-galactopyranosyl)-(1–4)-β-d-glucopyranoside
- carboxyadaGb3
- (2S,3R,4E)-2-(1-(3-carboxymethyl)-adamantanacetamido)-3-hydroxyl-4-octadecenyl-(α-d-galactotopyranosyl)-(1–4)-(β-galactopyranosyl)-(1–4)-β-d-glucopyranoside
- urea-adaGb3
- (2S,3R,4E)-2-(1-(3-(1,3-diisopropyl)-ureido)-adamantanacetamido)-3-hydroxyl-4-octadecenyl-(α-d-galactotopyranosyl)-(1–4)-(β-galactopyranosyl)-(1–4)-β-d-glucopyranoside
- OHEtadaGb3
- (2S,3R,4E)-2-(1-(3-(N-2-hydroxyethyl)-carbamoyl)-adamantanacetamido)-3-hydroxyl-4-octadecenyl-(α-d-galactotopyranosyl)-(1–4)-(β-galactopyranosyl)-(1–4)-β-d-glucopyranoside
- adabisGb3
- adamantylbisGb3
- GM1
- sialyl gangliotetraosyl ceramide
- BFA
- brefeldin A
- ER
- endoplasmic reticulum
- NBD
- 12-(N-methyl-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl))
- HUS
- hemolytic uremic syndrome
- TGN
- trans-Golgi network
- SPM
- sphingomyelin
- MβCD
- methyl-β-cyclodextrin
- CT
- cholera toxin
- CTB
- cholera toxin B subunit
- VT1B
- VT1 B-subunit
- PC
- phosphatidylcholine
- CD50
- dose required for 50% cell killing.
REFERENCES
- 1. Spitalnik P. F., Spitalnik S. L. (1995) The P blood group system. Biochemical, serological, and clinical aspects. Transfus. Med. Rev. 9, 110–122 [DOI] [PubMed] [Google Scholar]
- 2. Mangeney M., Richard Y., Coulaud D., Tursz T., Wiels J. (1991) CD77. An antigen of germinal center B cells entering apoptosis. Eur. J. Immunol. 21, 1131–1140 [DOI] [PubMed] [Google Scholar]
- 3. O'Brien A. D., Tesh V. L., Donohue-Rolfe A., Jackson M. P., Olsnes S., Sandvig K., Lindberg A. A., Keusch G. T. (1992) Shiga toxin. Biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180, 65–94 [DOI] [PubMed] [Google Scholar]
- 4. Karmali M. A., Gannon V., Sargeant J. M. (2010) Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 140, 360–370 [DOI] [PubMed] [Google Scholar]
- 5. Richardson S. E., Karmali M. A., Becker L. E., Smith C. R. (1988) The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum. Pathol. 19, 1102–1108 [DOI] [PubMed] [Google Scholar]
- 6. Müthing J., Schweppe C. H., Karch H., Friedrich A. W. (2009) Shiga toxins, glycosphingolipid diversity, and endothelial cell injury. Thromb. Haemost. 101, 252–264 [PubMed] [Google Scholar]
- 7. Goldwater P. N. (2007) Treatment and prevention of enterohemorrhagic Escherichia coli infection and hemolytic uremic syndrome. Expert Rev. Anti Infect. Ther. 5, 653–663 [DOI] [PubMed] [Google Scholar]
- 8. Bielaszewska M., Mellmann A., Zhang W., Köck R., Fruth A., Bauwens A., Peters G., Karch H. (2011) Characterization of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011. A microbiological study. Lancet Infect. Dis. 11, 671–676 [DOI] [PubMed] [Google Scholar]
- 9. Frank C., Werber D., Cramer J. P., Askar M., Faber M., an der Heiden M., Bernard H., Fruth A., Prager R., Spode A., Wadl M., Zoufaly A., Jordan S., Kemper M. J., Follin P., Müller L., King L. A., Rosner B., Buchholz U., Stark K., Krause G., and HUS Investigation Team (2011) Epidemic profile of Shiga toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365, 1771–1780 [DOI] [PubMed] [Google Scholar]
- 10. Karch H., Friedrich A. W., Gerber A., Zimmerhackl L. B., Schmidt M. A., Bielaszewska M. (2006) New aspects in the pathogenesis of enteropathic hemolytic uremic syndrome. Semin. Thromb. Hemost. 32, 105–112 [DOI] [PubMed] [Google Scholar]
- 11. Kawano K., Okada M., Haga T., Maeda K., Goto Y. (2008) Relationship between pathogenicity for humans and stx genotype in Shiga toxin-producing Escherichia coli serotype O157. Eur. J. Clin. Microbiol. Infect. Dis. 27, 227–232 [DOI] [PubMed] [Google Scholar]
