Abstract
Background
The purpose of our study was to determine the rate of carpal tunnel decompression (CTD) following local corticosteroid injection for carpal tunnel syndrome (CTS), as well as identifying predictors of requiring further intervention and eventual decompression.
Methods
All patients diagnosed with CTS in our unit over a 6-year period were prospectively assessed. Patients were diagnosed using a combination of clinical presentation and nerve conduction studies. Patients were managed with open carpal tunnel decompression or corticosteroid injection. There were 1,564 consecutive patients diagnosed with CTS over the study period, of whom 824 (53%) underwent a corticosteroid injection as their primary treatment. We performed a survivorship analysis of these patients and used Kaplan–Meier survivorship methodology to determine the 5-year rate of re-intervention. Risk factors for re-intervention were also determined.
Results
The overall 5-year Kaplan–Meier rate of secondary CTD was 15% at 1 year and 33% at 5 years. The need for secondary CTD was independently associated with female gender, diabetes mellitus and positive nerve conduction studies at diagnosis.
Conclusions
Steroid injection is an appropriate treatment in carefully selected patients. Those who are female, diabetic and have neurophysiological confirmation of diagnosis have the highest risk of relapse. These results may be used to guide initial treatment and counsel patients about the risk relapse.
Keywords: Carpal tunnel syndrome, Corticosteroid injection, Management
Introduction
Carpal tunnel syndrome (CTS) commonly presents as a chronic peripheral neuropathy affecting the median nerve as it traverses the wrist underneath the transverse carpal ligament [2, 21, 22]. Non-operative management may be employed in mild to moderate cases and includes wrist splints and local corticosteroid injection [2, 20]. Effective short-term symptomatic relief has been shown with local corticosteroid injection, although some literature questions the long-term efficacy of local corticosteroid injection [1, 7–9, 11, 18, 19]. The median nerve may also be surgically decompressed (carpal tunnel decompression, CTD), through division of the transverse carpal ligament, which can be performed using either open or endoscopic techniques, with effective comparable results [4, 6, 14, 23–25, 28]. Recent prospective randomised trials comparing corticosteroid injection with surgery have found contradictory results: Hui et al. demonstrated improved neurophysiological and symptomatic improvement at 20 weeks in the open decompression group, while Ly-Pen showed superior early results in the injection group, but similar outcomes at 1 year [13, 17]. There is limited data regarding long-term re-intervention rates following treatment with local corticosteroid injection [16]. These contradictory findings lead to difficulty when counselling patients about the advantages and disadvantages of different treatments for CTS. The aim of this study was to investigate the need for, and factors influencing, secondary CTD following corticosteroid injection for mild to moderate CTS using survivorship methodology.
Materials and Methods
All patients diagnosed with carpal tunnel syndrome in our unit from November 2004 to May 2010 were prospectively entered into an audit database. The setting was a regional hand service that was the sole provider of hand services to an entire health authority region of 363,365 patients. The diagnosis and severity of carpal tunnel syndrome was established through a combination of history, the Kamath questionnaire [5, 15], examination (muscle wasting, grip strength, sensation, Phalen’s test and Tinel sign positive) and neurophysiological testing. The Kamath questionnaire has demonstrated a positive predictive value of 90% in the diagnosis of CTS, using post-operative symptom relief as the gold standard comparison [15]. The original validation has recently been confirmed by a second study [5] that described a score of >6 as determining a clinical diagnosis of CTS, while <3 refuted it. It was reported that patients with scores of 3–6 should undergo nerve conduction studies (NCS). A disabilities of the arm, shoulder and hand (DASH) score was completed at this assessment [12]. NCS were performed by a specialist technician using a Dantec Keypoint portable nerve conduction machine (Dantec Dynamics, Bristol, UK), and reported as positive or negative by one consultant neurophysiologist. Neurophysiological testing was omitted if a patient was judged to have clinically severe disease with (1) thenar muscle atrophy, (2) abductor pollicus brevis (APB), weakness (3), worsening pain and dysaesthesia and (4) Tinel sign and Phalen test positive, along with an indication for urgent decompression. If the NCS was negative, a diagnosis of CTS was made if there was a consistent history of sensory disturbance in the median nerve distribution with the presence of the Tinel sign or a positive Phalen test. In this case, the NCS were determined to have a returned a false-negative result [3, 29]. NCS were performed in 774 (93%) patients. There were 50 patients who did not undergo NCS and this was on the basis of strong patient preference. Patients were counselled about the risks and benefits of open carpal tunnel decompression versus corticosteroid injection and were permitted to select their treatment. Those who suffered disease recurrence after an injection were offered the choice between open decompression (CTD) or a repeat injection.
