Abstract
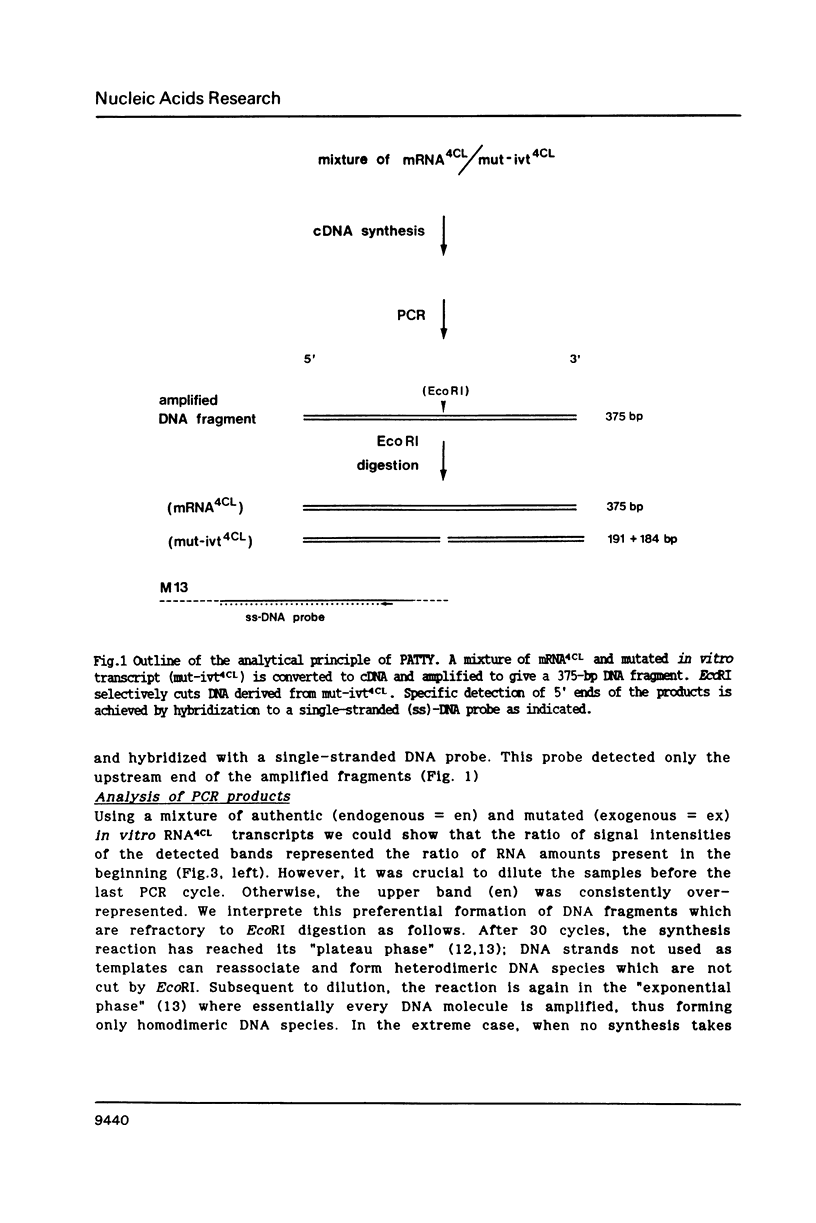
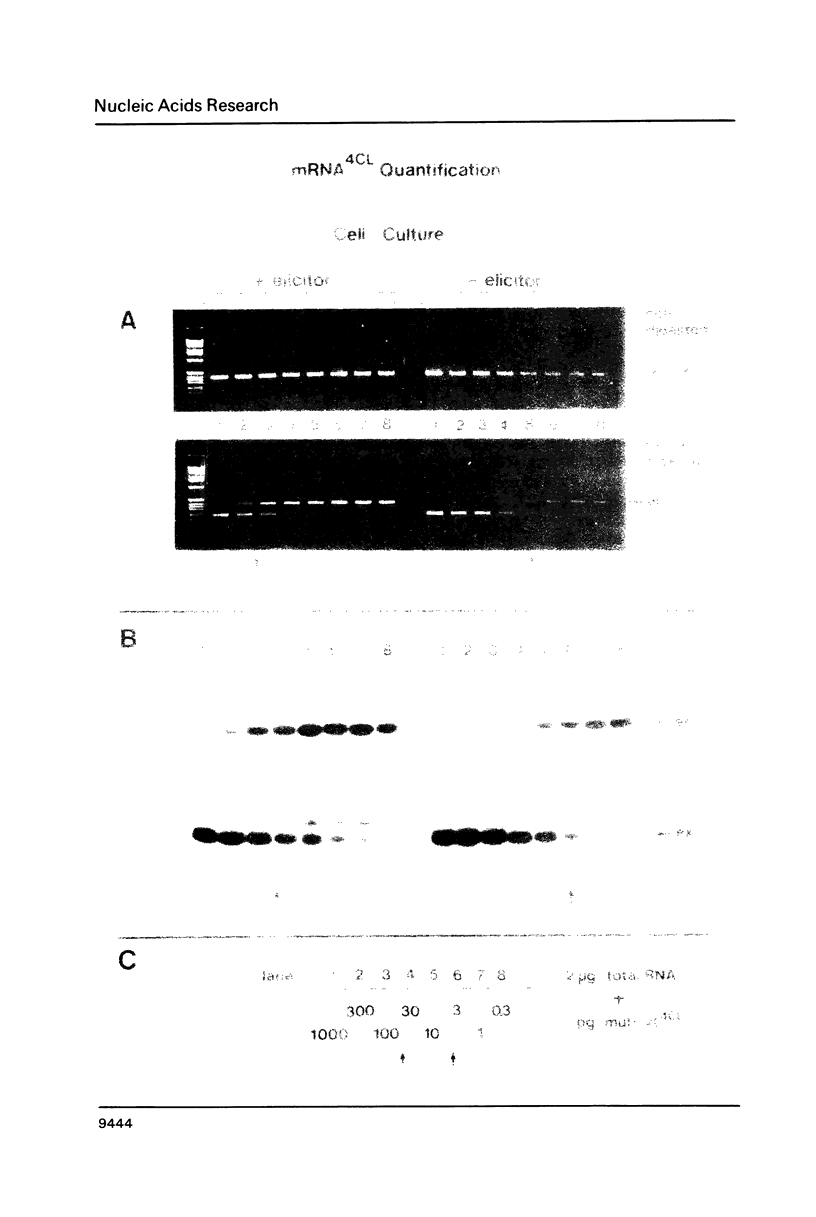
The polymerase chain reaction (PCR) is used as part of a new approach to the absolute quantification of mRNA. We describe a PCR aided transcript titration assay (PATTY) which is based on the co-amplification of an in vitro generated transcript differing by a single base exchange from the target mRNA. Identical portions of a total RNA sample are "spiked" with different amounts of this mutated standard RNA, converted to cDNA and amplified by PCR. Because the base exchange creates a novel restriction endonuclease site, the ratio of co-amplified DNA derived from target mRNA to amplified DNA derived from standard RNA can be determined after restriction endonuclease digestion and separation by gel electrophoresis. This method gives accurate results within 24 hours and is useful especially for the quantification of either low-abundance mRNA or more abundant mRNA present in very small amounts of total RNA. The low-abundance mRNA encoding 4-coumarate:CoA ligase (4CL) in cultured potato cells (Solanum tuberosum L.) was measured in a case study. About 100 molecules per assay could be accurately detected by the new method.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burbach J. P., Van Tol H. H., Bakkus M. H., Schmale H., Ivell R. Quantitation of vasopressin mRNA and oxytocin mRNA in hypothalamic nuclei by solution hybridization assays. J Neurochem. 1986 Dec;47(6):1814–1821. doi: 10.1111/j.1471-4159.1986.tb13093.x. [DOI] [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzemeier K. H., Cretin C., Kombrink E., Rohwer F., Taylor J., Scheel D., Hahlbrock K. Transient Induction of Phenylalanine Ammonia-Lyase and 4-Coumarate: CoA Ligase mRNAs in Potato Leaves Infected with Virulent or Avirulent Races of Phytophthora infestans. Plant Physiol. 1987 Sep;85(1):34–41. doi: 10.1104/pp.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saluz H., Jost J. P. Optimized genomic sequencing as a tool for the study of cytosine methylation in the regulatory region of the chicken vitellogenin II gene. Gene. 1986;42(2):151–157. doi: 10.1016/0378-1119(86)90291-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]