- 12. Tam P., Mahfoud R., Nutikka A., Khine A. A., Binnington B., Paroutis P., Lingwood C. (2008) Differential intracellular transport and binding of verotoxin 1 and verotoxin 2 to globotriaosylceramide-containing lipid assemblies. J. Cell. Physiol. 216, 750–763 [DOI] [PubMed] [Google Scholar]
- 13. Okuda T., Tokuda N., Numata S., Ito M., Ohta M., Kawamura K., Wiels J., Urano T., Tajima O., Furukawa K. (2006) Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J. Biol. Chem. 281, 10230–10235 [DOI] [PubMed] [Google Scholar]
- 14. Nyholm P. G., Magnusson G., Zheng Z., Norel R., Binnington-Boyd B., Lingwood C. A. (1996) Two distinct binding sites for globotriaosyl ceramide on verotoxins. Identification by molecular modeling and confirmation using deoxy analogues and a new glycolipid receptor for all verotoxins. Chem. Biol. 3, 263–275 [DOI] [PubMed] [Google Scholar]
- 15. Chark D., Nutikka A., Trusevych N., Kuzmina J., Lingwood C. (2004) Differential carbohydrate epitope recognition of globotriaosyl ceramide by verotoxins and monoclonal antibody: Role in human renal glomerular binding. Eur. J. Biochem. 271, 405–417 [DOI] [PubMed] [Google Scholar]
- 16. Mahfoud R., Manis A., Lingwood C. (2009) Fatty acid-dependent globotriaosyl ceramide receptor function in detergent-resistant model membranes. J. Lipid Res. 50, 1744–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan F., Proulx F., Lingwood C. A. (2009) Detergent-resistant globotriaosyl ceramide may define verotoxin/glomeruli-restricted hemolytic uremic syndrome pathology. Kidney Int. 75, 1209–1216 [DOI] [PubMed] [Google Scholar]
- 18. Yahi N., Aulas A., Fantini J. (2010) How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer's β amyloid peptide (Aβ1–40). PLoS One 5, e9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaiser H. J., Lingwood D., Levental I., Sampaio J. L., Kalvodova L., Rajendran L., Simons K. (2009) Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 106, 16645–16650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lingwood D., Binnington B., Róg T., Vattulainen I., Grzybek M., Coskun U., Lingwood C. A., Simons K. (2011) Cholesterol modulates glycolipid conformation and receptor activity. Nat. Chem. Biol. 7, 260–262 [DOI] [PubMed] [Google Scholar]
- 21. Mahfoud R., Manis A., Binnington B., Ackerley C., Lingwood C. A. (2010) A major fraction of glycosphingolipids in model and cellular cholesterol-containing membranes is undetectable by their binding proteins. J. Biol. Chem. 285, 36049–36059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falguières T., Mallard F., Baron C., Hanau D., Lingwood C., Goud B., Salamero J., Johannes L. (2001) Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 12, 2453–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith D. C., Sillence D. J., Falguières T., Jarvis R. M., Johannes L., Lord J. M., Platt F. M., Roberts L. M. (2006) The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect. Mol. Biol. Cell 17, 1375–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hooper N. (1999) Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review). Mol. Membr. Biol. 16, 145–156 [DOI] [PubMed] [Google Scholar]
- 25. Mahfoud R., Mylvaganam M., Lingwood C. A., Fantini J. (2002) A novel soluble analog of the HIV-1 fusion cofactor, globotriaosylceramide (Gb3), eliminates the cholesterol requirement for high affinity gp120/Gb3 interaction. J. Lipid Res. 43, 1670–1679 [DOI] [PubMed] [Google Scholar]