There were 1,564 patients diagnosed with CTS who had not had a previous injection or surgical treatment for carpal tunnel syndrome. They may however have received treatment from their primary care physician in the form of splint provision. All patients received a wrist splint while awaiting NCS and definitive management. After the initial consultation, 65 patients had resolution of symptoms with treatment with splintage alone and did not require further intervention. There were 675 who underwent primary open carpal tunnel decompression because of strong personal preference, or the opinion of the senior author regarding disease severity. Therefore, 824 (53%) patients received a primary corticosteroid injection. This group form the study group investigated in this paper.
The average waiting time for injection at our institution is 9 weeks. The injection was carried out as an outpatient procedure by the senior author or a staff-grade orthopaedic surgeon who used the same technique. Aseptic technique was observed. Two millilitres of local anaesthetic were instilled into the skin at the proximal wrist flexor crease through a 25-gauge 25-mm needle, just ulnar to the palmaris longus tendon. The retinaculum was not anaesthetised. Once this had taken effect, a second needle and syringe containing the steroid was inserted to the level of the retinaculum. No local anaesthetic was used in this injection as this could mask detection of inadvertent placement of the needle in the nerve. The needle was aimed distally at the same level through the retinaculum, at approximately 60° to the skin, aiming towards the ulnar border of the ring finger. If the patient experienced any excessive pain, paraesthesia or dysaesthesia, the needle was repositioned in a more ulnar direction. The steroid (20 mg methylprednisolone [depo-medrone], 0.5 ml) was then injected. Patients were given no specific post-injection regime, but continued with a wrist splint for use at night.
The mean age of the 824 patients was 50.2 years (range 19–92, SD 12.8). The mean body mass index was 29.7 kg/m2 (range 17–58, SD 5.9). There were 752 (91.3%) who were right-hand dominant and in 715 patients the dominant hand was affected (86.8%). There were 318 (38.6%) who had occupational exposure to vibratory tools. The Phalen test was positive in 702 (85.2%) patients and Tinel sign positive in 238 (28.9%).
Statistical Methods
Kaplan–Meier survivorship methodology was used to determine the cumulative hazard of CTD as a re-intervention. Patients were censored at the analysis date of May 2010 if they had not reached the endpoint in question. The at-risk population was determined on an annual basis by subtracting the number of patients reaching the end point and being censored from the total study population. This methodology predicts overall survivorship and endpoint hazard in an observational study where patients’ results are analysed at a variable period of follow-up. The log-rank test was used to compare survival curves. Chi-squared tests were used to compare differences in proportions of categorical variables (gender, bilaterality, vibration exposure, comoborbidities, nerve conduction studies positive/negative) between patients with and without disease recurrence. Student’s t tests were used to compare age between groups. Variables that had a p value ≤0.1 on bivariate testing were evaluated in a multivariable manner using the Cox proportional hazards test. This more lax significance level was selected as multivariable adjustment may render a variable more significant, once confounding effects are accounted for. A forward stepwise model was used to select the final model. Overall results were reported as an adjusted odds ratio with 95% confidence intervals for the presence of each variable.
Results
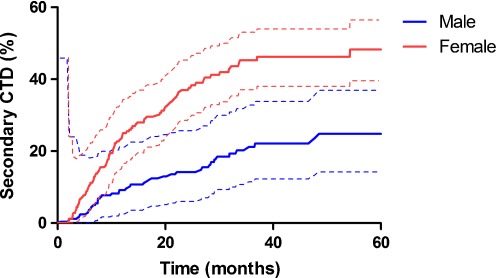
During the study period, there were 500 (61%) patients with a relapse of symptoms. Of these, 372 (45%) elected to receive a second injection. This successfully controlled symptoms in 308 patients. The remaining 64 underwent CTD. A further 128 did not wish a second injection and underwent CTD for their first relapse. Therefore, 192 (23%) out of 824 patients required a secondary CTD for relapse of symptoms (Table 1). Using survivorship methodology, the overall Kaplan–Meier rate of secondary CTD was 14.5% (95% CI 11.9 to 17) at 1 year and 33.2% (95% CI 28.7 to 37.8) at 5 years (Fig. 1). There was a significant difference in CTD rates between genders, with 9.1% (95% CI 6 to 12.2) of males progressing to CTD at 1 year compared with 18.7% (95% CI 15.0 to 22.5) of females (OR 2.08, 95% CI 1.34 to 3.27, p < 0.001). At 5 years 25.4% (95% CI 18.9 to 32.0) of males had undergone secondary CTD compared with 39.6% (95% CI 33.3 to 45.8) (OR 1.98 95% CI 1.41 to 2.80, p < 0.001).
Table 1.
Details of the 824 patients who received a primary corticosteroid injection
| Factor | No secondary CTD (n = 632) | Secondary CTD (n = 192) | P value |
|---|---|---|---|
| Age (years, mean, SD) | 50.1 (12.5) | 50.6 (13.7) | 0.646 |
| Gender (n, % of column total) | |||
| Male | 304 (48.1%) | 61 (31.8%) | <0.001 |
| Female | 328 (51.9%) | 131 (68.2%) | |
| Bilateral disease (n, %) | 438 (69.3%) | 144 (75%) | 0.148 |
| Hand dominance (n, %) | |||
| Left | 55 (8.7%) | 13 (6.8%) | 0.434 |
| Right | 574 (90.8%) | 179 (93.2%) | |
| Ambidextrous | 3 (0%) | 0 (0%) | |
| Dominant hand affected (n, %) | 541 (85.6%) | 175 (91.1%) | 0.051 |
| Exposure to vibration (n, %) | 253 (40%) | 65 (33.9%) | 0.128 |
| Claim (n, %) | 39 (6.2%) | 11 (5.7%) | 0.822 |
| Current smoker (n, %) | 164 (25.9%) | 59 (30.7%) | 0.195 |
| Comorbidity (n, %) | |||
| Menopause | 17 (2.7%) | 13 (6.8%) | 0.014 |
| IHD | 23 (3.6%) | 5 (2.6%) | 0.488 |
| Hypertension | 34 (5.4$) | 17 (8.9%) | 0.187 |
| Diabetes mellitus | 58 (9.2%) | 29 (33.3%) | 0.019 |
| Thyroid disease | 49 (7.8%) | 16 (8.3%) | 0.794 |
| Pregnancy | 3 (0.5%) | 2 (1%) | 0.331 |
| Osteoarthritis | 85 (13.4%) | 33 (17.2%) | 0.195 |
| Rheumatoid arthritis | 18 (2.8%) | 3 (1.6%) | 0.437 |
| Fracture | 6 (0.9%) | 1 (0.5%) | 1.000 |
| Examination findings (n, %) | |||
| Phalen’s sign positive | 534 (84.5%) | 169 (88%) | 0.227 |
| Tinel sign positive | 192 (30.4%) | 47 (24.5%) | 0.115 |
| Grip strength (kg, mean, SD) | 22.4 (14.4) | 19.4 (12.8) | 0.009 |
| DASH score (mean, SD) | 47.0 (22.8) | 51.4 (20.4) | 0.017 |
| Kamath score (mean, SD) | 6.85 (92.2) | 7.0 (2.1) | 0.393 |
| Nerve conduction studies (n, %) | |||
| Not performed | 39 (6.2%) | 11 (5.7%) | <0.001 |
| Positive | 524 (82.9%) | 178 (92.7%) | |
| Negative | 69 (10.9%) | 3 (1.6%) | |
Fig. 1.