- 26. Sandvig K., Olsnes S., Brown J. E., Petersen O. W., van Deurs B. (1989) Endocytosis from coated pits of Shiga toxin. A glycolipid-binding protein from Shigella dysenteriae 1. J. Cell Biol. 108, 1331–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nichols B. J., Kenworthy A. K., Polishchuk R. S., Lodge R., Roberts T. H., Hirschberg K., Phair R. D., Lippincott-Schwartz J. (2001) Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sandvig K., Garred O., Prydz K., Kozlov J. V., Hansen S. H., van Deurs B. (1992) Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 358, 510–512 [DOI] [PubMed] [Google Scholar]
- 29. Tam P. J., Lingwood C. A. (2007) Membrane cytosolic translocation of verotoxin A1 subunit in target cells. Microbiology 153, 2700–2710 [DOI] [PubMed] [Google Scholar]
- 30. Hoey D. E., Sharp L., Currie C., Lingwood C. A., Gally D. L., Smith D. G. (2003) Verotoxin 1 binding to intestinal crypt epithelial cells results in localization to lysosomes and abrogation of toxicity. Cell. Microbiol. 5, 85–97 [DOI] [PubMed] [Google Scholar]
- 31. Pagano R. E., Puri V., Dominguez M., Marks D. L. (2000) Membrane traffic in sphingolipid storage diseases. Traffic 1, 807–815 [DOI] [PubMed] [Google Scholar]
- 32. Johannes L., Pezo V., Mallard F., Tenza D., Wiltz A., Saint-Pol A., Helft J., Antony C., Benaroch P. (2003) Effects of HIV-1 Nef on retrograde transport from the plasma membrane to the endoplasmic reticulum. Traffic 4, 323–332 [DOI] [PubMed] [Google Scholar]
- 33. Kiarash A., Boyd B., Lingwood C. A. (1994) Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J. Biol. Chem. 269, 11138–11146 [PubMed] [Google Scholar]
- 34. Boyd B., Magnusson G., Zhiuyan Z., Lingwood C. A. (1994) Lipid modulation of glycolipid receptor function. Availability of Gal(α1–4)Gal disaccharide for verotoxin binding in natural and synthetic glycolipids. Eur. J. Biochem. 223, 873–878 [DOI] [PubMed] [Google Scholar]
- 35. Arab S., Lingwood C. A. (1996) Influence of phospholipid chain length on verotoxin/globotriaosyl ceramide binding in model membranes. Comparison of a supported bilayer film and liposomes. Glycoconj. J. 13, 159–166 [DOI] [PubMed] [Google Scholar]
- 36. Lingwood C. A. (1996) Aglycone modulation of glycolipid receptor function. Glycoconj. J. 13, 495–503 [DOI] [PubMed] [Google Scholar]
- 37. Sandvig K., van Deurs B. (2002) Transport of protein toxins into cells. Pathways used by ricin, cholera toxin, and Shiga toxin. FEBS Lett. 529, 49–53 [DOI] [PubMed] [Google Scholar]
- 38. Lencer W. I., Tsai B. (2003) The intracellular voyage of cholera toxin. Going retro. Trends Biochem. Sci. 28, 639–645 [DOI] [PubMed] [Google Scholar]
- 39. Pagano R. E., Watanabe R., Wheatley C., Dominguez M. (2000) Applications of BODIPY-sphingolipid analogs to study lipid traffic and metabolism in cells. Methods Enzymol. 312, 523–534 [DOI] [PubMed] [Google Scholar]
- 40. Kitov P. I., Sadowska J. M., Mulvey G., Armstrong G. D., Ling H., Pannu N. S., Read R. J., Bundle D. R. (2000) Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 403, 669–672 [DOI] [PubMed] [Google Scholar]
- 41. Nishikawa K., Matsuoka K., Watanabe M., Igai K., Hino K., Hatano K., Yamada A., Abe N., Terunuma D., Kuzuhara H., Natori Y. (2005) Identification of the optimal structure required for a Shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J. Infect. Dis. 191, 2097–2105 [DOI] [PubMed] [Google Scholar]
- 42. Kitov P. I., Mulvey G. L., Griener T. P., Lipinski T., Solomon D., Paszkiewicz E., Jacobson J. M., Sadowska J. M., Suzuki M., Yamamura K., Armstrong G. D., Bundle D. R. (2008) In vivo supramolecular templating enhances the activity of multivalent ligands. A potential therapeutic against the Escherichia coli O157 AB5 toxins. Proc. Natl. Acad. Sci. U.S.A. 105, 16837–16842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. St Hilaire P. M., Boyd M. K., Toone E. J. (1994) Interaction of the Shiga-like toxin type 1 B-subunit with its carbohydrate receptor. Biochemistry 33, 14452–14463 [DOI] [PubMed] [Google Scholar]