Kaplan–Meier cumulative hazard proportion (±95% confidence interval) of secondary CTD after injection for CTS by gender (log-rank p < 0.001)
Overall females with positive nerve conduction studies had a higher rate of secondary CTD compared with males (OR 3.89, 95% CI 2.69 to 5.68, p < 0.001). Grip strength was poor (p = 0.009) in those subsequently needing CTD and the initial DASH score was worse (p = 0.017). On bivariate testing, diabetic status just failed to reach statistical significance (Table 1). A multivariable Cox regression model showed that secondary CTD was independently associated with female gender (OR 2.06, 95% CI 1.50 to 2.83, p < 0.001), diabetes mellitus (OR 1.58, 95% CI 1.05 to 2.39, p = 0.029) and the presence of positive nerve conduction results (OR 7.62, 95% CI 2.43 to 23.9, p = 0.001). After multivariable adjustment, the DASH score and grip strength were not significantly different between groups.
Discussion
Our study has shown that following steroid injection, 33% of patients at risk at 5 years required secondary CTD. The independent factors associated with requirement for secondary CTD were female gender, diabetes and positive nerve conduction studies. The finding on bivariate tests (Table 1) that DASH score was worse and grip strength poorer in patients requiring secondary CTD was not significant after multivariable testing. One explanation for this finding could be the confounding factor of gender. When adjusted for gender, the effects of grip strength and DASH score were not significant. Patients with evidence of severe disease (thenar muscle atrophy and APB weakness) were excluded from this study. These results will allow clinicians to engage in an informed discussion with patients with mild to moderate disease when discussing treatment options. Patients falling into groups with higher risk of relapse may benefit from earlier surgical management, while patients with a lower risk of recurrence may avoid the risks of surgery by opting for corticosteroid injection.
Although the literature supports the short-term symptomatic relief of carpal tunnel syndrome through the use of local corticosteroid injection, its long-term efficacy is questioned [1, 8, 9, 11, 18, 19]. A recent Cochrane review concluded that local corticosteroid injections provided greater clinical symptomatic relief at 1 month compared to placebo, but no significant relief was noted beyond that [18]. This study examined 12 studies including 671 patients and concluded that further research was required to determine the length of benefit for mild and moderate CTS. Our study examines a larger series of patients who have undergone pragmatic treatment with a steroid injection. Other studies have found relief for up to 3 months and even 1 year following injection [7, 17]. We have found that almost 63% of our patients gained effective long-term treatment from primary local corticosteroid injection, and avoided the potential complications of surgery. However, corticosteroid injection can lead to iatrogenic median nerve injury [2] although no cases occurred during our series. The discrepancies in the literature are possibly related to the methods for determining patient outcome, with one of the common criticisms in the literature related to the discrepancy in agreed outcome criteria [2]. Our primary outcome was the need for CTD determined by the recurrence of symptoms, with subjective symptom severity having been shown to be a key in determining a patient’s willingness to undergo CTD [10]. Marshall et al. could find no evidence in their systematic review to support a second local corticosteroid injection [18]. Our study may be at variance with these previous studies through patient selection. Patients with severe disease were excluded from this study as they underwent primary CTD. This may suggest that injection is more suitable for and successful in less severe disease. Such sub-group effects may not be apparent in systematic reviews.
A recent prospective randomised trial advocated the use of primary CTD over corticosteroid injection, with superior results seen in both symptomatology and nerve conduction studies, but not grip strength [13]. Good or excellent outcome following open CTD is seen in 70–90% of patients, with similar excellent long-term results and low re-intervention rates recently shown in elderly patients [4, 7, 16, 26, 28]. However, there is limited evidence to support the use of CTD in patients with mild symptoms and it is associated with a greater risk of complications when compared to non-operative management [27]. A prospective randomised trial in 163 patients with carpal tunnel syndrome examined the effects of surgical decompression versus local corticosteroid injection and concluded that local corticosteroid injection was superior to CTD for early symptomatic relief, but showed comparable results to surgery at 1 year [17].
The main strengths of our observational study are the inclusion of a large number of patients, the use of prospective audit data and the use of survivorship methodology, which has allowed prediction of the longer term need for secondary CTD and identification of a group of patients who may benefit from steroid injection and avoid surgery.
The primary limitation to our study is that patients could select their own treatment leading to potential selection bias. Patients undergoing steroid injections were provided with splintage after their initial visit to clinic, and prior to injection. Patients who failed to respond to splint treatment underwent injection (n = 65). This low rate of response to splintage may reflect treatment prior to referral, as many patients may have already been managed with a splint, by their primary care physician. Such patients will never have been considered by this study. They were advised to continue splintage after the injection and this may have contributed to the treatment effect. This potential selection bias may also result in “lead-time” bias. Patients in the primary injection group may be more likely to be at earlier stage of disease and may relapse at a later stage, beyond the period of this study. We consider this unlikely as the Kaplan–Meier graphs (Fig. 1) demonstrate the rate of CTD to have levelled off. It is therefore important for this study group to be followed up in the future to determine if there is evidence of later relapse. This study was also deliberately not stratified by neurophysiological parameters for several reasons. Patients with signs of severe disease were excluded after clinical examination to allow early CTD. Neurophysiological testing may result in false-negative results in cases of true disease. We follow a pragmatic approach in these cases and offer treatment if patients fulfil our clinical diagnostic criteria. This demonstrates inherent difficulties in constructing studies of the outcome of treatment of CTS. Patients can be included and excluded on the basis of clinical history and examination findings. In isolation, these have poor positive and negative predictive values. Questionnaires can be used to codify the history element of this procedure. The gold standard for epidemiological studies has been electrophysiological testing, but as discussed, even this is not fully sensitive. The cutoff values for abnormal tests vary between studies, countries, testing equipment and depend on the clinical balance between an optimal false-positive and negative rate [4, 5]. We have used a pragmatic approach to patient inclusion. We have used a combination of history and examination findings, along with NCS to study a group of patients which is representative of the whole range of patients that are diagnosed with CTS in clinical practice. The description of nerve conduction testing as overall negative or positive allows application of these results to a wider group of patients, in clinical circumstances with different neurophysiological protocols and access to testing.
The findings of the present study indicate that two thirds of patients having primary corticosteroid injection for carpal tunnel syndrome have not required CTD within 5 years. These findings, along with the risk factors identified, can help inform patients of their likely outcome following primary corticosteroid injection. This information can aid in patient-oriented consultation and improve informed decision making with regards to primary treatment choice, with early CTD offered to higher risk patients (particularly diabetics and females with positive NCS). Future investigations should include sufficiently powered, severity stratified, long-term randomised controlled trials to determine the efficacy of primary corticosteroid injection and primary decompression for patients with CTS.
Acknowledgments
Disclosure
There are no conflicts of interest, commercial associations, or intent of financial gain regarding this research for any of the authors.
Contributor Information
Paul J. Jenkins, Phone: +44-1383-623623, FAX: +44-131-2423407, Email: paul@jenkinsnet.org.uk
Andrew D. Duckworth, Email: andrew.duckworth@yahoo.co.uk
References
- 1.Agarwal V, Singh R, Sachdev A, et al. A prospective study of the long-term efficacy of local methyl prednisolone acetate injection in the management of mild carpal tunnel syndrome. Rheumatology (Oxford) 2005;44:647–650. doi: 10.1093/rheumatology/keh571. [DOI] [PubMed] [Google Scholar]
- 2.Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J. 2008;77:6–17. [PMC free article] [PubMed] [Google Scholar]