- 44. Mylvaganam M., Lingwood C. (1999) Adamantyl globotriaosyl ceramide. A monovalent soluble mimic which inhibits verotoxin binding to its glycolipid receptor. Biochem. Biophys. Res. Commun. 257, 391–394 [DOI] [PubMed] [Google Scholar]
- 45. Mylvaganam M., Lingwood C. A. (2003) A preamble to aglycone reconstruction for membrane-presented glycolipids. in Carbohydrate-based Drug Discovery (Wong C.-H., ed) pp. 761–780, Wiley-VCH Press, Weinheim, Germany [Google Scholar]
- 46. Rutjes N. W., Binnington B. A., Smith C. R., Maloney M. D., Lingwood C. A. (2002) Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse animal model. Kidney Int. 62, 832–845 [DOI] [PubMed] [Google Scholar]
- 47. Petric M., Karmali M. A., Richardson S., Cheung R. (1987) Purification and biological properties of Escherichia coli verocytotoxin FEMS Microbiol. Lett. 41, 63–68 [Google Scholar]
- 48. Niu S. L., Litman B. J. (2002) Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system. Effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 83, 3408–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Halling K. K., Ramstedt B., Nyström J. H., Slotte J. P., Nyholm T. K. (2008) Cholesterol interactions with fluid-phase phospholipids. Effect on the lateral organization of the bilayer. Biophys. J. 95, 3861–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wolf A. A., Jobling M. G., Saslowsky D. E., Kern E., Drake K. R., Kenworthy A. K., Holmes R. K., Lencer W. I. (2008) Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster ganglioside GM1 molecules. Infect. Immun. 76, 1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khine A. A., Tam P., Nutikka A., Lingwood C. A. (2004) Brefeldin A and filipin distinguish two globotriaosyl ceramide/verotoxin-1 intracellular trafficking pathways involved in Vero cell cytotoxicity. Glycobiology 14, 701–712 [DOI] [PubMed] [Google Scholar]
- 52. Sandvig K., van Deurs B. (1994) Endocytosis and intracellular sorting of ricin and Shiga toxin. FEBS Lett. 346, 99–102 [DOI] [PubMed] [Google Scholar]
- 53. Veiga M. P., Arrondo J. L., Goñi F. M., Alonso A., Marsh D. (2001) Interaction of cholesterol with sphingomyelin in mixed membranes containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies. A possible stabilization of gel-phase sphingolipid domains by cholesterol. Biochemistry 40, 2614–2622 [DOI] [PubMed] [Google Scholar]
- 54. Majoul I., Schmidt T., Pomasanova M., Boutkevich E., Kozlov Y., Söling H. D. (2002) Differential expression of receptors for Shiga and cholera toxin is regulated by the cell cycle. J. Cell Sci. 115, 817–826 [DOI] [PubMed] [Google Scholar]
- 55. Rosales Fritz V. M., Daniotti J. L., Maccioni H. J. (1997) Chinese hamster ovary cells lacking GM1 and GD1a synthesize gangliosides upon transfection with human GM2 synthase. Biochim. Biophys. Acta 1354, 153–158 [DOI] [PubMed] [Google Scholar]
- 56. Lingwood C. A., Binnington B., Manis A., Branch D. R. (2010) Globotriaosyl ceramide receptor function. Where membrane structure and pathology intersect. FEBS Lett. 584, 1879–1886 [DOI] [PubMed] [Google Scholar]
- 57. Lingwood C. A., Manis A., Mahfoud R., Khan F., Binnington B., Mylvaganam M. (2010) New aspects of the regulation of glycosphingolipid receptor function. Chem. Phys. Lipids 163, 27–35 [DOI] [PubMed] [Google Scholar]
- 58. Sandvig K., Bergan J., Dyve A. B., Skotland T., Torgersen M. L. (2009) Endocytosis and retrograde transport of Shiga toxin. Toxicon 56, 1181–1185 [DOI] [PubMed] [Google Scholar]
- 59. Arab S., Rutka J., Lingwood C. (1999) Verotoxin induces apoptosis and the complete, rapid, long-term elimination of human astrocytoma xenografts in nude mice. Oncol. Res. 11, 33–39 [PubMed] [Google Scholar]
- 60. Salhia B., Rutka J. T., Lingwood C., Nutikka A., Van Furth W. R. (2002) The treatment of malignant meningioma with verotoxin. Neoplasia 4, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. LaCasse E. C., Bray M. R., Patterson B., Lim W. M., Perampalam S., Radvanyi L. G., Keating A., Stewart A. K., Buckstein R., Sandhu J. S., Miller N., Banderjee D., Singh D., Belch A. R., Pilarski L. M., Gariépy J. (1999) Shiga-like toxin I receptor on human breast cancer, lymphoma, and myeloma and absence from CD34+ hematopoietic stem cells: Implications for ex vivo tumor purging and autologous stem cell transplantation. Blood 94, 2901–2910 [PubMed] [Google Scholar]
- 62. Falguières T., Maak M., von Weyhern C., Sarr M., Sastre X., Poupon M. F., Robine S., Johannes L., Janssen K. P. (2008) Human colorectal tumors and metastases express Gb3 and can be targeted by an intestinal pathogen-based delivery tool. Mol. Cancer Ther. 7, 2498–2508 [DOI] [PubMed] [Google Scholar]
- 63. Amessou M., Carrez D., Patin D., Sarr M., Grierson D. S., Croisy A., Tedesco A. C., Maillard P., Johannes L. (2008) Retrograde delivery of photosensitizer (TPPp-O-β-GluOH)3 selectively potentiates its photodynamic activity. Bioconjug. Chem. 19, 532–538 [DOI] [PubMed] [Google Scholar]
- 64. D̸evenica D., Čikeš Čulić V., Vuica A., Markotić A. (2011) Biochemical, pathological and oncological relevance of Gb3Cer receptor. Med. Oncol. 28, S675–S684 [DOI] [PubMed] [Google Scholar]
- 65. Heath-Engel H. M., Lingwood C. A. (2003) Verotoxin sensitivity of ECV304 cells in vitro and in vivo in a xenograft tumor model. VT1 as a tumor neovascular marker. Angiogenesis 6, 129–141 [DOI] [PubMed] [Google Scholar]
- 66. Johansson D., Kosovac E., Moharer J., Ljuslinder I., Brännström T., Johansson A., Behnam-Motlagh P. (2009) Expression of verotoxin-1 receptor Gb3 in breast cancer tissue and verotoxin-1 signal transduction to apoptosis. BMC Cancer 9, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lund N., Olsson M. L., Ramkumar S., Sakac D., Yahalom V., Levene C., Hellberg A., Ma X. Z., Binnington B., Jung D., Lingwood C. A., Branch D. R. (2009) The human pk histo-blood group antigen provides protection against HIV-1 infection. Blood 113, 4980–4991 [DOI] [PubMed] [Google Scholar]
- 68. Sillence D. J., Puri V., Marks D. L., Butters T. D., Dwek R. A., Pagano R. E., Platt F. M. (2002) Glucosylceramide modulates membrane traffic along the endocytic pathway. J. Lipid Res. 43, 1837–1845 [DOI] [PubMed] [Google Scholar]
- 69. Patterson G. H., Hirschberg K., Polishchuk R. S., Gerlich D., Phair R. D., Lippincott-Schwartz J. (2008) Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell 133, 1055–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang H., Abraham N., Khan L. A., Hall D. H., Fleming J. T., Göbel V. (2011) Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat. Cell Biol. 13, 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lingwood C. A., Sadacharan S., Abul-Milh A., Mylvaganam M., Peter M. (2006) Soluble adamantyl glycosphingolipid analogs as probes of glycosphingolipid function. in Glycobiology Protocols (Braukhausen I., ed) pp. 305–320, Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 72. Kamani M., Mylvaganam M., Tian R., Rigat B., Binnington B., Lingwood C. (2011) Adamantyl glycosphingolipids provide a new approach to the selective regulation of cellular glycosphingolipid metabolism. J. Biol. Chem. 286, 21413–21426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKenzie J., Johannes L., Taguchi T., Sheff D. (2009) Passage through the Golgi is necessary for Shiga toxin B subunit to reach the endoplasmic reticulum. FEBS J. 276, 1581–1595 [DOI] [PubMed] [Google Scholar]