- 3.Atroshi I, Gummesson C, Johnsson R, et al. Diagnostic properties of nerve conduction tests in population-based carpal tunnel syndrome. BMC Musculoskelet Disord. 2003;4:9. doi: 10.1186/1471-2474-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badger SA, O’Donnell ME, Sherigar JM, et al. Open carpal tunnel release—still a safe and effective operation. Ulster Med J. 2008;77:22–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Bridges MJ, Robertson DC, Chuck AJ. Predicting the result of nerve conduction tests in carpal tunnel syndrome using a questionnaire. Hand Surg. 2011;16:39–42. doi: 10.1142/S0218810411005047. [DOI] [PubMed] [Google Scholar]
- 6.Brown RA, Gelberman RH, Seiler JG, III, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75:1265–1275. doi: 10.2106/00004623-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int. 2002;22:33–37. doi: 10.1007/s00296-002-0184-0. [DOI] [PubMed] [Google Scholar]
- 8.Gelberman RH, Aronson D, Weisman MH. Carpal-tunnel syndrome. Results of a prospective trial of steroid injection and splinting. J Bone Joint Surg Am. 1980;62:1181–1184. [PubMed] [Google Scholar]
- 9.Giannini F, Passero S, Cioni R, et al. Electrophysiologic evaluation of local steroid injection in carpal tunnel syndrome. Arch Phys Med Rehabil. 1991;72:738–742. [PubMed] [Google Scholar]
- 10.Gong HS, Baek GH, Oh JH, et al. Factors affecting willingness to undergo carpal tunnel release. J Bone Joint Surg Am. 2009;91:2130–2136. doi: 10.2106/JBJS.H.01221. [DOI] [PubMed] [Google Scholar]
- 11.Green DP. Diagnostic and therapeutic value of carpal tunnel injection. J Hand Surg Am. 1984;9:850–854. doi: 10.1016/s0363-5023(84)80065-9. [DOI] [PubMed] [Google Scholar]
- 12.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11. doi: 10.1186/1471-2474-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui AC, Wong S, Leung CH, et al. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology. 2005;64:2074–2078. doi: 10.1212/01.WNL.0000169017.79374.93. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez DF, Gibbs SR, Clapper AT. Endoscopic treatment of carpal tunnel syndrome: a critical review. J Neurosurg. 1998;88:817–826. doi: 10.3171/jns.1998.88.5.0817. [DOI] [PubMed] [Google Scholar]
- 15.Kamath V, Stothard J. A clinical questionnaire for the diagnosis of carpal tunnel syndrome. J Hand Surg Br. 2003;28:455–459. doi: 10.1016/S0266-7681(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 16.Katz JN, Losina E, Amick BC, III, et al. Predictors of outcomes of carpal tunnel release. Arthritis Rheum. 2001;44:1184–1193. doi: 10.1002/1529-0131(200105)44:5<1184::AID-ANR202>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Ly-Pen D, Andreu JL, de Blas G, et al. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled clinical trial. Arthritis Rheum. 2005;52:612–619. doi: 10.1002/art.20767. [DOI] [PubMed] [Google Scholar]
- 18.Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;CD001554. [DOI] [PubMed]
- 19.McGrath MH. Local steroid therapy in the hand. J Hand Surg Am. 1984;9:915–921. doi: 10.1016/s0363-5023(84)80080-5. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;CD003219. [DOI] [PMC free article] [PubMed]
- 21.Phalen GS. The carpal tunnel syndrome. Instr Course Lect. 1957;14:142–148. [PubMed] [Google Scholar]
- 22.Phalen GS. The carpal-tunnel syndrome. Seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg Am. 1966;48:211–228. [PubMed] [Google Scholar]
- 23.Scholten RJ, Mink vdM, Uitdehaag BM, et al. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;CD003905. [DOI] [PMC free article] [PubMed]
- 24.Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114:1137–1146. doi: 10.1097/01.PRS.0000135850.37523.D0. [DOI] [PubMed] [Google Scholar]
- 25.Thoma A, Wong VH, Sprague S, et al. A cost-utility analysis of open and endoscopic carpal tunnel release. Can J Plast Surg. 2006;14:15–20. doi: 10.1177/229255030601400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner A, Kimble F, Gulyas K, et al. Can the outcome of open carpal tunnel release be predicted?: a review of the literature. ANZ J Surg. 2010;80:50–54. doi: 10.1111/j.1445-2197.2009.05175.x. [DOI] [PubMed] [Google Scholar]
- 27.Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;CD001552. [DOI] [PMC free article] [PubMed]
- 28.Weber RA, DeSalvo DJ, Rude MJ. Five-year follow-up of carpal tunnel release in patients over age 65. J Hand Surg Am. 2010;35:207–211. doi: 10.1016/j.jhsa.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Werner RA, Andary M. Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol. 2002;113:1373–1381. doi: 10.1016/S1388-2457(02)00169-4. [DOI] [PubMed] [Google Scholar]