- 74. Lingwood D., Ries J., Schwille P., Simons K. (2008) Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc. Natl. Acad. Sci. U.S.A. 105, 10005–10010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schwarzmann G. (2001) Uptake and metabolism of exogenous glycosphingolipids by cultured cells. Semin. Cell Dev. Biol. 12, 163–171 [DOI] [PubMed] [Google Scholar]
- 76. Soltyk A. M., MacKenzie C. R., Wolski V. M., Hirama T., Kitov P. I., Bundle D. R., Brunton J. L. (2002) A mutational analysis of the globotriaosylceramide-binding sites of verotoxin VT1. J. Biol. Chem. 277, 5351–5359 [DOI] [PubMed] [Google Scholar]
- 77. Windschiegl B., Orth A., Römer W., Berland L., Stechmann B., Bassereau P., Johannes L., Steinem C. (2009) Lipid reorganization induced by Shiga toxin clustering on planar membranes. PLoS One 4, e6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schapiro F. B., Lingwood C., Furuya W., Grinstein S. (1998) pH-independent retrograde targeting of glycolipids to the Golgi complex. Am. J. Physiol. 274, C319–C332 [DOI] [PubMed] [Google Scholar]
- 79. Lauvrak S. U., Torgersen M. L., Sandvig K. (2004) Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J. Cell Sci. 117, 2321–2331 [DOI] [PubMed] [Google Scholar]
- 80. Saint-Pol A., Yélamos B., Amessou M., Mills I. G., Dugast M., Tenza D., Schu P., Antony C., McMahon H. T., Lamaze C., Johannes L. (2004) Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev. Cell 6, 525–538 [DOI] [PubMed] [Google Scholar]
- 81. Rodal S. K., Skretting G., Garred O., van Deurs B., Sandvig K. (1999) Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10, 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Le P. U., Nabi I. R. (2003) Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 116, 1059–1071 [DOI] [PubMed] [Google Scholar]
- 83. Badizadegan K., Wheeler H. E., Fujinaga Y., Lencer W. I. (2004) Trafficking of cholera toxin-ganglioside GM1 complex into Golgi and induction of toxicity depend on actin cytoskeleton. Am. J. Physiol. Cell Physiol. 287, C1453–C1462 [DOI] [PubMed] [Google Scholar]
- 84. Spilsberg B., Van Meer G., Sandvig K. (2003) Role of lipids in the retrograde pathway of ricin intoxication. Traffic 4, 544–552 [DOI] [PubMed] [Google Scholar]
- 85. Bacia K., Schwille P., Kurzchalia T. (2005) Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. U.S.A. 102, 3272–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Puri V., Watanabe R., Dominguez M., Sun X., Wheatley C. L., Marks D. L., Pagano R. E. (1999) Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nat. Cell Biol. 1, 386–388 [DOI] [PubMed] [Google Scholar]
- 87. Hall A., Róg T., Karttunen M., Vattulainen I. (2010) Role of glycolipids in lipid rafts. A view through atomistic molecular dynamics simulations with galactosylceramide. J. Phys. Chem. B 114, 7797–7807 [DOI] [PubMed] [Google Scholar]
- 88. Nyholm P. G., Pascher I. (1993) Orientation of the saccharide chains of glycolipids at the membrane surface. Conformational analysis of the glucose-ceramide and the glucose-glyceride linkages using molecular mechanics (MM3). Biochemistry 32, 1225–1234 [DOI] [PubMed] [Google Scholar]
- 89. Calander N., Karlsson K. A., Nyholm P. G., Pascher I. (1988) On the dissection of binding epitopes on carbohydrate receptors for microbes using molecular modeling. Biochimie 70, 1673–1682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.